A Standardized Porcine Model for Partial-Thickness Wound Healing Studies: Design, Characterization, Model Validation, and Histological Insights
Abstract
:1. Introduction
2. Results
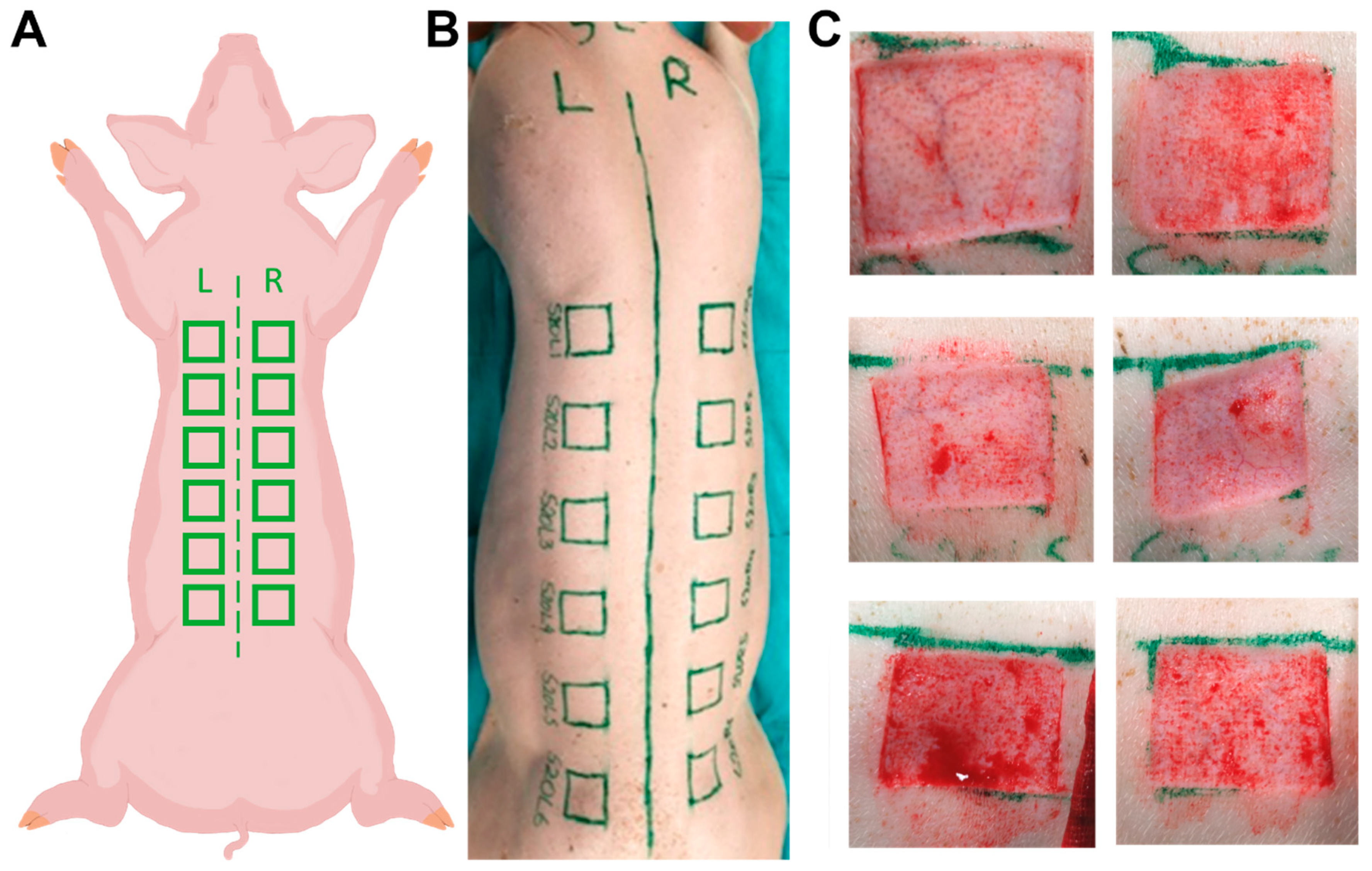
2.1. Animals and Wounds
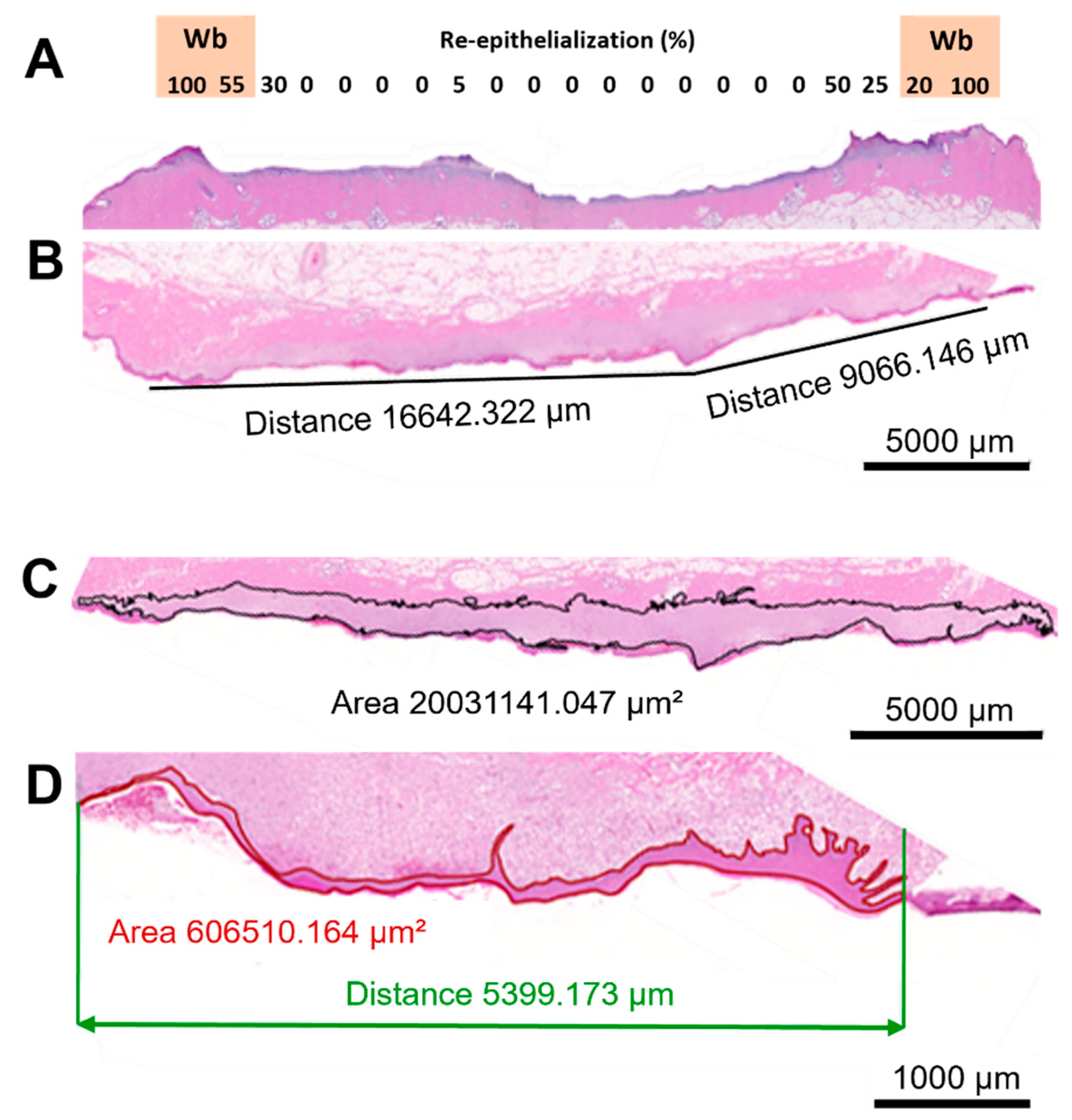
2.2. Histological Assessment
2.2.1. General
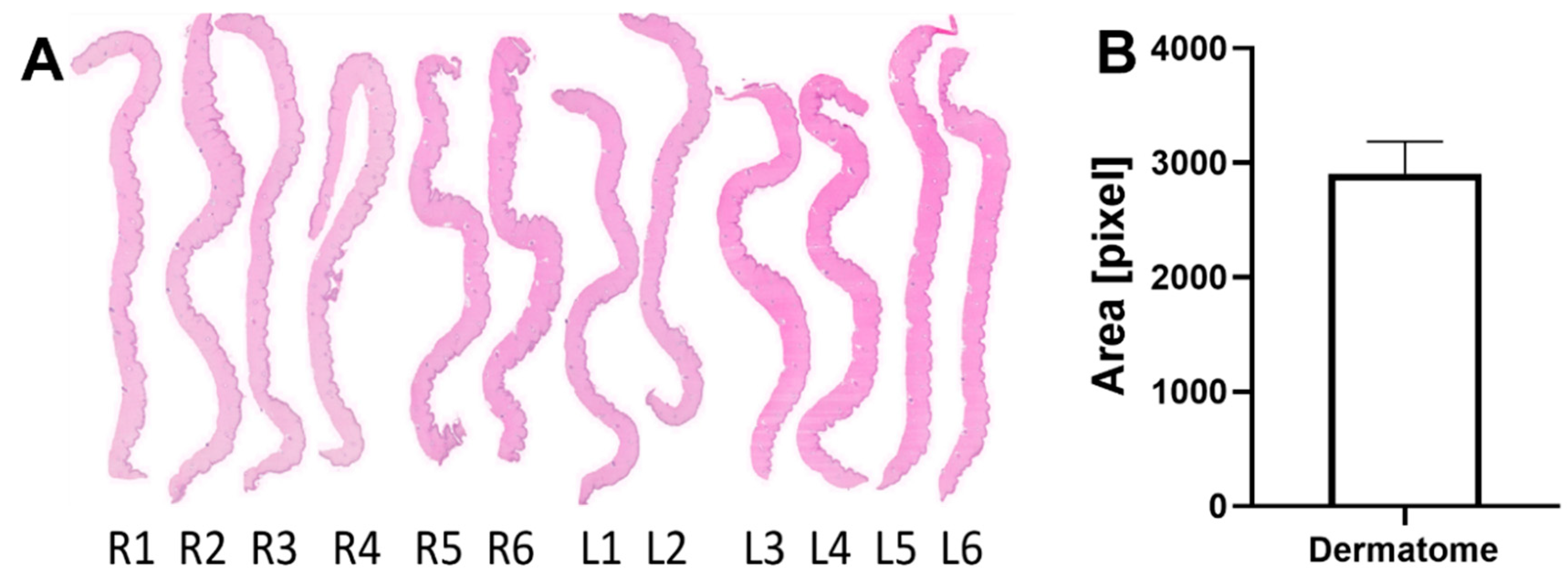
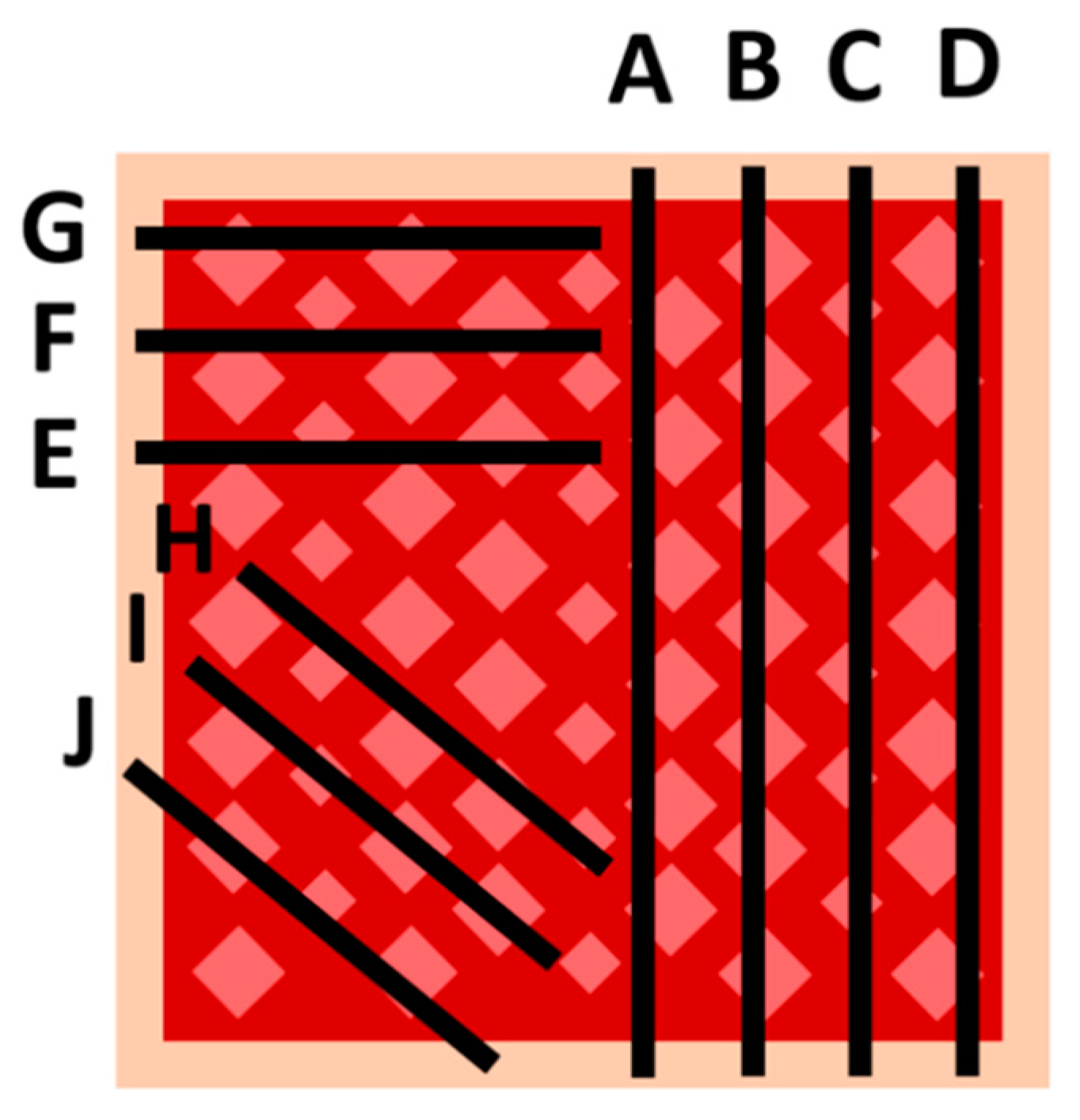
2.2.2. Dermatome Samples
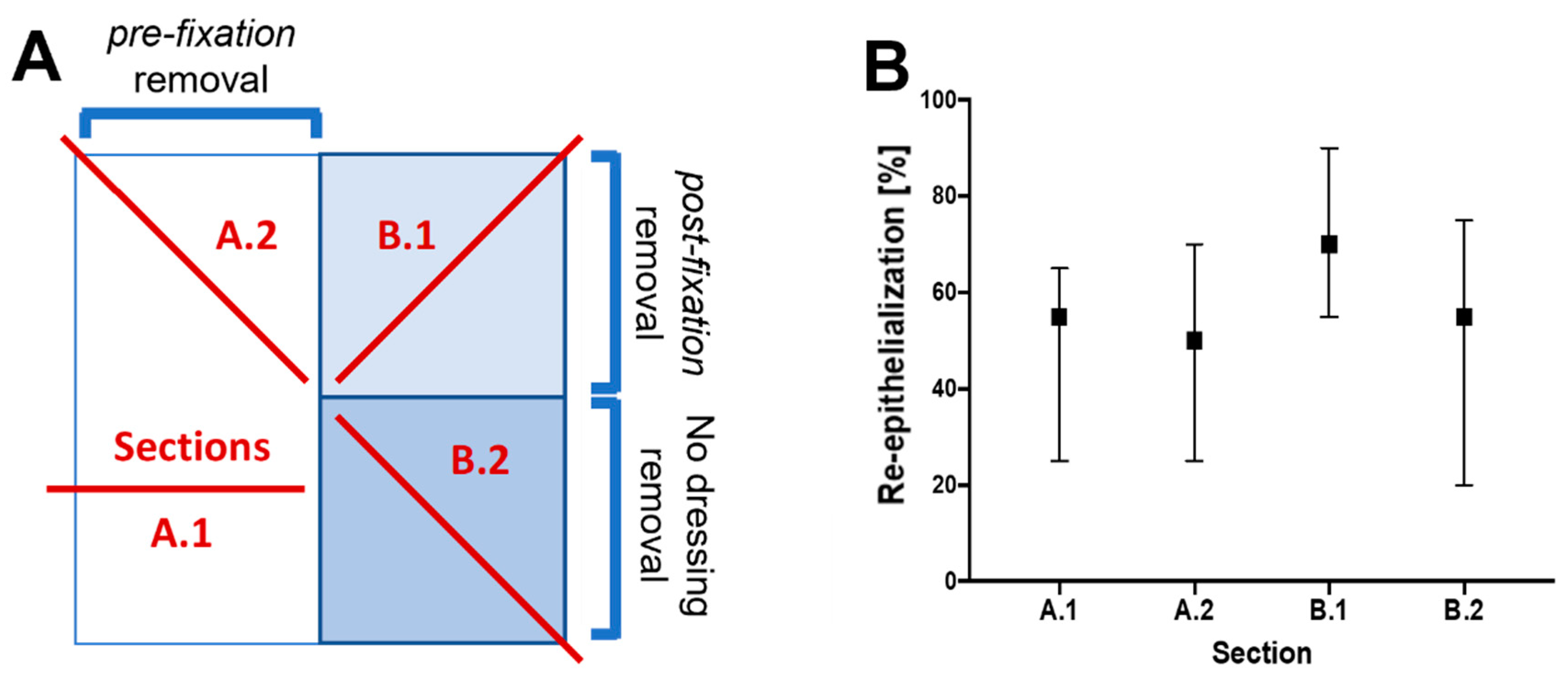
2.2.3. Sampling Procedure Effects and Wound Sample Position
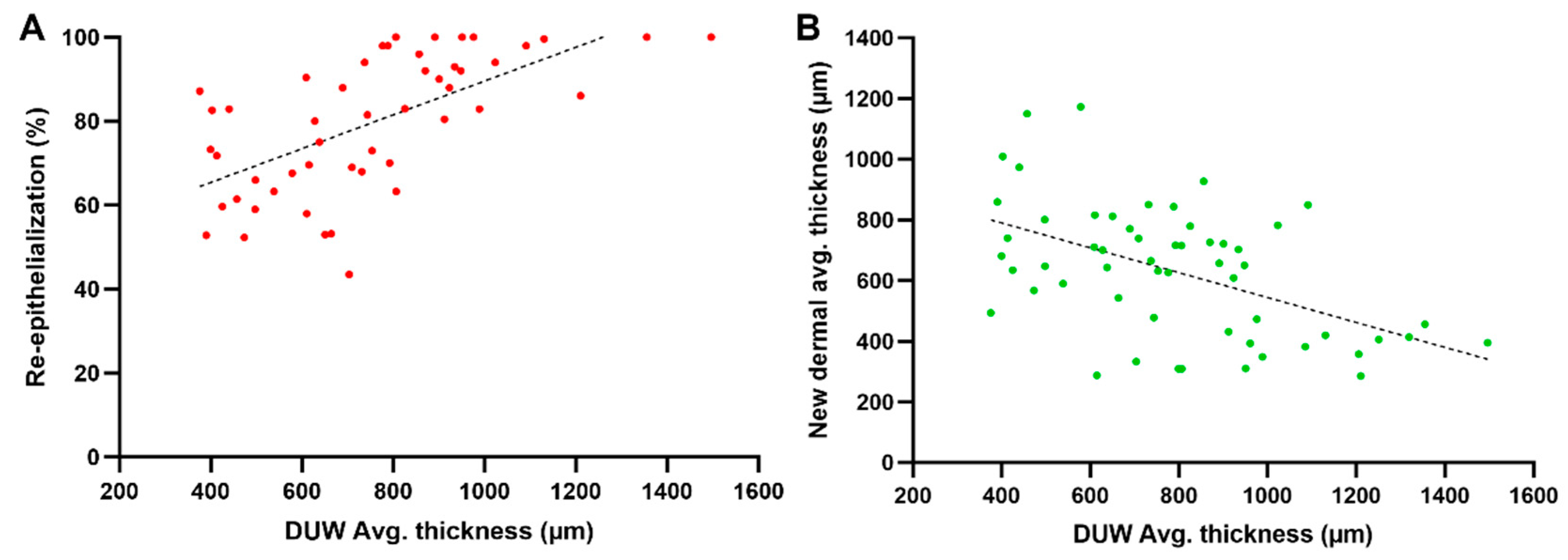
2.2.4. Body Mass Influence on the Dermal Tissue and Residual Dermal Tissue
2.2.5. Wound Position Effects
2.2.6. Influence of the Residual Dermis under the Wound on Healing Parameters
3. Discussion
3.1. Animal Selection and Wound Setting
3.2. Histological Assessment
3.3. Dermatome Samples
3.4. Sampling and Histology within the Wound
3.5. Body Mass Influence on the Dermal Tissue and Residual Dermal Tissue
3.6. Position Effects
3.7. Influence of the Residual Dermis under the Wound on Healing Parameters
3.8. Comparable Studies
3.9. Limitations
4. Materials and Methods
4.1. Animal Wound Model Development
4.2. Histological Analysis
4.2.1. General
4.2.2. Sampling and Histology within the Wound
