Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach
Abstract
1. Introduction
2. Results
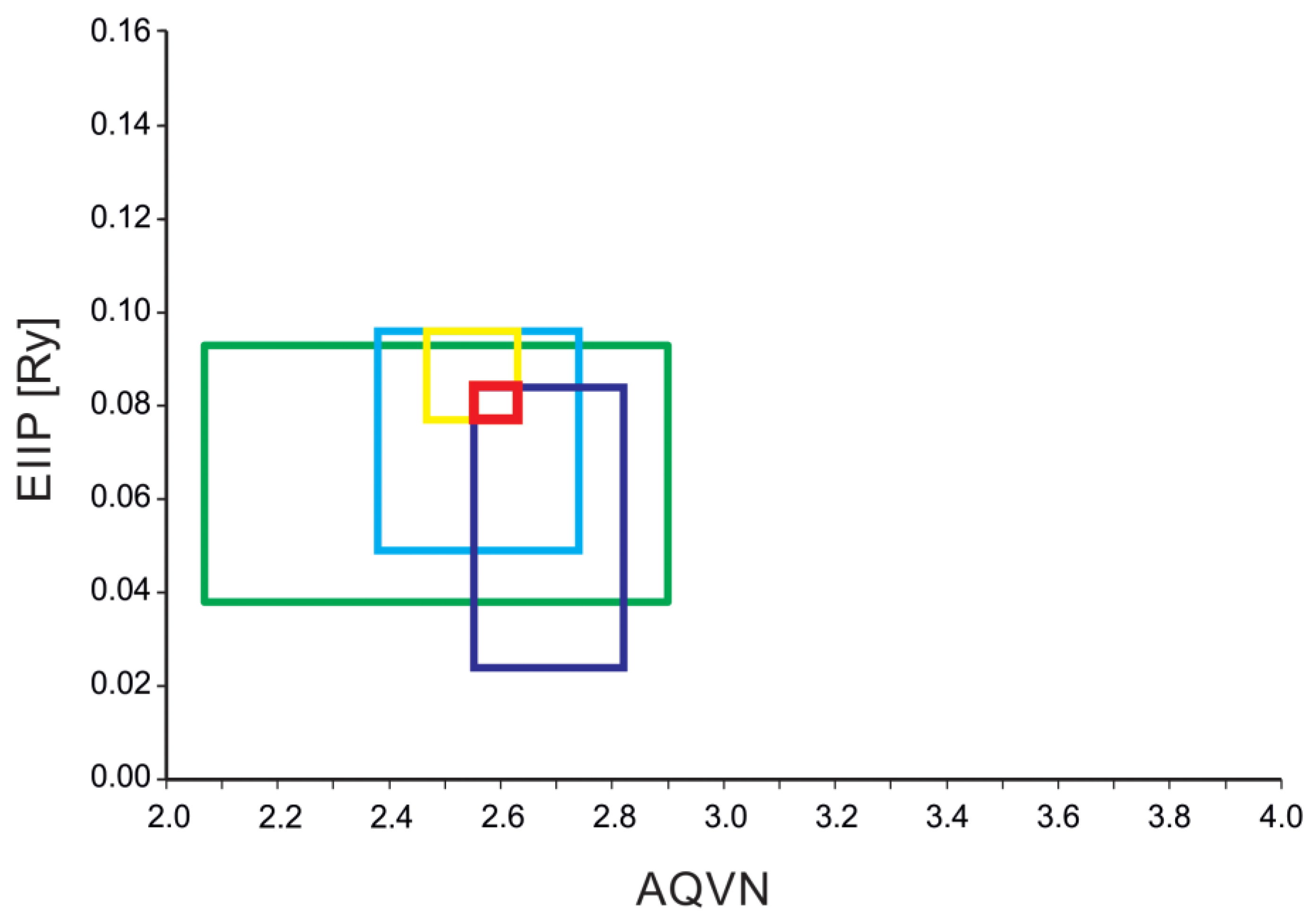
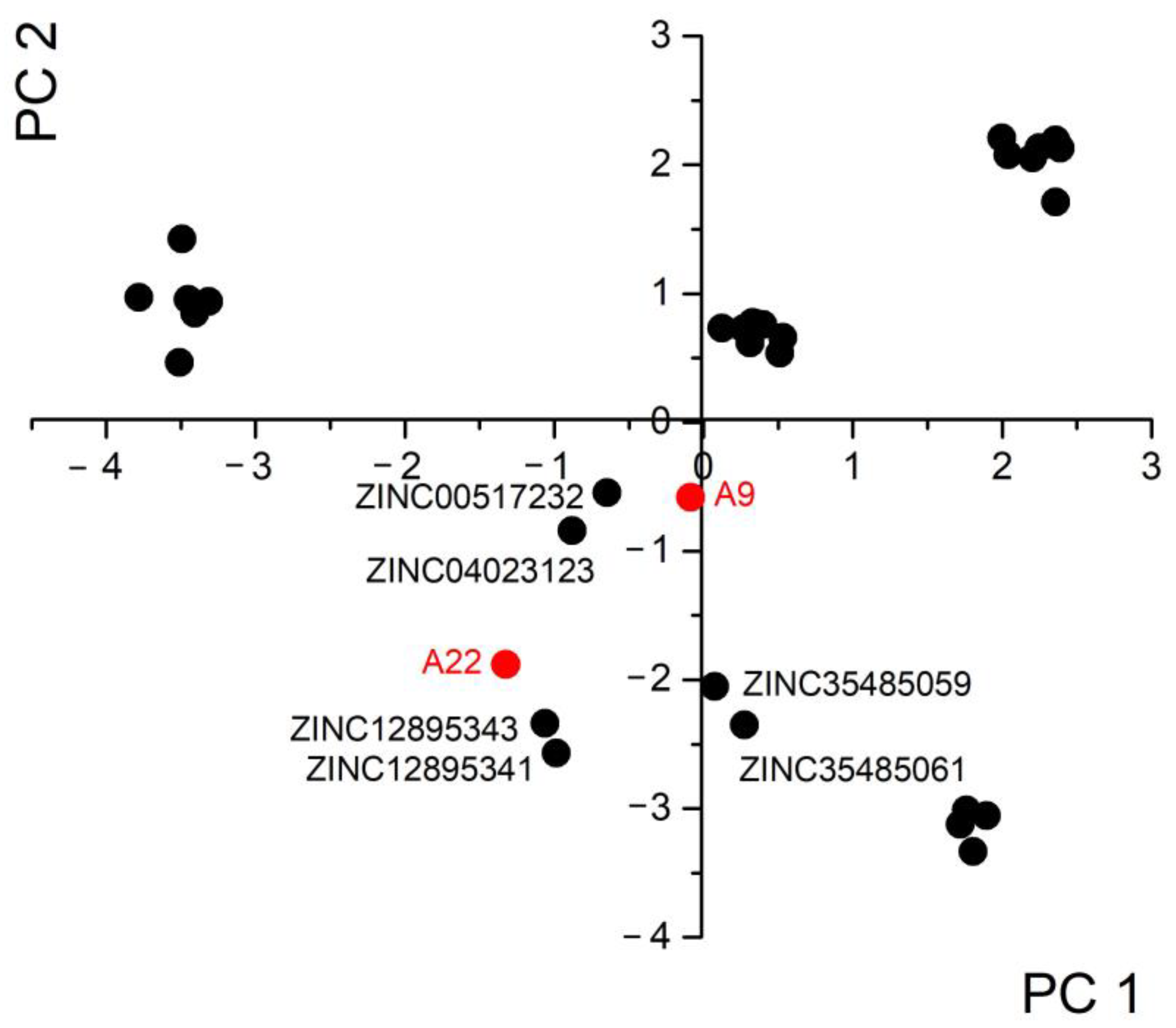
