Structure and Composition of Spermatozoa Fibrous Sheath in Diverse Groups of Metazoa
Abstract
:1. Introduction

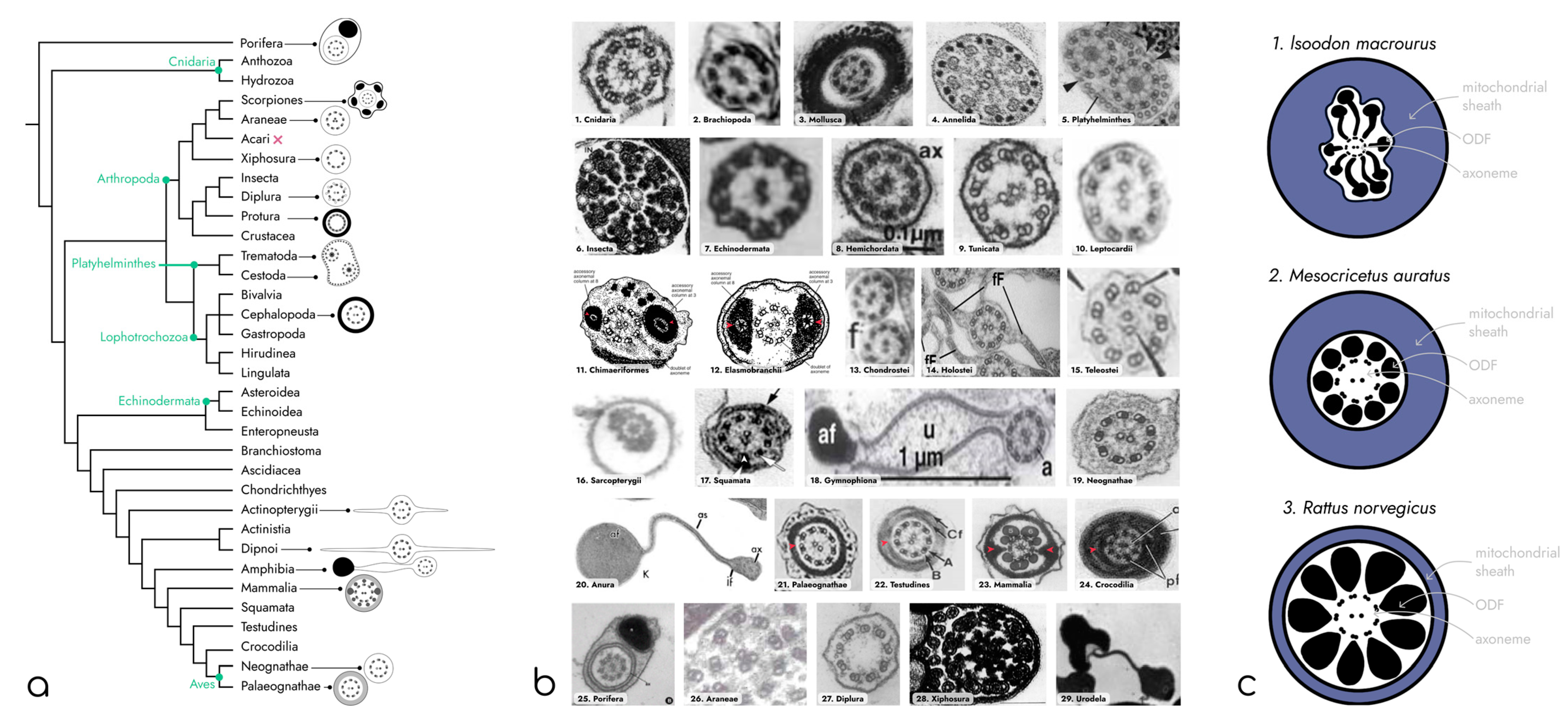
2. Diversity of the Structure of Sperm Flagella in Metazoa
2.1. Unusual Sperm Shapes
2.2. Diversity of Axoneme Anchoring Structures
2.3. Diversity of Axoneme Organization
2.4. Structures Surrounding Axoneme
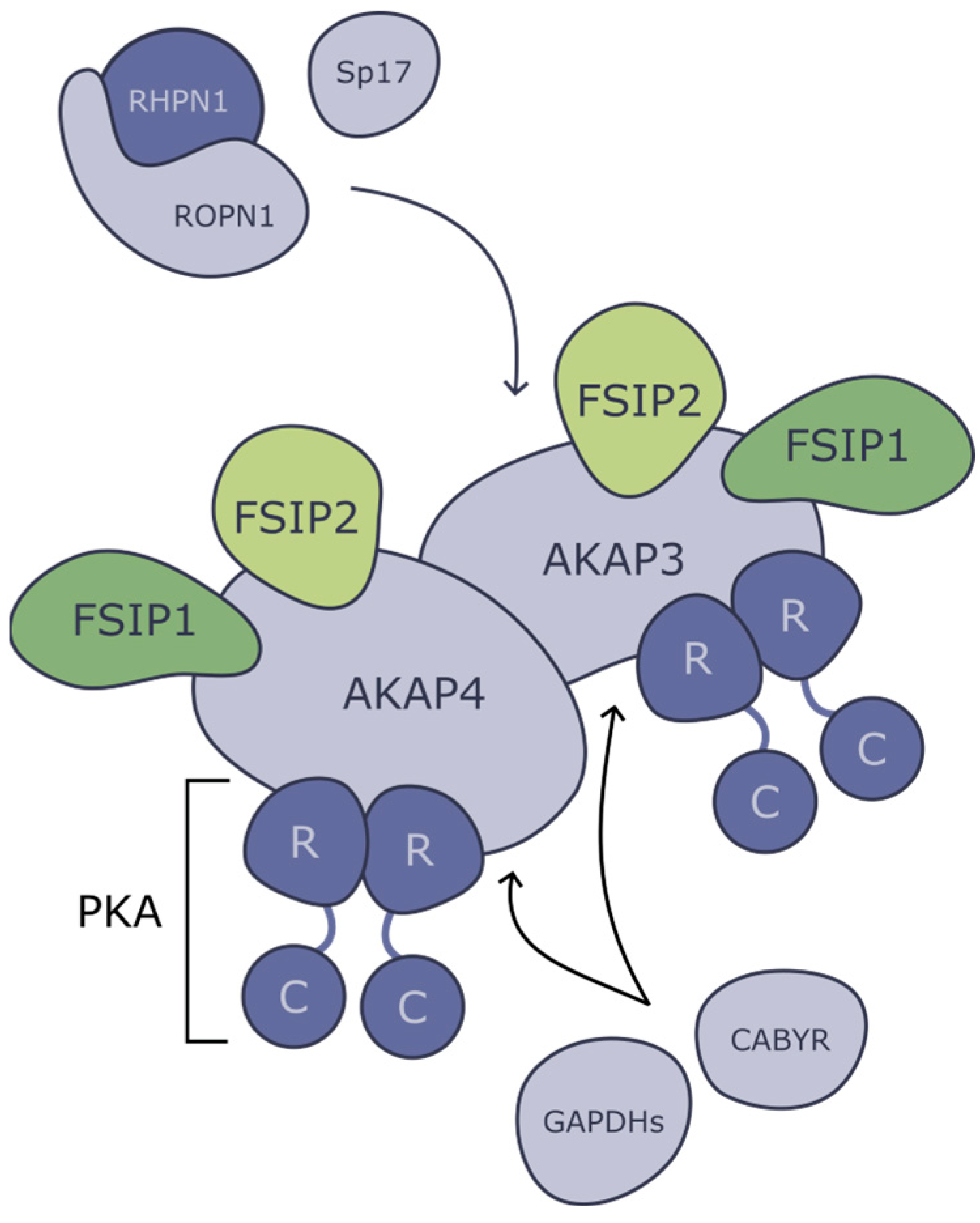
3. Proteins of Fibrous Sheath and Associated Structures
3.1. Protein Kinase-Associated Proteins
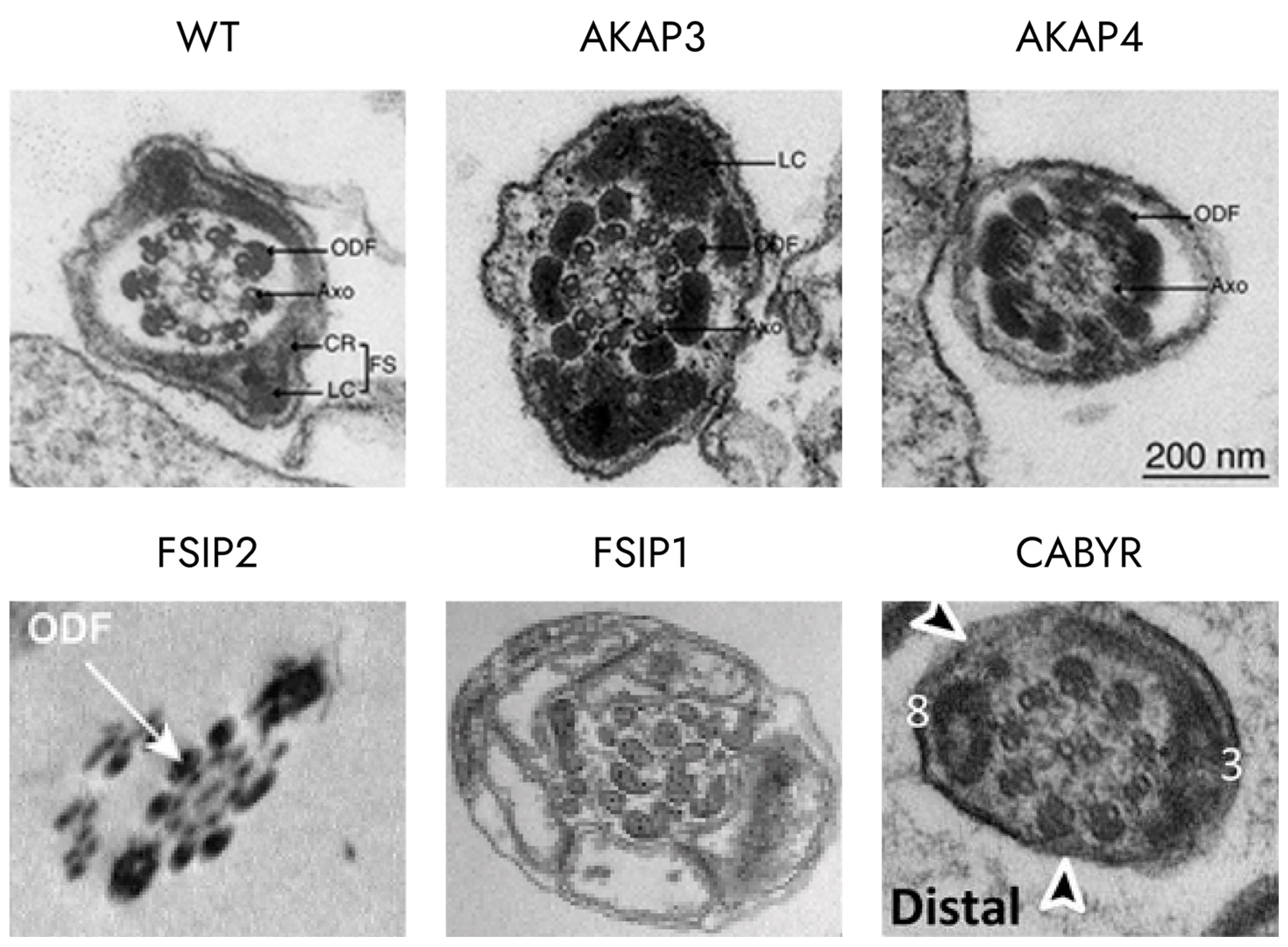
3.2. Glycolytic Enzymes
3.3. Intraflagellar Transport
3.4. Post-Translational Modifications
3.5. Protectors from Oxidative Stress
3.6. Proteins Affecting the Positioning of Longitudinal Columns
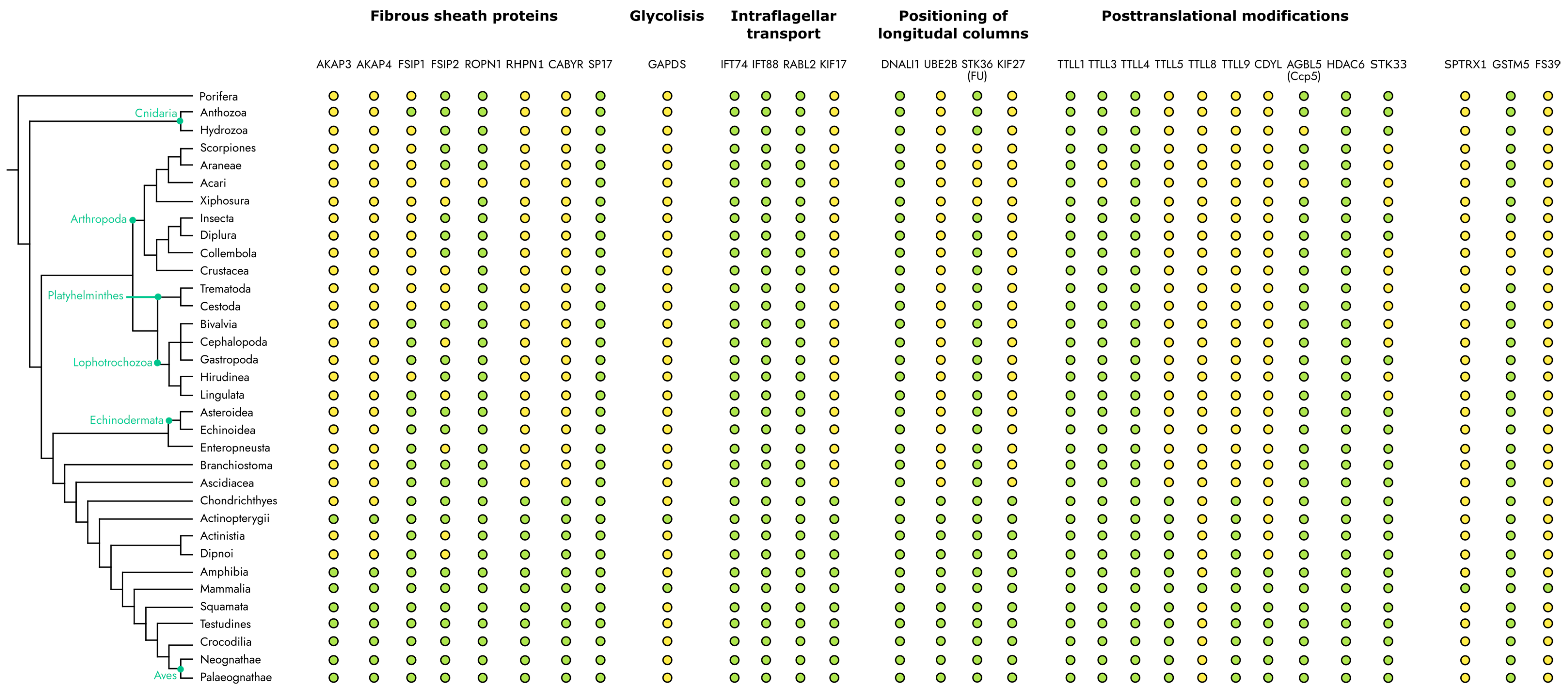
4. Evolutionary Distribution of the Fibrous Sheath Associated Proteins among Metazoa
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baccetti, B.; Afzelius, B.A. The Biology of the Sperm Cell; Monographs in developmental biology; S. Karger AG: Basel, Switzerland, 1976; 254p. [Google Scholar]
- Inaba, K. Molecular Architecture of the Sperm Flagella: Molecules for Motility and Signaling. Zool. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.W. The Mammalian Spermatozoon. Dev. Biol. 1975, 44, 394–436. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted Disruption of the Akap4 Gene Causes Defects in Sperm Flagellum and Motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Z.; Ping, P.; Wang, G.; Yuan, X.; Sun, F. Outer Dense Fibers Stabilize the Axoneme to Maintain Sperm Motility. J. Cell. Mol. Med. 2018, 22, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.C.; Hirth, F. Phylogenetic Tracing of Midbrain-Specific Regulatory Sequences Suggests Single Origin of Eubilaterian Brains. Sci. Adv. 2023, 9, eade8259. [Google Scholar] [CrossRef] [PubMed]
- Franzén, Å. Ultrastructure of Spermatozoa and Spermiogenesis in the Hydrozoan Cordylophora caspia with Comments on Structure and Evolution of the Sperm in the Cnidaria and the Porifera. Invertebr. Reprod. Dev. 1996, 29, 19–26. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Reunov, A.A. Ultrastructure of the Spermatozoon and Spermatogenesis of the Brachiopods Discinisca tenuis (Inarticulata) and Kraussina Rubra (Articulata). Invertebr. Reprod. Dev. 1994, 25, 23–31. [Google Scholar] [CrossRef]
- Healy, J.M. An Ultrastructural Study of Basommatophoran Spermatozoa (Mollusca, Gastropoda). Zool. Scr. 1983, 12, 57–66. [Google Scholar] [CrossRef]
- Braidotti, P.; Ferraguti, M. Two Sperm Types in the Spermatozeugmata of Tubifex tubifex (Annelida, Oligochaeta). J. Morphol. 1982, 171, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Świderski, Z.; Sripa, B.; Ribas, A. Ultrastructural Characters of the Spermatozoon of the Liver Fluke Opisthorchis Viverrini (Poirier, 1886) (Opisthorchiidae). Parasitol. Res. 2017, 116, 2499–2506. [Google Scholar] [CrossRef]
- Dallai, R.; Afzelius, B.A. Sperm Flagellum of Dacus Oleae (Gmelin) (Tephritidae) and Drosophila Melanogaster Meigen (Drosophilidae) (Diptera). Int. J. Insect Morphol. Embryol. 1991, 20, 215–222. [Google Scholar] [CrossRef]
- Dehn, P.F.; Hinsch, G.W. The Ultrastructural Organization of the Mature Spermatozoon of Luidia Clathrata (Say) (Echinodermata: Asteroidea). Gamete Res. 1981, 4, 547–553. [Google Scholar] [CrossRef]
- Lester, S.M. Ultrastructure of Adult Gonads and Development and Structure of the Larva of Rhabdopleura normani (Hemichordata: Pterobranchia). Acta Zool. 1988, 69, 95–109. [Google Scholar] [CrossRef]
- Jamieson, B.G.M. Spermatozoal Ultrastructure in Branchiostoma moretonensis Kelly, a Comparison with B. lanceolatum (Cephalochordata) and with Other Deuterostomes. Zool. Scr. 1984, 13, 223–229. [Google Scholar] [CrossRef]
- Stanley, H.P. The Fine Structure of Spermatozoa of Hydrolagus Colliei (Chondrichthyes, Holocephali). J. Ultrastruct. Res. 1983, 83, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.P. Fine Structure of Spermiogenesis in the Elasmobranch Fish Squalus Suckleyi. II. Late Stages of Differentiation and Structure of the Mature Spermatozoon. J. Ultrastruct. Res. 1971, 36, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Mattei, X. Contribution à l’étude de La Spermiogenèse et Des Spermatozoïdes de Poissons Par Les Méthodes de La Microscopie Électronique; Faculté Des Sciences, Université de Montpellier: Montpellier, France, 1969. [Google Scholar]