4.2.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A.A.H. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Parnell, L.K.S.; Volk, S.W. The evolution of animal models in wound healing research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Vlig, M.; Boekema, B.K.H.L.; Ulrich, M.M.W. Porcine models. In Biomaterials for Skin Repair and Regeneration; Woodhead Publishing: Cambridge, UK, 2019; pp. 297–330. [Google Scholar]
- Meyer, W.; Schwarz, R.; Neurand, K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr. Probl. Dermatol. 1978, 7, 39–52. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, G.; El-Amin, I.; Mendenhall, V. Animal models for wound healing. In Skin Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2016; pp. 387–400. [Google Scholar]
- Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple: Animal models of wound healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [PubMed]
- Sami, D.G.; Abdellatif, A. Chronic wounds: An overview of wound healing and experimental models for wound studies. In Wound Healing Research; The Japanese Society for Wound Healing: Tokyo, Japan, 2021; pp. 431–458. [Google Scholar]
- Kuo, T.Y.; Huang, C.C.; Shieh, S.J.; Wang, Y.B.; Lin, M.J.; Wu, M.C.; Huang, L.L. Skin wound healing assessment via an optimized wound array model in miniature pigs. Sci. Rep. 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, W.; Lange, P.M.; Stirtz, T.; Iancu, C.; Heidemann, E. Isolation and characterization of the large cyanogen bromide peptides from the α1- and α2-chains of pig skin collagen. FEBS Lett. 1971, 16, 63–67. [Google Scholar] [CrossRef]
