Transcriptome and Metabolome Analyses of Leaves from Cutting Rejuvenation of Ancient Cinnamomum camphora
Abstract
1. Introduction
2. Results
2.1. The Leaf Phenotype and Tissue Structure of Ancient C. camphora and Its Cutting Plantlets
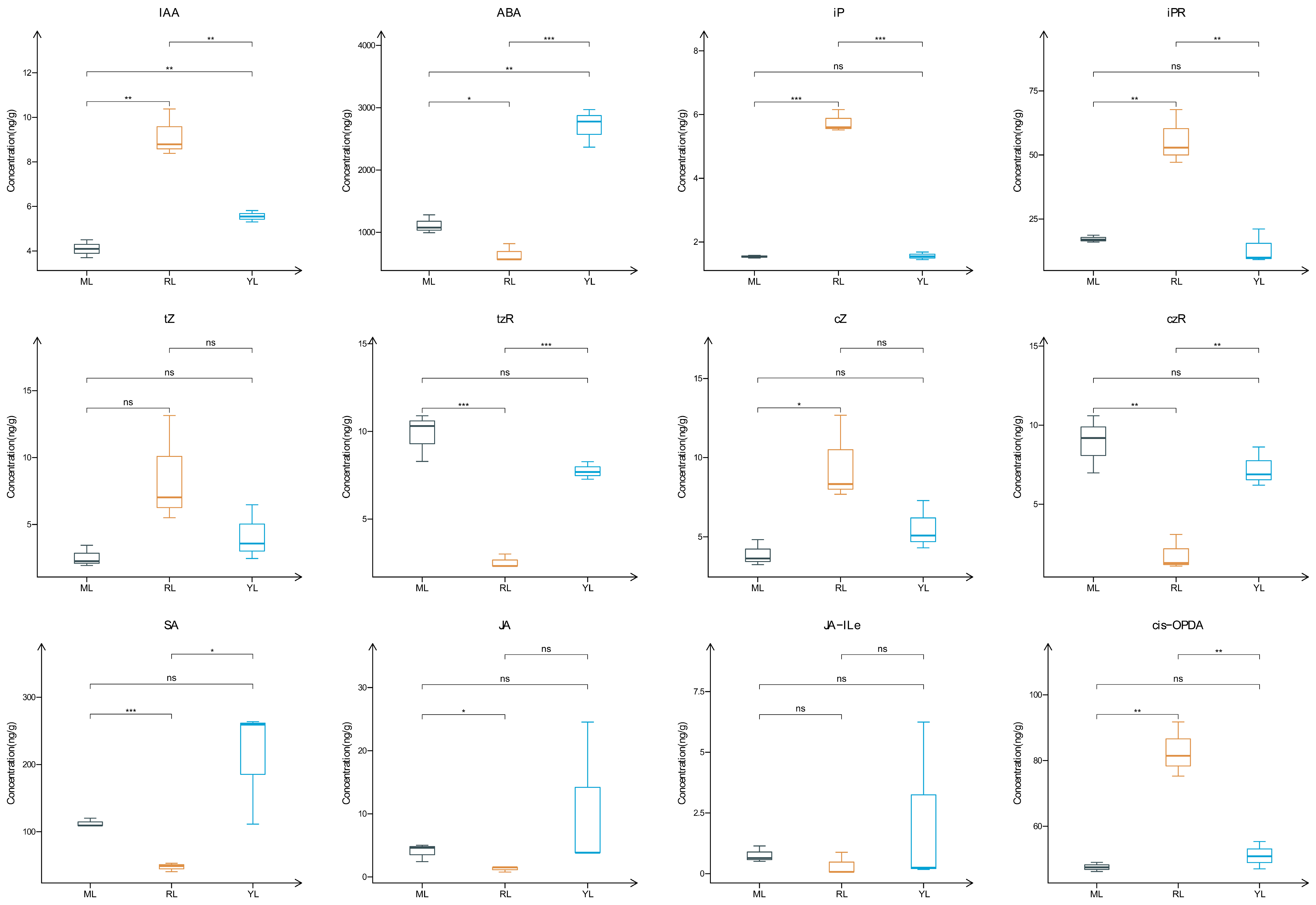
2.2. Analysis of Plant Hormone Contents in the Leaves of an Ancient C. camphora and Its Cutting Plantlets
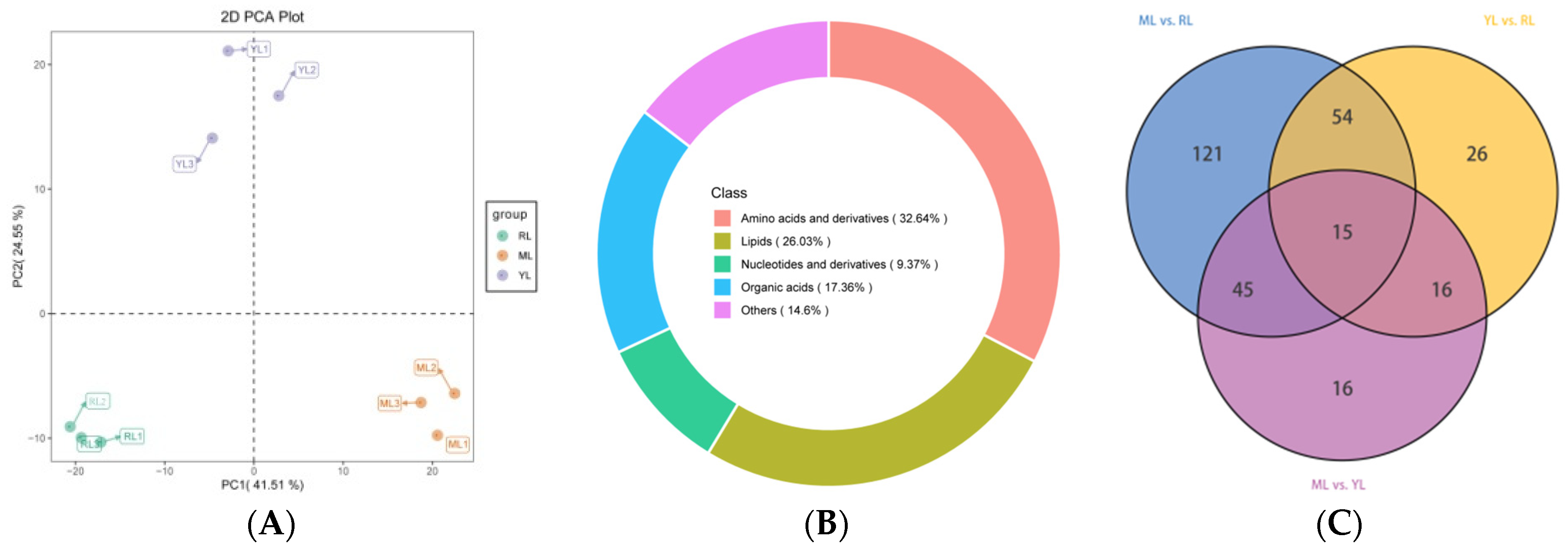
2.3. Identification of DAMs in the Leaves of Ancient C. camphora and Its Cutting Plantlets
2.4. DEG Identification and Analysis in the Leaves of an Ancient C. camphora and Its Cutting Plantlets
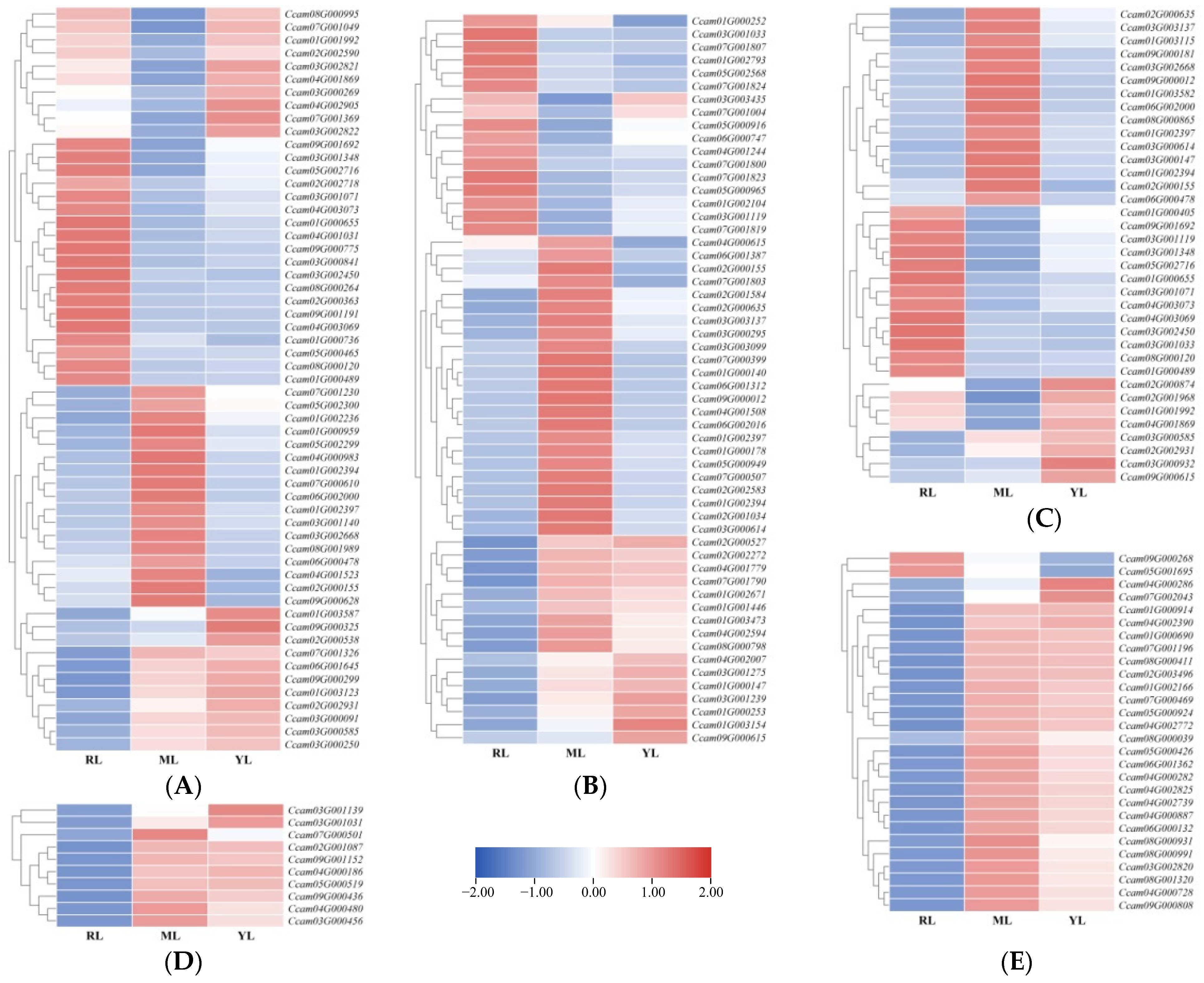
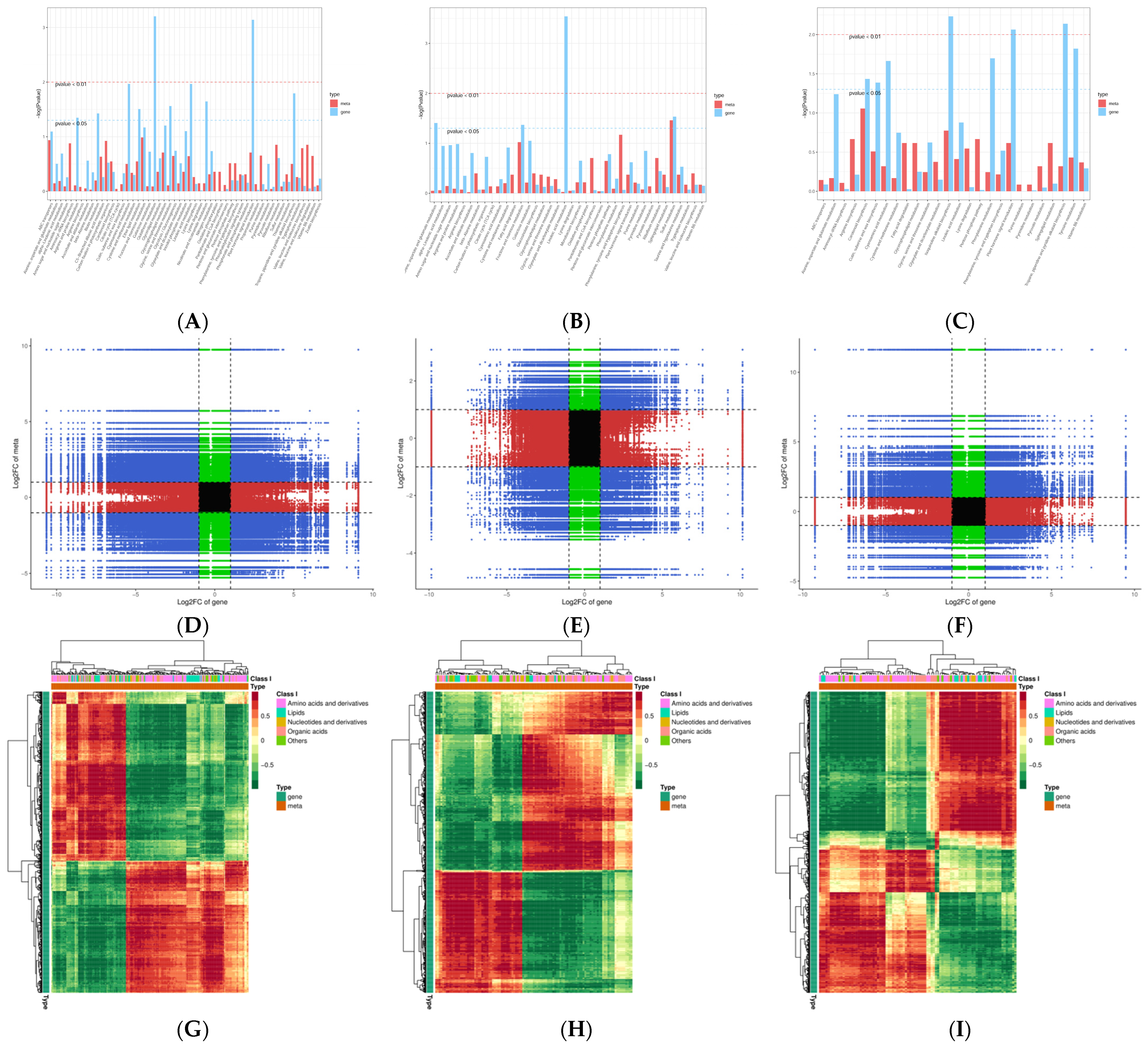
2.5. The Sequence Data Conjoint Analysis of DEGs and DAMs
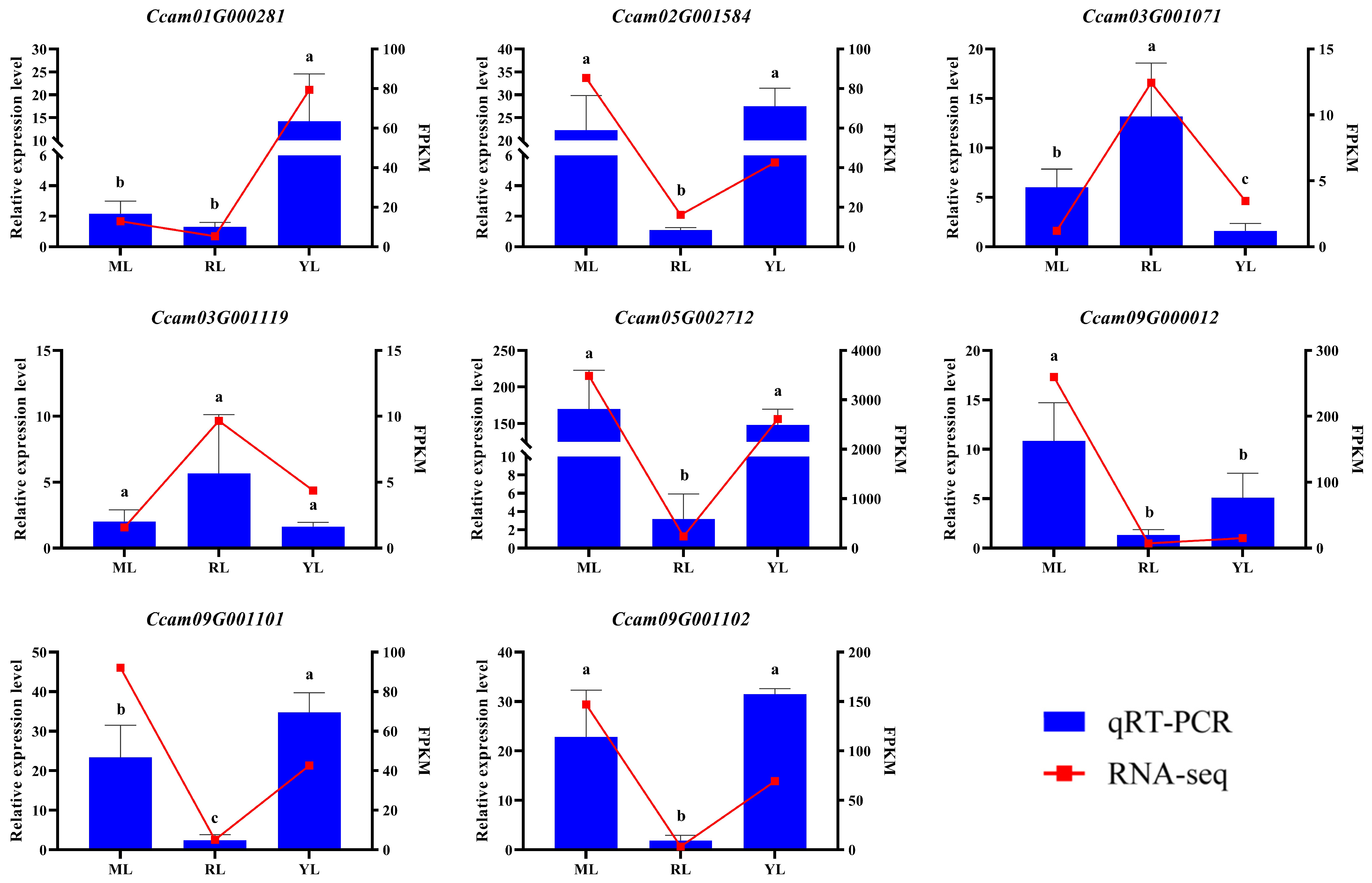
2.6. qRT–PCR Verification of DEGs
3. Discussion
3.1. Leaf Differences between Juvenile and Mature Plants
3.2. Plant Hormone Contents and Gene Expression Regulatory Function
3.3. Identification of Differential Accumulated Primary Metabolites
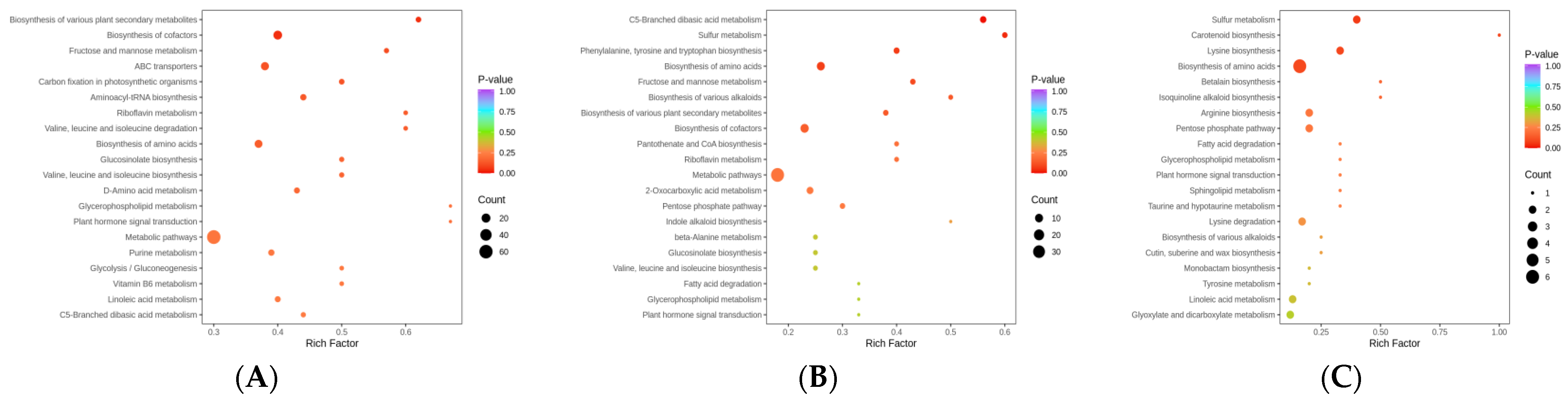
3.4. Gene Identification of Metabolic Pathways Related to Stress Resistance
4. Materials and Methods
4.1. Plant Materials and Cutting Method