2.1. The Principal Component Analysis (PCA) Model
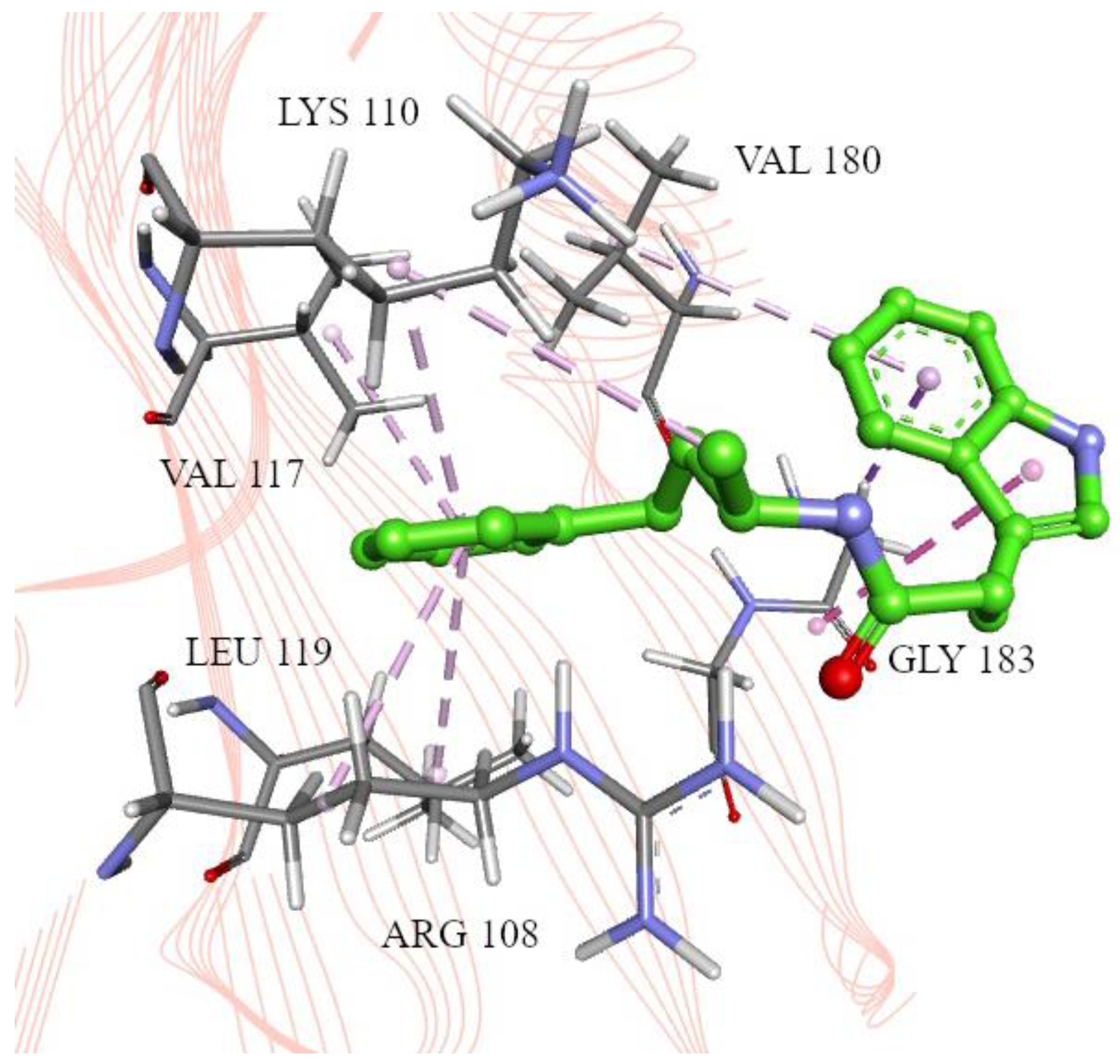
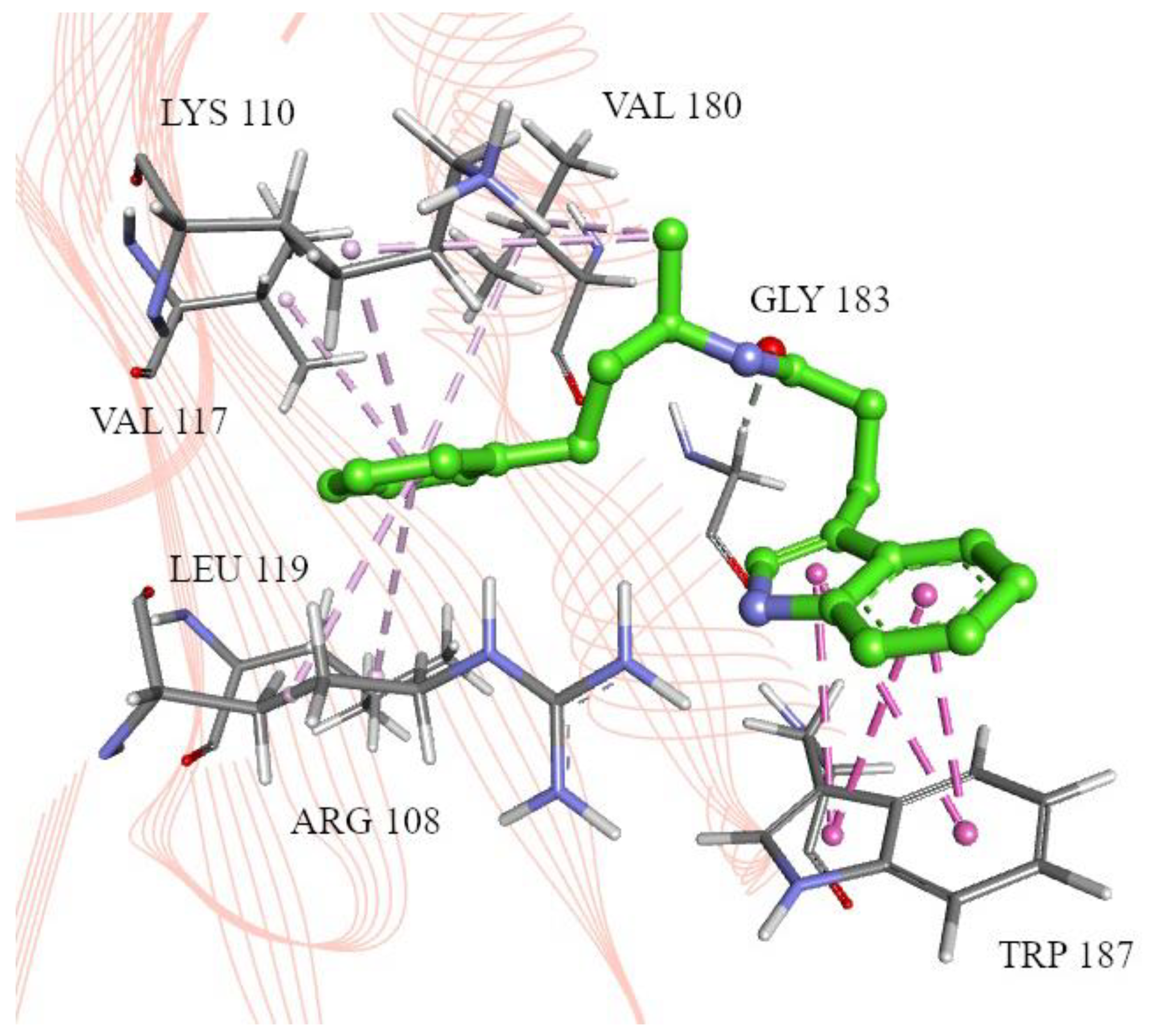
2.2. Molecular Docking
2.3. The Absorption, Distribution, Metabolism, Elimination, and Toxicity (ADMET) Prediction