- Thomas, A.; Farah, K.; Millis, R.M. Epigenetic Influences on Wound Healing and Hypertrophic-Keloid Scarring: A Review for Basic Scientists and Clinicians. Cureus 2022, 14, e23503. [Google Scholar] [CrossRef]
- Bourgeois, J.; Beer, J.; Jacob, L.; Henry, M. Scarring and Dyschromias in Fitzpatrick Skin Type IV-VI: A Review of Dermatologic Treatment Protocols. J. Drugs Dermatol. 2023, 22, 7253. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Ashrafi, M.; Hague, A.; Baguneid, M.; Alonso-Rasgado, T.; Bayat, A. Wound healing and cutaneous scarring models of the human skin. In Skin Tissue Models for Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2018; pp. 201–221. [Google Scholar]
- Wilhelm, K.P.; Wilhelm, D.; Bielfeldt, S. Models of wound healing: An emphasis on clinical studies. Skin Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.W.; Walker, J.T.; Tinney, D.; Grynyshyn, M.; El-Warrak, A.; Truscott, E.; Flynn, L.E. The pig as a model system for investigating the recruitment and contribution of myofibroblasts in skin healing. Wound Repair Regen. 2022, 30, 45–63. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: North Yorkshire, UK, 1959. [Google Scholar]
- Rakita, A.; Nikolic, N.; Mildner, M.; Matiasek, J.; Elbe-Bürger, A. Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Fink, J.; Eberl, A.; Prugger, E.-M.; Kolb, D.; Luze, H.; Schwingenschuh, S.; Birngruber, T.; Magnes, C.; Mautner, S.I.; et al. A novel human ex vivo skin model to study early local responses to burn injuries. Sci. Rep. 2021, 11, 364. [Google Scholar] [CrossRef]