4.2. Leaf Cytological Structural Observation
4.3. Metabolome Analysis
4.3.1. Plant Hormone Metabolome Sequencing and Analysis
4.3.2. Primary Metabolome Sequencing and Analysis
4.4. RNA Extraction and Transcriptome Sequencing
4.5. Quantitative Real-Time PCR (qRT–PCR) Analysis
4.6. Data Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Gu, R. A review on the rejuvenation of mature trees. Chin. Bull. Bot. 2005, 22, 753–760. [Google Scholar]
- Poethig, R.S. Phase change and the regulation of developmental timing in plants. Science 2003, 301, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S. The past, present, and future of vegetative phase change. Plant Physiol. 2010, 154, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Heide, O.M. Juvenility, maturation and rejuvenation in plants: Adventitious bud formation as a novel rejuvenation process. J. Hortic. Sci. Biotechnol. 2018, 94, 2–11. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, S.; Tong, M.; Lu, J.; Wang, T.; Xu, Y.; Li, W.; Wang, L. Physiological and genetic analysis of leaves from the resprouters of an old Ginkgo biloba tree. Forests 2021, 12, 1255. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, L.; Lu, J.; Shen, N.; Wang, L.; Liu, S.; Wang, Q.; Yu, W.; Kato-Noguchi, H.; Li, W.; et al. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic. Res. 2022, 9, uhab018. [Google Scholar] [CrossRef]