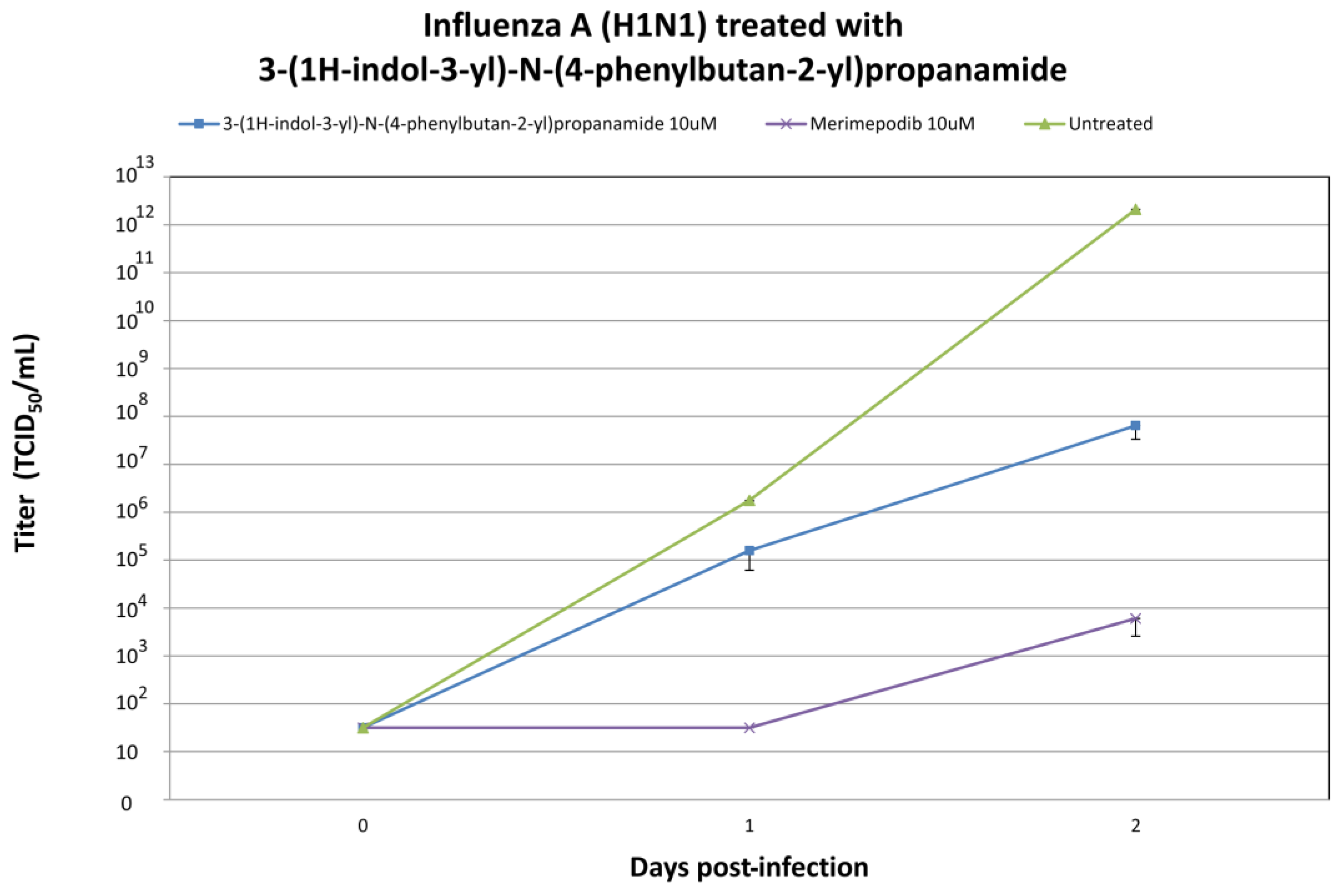
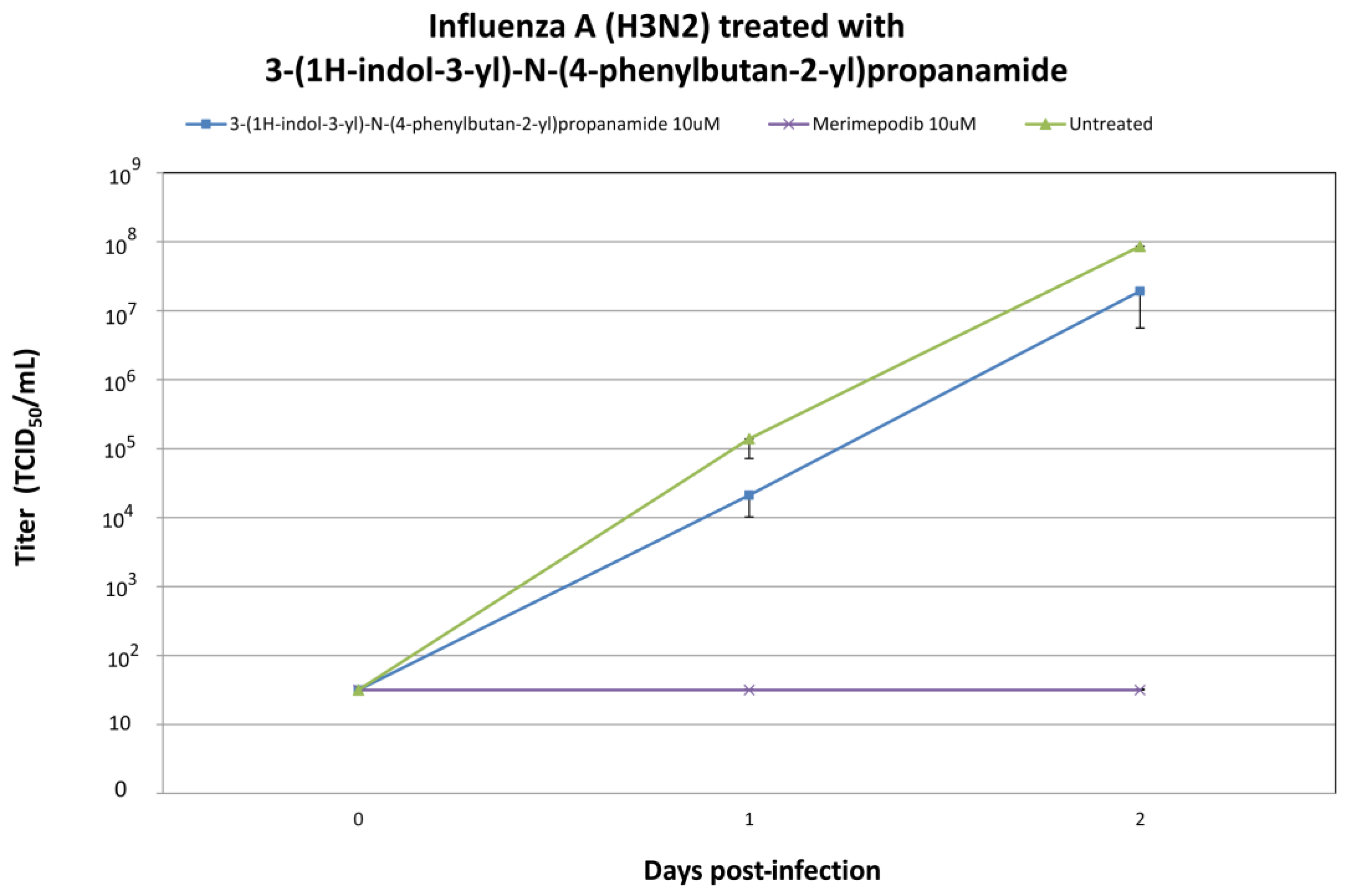
2.4. In Vitro Efficacy Testing of 3-(1H-Indol-3-yl)-N-(4-phenylbutan-2-yl)propanamide against H1N1 and H3N2 Influenza A Viruses
3. Discussion
4. Materials and Methods
4.1. Data Preparation
4.2. EIIP/AQVN Filter
4.3. PCA Model
4.4. Molecular Docking
4.5. ADMET Prediction
4.6. In Vitro Efficacy Testing against H1N1 and H3N2 Influenza A Viruses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Influenza. Factsheet; March. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 21 January 2024).