- Tuca, A.C.; Bernardelli de Mattos, I.; Funk, M.; Winter, R.; Palackic, A.; Groeber-Becker, F.; Kruse, D.; Kukla, F.; Lemarchand, T.; Kamolz, L.-P. Orchestrating the dermal/epidermal tissue ratio during wound healing by controlling the moisture content. Biomedicines 2022, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; McClain, S.A. Development of a porcine excisional wound model. Acad. Emerg. Med. 2003, 10, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.H.; Sedlak, C.; Kaser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmuller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Stone, R.; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117 (Suppl. S7), 1e-S–32e-S. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian Delavary, B.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.R.; Alvarez, O.M.; Bere, E.W., Jr.; Eaglstein, W.H.; Katz, S.I. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J. Investig. Dermatol. 1981, 77, 240–243. [Google Scholar] [CrossRef]
- Pirone, L.A.; Bolton, L.L.; Monte, K.A.; Shannon, R.J. Effect of calcium alginate dressings on partial-thickness wounds in swine. J. Investig. Surg. 1992, 5, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Wright, J.B.; Lam, K.; Burrell, R.E. Healing of porcine donor sites covered with silver-coated dressings. Eur. J. Surg. 2000, 166, 486–489. [Google Scholar] [CrossRef]
- Singer, A.J.; Nable, M.; Cameau, P.; Singer, D.D.; Mcclain, S.A.; Bs, P.C. Evaluation of a new liquid occlusive dressing for excisional wounds. Wound Repair Regen. 2003, 11, 181–187. [Google Scholar] [CrossRef]
- Singer, A.J.; Wang, Z.; McClain, S.A.; Pan, Y. Optical coherence tomography: A noninvasive method to assess wound reepithelialization. Acad. Emerg. Med. 2007, 14, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Faucher, L.D.; Kleinbeck, K.R.; Kao, W.J. Multifunctional photopolymerized semiinterpenetrating network (sIPN) system containing bupivacaine and silver sulfadiazine is an effective donor site treatment in a swine model. J. Burn Care Res. 2010, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Peura, M.; Kaartinen, I.; Suomela, S.; Hukkanen, M.; Bizik, J.; Harjula, A.; Kankuri, E.; Vuola, J. Improved skin wound epithelialization by topical delivery of soluble factors from fibroblast aggregates. Burns 2012, 38, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Masella, P.C.; Balent, E.M.; Carlson, T.L.; Lee, K.W.; Pierce, L.M. Evaluation of Six Split-thickness Skin Graft Donor-site Dressing Materials in a Swine Model. Plastic and Reconstructive. Surg. Glob. Open 2013, 1, e84. [Google Scholar] [CrossRef]