- Cannon, C.H.; Piovesan, G.; Munné-Bosch, S. Old and ancient trees are life history lottery winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 2022, 8, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, G.; Cannon, C.H.; Liu, J.; Munné-Bosch, S. Ancient trees: Irreplaceable conservation resource for ecosystem restoration. Trends Ecol. Evol. 2022, 37, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Blicharska, M.; Mikusiński, G. Incorporating social and cultural significance of large old trees in conservation policy. Conserv. Biol. 2014, 28, 1558–1567. [Google Scholar] [CrossRef]
- Winkelmann, T.; Spethmann, W.; Seegert, A. Propagation of 285-year-old alley linden (Tilia × vulgaris) trees via long cuttings. Eur. J. Hortic. Sci. 2020, 85, 160–168. [Google Scholar] [CrossRef]
- Khan, M.; Rozhon, W.; Poppenberger, B. The role of hormones in the aging of plants—A mini-review. Gerontology 2014, 60, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Explant rejuvenation in the clonal propagation of woody plants. Plant Cell Tissue Organ Cult. 2023, 154, 209–212. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, N.; Zhang, X.; Zhang, Y.; Wang, Y.; Wu, T.; Xu, X.; Han, Z. The lose of juvenility elicits adventitious rooting recalcitrance in apple rootstocks. Plant Cell Tissue Organ Cult. 2014, 119, 51–63. [Google Scholar] [CrossRef]
- Jayaraman, J.; Miret, J.A.; Munné-Bosch, S.; Dijkwel, P.P. Redox and hormone profiling of a Nicotiana tabacum dedifferentiated protoplast culture suggests a role for a cytokinin and gibberellins in plant totipotency. Plant Cell Tissue Organ Cult. 2016, 124, 295–306. [Google Scholar] [CrossRef]
- Vining, K.J.; Romanel, E.; Jones, R.C.; Klocko, A.; Alves-Ferreira, M.; Hefer, C.A.; Amarasinghe, V.; Dharmawardhana, P.; Naithani, S.; Ranik, M.; et al. The floral transcriptome of Eucalyptus grandis. New Phytol. 2015, 206, 1406–1422. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Qu, C.; Yang, J.; Zhang, W.; Ji, Y. Transcriptome changes in the phenylpropanoid pathway in senescing leaves of Toona sinensis. Acta Physiol. Plant. 2019, 41, 126. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, Y.; Wang, G.; Wang, T.; Cao, F. Integrated analysis of the transcriptome and metabolome in young and mature leaves of Ginkgo biloba L. Ind. Crops Prod. 2020, 143, 111906. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, B.; Yang, L.; Wang, D. Integrated analysis of the transcriptome and metabolome in young and mature leaves of Yunnanopilia longistaminea. Plant Biotechnol. Rep. 2022, 16, 553–564. [Google Scholar] [CrossRef]
- Suntichaikamolkul, N.; Tantisuwanichkul, K.; Prombutara, P.; Kobtrakul, K.; Zumsteg, J.; Wannachart, S.; Schaller, H.; Yamazaki, M.; Saito, K.; De-Eknamkul, W.; et al. Transcriptome analysis of Pueraria candollei var. mirifica for gene discovery in the biosyntheses of isoflavones and miroestrol. BMC Plant Biol. 2019, 19, 581. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Lin, Y.; Zhu, H.; Tang, L.; Wang, X.; Wang, X. Comparative transcriptome and weighted correlation network analyses reveal candidate genes involved in chlorogenic acid biosynthesis in sweet potato. Sci. Rep. 2022, 12, 2770. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Gao, L.; Fang, Z.; Lei, T.; Xing, D.; Ding, Y.; Rashid, A.; Zhuang, J.; Zhang, Q.; Gu, C.; et al. A flavonoid metabolon: Cytochrome b5 enhances B-ring trihydroxylated flavan-3-ols synthesis in tea plants. Plant J. 2024, 118, 1793–1814. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lu, D.; Wang, S.; Lu, Z.; Liu, W.; Wang, X.; Fang, Z.; He, X. Integrated transcriptomic and metabolomic analysis provides insight into the regulation of leaf senescence in rice. Sci. Rep. 2021, 11, 14083. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gordon, M.I.; Amarasinghe, V.; Strauss, S.H. Extensive transcriptome changes during seasonal leaf senescence in field-grown black cottonwood (Populus trichocarpa Nisqually-1). Sci. Rep. 2020, 10, 6581. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-X.; Liu, K.; Chen, Y.; Huang, X.; Liang, W.; Li, B.; Shen, Y.; Liang, H. Comprehensive transcriptome and metabolome analysis of Lithocarpus polystachyus leaf revealed key genes in flavonoid biosynthesis pathways. J. Am. Soc. Hortic. Sci. 2021, 146, 147–157. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Shen, C.-H.; Lin, W.-D.; Chu, H.-A.; Huang, B.-L.; Kuo, C.-I.; Yeh, K.-W.; Huang, L.-C.; Chang, I.-F. Small RNAs of Sequoia sempervirens during rejuvenation and phase change. Plant Biol. 2013, 15, 27–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Cao, S.; Guo, Q.; Sun, Y.; Niu, D.; Long, C.; Fan, Y.; Li, Y. The RpTOE1-RpFT module is involved in rejuvenation during root-based vegetative propagation in Robinia pseudoacacia. Int. J. Mol. Sci. 2022, 23, 5079. [Google Scholar] [CrossRef]
