High-Intensity Focused Ultrasound Increases Facial Adipogenesis in a Swine Model via Modulation of Adipose-Derived Stem Cell Cilia
Abstract
:1. Introduction
2. Results
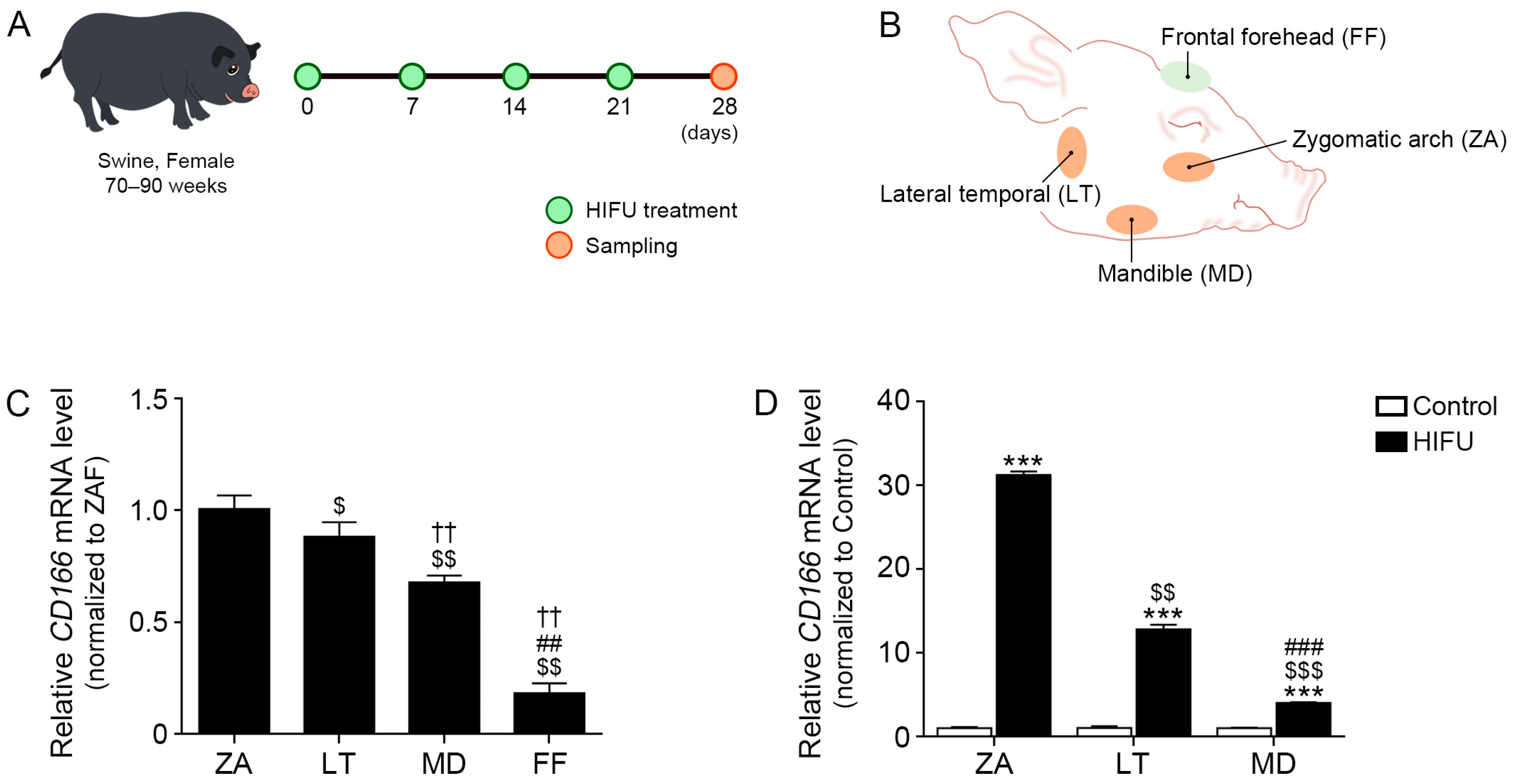
2.1. CD166 Expression Differs Depending on the Location of Subcutaneous Adipose Tissue
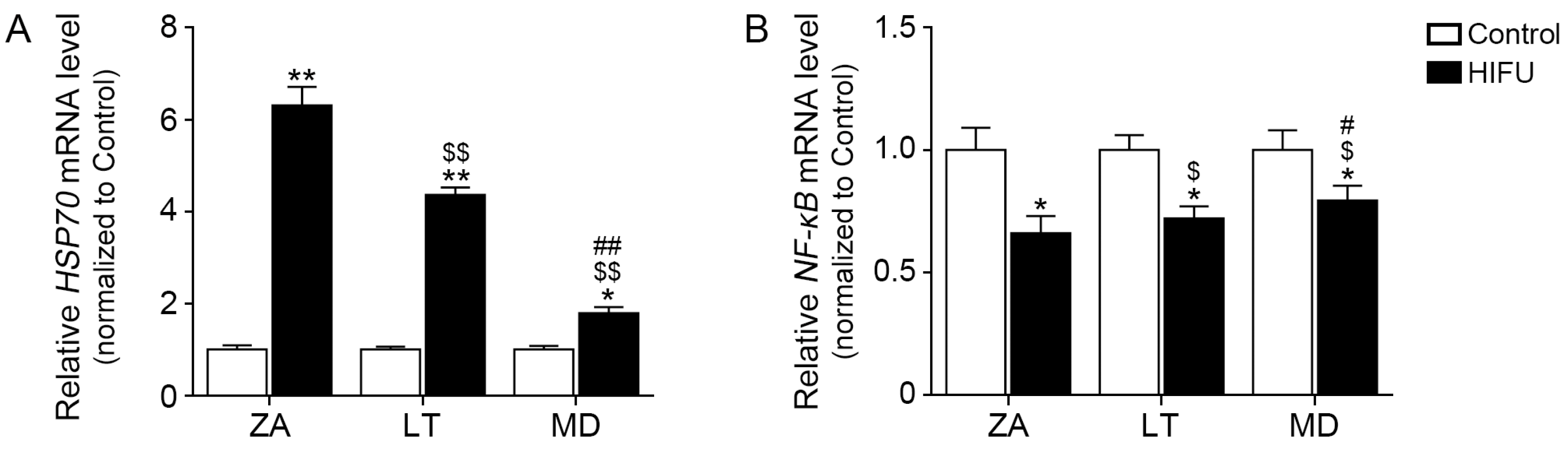
2.2. HIFU Increases HSP70 Expression and Decreases NF-κB Expression in Facial Subcutaneous Adipose Tissue
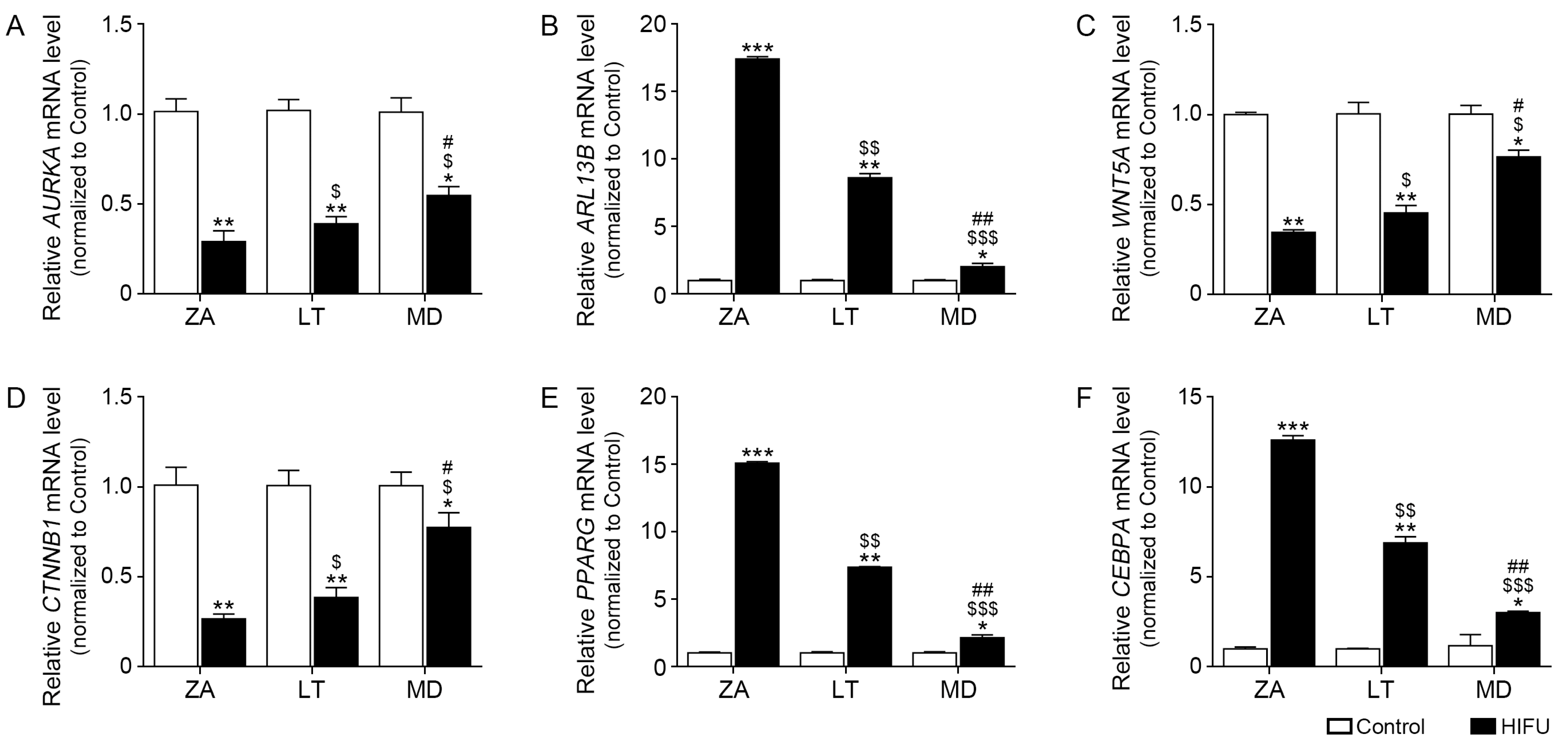
2.3. HIFU Decreases Cilia Disassembly-Related Factors and Increases Cilia Length Increasing Factors in Facial Subcutaneous Adipose Tissue
2.4. HIFU Application Increases Adipogenesis Factors
2.5. HIFU Increases the Thickness of Facial Adipose Tissue and Number of Adipocytes
3. Discussion
4. Materials and Methods
4.1. HIFU System
4.2. Animal Experiments and HIFU Application
4.3. MRI Scan and Analysis
4.4. RNA Extraction and cDNA Synthesis
4.5. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
4.6. Preparation of Paraffin-Embedded Tissue and Hematoxylin and Eosin Staining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donofrio, L.M. Fat distribution: A morphologic study of the aging face. Dermatol. Surg. 2000, 26, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Vashisht, K.R.; Sushruth, P.R.; Saka, D.V. Monopolar radiofrequency for reposing drooping fat planes and facial rejuvenation: A prospective study on 30 subjects towards true antiaging. J. Cosmet. Dermatol. 2022, 21, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Haddock, N.T.; Saadeh, P.B.; Boutros, S.; Thorne, C.H. The tear trough and lid/cheek junction: Anatomy and implications for surgical correction. Plast. Reconstr. Surg. 2009, 123, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Pessa, J.E.; Ristow, B. The youthful cheek and the deep medial fat compartment. Plast. Reconstr. Surg. 2008, 121, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Collawn, S.S.; Vasconez, L.O.; Gamboa, M.; Guzman-Stein, G.; Carriquiry, C. Subcutaneous approach for elevation of the malar fat pad through a prehairline incision. Plast. Reconstr. Surg. 1996, 97, 836–841. [Google Scholar] [CrossRef]