- Grandi, G.; Astolfi, G.; Chicca, M.; Pezzi, M. Ultrastructural Investigations on Spermatogenesis and Spermatozoan Morphology in the Endangered Adriatic Sturgeon, Acipenser naccarii (Chondrostei, Acipenseriformes). J. Morphol. 2018, 279, 1376–1396. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Emerson, C.J.; Crim, L.W. Ultrastructure of the Spermatozoa and Eggs of the Ocean Pout (Macrozoarces americanus L.), an Internally Fertilizing Marine Fish. Mol. Reprod. Dev. 1995, 42, 58–64. [Google Scholar] [CrossRef]
- Millot, J.; Tuzet, O. La Spermatogenése de Latimeria Chalumnæ Smith (Crossoptérygien Coelacanthidé). Ann. Sci. Nat. Zool. 1959, 12, 61–69. [Google Scholar]
- Tavares-Bastos, L.; Cunha, L.D.; Colli, G.R.; Báo, S.N. Ultrastructure of Spermatozoa of Scolecophidian Snakes (Lepidosauria, Squamata). Acta Zool. 2007, 88, 189–197. [Google Scholar] [CrossRef]
- Fawcett, D.W. A Comparative View of Sperm Ultrastructure. Biol. Reprod. Suppl. 1970, 2, 90–127. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T. Observations on the Fine Structure of the Developing Spermatid in the Domestic Chicken. J. Cell Biol. 1962, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.C.; Garda, A.A.; Teixeira, R.D.; Colli, G.R.; Báo, S.N. Comparative Analysis of the Sperm Ultrastructure of Three Species of Phyllomedusa (Anura, Hylidae). Acta Zool. 2004, 85, 257–262. [Google Scholar] [CrossRef]
- Soley, J.T. Ultrastructure of Ostrich (Struthio Camelus) Spermatozoa: I. Transmission Electron Microscopy. Onderstepoort J. Vet. Res. 1993, 60, 119–130. [Google Scholar] [PubMed]
- Hess, R.A.; Thurston, R.J.; Gist, D.H. Ultrastructure of the Turtle Spermatozoon. Anat. Rec. 1991, 229, 473–481. [Google Scholar] [CrossRef] [PubMed]
- San Agustin, J.T.; Pazour, G.J.; Witman, G.B. Intraflagellar Transport Is Essential for Mammalian Spermiogenesis but Is Absent in Mature Sperm. Mol. Biol. Cell 2015, 26, 4358–4372. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, B.G.M.; Scheltinga, D.M.; Tucker, A.D. The Ultrastructure of Spermatozoa of the Australian Freshwater Crocodile, Crocodylus Johnstoni Krefft, 1873 (Crocodylidae, Reptilia). J. Submicrosc. Cytol. Pathol. 1997, 29, 265–274. [Google Scholar]
- Riesgo, A.; Maldonado, M. An Unexpectedly Sophisticated, V-Shaped Spermatozoon in Demospongiae (Porifera): Reproductive and Evolutionary Implications: Modified sperm in porifera. Biol. J. Linn. Soc. 2009, 97, 413–426. [Google Scholar] [CrossRef]
- Alberti, G. Comparative Spermatology of Chelicerata: Review and Perspective. Mém. Muséum Natl. Hist. Nat. 1995, 166, 203–230. [Google Scholar]
- Yamamichi, Y.; Sekiguchi, K. Axoneme Patterns of Spermatozoa of Asian Horseshoe Crabs. Experientia 1982, 38, 1219–1220. [Google Scholar] [CrossRef]
- Bareth, C. An Ultrastructural Study of the Spermatids of Campodea C. remyi Denis (Diplura Campodeidea) at the Bundle Stage. Cell Tissue Res. 1974, 149, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Sever, D.M.; Tait, C.K.; Diller, L.V.; Burkholder, L. Ultrastructure of the Annual Cycle of Female Sperm Storage in Spermathecae of the Torrent Salamander, Rhyacotriton variegatus (Amphibia: Rhyacotritonidae). J. Morphol. 2004, 261, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.; Leigh, C.; Breed, W. Ultrastructure and Motility of Spermatozoa in the Male Reproductive Tract of Perameloid Marsupials. Reprod. Fertil. Dev. 1995, 7, 1141. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.-H.; Zhao, W.-L.; Wang, G.-S.; Sun, F. Comparative Analysis of Mammalian Sperm Ultrastructure Reveals Relationships between Sperm Morphology, Mitochondrial Functions and Motility. Reprod. Biol. Endocrinol. 2019, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Bré, M.-H.; Redeker, V.; Quibell, M.; Darmanaden-Delorme, J.; Bressac, C.; Cosson, J.; Huitorel, P.; Schmitter, J.-M.; Rossier, J.; Johnson, T.; et al. Axonemal Tubulin Polyglycylation Probed with Two Monoclonal Antibodies: Widespread Evolutionary Distribution, Appearance during Spermatozoan Maturation and Possible Function in Motility. J. Cell Sci. 1996, 109, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, C.; Caroti, D.; Bré, M.; Levilliers, N.; Dallai, R. Tubulin Glycylation and Glutamylation Deficiencies in Unconventional Insect Axonemes. Cell Motil. 2005, 61, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Reunov, A.; Klepal, W. Ultrastructural Study of Spermatogenesis in Phoronopsis harmeri (Lophophorata, Phoronida). Helgol. Mar. Res. 2004, 58, 1–10. [Google Scholar] [CrossRef]
- Ferraguti, M. The Comparative Ultrastructure of Sperm Flagella Central Sheath in Clitellata Reveals a New Autapomorphy of the Group. Zool. Scr. 1984, 13, 201–207. [Google Scholar] [CrossRef]
- Jamieson, B.G.M. The Ultrastructure and Phylogeny of Insect Spermatozoa, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0-521-27941-3. [Google Scholar]