- Bridges, C.; Peasah, S.; Meltzer, M. The control of influenza and cost effectiveness of interventions. In Influenza Textbook; Webster, R.G., Monto, A.S., Braciale, T.J., Lamb, R.A., Eds.; Wiley-Blackwell: Hoboken, NY, USA, 2013; pp. 419–433. [Google Scholar]
- Heo, Y.A. Baloxavir: First global approval. Drugs 2018, 78, 693–697. [Google Scholar] [CrossRef]
- Ison, M.G. Antiviral treatments. Clin. Chest Med. 2017, 38, 139–153. [Google Scholar] [CrossRef]
- Hurt, A.C.; Besselaar, T.G.; Daniels, R.S.; Ermetal, B.; Fry, A.; Gubareva, L.; Huang, W.; Lackenby, A.; Lee, R.T.; Lo, J.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2014–2015. Antivir. Res. 2016, 132, 178–185. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Terrier, O.; Rosa-Calatrava, M. Drug Repurposing Approaches for the Treatment of Influenza Viral Infection: Reviving Old Drugs to Fight Against a Long-Lived Enemy. Front. Immunol. 2019, 10, 531. [Google Scholar] [CrossRef]
- Tran, D.H.; Sugamata, R.; Hirose, T.; Suzuki, S.; Noguchi, Y.; Sugawara, A.; Ito, F.; Yamamoto, T.; Kawachi, S.; Akagawa, K.S.; et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1) pdm09 virus infection by interfering with virus internalization process. J. Antibiot. 2019, 72, 759–768. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.K.; Ko, J.; Park, C.J.; Kim, M.; Jeong, Y.; Hong, S.; Varani, G.; Choi, B.S. Aminoglycoside antibiotics bind to the influenza A virus RNA promoter. Mol. BioSystems 2012, 8, 2857–2859. [Google Scholar] [CrossRef] [PubMed]
- Rosário-Ferreira, N.; Preto, A.J.; Melo, R.; Moreira, I.S.; Brito, R.M.M. The Central Role of Non-Structural Protein 1 (NS1) in Influenza Biology and Infection. Int. J. Mol. Sci. 2020, 21, 1511. [Google Scholar] [CrossRef]
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef]
- Trigueiro-Louro, J.M.; Correia, V.; Santos, L.A.; Guedes, R.C.; Brito, R.M.M.; Rebelo-de-Andrade, H. To hit or not to hit: Large-scale sequence analysis and structure characterization of influenza A NS1 unlocks new antiviral target potential. Virology 2019, 535, 297–307. [Google Scholar] [CrossRef]
- Trigueiro-Louro, J.; Santos, L.A.; Almeida, F.; Correia, V.; Brito, R.M.M.; Rebelo-de-Andrade, H. NS1 protein as a novel antiinfluenza target: Map-and-mutate antiviral rationale reveals new putative druggable hot spots with an important role on viral replication. Virology 2022, 565, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.A. The influenza virus NS1 protein as a therapeutic target. Antivir. Res. 2013, 99, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Fabrican, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. Suppl. 2001, 109, 69–75. [Google Scholar]
- Drewry, D.H.; Macarron, R. Enhancements of screening collections to address areas of unmet medical need: An industry perspective. Curr. Opin. Chem. Biol. 2010, 14, 289–298. [Google Scholar] [CrossRef]
- McInnes, C. Virtual screening strategies in drug discovery. Curr. Opin. Chem. Biol. 2007, 11, 494–502. [Google Scholar] [CrossRef]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. ZINC: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Jablonski, J.J.; Basu, D.; Engel, D.A.; Geysen, H.M. Design, synthesis, and evaluation of novel small molecule inhibitors of the influenza virus protein NS1. Bioorg. Med. Chem. 2012, 20, 487–497. [Google Scholar] [CrossRef][Green Version]
- Duran, A.; Zamora, I.; Pastor, M. Suitability of GRIND-based principal properties for the description of molecular similarity and ligand-based virtual screening. J. Chem. Inf. Model. 2009, 49, 2129–2138. [Google Scholar] [CrossRef]
- Sencanski, M.; Radosevic, D.; Perovic, V.; Gemovic, B.; Stanojevic, M.; Veljkovic, N.; Glisic, S. Natural Products as Promising Ther-apeutics for Treatment of Influenza Disease. Curr. Pharm. Des. 2015, 21, 5573–5588. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Ma, L.C.; Xiao, R.; Radvansky, B.; Aramini, J.; Zhao, L.; Marklund, J.; Kuo, R.L.; Twu, K.Y.; Arnold, E.; et al. Structural basis for suppression of a host antiviral response by influenza A virus. Proc. Natl. Acad. Sci. USA 2008, 105, 13093–13098. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, A.B.; Jureka, A.S.; Falahat, S.M.; Green, T.J.; Petit, C.M. Structural analyses reveal the mechanism of inhibition of influenza virus NS1 by two antiviral compounds. J. Biol. Chem. 2018, 293, 14659–14668. [Google Scholar] [CrossRef] [PubMed]
- Kelder, J.; Grootenhuis, P.D.; Bayada, D.M.; Delbressine, L.P.; Ploemen, J.P. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond Rules: The De-velopment of a Central Nervous System Multiparameter Optimization (CNS MPO) Ap-proach To Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graphics Mod. 1999, 1, 57–61. [Google Scholar]
- Tong, X.; Smith, J.; Bukreyeva, N.; Koma, T.; Manning, J.T.; Kalkeri, R.; Kwong, A.D.; Paessler, S. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antivir. Res. 2018, 149, 34–40. [Google Scholar] [CrossRef]