- Flowers, R.S. Tear trough implants for correction of tear trough deformity. Clin. Plast. Surg. 1993, 20, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.T.; Hutmacher, D.W.; Chew, F.T.; Lim, T.C. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J. Dermatol. Sci. 2005, 37, 169–176. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Sbarbati, A. Adipose-derived mesenchymal stem cells: Past, present, and future. Aesthetic Plast. Surg. 2009, 33, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Mojallal, A.; Lequeux, C.; Shipkov, C.; Breton, P.; Foyatier, J.L.; Braye, F.; Damour, O. Improvement of skin quality after fat grafting: Clinical observation and an animal study. Plast. Reconstr. Surg. 2009, 124, 765–774. [Google Scholar] [CrossRef]
- Mailey, B.; Saba, S.; Baker, J.; Tokin, C.; Hickey, S.; Wong, R.; Wallace, A.M.; Cohen, S.R. A comparison of cell-enriched fat transfer to conventional fat grafting after aesthetic procedures using a patient satisfaction survey. Ann. Plast. Surg. 2013, 70, 410–415. [Google Scholar] [CrossRef]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 2015, 135, 999–1009. [Google Scholar] [CrossRef]
- Zhu, M.; Heydarkhan-Hagvall, S.; Hedrick, M.; Benhaim, P.; Zuk, P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J. Vis. Exp. 2013, 79, e50585. [Google Scholar]
- Carvalho, P.P.; Gimble, J.M.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng. Part. C Methods 2013, 19, 473–478. [Google Scholar] [CrossRef]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; Da Ponte, A.; Tessitori, G.; et al. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. Ther. 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; White, L.E.; Martin, N.; Witherspoon, J.; Yoo, S.; West, D.P. Ultrasound tightening of facial and neck skin: A rater-blinded prospective cohort study. J. Am. Acad. Dermatol. 2010, 62, 262–269. [Google Scholar] [CrossRef]
- Suh, D.H.; Shin, M.K.; Lee, S.J.; Rho, J.H.; Lee, M.H.; Kim, N.I.; Song, K.Y. Intense focused ultrasound tightening in Asian skin: Clinical and pathologic results. Dermatol. Surg. 2011, 37, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, E.; Kim, J.; Ro, Y.; Ko, J. High-Intensity Focused Ultrasound for the Treatment of Wrinkles and Skin Laxity in Seven Different Facial Areas. Ann. Dermatol. 2015, 27, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Brobst, R.W.; Ferguson, M.; Perkins, S.W. Ulthera: Initial and six month results. Facial Plast. Surg. Clin. N. Am. 2012, 20, 163–176. [Google Scholar] [CrossRef]
- Oh, S.; Kim, H.M.; Batsukh, S.; Sun, H.J.; Kim, T.; Kang, D.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 8866. [Google Scholar] [CrossRef]