- Dallai, R.; Bellon, P.L.; Lanzavecchia, S.; Afzelius, B.A. Sperm Axoneme in Some Apterygote Insects Examined by Computer-aided Image Analysis. Acta Zool. 1992, 73, 109–114. [Google Scholar] [CrossRef]
- Franzén, A. On Spermiogenesis, Morphology of the Spermatozoon, and Biology of Fertilization among Invertebrates. Zool Bidr. 1956, 31, 355–482. [Google Scholar]
- Healy, J.M. Ultrastructure of Spermiogenesis of Philippia (Psilaxis) Oxytropis, with Special Reference to the Taxonomic Position of the Architectonicidae (Gastropoda). Zoomorphology 1982, 101, 197–214. [Google Scholar] [CrossRef]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous Sheath of Mammalian Spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, R.M. Spermatozoa of Ciona Intestinalis and Analysis of Ascidian Fertilization. J. Morphol. 1977, 152, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Quagio-Grassiotto, I.; Baicere-Silva, C.M.; Santana, J.C.D.O.; Mirande, J.M. Spermiogenesis and Sperm Ultrastructure as Sources of Phylogenetic Characters. The Example of Characid Fishes (Teleostei: Characiformes). Zool. Anz. 2020, 289, 77–86. [Google Scholar] [CrossRef]
- Hearly, J.; Jamieson, B.G.M. The Ultrastructure of Spermatogenesis and Epididymal Spermatozoa of the Tuatara Sphenodon punctatus (Sphenodontida, Amniota). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 344, 187–199. [Google Scholar] [CrossRef]
- Retzius, G. Die Spermien Der: Amphibien. Biol. Untersuchungen Neue Folge 1906, 13, 49–70. [Google Scholar]
- Kierszenbaum, A.L. Sperm Axoneme: A Tale of Tubulin Posttranslation Diversity. Mol. Reprod. Dev. 2002, 62, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.B.; Gibbons, I.R. Adenosine Triphosphate-Induced Motility and Sliding of Filaments in Mammalian Sperm Extracted with Triton X-100. J. Cell Biol. 1975, 65, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.B.; Lesich, K.A. Functional Anatomy of the Mammalian Sperm Flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef]
- Woolley, D.M.; Carter, D.A.; Tilly, G.N. Compliance in the Neck Structures of the Guinea Pig Spermatozoon, as Indicated by Rapid Freezing and Electron Microscopy. J. Anat. 2008, 213, 336–341. [Google Scholar] [CrossRef]
- Lindemann, C.B. Functional Significance of the Outer Dense Fibers of Mammalian Sperm Examined by Computer Simulations with the Geometric Clutch Model. Cell Motil. Cytoskeleton 1996, 34, 258–270. [Google Scholar] [CrossRef]
- Brokaw, C.J. Regulation of Sperm Flagellar Motility by Calcium and cAMP-dependent Phosphorylation. J. Cell. Biochem. 1987, 35, 175–184. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, C.J.; Han, H.-L.; Lawrie, H.; Mack, S.R.; Zaneveld, L.J.D. Modulation of the Human Sperm Acrosome Reaction by Effectors of the Adenylate Cyclase/Cyclic AMP Second-messenger Pathway. J. Exp. Zool. 1991, 258, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Baro Graf, C.; Ritagliati, C.; Stival, C.; Luque, G.M.; Gentile, I.; Buffone, M.G.; Krapf, D. Everything You Ever Wanted to Know about PKA Regulation and Its Involvement in Mammalian Sperm Capacitation. Mol. Cell. Endocrinol. 2020, 518, 110992. [Google Scholar] [CrossRef]
- Gazo, I.; Dietrich, M.A.; Prulière, G.; Shaliutina-Kolešová, A.; Shaliutina, O.; Cosson, J.; Chenevert, J. Protein Phosphorylation in Spermatozoa Motility of Acipenser Ruthenus and Cyprinus Carpio. Reproduction 2017, 154, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Loza-Huerta, A.; Pacheco-Castillo, H.; Darszon, A.; Beltrán, C. Crosstalk between Protein Kinases A and C Regulates Sea Urchin Sperm Motility. Zygote 2022, 30, 398–409. [Google Scholar] [CrossRef]
- Das, R.; Esposito, V.; Abu-Abed, M.; Anand, G.S.; Taylor, S.S.; Melacini, G. cAMP Activation of PKA Defines an Ancient Signaling Mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Landmark, B.F.; Oyen, O.; Skalhegg, B.S.; Fauske, B.; Jahnsen, T.; Hansson, V. Cellular Location and Age-Dependent Changes of the Regulatory Subunits of cAMP-Dependent Protein Kinase in Rat Testis. Reproduction 1993, 99, 323–334. [Google Scholar] [CrossRef]
- Bertherat, J.; Horvath, A.; Groussin, L.; Grabar, S.; Boikos, S.; Cazabat, L.; Libe, R.; René-Corail, F.; Stergiopoulos, S.; Bourdeau, I.; et al. Mutations in Regulatory Subunit Type 1A of Cyclic Adenosine 5′-Monophosphate-Dependent Protein Kinase (PRKAR1A): Phenotype Analysis in 353 Patients and 80 Different Genotypes. J. Clin. Endocrinol. Metab. 2009, 94, 2085–2091. [Google Scholar] [CrossRef]
- Agustin, J.T.S.; Wilkerson, C.G.; Witman, G.B. The Unique Catalytic Subunit of Sperm cAMP-Dependent Protein Kinase Is the Product of an Alternative Cα mRNA Expressed Specifically in Spermatogenic Cells. Mol. Biol. Cell 2000, 11, 3031–3044. [Google Scholar] [CrossRef]