- Xue, Z.; Gao, H.; Zhao, S. Effects of cadmium on the photosynthetic activity in mature and young leaves of soybean plants. Environ. Sci. Pollut. Res. 2014, 21, 4656–4664. [Google Scholar] [CrossRef]
- Fang, Y.; Mei, H.; Zhou, B.; Xiao, X.; Yang, M.; Huang, Y.; Long, X.; Hu, S.; Tang, C. De novo transcriptome analysis reveals distinct defense mechanisms by young and mature leaves of Hevea brasiliensis (Para rubber tree). Sci. Rep. 2016, 6, 33151. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Luo, Y.; Rao, G. Metabolome and transcriptome analyses reveal tissue-specific variations in gene expression and metabolites of olive. J. Plant Biol. 2020, 63, 73–82. [Google Scholar]
- Feng, Z.; Ji, S.; Cui, D. Integration of the metabolomic and transcriptome analysis reveals the remarkable compounds of G. bicolor young and mature leaves under different iron nutrient conditions. Int. J. Mol. Sci. 2022, 23, 1160. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lian, H.; Zhang, Q.; Cai, Y.; He, B. Camphor Tree Resources and Utilization; IUFRO World Series; Asia and the Pacific Workshop: Vienna, Austria, 2012; Volume 30, pp. 59–61. [Google Scholar]