- Mauskar, N.A.; Sood, S.; Travis, T.E.; Matt, S.E.; Mino, M.J.; Burnett, M.-S.; Moffatt, L.T.; Fidler, P.; Epstein, S.E.; Jordan, M.H.; et al. Donor site healing dynamics. J. Burn Care Res. 2013, 34, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Travis, T.E.; Mauskar, N.A.; Mino, M.J.; Prindeze, N.; Moffatt, L.T.; Fidler, P.E.; Jordan, M.H.; Shupp, J.W. Commercially available topical platelet-derived growth factor as a novel agent to accelerate burn-related wound healing. J. Burn Care Res. 2014, 35, e321–e329. [Google Scholar] [CrossRef] [PubMed]
- Wlaschin, K.F.; Ninkovic, J.; Griesgraber, G.W.; Atan, S.C.; Young, A.J.; Pereira, J.M.; Solberg, M.J.; Smith, G.; Parks, P.J.; McNulty, A.K.; et al. The impact of first-aid dressing design on healing of porcine partial thickness wounds. Wound Repair Regen. 2019, 27, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, J.L.; Rath, R.; Held, M.; Werner, J.-O.; Petersen, W.; Schaller, H.-E.; Rahmanian-Schwarz, A. Gelatin-collagen nonwoven scaffold provides an alternative to Suprathel for treatment of superficial skin defects. Adv. Ski. Wound Care 2019, 32, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.M.; Stevenson, R.S.; Railer, R.F.; Clark, O.E.; Whitten, K.A.; Lee-Stubbs, R.B.; Anderson, D.R. Impairment of wound healing by reactive skin decontamination lotion (RSDL®) in a Gottingen minipig® model. Cutan. Ocul. Toxicol. 2020, 39, 143–157. [Google Scholar] [CrossRef]