- San Agustin, J.T.; Witman, G.B. Differential Expression of the Cs and Cα1 Isoforms of the Catalytic Subunit of Cyclic 3′,5′-Adenosine Monophosphate-Dependent Protein Kinase in Testicular Cells. Biol. Reprod. 2001, 65, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Desseyn, J.-L.; Burton, K.A.; McKnight, G.S. Expression of a Nonmyristylated Variant of the Catalytic Subunit of Protein Kinase A during Male Germ-Cell Development. Proc. Natl. Acad. Sci. USA 2000, 97, 6433–6438. [Google Scholar] [CrossRef]
- Skålhegg, B.S.; Huang, Y.; Su, T.; Idzerda, R.L.; McKnight, G.S.; Burton, K.A. Mutation of the Cα Subunit of PKA Leads to Growth Retardation and Sperm Dysfunction. Mol. Endocrinol. 2002, 16, 630–639. [Google Scholar] [CrossRef]
- Wong, W.; Scott, J.D. AKAP Signalling Complexes: Focal Points in Space and Time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar] [CrossRef]
- Itoh, A.; Inaba, K.; Ohtake, H.; Fujinoki, M.; Morisawa, M. Characterization of a cAMP-Dependent Protein Kinase Catalytic Subunit from Rainbow Trout Spermatozoa. Biochem. Biophys. Res. Commun. 2003, 305, 855–861. [Google Scholar] [CrossRef]
- Zilli, L.; Schiavone, R.; Storelli, C.; Vilella, S. Molecular Mechanisms Determining Sperm Motility Initiation in Two Sparids (Sparus aurata and Lithognathus mormyrus). Biol. Reprod. 2008, 79, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yang, L.; Zhang, L.; Qi, H. Lack of AKAP3 Disrupts Integrity of the Subcellular Structure and Proteome of Mouse Sperm and Causes Male Sterility. Development 2020, 147, dev181057. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Eddy, E.M. Single Amino Acids Determine Specificity of Binding of Protein Kinase A Regulatory Subunits by Protein Kinase A Anchoring Proteins. J. Biol. Chem. 1999, 274, 29057–29062. [Google Scholar] [CrossRef]
- Brown, P.R.; Miki, K.; Harper, D.B.; Eddy, E.M. A-Kinase Anchoring Protein 4 Binding Proteins in the Fibrous Sheath of the Sperm Flagellum. Biol. Reprod. 2003, 68, 2241–2248. [Google Scholar] [CrossRef]
- Fang, X.; Gamallat, Y.; Chen, Z.; Mai, H.; Zhou, P.; Sun, C.; Li, X.; Li, H.; Zheng, S.; Liao, C.; et al. Hypomorphic and Hypermorphic Mouse Models of Fsip2 Indicate Its Dosage-Dependent Roles in Sperm Tail and Acrosome Formation. Development 2021, 148, dev199216. [Google Scholar] [CrossRef]
- Martinez, G.; Kherraf, Z.-E.; Zouari, R.; Fourati Ben Mustapha, S.; Saut, A.; Pernet-Gallay, K.; Bertrand, A.; Bidart, M.; Hograindleur, J.P.; Amiri-Yekta, A.; et al. Whole-Exome Sequencing Identifies Mutations in FSIP2 as a Recurrent Cause of Multiple Morphological Abnormalities of the Sperm Flagella. Hum. Reprod. 2018, 33, 1973–1984. [Google Scholar] [CrossRef]
- Liu, W.; Wu, H.; Wang, L.; Yang, X.; Liu, C.; He, X.; Li, W.; Wang, J.; Chen, Y.; Wang, H.; et al. Homozygous Loss-of-Function Mutations in FSIP2 Cause Male Infertility with Asthenoteratospermia. J. Genet. Genom. 2019, 46, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Tang, D.; Yu, H.; Geng, H.; Zhou, Y.; Shao, Z.; Li, K.; Gao, Y.; Guo, S.; Xu, C.; et al. Novel FSIP2 Variants Induce Super-Length Mitochondrial Sheath and Asthenoteratozoospermia in Humans. Int. J. Biol. Sci. 2023, 19, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Gamallat, Y.; Fang, X.; Mai, H.; Liu, X.; Li, H.; Zhou, P.; Han, D.; Zheng, S.; Liao, C.; Yang, M.; et al. Bi-Allelic Mutation in Fsip1 Impairs Acrosome Vesicle Formation and Attenuates Flagellogenesis in Mice. Redox Biol. 2021, 43, 101969. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Nakamura, K.; Kato, T.; Watanabe, N.; Ishizaki, T.; Kimura, K.; Mizoguchi, A.; Narumiya, S. Ropporin, a Sperm-Specific Binding Protein of Rhophilin, That Is Localized in the Fibrous Sheath of Sperm Flagella. J. Cell Sci. 2000, 113, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.E.; Dudiki, T.; Vijayaraghavan, S.; Carr, D.W. Loss of R2D2 Proteins ROPN1 and ROPN1L Causes Defects in Murine Sperm Motility, Phosphorylation, and Fibrous Sheath Integrity. Biol. Reprod. 2013, 88, 41. [Google Scholar] [CrossRef]
- Pelloni, M.; Paoli, D.; Majoli, M.; Pallotti, F.; Carlini, T.; Lenzi, A.; Lombardo, F. Molecular Study of Human Sperm RNA: Ropporin and CABYR in Asthenozoospermia. J. Endocrinol. Investig. 2018, 41, 781–787. [Google Scholar] [CrossRef]
- Frintrop, L.; Wiesehöfer, C.; Stoskus, A.; Hilken, G.; Dubicanac, M.; Von Ostau, N.E.; Rode, S.; Elgeti, J.; Dankert, J.T.; Wennemuth, G. cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement. Int. J. Mol. Sci. 2022, 23, 10607. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Mandal, A.; Wolkowicz, M.J.; Sen, B.; Westbrook, V.A.; Shetty, J.; Coonrod, S.A.; Klotz, K.L.; Kim, Y.-H.; Bush, L.A.; et al. CABYR, a Novel Calcium-Binding Tyrosine Phosphorylation-Regulated Fibrous Sheath Protein Involved in Capacitation. Dev. Biol. 2002, 242, 236–254. [Google Scholar] [CrossRef]