- Markland, W.; McQuaid, T.J.; Jain, J.; Kwong, A.D. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: A comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 2000, 44, 859–866. [Google Scholar] [CrossRef]
- Nemeroff, M.E.; Barabino, S.M.; Li, Y.; Keller, W.; Krug, R.M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol. Cell 1998, 1, 991–1000. [Google Scholar] [CrossRef]
- Veljkovic, N.; Glisic, S.; Perovic, V.; Veljkovic, V. The role of long-range intermolecular interactions in discovery of new drugs. Expert. Opin. Drug Discov. 2011, 6, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Matejin, S.; Bukreyeva, N.; Radosevic, D.; Sencanski, M.; Mantlo, E.; Veljkovic, V.; Glisic, S.; Paessler, S. In vitro anti-influenza activ-ity of in silico repurposed candidate drug cycrimine. Antivir. Ther. 2019, 24, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Sencanski, M.; Perovic, V.; Milicevic, J.; Todorovic, T.; Prodanovic, R.; Veljkovic, V.; Paessler, S.; Glisic, S. Identification of SARS-CoV-2 Papain-like Protease (PLpro) Inhibitors Using Combined Computational Approach. ChemistryOpen 2022, 11, e202100248. [Google Scholar] [CrossRef] [PubMed]
- Radosevic, D.; Sencanski, M.; Perovic, V.; Veljkovic, N.; Prljic, J.; Veljkovic, V.; Mantlo, E.; Bukreyeva, N.; Paessler, S.; Glisic, S. Virtual Screen for Repurposing of Drugs for Candidate Influenza a M2 Ion-Channel Inhibitors. Front. Cell Infect. Microbiol. 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Paessler, S.; Huang, C.; Sencanski, M.; Veljkovic, N.; Perovic, V.; Glisic, S.; Veljkovic, V. Ibuprofen as a template molecule for drug design against Ebola virus. Front. Biosci. (Landmark Ed). 2018, 23, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Glisic, S.; Perovic, V.; Paessler, S.; Veljkovic, N.; Nicolson, G.L. Simple Chemoinformatics Criterion Using Electron Donor-Acceptor Molecular Characteristics for Selection of Antibiotics Against Multi-Drug-Resistant Bacteria. Discoveries 2016, 4, e64. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Togawa, Y.; Shindo, N. Bacterial and viral infections associated with influenza. Influenza Other Respir. Viruses 2013, 7 (Suppl. S2), 105–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. NPBS database: A chemical data resource with relational data between natural products and biological sources. Database 2020, 2022, baaa102. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shimura, N.; Otabe, Y.; Hirai-Morita, A.; Nakamura, Y.; Ono, N.; Ul-Amin, M.A.; Kanaya, S. KNApSAcK-3D: A three-dimensional structure database of plant metabolites. Plant Cell Physiol. 2013, 54, e4. [Google Scholar] [CrossRef]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of open natural products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, [BIOVIA Draw]; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- 3M5R. Crystal Structure of Swine Flu Virus NS1 Effector Domain from H1N1 Influenza A/California/07/2009. Available online: https://www.rcsb.org/structure/3M5R (accessed on 20 January 2024).
- Veljkovic, V. A Theoretical Approach to Preselection of Carcinogens and Chemical Carcinogenesis; Gordon & Breach: New York, NY, USA, 1980. [Google Scholar]
- Veljkovic, V.; Slavic, I. Simple general-model pseudopotential. Phys. Rev. Lett. 1972, 29, 105–107. [Google Scholar] [CrossRef]
- Pastor, M.; Cruciani, G.; McLay, I.; Pickett, S.; Clementi, S. GRid-INdependent descriptors (GRIND): A novel class of alignment-independent three-dimensional molecular descriptors. J. Med. Chem. 2000, 43, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Martínez, G.C.; Pastor, M. Development and validation of AMANDA, a new algorithm for selecting highly relevant regions in molecular interaction fields. J. Chem. Inf. Model. 2008, 48, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput.-Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef] [PubMed]
- MOPAC 2016, U. James J. P. Stewart, Stewart Computational Chemistry, Colorado Springs, CO, 2016. Available online: http://openmopac.net/ (accessed on 3 March 2024).
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger LLC. QikProp. 2021. Available online: https://www.schrodinger.com/products/qikprop (accessed on 7 March 2024).
- ChemAxon Ltd. MarvinSketch. 2022. Available online: https://chemaxon.com/products/marvin (accessed on 3 March 2024).
- Basu, D.; Walkiewicz, M.P.; Frieman, M.; Baric, R.S.; Auble, D.T.; Engel, D.A. Novel Influenza Virus NS1 Antagonists Block Replication and Restore Innate Immune Function. J. Virol. 2009, 83, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ebi.ac.uk/chembl/g/#browse/targets/filter/_metadata.related_compounds.all_chembl_ids%3A(%22CHEMBL1739790%22%20OR%20%22CHEMBL1698845%22) (accessed on 3 March 2024).
- Available online: https://www.ebi.ac.uk/chembl/g/#browse/targets/filter/_metadata.related_compounds.all_chembl_ids%3A(%22CHEMBL1608529%22) (accessed on 3 March 2024).
- Available online: https://www.ebi.ac.uk/chembl/g/#browse/targets/filter/_metadata.related_compounds.all_chembl_ids%3A(%22CHEMBL1311154%22) (accessed on 3 March 2024).