- Nakano, T.; Sakamoto, M.; Katayama, Y.; Shimizu, Y.; Inoie, M.; Li, Y.; Yamanaka, H.; Tsuge, I.; Saito, S.; Morimoto, N. Dried human-cultured epidermis accelerates wound healing in a porcine partial-thickness skin defect model. Regen. Ther. 2023, 22, 203–209. [Google Scholar] [CrossRef]


| Year | Author | Number of Wounds per Animal | Weight Range (kg) | Wound Area (cm) | Wound Depth (mm) | Biopsy Size |
|---|---|---|---|---|---|---|
| 1981 | Stanley et al. [32] | not specified | 5–9 | 0.7 × 1 | 0.3 | not specified |
| 1992 | Pirone et al. [33] | 12 | 15–20 | 2.2 × 2.2 | 0.5 | not specified |
| 2000 | Olson et al. [34] | 72 | 20–25 | 1 × 2 | 0.4 | 2 × 3 cm |
| 2003 | Singer et al. [23] | ~38 | 20–30 | 2.5 × 2.5 | 0.6 | not specified |
| 2003 | Singer et al. [35] | 32 | 20–30 | 2.5 × 2.5 | 0.3–0.9 | not specified |
| 2007 | Singer et al. [36] | 10 | 40 | 2.5 × 2.5 | 0.6 | 1.25 × 1.25 cm |
| 2010 | Faucher et al. [37] | 2 | 18.14 | not specified | 0.56 & 0.76 | not specified |
| 2012 | Peura et al. [38] | 8 | 18–26 | 4 × 5 | 0.76 | 5 × 6 cm |
| 2013 | Masella et al. [39] | 24 | 25 | 4 × 2 | 0.15 | 2.5 × 5.5 cm |
| 2013 | Mauskar et al. [40] | 12 | 30–55 | 7.6 × 7.6 | ~1.5 | 9 times 3 mm punch |
| 2014 | Travis et al. [41] | 6 | 30–55 | 7.6 × 7.6 | ~1.5 | 9 times 3 mm punch |
| 2019 | Wlaschin et al. [42] | 4 | 28–32 | 2.5 × 2.5 | 0.5 | 0.8 × 5 cm |
| 2019 | Schiefer et al. [43] | 3 | 25.8 (±2.5) | 2.4 × 2.4 | 0.5 | not specified |
| 2020 | Connolly et al. [44] | 6 | 10–15 | 5 × 5 | 0.1 | 5 × 5 cm |