- Li, Y.-F.; He, W.; Mandal, A.; Kim, Y.-H.; Digilio, L.; Klotz, K.; Flickinger, C.J.; Herr, J.C.; Herr, J.C. CABYR Binds to AKAP3 and Ropporin in the Human Sperm Fibrous Sheath. Asian J. Androl. 2011, 13, 266–274. [Google Scholar] [CrossRef]
- Young, S.A.M.; Miyata, H.; Satouh, Y.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CABYR Is Essential for Fibrous Sheath Integrity and Progressive Motility in Mouse Spermatozoa. J. Cell Sci. 2016, 129, 4379–4387. [Google Scholar] [CrossRef] [PubMed]
- Zilli, L.; Schiavone, R.; Storelli, C.; Vilella, S. Molecular Mechanism Regulating Axoneme Activation in Marine Fish: A Review. Int. Aquat. Res. 2012, 4, 2. [Google Scholar] [CrossRef]
- Satarić, M.V.; Nemeš, T.; Sekulić, D.; Tuszynski, J.A. How Signals of Calcium Ions Initiate the Beats of Cilia and Flagella. Biosystems 2019, 182, 42–51. [Google Scholar] [CrossRef]
- Chiriva-Internati, M.; Gagliano, N.; Donetti, E.; Costa, F.; Grizzi, F.; Franceschini, B.; Albani, E.; Levi-Setti, P.E.; Gioia, M.; Jenkins, M.; et al. Sperm Protein 17 Is Expressed in the Sperm Fibrous Sheath. J. Transl. Med. 2009, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Yamasaki, N.; O’Rand, M.G. Sequence of a Rabbit Sperm Zona Pellucida Binding Protein and Localization during the Acrosome Reaction. Dev. Biol. 1994, 165, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Lea, I.A.; Widgren, E.E.; O’Rand, M.G. Association of Sperm Protein 17 with A-Kinase Anchoring Protein 3 in Flagella. Reprod. Biol. Endocrinol. 2004, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.D.; Hillhouse, E.W.; Vlad, M. Developmental Expression and Characterization of FS39, a Testis Complementary DNA Encoding an Intermediate Filament-Related Protein of the Sperm Fibrous Sheath1. Biol. Reprod. 2001, 65, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, S.; Hecht, N.B.; Goldberg, E.; Boatright, K.M.; Reed, J.C.; Millán, J.L. Testis-Specific Cytochrome c -Null Mice Produce Functional Sperm but Undergo Early Testicular Atrophy. Mol. Cell. Biol. 2002, 22, 5554–5562. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Ford, W.C. The Role of Glucose in Supporting Motility and Capacitation in Human Spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar] [CrossRef]
- Tanii, I.; Yagura, T.; Inagaki, N.; Nakayama, T.; Imaizumi, K.; Yoshinaga, K. Preferential Localization of Rat GAPDS on the Ribs of Fibrous Sheath of Sperm Flagellum and Its Expression during Flagellar Formation. ACTA Histochem. Cytochem. 2007, 40, 19–26. [Google Scholar] [CrossRef]
- Welch, J.E.; Barbee, R.R.; Magyar, P.L.; Bunch, D.O.; O’Brien, D.A. Expression of the Spermatogenic Cell-specific Glyceraldehyde 3-phosphate Dehydrogenase (GAPDS) in Rat Testis. Mol. Reprod. Dev. 2006, 73, 1052–1060. [Google Scholar] [CrossRef]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-Phosphate Dehydrogenase-S, a Sperm-Specific Glycolytic Enzyme, Is Required for Sperm Motility and Male Fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506. [Google Scholar] [CrossRef]
- Westhoff, D.; Kamp, G. Glyceraldehyde 3-Phosphate Dehydrogenase Is Bound to the Fibrous Sheath of Mammalian Spermatozoa. J. Cell Sci. 1997, 110 Pt 15, 1821–1829. [Google Scholar] [CrossRef]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple Glycolytic Enzymes Are Tightly Bound to the Fibrous Sheath of Mouse Spermatozoa1. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Feiden, S.; Stypa, H.; Wolfrum, U.; Wegener, G.; Kamp, G. A Novel Pyruvate Kinase (PK-S) from Boar Spermatozoa Is Localized at the Fibrous Sheath and the Acrosome. Reprod. Camb. Engl. 2007, 134, 81–95. [Google Scholar] [CrossRef]
- Nakamura, N.; Mori, C.; Eddy, E.M. Molecular Complex of Three Testis-Specific Isozymes Associated with the Mouse Sperm Fibrous Sheath: Hexokinase 1, Phosphofructokinase M, and Glutathione S-Transferase Mu Class 51. Biol. Reprod. 2010, 82, 504–515. [Google Scholar] [CrossRef]
- Lacey, S.E.; Foster, H.E.; Pigino, G. The Molecular Structure of Anterograde Intraflagellar Transport Trains. Mol. Biol. 2022. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, T.; Huang, Q.; Zhang, S.; Li, W.; Zhang, L.; Hess, R.A.; Pazour, G.J.; Zhang, Z. Intraflagellar Transport Protein 74 Is Essential for Spermatogenesis and Male Fertility in Mice. Biol. Reprod. 2019, 101, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, Z.; Zhu, T.; Jin, M.; Meng, D.; He, R.; Cao, Z.; Shen, Y.; Lu, C.; Cai, R.; et al. Disrupted Intraflagellar Transport Due to IFT74 Variants Causes Joubert Syndrome. Genet. Med. 2021, 23, 1041–1049. [Google Scholar] [CrossRef]