- Ritter, A.; Roth, S.; Kreis, N.N.; Friemel, A.; Hoock, S.C.; Souto, A.S.; Eichbaum, C.; Neuhoff, A.; Chen, Q.; Solbach, C.; et al. Primary Cilia in Trophoblastic Cells: Potential Involvement in Preeclampsia. Hypertension 2020, 76, 1491–1505. [Google Scholar] [CrossRef]
- Corbit, K.C.; Shyer, A.E.; Dowdle, W.E.; Gaulden, J.; Singla, V.; Reiter, J.F. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008, 10, 70–76, Erratum in Nat. Cell Biol. 2008, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.K.; Kim, G.H.; Kwon, J.H.; Kim, M.; Choi, S.J.; Oh, W.; Um, S.; Jin, H.J. Primary Cilia Mediate Wnt5a/β-catenin Signaling to Regulate Adipogenic Differentiation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Following Calcium Induction. Tissue Eng. Regen. Med. 2020, 17, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.K.; Kwon, J.H.; Kim, M.; Kim, G.H.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jin, H.J.; Jeon, H.B. Intracellular Calcium Determines the Adipogenic Differentiation Potential of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells via the Wnt5a/β-Catenin Signaling Pathway. Stem Cells Int. 2018, 2018, 6545071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Yan, Y.P.; Qi, K.M.; Wang, J.Q.; Liu, Z.F. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast. Reconstr. Surg. 2002, 109, 2509–2520. [Google Scholar] [CrossRef]
- Rey, R.M. The Fat of Bichat as Additional Source for Head and Face Fat Transfer Procedures. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2816. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Ewers, R. Application of the buccal fat pad in oral reconstruction. J. Oral. Maxillofac. Surg. 2000, 58, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pelo, S.; Moro, A.; Mosca, R.; Boniello, R. L’utilizzo della bolla di Bichat nella terapia chirurgica dell’anchilosi dell’articolazione temporomandibolare. Prime valutazioni [The use of Bichat’s fat pad in temporomandibular joint surgery. First evaluation]. Minerva Stomatol. 2000, 49, 129–134. [Google Scholar] [PubMed]
- Rapidis, A.D.; Alexandridis, C.A.; Eleftheriadis, E.; Angelopoulos, A.P. The use of the buccal fat pad for reconstruction of oral defects: Review of the literature and report of 15 cases. J. Oral. Maxillofac. Surg. 2000, 58, 158–163. [Google Scholar] [CrossRef]