| 2023 | Nakano et al. [45] | 4 | ~27.5 | 8 × 10 | 1.5 | 6 times 5 mm punch |
| Present study | Tuca et al. | 12 | 24–57 | 3x3 | 1.2 | 4 × 4 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuca, A.-C.; Bernardelli de Mattos, I.; Funk, M.; Markovic, D.; Winter, R.; Lemarchand, T.; Kniepeiss, D.; Spendel, S.; Hartmann, B.; Ottoman, C.; et al. A Standardized Porcine Model for Partial-Thickness Wound Healing Studies: Design, Characterization, Model Validation, and Histological Insights. Int. J. Mol. Sci. 2024, 25, 7658. https://doi.org/10.3390/ijms25147658
Tuca A-C, Bernardelli de Mattos I, Funk M, Markovic D, Winter R, Lemarchand T, Kniepeiss D, Spendel S, Hartmann B, Ottoman C, et al. A Standardized Porcine Model for Partial-Thickness Wound Healing Studies: Design, Characterization, Model Validation, and Histological Insights. International Journal of Molecular Sciences. 2024; 25(14):7658. https://doi.org/10.3390/ijms25147658
Chicago/Turabian StyleTuca, Alexandru-Cristian, Ives Bernardelli de Mattos, Martin Funk, Danijel Markovic, Raimund Winter, Thomas Lemarchand, Daniela Kniepeiss, Stephan Spendel, Bernd Hartmann, Christian Ottoman, and et al. 2024. "A Standardized Porcine Model for Partial-Thickness Wound Healing Studies: Design, Characterization, Model Validation, and Histological Insights" International Journal of Molecular Sciences 25, no. 14: 7658. https://doi.org/10.3390/ijms25147658