- Lorès, P.; Kherraf, Z.-E.; Amiri-Yekta, A.; Whitfield, M.; Daneshipour, A.; Stouvenel, L.; Cazin, C.; Cavarocchi, E.; Coutton, C.; Llabador, M.-A.; et al. A Missense Mutation in IFT74, Encoding for an Essential Component for Intraflagellar Transport of Tubulin, Causes Asthenozoospermia and Male Infertility without Clinical Signs of Bardet–Biedl Syndrome. Hum. Genet. 2021, 140, 1031–1043. [Google Scholar] [CrossRef]
- Tasaki, K.; Zhou, Z.; Ishida, Y.; Katoh, Y.; Nakayama, K. Compound Heterozygous IFT81 Variations in a Skeletal Ciliopathy Patient Cause Bardet–Biedl Syndrome-like Ciliary Defects. Hum. Mol. Genet. 2023, 32, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Perrault, I.; Halbritter, J.; Porath, J.D.; Gérard, X.; Braun, D.A.; Gee, H.Y.; Fathy, H.M.; Saunier, S.; Cormier-Daire, V.; Thomas, S.; et al. IFT81, Encoding an IFT-B Core Protein, as a Very Rare Cause of a Ciliopathy Phenotype. J. Med. Genet. 2015, 52, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Boegholm, N.; Petriman, N.A.; Loureiro-López, M.; Wang, J.; Vela, M.I.S.; Liu, B.; Kanie, T.; Ng, R.; Jackson, P.K.; Andersen, J.S.; et al. The IFT81-IFT74 Complex Acts as an Unconventional RabL2 GTPase -activating Protein during Intraflagellar Transport. EMBO J. 2023, 42, e111807. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Hagiya, Y.; Kubo, T.; Takei, R.; Katoh, Y.; Nakayama, K. RABL2 Interacts with the Intraflagellar Transport-B Complex and CEP19 and Participates in Ciliary Assembly. Mol. Biol. Cell 2017, 28, 1652–1666. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-T.; Hong, S.-R.; He, K.; Ling, K.; Shaiv, K.; Hu, J.; Lin, Y.-C. The Emerging Roles of Axonemal Glutamylation in Regulation of Cilia Architecture and Functions. Front. Cell Dev. Biol. 2021, 9, 622302. [Google Scholar] [CrossRef] [PubMed]
- Strzyz, P. Sperm Going in Circles. Nat. Rev. Mol. Cell Biol. 2021, 22, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Gadadhar, S.; Alvarez Viar, G.; Hansen, J.N.; Gong, A.; Kostarev, A.; Ialy-Radio, C.; Leboucher, S.; Whitfield, M.; Ziyyat, A.; Touré, A.; et al. Tubulin Glycylation Controls Axonemal Dynein Activity, Flagellar Beat, and Male Fertility. Science 2021, 371, eabd4914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Chen, J.; Wu, B.; Tang, S.; Zhang, F.; Liu, C.; Wang, L. Dnali1 Is Required for Sperm Motility and Male Fertility in Mice. Basic Clin. Androl. 2023, 33, 32. [Google Scholar] [CrossRef] [PubMed]
- Escalier, D. New Insights into the Assembly of the Periaxonemal Structures in Mammalian Spermatozoa. Biol. Reprod. 2003, 69, 373–378. [Google Scholar] [CrossRef]
- Nozawa, Y.I.; Yao, E.; Gacayan, R.; Xu, S.-M.; Chuang, P.-T. Mammalian Fused Is Essential for Sperm Head Shaping and Periaxonemal Structure Formation during Spermatogenesis. Dev. Biol. 2014, 388, 170–180. [Google Scholar] [CrossRef]
- Yogo, K. Molecular Basis of the Morphogenesis of Sperm Head and Tail in Mice. Reprod. Med. Biol. 2022, 21, e12466. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Hansen, G.; Fontenot, G.; Read, R. Tubulin Tyrosine Ligase-like 1 Deficiency Results in Chronic Rhinosinusitis and Abnormal Development of Spermatid Flagella in Mice. Vet. Pathol. 2010, 47, 703–712. [Google Scholar] [CrossRef]
- Ikegami, K.; Sato, S.; Nakamura, K.; Ostrowski, L.E.; Setou, M. Tubulin Polyglutamylation Is Essential for Airway Ciliary Function through the Regulation of Beating Asymmetry. Proc. Natl. Acad. Sci. USA 2010, 107, 10490–10495. [Google Scholar] [CrossRef]
- Konno, A.; Ikegami, K.; Konishi, Y.; Yang, H.-J.; Abe, M.; Yamazaki, M.; Sakimura, K.; Yao, I.; Shiba, K.; Inaba, K.; et al. Ttll9-/- Mice Sperm Flagella Show Shortening of Doublet 7, Reduction of Doublet 5 Polyglutamylation and a Stall in Beating. J. Cell Sci. 2016, 129, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, J.; Rogowski, K.; Miro, J.; Lacroix, B.; Eddé, B.; Janke, C. A Targeted Multienzyme Mechanism for Selective Microtubule Polyglutamylation. Mol. Cell 2007, 26, 437–448. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Rong, Y.; Bansal, P.K.; Wei, P.; Guo, H.; Morgan, J.I. TTLL1 and TTLL4 Polyglutamylases Are Required for the Neurodegenerative Phenotypes in Pcd Mice. PLoS Genet. 2022, 18, e1010144. [Google Scholar] [CrossRef]