- Amaral, M.F.D.; Guerra, L.A.P.; Gerbi, M.E.M.D.M.; Melo, A.R.; Gondim, D.G.D.A.; Junior, R.M.; De Melo, S.L.; Neto, A.P.D.S. Oroantral fistulas closure using Bichat’s fat pad. RGO-Rev. Gaúcha Odontol. 2014, 62, 437–442. [Google Scholar] [CrossRef]
- Conti, G.; Bertossi, D.; Dai Prè, E.; Cavallini, C.; Scupoli, M.T.; Ricciardi, G.; Parnigotto, P.; Saban, Y.; Sbarbati, A.; Nocini, P. Regenerative potential of the Bichat fat pad determined by the quantification of multilineage differentiating stress enduring cells. Eur. J. Histochem. 2018, 62, 2900. [Google Scholar] [CrossRef]
- Peng, Q.; Alipour, H.; Porsborg, S.; Fink, T.; Zachar, V. Evolution of ASC Immunophenotypical Subsets During Expansion In Vitro. Int. J. Mol. Sci. 2020, 21, 1408. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Toh, M.T.; Narasimhan, V.; Thamilselvam, S.K.; Choksi, S.P.; Roy, S. A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation. Dev. Biol. 2015, 397, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Q.; Liu, J.; Zhang, W. CTNNB1 Alternation Is a Potential Biomarker for Immunotherapy Prognosis in Patients With Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 759565. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, J.; Zhu, T.; Shen, Y.; Tang, X.; Fang, L.; Xu, Y. Cross-Talking Between PPAR and WNT Signaling and its Regulation in Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in Adipose-Derived Stem Cells Isolation, Characterization, and Application in Regenerative Tissue Engineering. Stem Cells Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Kreis, N.N.; Hoock, S.C.; Roth, S.; Kielland-Kaisen, U.; Brüggmann, D.; Solbach, C.; Louwen, F.; Yuan, J. Primary Cilia Are Dysfunctional in Obese Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rep. 2018, 10, 583–599. [Google Scholar] [CrossRef]
- Laudes, M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J. Mol. Endocrinol. 2011, 46, R65–R72. [Google Scholar]
- Mok, C.A.; Héon, E.; Zhen, M. Ciliary dysfunction and obesity. Clin. Genet. 2010, 77, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Marion, V.; Stoetzel, C.; Schlicht, D.; Messaddeq, N.; Koch, M.; Flori, E.; Danse, J.M.; Mandel, J.-L.; Dollfus, H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Lee, J.S.; Kim, M.; Ahn, B.Y.; Jung, H.S.; Lee, H.M.; Kim, J.-W.; Park, K.S. Regulation of Wnt/β-catenin signaling by CCAAT/enhancer binding protein β during adipogenesis. Obesity 2012, 20, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, P.; Prodromou, N.V.; Chapple, J.P. Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J. Endocrinol. 2009, 203, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Kouzmenko, A.P.; Kato, S. PPAR-gamma Signaling Crosstalk in Mesenchymal Stem Cells. PPAR Res. 2010, 2010, 341671. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Bree, A.J.; Yao, Y.; Du, B.; Hemati, N.; Martinez-Santibañez, G.; MacDougald, O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012, 50, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Cao, L.; David, V.; Quarles, L.D.; Xiao, Z. Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis. PLoS ONE 2010, 5, e15240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122 Pt 15, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.T.; Clement, C.A.; Satir, P.; Pedersen, L.B. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J. Pathol. 2012, 226, 172–184. [Google Scholar] [CrossRef]
- Lu, D.; Carson, D.A. Repression of beta-catenin signaling by PPAR gamma ligands. Eur. J. Pharmacol. 2010, 636, 198–202. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.; Lynch, L.; Cawood, T.J.; Kwasnik, A.; Nolan, N.; Geoghegan, J.; McCormick, A.; O’Farrelly, C.; O’Shea, D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE 2010, 5, e9997. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Liu, B. Do adipose tissue macrophages promote insulin resistance or adipose tissue remodelling in humans? Horm. Mol. Biol. Clin. Investig. 2014, 20, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Tomaru, U.; Miyoshi, A.; Ito, T.; Fukaya, S.; Miyoshi, H.; Atsumi, T.; Ishizu, A. Overexpression of TNF-α converting enzyme promotes adipose tissue inflammation and fibrosis induced by high fat diet. Exp. Mol. Pathol. 2014, 97, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Hacohen, N.; Golub, T.R.; Van Parijs, L.; Lodish, H.F. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: Nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 2002, 51, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Marco, L.; Chacón, M.R.; Maymó-Masip, E.; Barroso, E.; Salvadó, L.; Wabitsch, M.; Garrido-Sánchez, L.; Tinahones, F.J.; Palomer, X.; Vendrell, J.; et al. TNF-α inhibits PPARβ/δ activity and SIRT1 expression through NF-κB in human adipocytes. Biochim. Biophys. Acta 2012, 