- Cho, E.J.; Xia, S.; Ma, L.C.; Robertus, J.; Krug, R.M.; Anslyn, E.V. Identification of Influenza Virus Inhibitors Targeting NS1A Utilizing Fluorescence Polarization–Based High-Throughput Assay. J. Biomol. Screen. 2012, 17, 448–459. [Google Scholar] [CrossRef]
- Nayak, M.K.; Agrawal, A.S.; Bose, S.; Naskar, S.; Bhowmick, R.; Chakrabarti, S. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 2014, 69, 1298–1310. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Zhang, R.; Chan, C.C.; Xi, B.; Zhu, Z.; Yang, J.; Poon, V.K.; Zhou, J.; Chen, M.; et al. CL-385319 inhibits H5N1 avian influenza A virus infection by blocking viral entry. Eur. J. Pharmacol. 2011, 660, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Plotch, S.J.; O‘Hara, B.; Morin, J.; Palant, O.; LaRocque, J.; Bloom, J.D.; Lang, S.A., Jr.; DiGrandi, M.J.; Bradley, M.; Nilakantan, R.; et al. Inhibition of influenza A virus replication by compounds interfering with the fusogenic function of the viral hemagglutinin. J. Virol. 1999, 73, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Kerry, P.S.; Stevens, D.J.; Steinhauer, D.A.; Martin, S.R.; Gamblin, S.J.; Skehel, J.J. Structure of influenza hemagglutinin in complex with an inhibitor of membrane fusion. Proc. Natl. Acad. Sci. USA 2008, 105, 17736–17741. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Colonno, R.; Krystal, M. Characterization of a hemagglutinin-specific inhibitor of influenza A virus. Virology 1996, 226, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, X.; Liu, S. Novel hemagglutinin-based influenza virus inhibitors. J. Thorac. Dis. 2013, 5 (Suppl. S2), S149–S159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Y.; Li, S.; Li, H.; Qiu, Z.; Lee, C.; Lu, H.; Lin, X.; Zhao, R.; Chen, L.; et al. Inhibition of influenza A virus (H1N1) fusion by benzenesulfonamide derivatives targeting viral hemagglutinin. PLoS ONE 2011, 6, e29120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Leneva, I.A.; Russell, R.J.; Boriskin, Y.S.; Hay, A.J. Characteristics of arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009, 81, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Shien, J.H.; Tiley, L.; Chiou, S.S.; Wang, S.Y.; Chang, T.J.; Hsu, W.L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Walkiewicz, M.P.; Basu, D.; Jablonski, J.J.; Geysen, H.M.; Engel, D.A. Novel inhibitor of influenza non-structural protein 1 blocks multi-cycle replication in an RNase L-dependent manner. J. Gen. Virol. 2011, 92, 60–70. [Google Scholar] [CrossRef] [PubMed]

| Component | SSX | SSXacc | VarX | VarXacc |
|---|---|---|---|---|
| 1 | 27.45 | 27.45 | 24.99 | 24.99 |
| 2 | 22.81 | 50.26 | 21.78 | 46.77 |
| 3 | 11.14 | 61.40 | 10.43 | 57.20 |
| 4 | 8.87 | 70.28 | 8.61 | 65.81 |
| 5 | 4.52 | 74.80 | 4.08 | 69.89 |
| ZINC ID | Vina Docking Energy (kcal/mol) | PA22 |
|---|---|---|
| ZINC12895343 | −6.6 | 0.529736751 |
| ZINC12895341 | −6.3 | 0.768482715 |
| ZINC04023123 | −5.6 | 1.131189207 |
| ZINC35485059 | −6.8 | 1.409582845 |
| ZINC00517232 | −5.9 | 1.49712743 |
| ZINC35485061 | −6.5 | 1.667747682 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perovic, V.; Stevanovic, K.; Bukreyeva, N.; Paessler, S.; Maruyama, J.; López-Serrano, S.; Darji, A.; Sencanski, M.; Radosevic, D.; Berardozzi, S.; et al. Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach. Int. J. Mol. Sci. 2024, 25, 4911. https://doi.org/10.3390/ijms25094911
Perovic V, Stevanovic K, Bukreyeva N, Paessler S, Maruyama J, López-Serrano S, Darji A, Sencanski M, Radosevic D, Berardozzi S, et al. Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach. International Journal of Molecular Sciences. 2024; 25(9):4911. https://doi.org/10.3390/ijms25094911
Chicago/Turabian StylePerovic, Vladimir, Kristina Stevanovic, Natalya Bukreyeva, Slobodan Paessler, Junki Maruyama, Sergi López-Serrano, Ayub Darji, Milan Sencanski, Draginja Radosevic, Simone Berardozzi, and et al. 2024. "Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach" International Journal of Molecular Sciences 25, no. 9: 4911. https://doi.org/10.3390/ijms25094911
APA StylePerovic, V., Stevanovic, K., Bukreyeva, N., Paessler, S., Maruyama, J., López-Serrano, S., Darji, A., Sencanski, M., Radosevic, D., Berardozzi, S., Botta, B., Mori, M., & Glisic, S. (2024). Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach. International Journal of Molecular Sciences, 25(9), 4911. https://doi.org/10.3390/ijms25094911