- Lee, G.-S.; He, Y.; Dougherty, E.J.; Jimenez-Movilla, M.; Avella, M.; Grullon, S.; Sharlin, D.S.; Guo, C.; Blackford, J.A.; Awasthi, S.; et al. Disruption of Ttll5/Stamp Gene (Tubulin Tyrosine Ligase-like Protein 5/SRC-1 and TIF2-Associated Modulatory Protein Gene) in Male Mice Causes Sperm Malformation and Infertility. J. Biol. Chem. 2013, 288, 15167–15180. [Google Scholar] [CrossRef]
- Giordano, T.; Gadadhar, S.; Bodakuntla, S.; Straub, J.; Leboucher, S.; Martinez, G.; Chemlali, W.; Bosc, C.; Andrieux, A.; Bieche, I.; et al. Loss of the Deglutamylase CCP5 Perturbs Multiple Steps of Spermatogenesis and Leads to Male Infertility. J. Cell Sci. 2019, 132, jcs226951. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Wei, P.; Morgan, J.I. Role of Cytosolic Carboxypeptidase 5 in Neuronal Survival and Spermatogenesis. Sci. Rep. 2017, 7, 41428. [Google Scholar] [CrossRef]
- Bhagwat, S.; Dalvi, V.; Chandrasekhar, D.; Matthew, T.; Acharya, K.; Gajbhiye, R.; Kulkarni, V.; Sonawane, S.; Ghosalkar, M.; Parte, P. Acetylated α-Tubulin Is Reduced in Individuals with Poor Sperm Motility. Fertil. Steril. 2014, 101, 95–104.e3. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Caron, C.; Matthias, G.; Hess, D.; Khochbin, S.; Matthias, P. HDAC-6 Interacts with and Deacetylates Tubulin and Microtubules in Vivo. EMBO J. 2003, 22, 1168–1179. [Google Scholar] [CrossRef]
- Parab, S.; Shetty, O.; Gaonkar, R.; Balasinor, N.; Khole, V.; Parte, P. HDAC6 Deacetylates Alpha Tubulin in Sperm and Modulates Sperm Motility in Holtzman Rat. Cell Tissue Res. 2015, 359, 665–678. [Google Scholar] [CrossRef]
- Parab, S.; Dalvi, V.; Mylavaram, S.; Kishore, A.; Idicula-Thomas, S.; Sonawane, S.; Parte, P. Tubulin Acetylation: A Novel Functional Avenue for CDYL in Sperm. Cytoskeleton 2017, 74, 331–342. [Google Scholar] [CrossRef]
- Jassim, A.; Gillott, D.J.; Al-Zuhdi, Y. Human Sperm Tall Fibrous Sheath Undergoes Phosphorylation during Its Development. Hum. Reprod. 1991, 6, 1135–1142. [Google Scholar] [CrossRef]
- Inaba, K.; Kagami, O.; Ogawa, K. Tctex2-Related Outer Arm Dynein Light Chain Is Phosphorylated at Activation of Sperm Motility. Biochem. Biophys. Res. Commun. 1999, 256, 177–183. [Google Scholar] [CrossRef]
- Bracho, G.E.; Fritch, J.J.; Tash, J.S. Identification of Flagellar Proteins That Initiate the Activation of Sperm Motilityin Vivo. Biochem. Biophys. Res. Commun. 1998, 242, 231–237. [Google Scholar] [CrossRef]
- Dey, C.S.; Brokaw, C.J. Activation of Dona Sperm Motility: Phosphorylation of Dynein Polypeptides and Effects of a Tyrosine Kinase Inhibitor. J. Cell Sci. 1991, 100, 815–824. [Google Scholar] [CrossRef]
- Chung, J.-J.; Shim, S.-H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally Distinct Ca2+ Signaling Domains of Sperm Flagella Orchestrate Tyrosine Phosphorylation and Motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Carrera, A.; Moos, J.; Ning, X.P.; Gerton, G.L.; Tesarik, J.; Kopf, G.S.; Moss, S.B. Regulation of Protein Tyrosine Phosphorylation in Human Sperm by a Calcium/Calmodulin-Dependent Mechanism: Identification of A Kinase Anchor Proteins as Major Substrates for Tyrosine Phosphorylation. Dev. Biol. 1996, 180, 284–296. [Google Scholar] [CrossRef]
- Jha, K.N.; Shivaji, S. Identification of the Major Tyrosine Phosphorylated Protein of Capacitated Hamster Spermatozoa as a Homologue of Mammalian Sperm a Kinase Anchoring Protein. Mol. Reprod. Dev. 2002, 61, 258–270. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Chen, H.; Cui, Y.; Situ, C.; Yao, L.; Zhang, X.; Lu, S.; Liu, L.; Li, L.; et al. STK33 Phosphorylates Fibrous Sheath Protein AKAP3/4 to Regulate Sperm Flagella Assembly in Spermiogenesis. Mol. Cell. Proteomics 2023, 22, 100564. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive Oxygen Species and Sperm Physiology in: Reviews of Reproduction. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of Reactive Oxygen Species in Male Infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Batruch, I.; Lecker, I.; Kagedan, D.; Smith, C.R.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Proteomic Analysis of Seminal Plasma from Normal Volunteers and Post-Vasectomy Patients Identifies over 2000 Proteins and Candidate Biomarkers of the Urogenital System. J. Proteome Res. 2011, 10, 941–953. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive Oxygen Species and Sperm Cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vizuete, A.; Ljung, J.; Damdimopoulos, A.E.; Gustafsson, J.Å.; Oko, R.; Pelto-Huikko, M.; Spyrou, G. Characterization of Sptrx, a Novel Member of the Thioredoxin Family Specifically Expressed in Human Spermatozoa. J. Biol. Chem. 2001, 276, 31567–31574. [Google Scholar] [CrossRef]
- Jiménez, A.; Johansson, C.; Ljung, J.; Sagemark, J.; Berndt, K.D.; Ren, B.; Tibbelin, G.; Ladenstein, R.; Kieselbach, T.; Holmgren, A.; et al. Human Spermatid-specific Thioredoxin-1 (Sptrx-1) Is a Two-domain Protein with Oxidizing Activity. FEBS Lett. 2002, 530, 79–84. [Google Scholar] [CrossRef]