1821, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Debeer, S.; Le Luduec, J.B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.F.; Dubois, B.; Kanitakis, J. Comparative histology and immunohistochemistry of porcine versus human skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.G.; Zheng, Z.; Park, H.J.; Yoon, J.H.; Oh, W.; Lee, C.W.; Cho, S.B. Coagulation and ablation patterns of high-intensity focused ultrasound on a tissue-mimicking phantom and cadaveric skin. Lasers Med. Sci. 2015, 30, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Umair, Z.; Baek, M.O.; Song, J.; An, S.; Chon, S.J.; Yoon, M.S. MicroRNA-4516 in Urinary Exosomes as a Biomarker of Premature Ovarian Insufficiency. Cells 2022, 11, 2797. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, H.Y.; Jang, J.T.; Hong, S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules 2023, 28, 8099. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Kim, H.M.; Oh, S.; Batsukh, S.; Lee, S.; Oh, M.; Lee, J.; Lee, R.; Kim, J.W.; Oh, S.M.; et al. High-Intensity Focused Ultrasound Increases Facial Adipogenesis in a Swine Model via Modulation of Adipose-Derived Stem Cell Cilia. Int. J. Mol. Sci. 2024, 25, 7648. https://doi.org/10.3390/ijms25147648
Byun K-A, Kim HM, Oh S, Batsukh S, Lee S, Oh M, Lee J, Lee R, Kim JW, Oh SM, et al. High-Intensity Focused Ultrasound Increases Facial Adipogenesis in a Swine Model via Modulation of Adipose-Derived Stem Cell Cilia. International Journal of Molecular Sciences. 2024; 25(14):7648. https://doi.org/10.3390/ijms25147648
Chicago/Turabian StyleByun, Kyung-A, Hyoung Moon Kim, Seyeon Oh, Sosorburam Batsukh, Sangsu Lee, Myungjune Oh, Jeongwoo Lee, Ran Lee, Jae Woo Kim, Seung Min Oh, and et al. 2024. "High-Intensity Focused Ultrasound Increases Facial Adipogenesis in a Swine Model via Modulation of Adipose-Derived Stem Cell Cilia" International Journal of Molecular Sciences 25, no. 14: 7648. https://doi.org/10.3390/ijms25147648
APA StyleByun, K.-A., Kim, H. M., Oh, S., Batsukh, S., Lee, S., Oh, M., Lee, J., Lee, R., Kim, J. W., Oh, S. M., Kim, J., Kim, G., Park, H. J., Hong, H., Lee, J., An, S.-H., Oh, S. S., Jung, Y.-S., Son, K. H., & Byun, K. (2024). High-Intensity Focused Ultrasound Increases Facial Adipogenesis in a Swine Model via Modulation of Adipose-Derived Stem Cell Cilia. International Journal of Molecular Sciences, 25(14), 7648. https://doi.org/10.3390/ijms25147648