- Yu, Y.; Oko, R.; Miranda-Vizuete, A. Developmental Expression of Spermatid-Specific Thioredoxin-1 Protein: Transient Association to the Longitudinal Columns of the Fibrous Sheath During Sperm Tail Formation1. Biol. Reprod. 2002, 67, 1546–1554. [Google Scholar] [CrossRef]
- Llavanera, M.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Fernández-Fuertes, B.; Yeste, M. The Triple Role of Glutathione S-Transferases in Mammalian Male Fertility. Cell. Mol. Life Sci. 2020, 77, 2331–2342. [Google Scholar] [CrossRef]
- Fulcher, K.D.; Welch, J.E.; Klapper, D.G.; O’Brien, D.A.; Eddy, E.M. Identification of a unique μ-class glutathione S-transferase in mouse spermatogenic cells. Mol. Reprod. Dev. 1995, 42, 415–424. [Google Scholar] [CrossRef]
- Mandal, A.; Naaby-Hansen, S.; Wolkowicz, M.J.; Klotz, K.; Shetty, J.; Retief, J.D.; Coonrod, S.A.; Kinter, M.; Sherman, N.; Cesar, F.; et al. FSP95, A Testis-Specific 95-Kilodalton Fibrous Sheath Antigen That Undergoes Tyrosine Phosphorylation in Capacitated Human Spermatozoa1. Biol. Reprod. 1999, 61, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Li, Y.; Li, K.; Xu, C.; Gao, Y.; Lv, M.; Guo, R.; Xu, Y.; Zhou, P.; et al. DNALI1 Deficiency Causes Male Infertility with Severe Asthenozoospermia in Humans and Mice by Disrupting the Assembly of the Flagellar Inner Dynein Arms and Fibrous Sheath. Cell Death Dis. 2023, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, D.; Tegenfeldt, F.; Manni, M.; Seppey, M.; Berkeley, M.; Kriventseva, E.V.; Zdobnov, E.M. OrthoDB V11: Annotation of Orthologs in the Widest Sampling of Organismal Diversity. Nucleic Acids Res. 2023, 51, D445–D451. [Google Scholar] [CrossRef] [PubMed]

| Protein | Cellular Localization | Function |
|---|---|---|
| Protein-kinase A PKA | Fibrous sheath | Phosphorylation of different targets, signaling |
| PKA-associated protein 3 AKAP3 | Structural protein of circumferential ribs | |
| PKA-associated protein 4 AKAP4 | Structural protein of circumferential ribs and longitudinal columns | |
| FS-interacting protein 1 FSIP1 | Interaction with IFT machinery | |
| FS-interacting protein 2 FSIP2 | Structural protein of FS. Possibly involved in control of mitochondria | |
| Ropporin | Binding of rhophilin | |
| Rhophilin | Putative target of small GTPase Rho | |
| Ca2+-binding Y-phosphorylation-regulated protein CABYR | Calcium signaling during capacitation, FS development | |
| Sp17 | Capacitation, structural protein of the FS | |
| FS39 | Structural protein of the FS | |
| Glyceraldehyde 3-phosphate dehydrogenase-S GAPDS | Glycolytic enzyme | |
| IFT74, IFT81 | Intraflagellar transport | Core components of the IFT complex. Transport of β-tubulin |
| Rab-like 2 RABL2 | Small GTPase. Initiation of anterograde IFT | |
| IFT88 | ||
| Tubulin tyrosine ligase-like 3, 8 TTLL3, TTLL8 | Axoneme | Tubulin glycylation |
| Tubulin tyrosine ligase-like 1, 9, 4, 5 TTLL1, TTLL9, TTLL4, TTLL5 | Tubulin polyglutamylation | |
| Cytoplasmic carboxypeptidase 5 CCP5 (AGBL5) | Tubulin deglutamination | |
| Histone deacetylase-6 HDAC6 | Tubulin deacetylation | |
| Chromodomain Y Like CDYL | ||
| Serine/Threonine kinase 33 STK33 | Phosphorylation of AKAP3 and AKAP4 | |
| Sperm-specific Thioredoxin-1 SPTRX-1 | Thioredoxin. Protection from oxidative stress, regulation of disulfide bond formation, FS formation | |
| Glutathione S-transferase mu class GSTm5 | Detoxification | |
| Ubiquitin-conjugating enzyme E2 B UBE2B | Positioning of longitudinal columns | |
| Dynein Axonemal Light Intermediate Chain 1 Dnali1 | Dynein-associated transport and the assembly of AKAP3 and AKAP4 | |
| Fused Fu | Kinesin transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guseva, E.A.; Buev, V.S.; Mirzaeva, S.E.; Pletnev, P.I.; Dontsova, O.A.; Sergiev, P.V. Structure and Composition of Spermatozoa Fibrous Sheath in Diverse Groups of Metazoa. Int. J. Mol. Sci. 2024, 25, 7663. https://doi.org/10.3390/ijms25147663
Guseva EA, Buev VS, Mirzaeva SE, Pletnev PI, Dontsova OA, Sergiev PV. Structure and Composition of Spermatozoa Fibrous Sheath in Diverse Groups of Metazoa. International Journal of Molecular Sciences. 2024; 25(14):7663. https://doi.org/10.3390/ijms25147663
Chicago/Turabian StyleGuseva, Ekaterina A., Vitaly S. Buev, Sabina E. Mirzaeva, Philipp I. Pletnev, Olga A. Dontsova, and Petr V. Sergiev. 2024. "Structure and Composition of Spermatozoa Fibrous Sheath in Diverse Groups of Metazoa" International Journal of Molecular Sciences 25, no. 14: 7663. https://doi.org/10.3390/ijms25147663