- Zhong, Y.; Yang, A.; Li, Z.; Zhang, H.; Liu, L.; Wu, Z.; Li, Y.; Liu, T.; Xu, M.; Yu, F. Genetic diversity and population genetic structure of Cinnamomum camphora in south China revealed by EST-SSR markers. Forests 2019, 10, 1019. [Google Scholar] [CrossRef]
- Gong, X.; Shen, T.; Li, X.; Lin, H.; Chen, C.; Li, H.; Wu, Z.; Liu, Q.; Xu, M.; Zhang, B.; et al. Genome-wide characterization and analysis of bHLH transcription factors related to anthocyanin biosynthesis in Cinnamomum camphora (‘Gantong 1’). Int. J. Mol. Sci. 2023, 24, 3498. [Google Scholar] [CrossRef]
- Shen, T.; Qi, H.; Luan, X.; Xu, W.; Yu, F.; Zhong, Y.; Xu, M. The chromosome-level genome sequence of the camphor tree provides insights into Lauraceae evolution and terpene biosynthesis. Plant Biotechnol. J. 2022, 20, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, W. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2016, 63, 1049–1061. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y. China Species Red List; Higer Education Press: Beijing, China, 2004. [Google Scholar]
- Nolan, V.; Reader, T.; Gilbert, F.; Atkinson, N. The ancient tree inventory: A summary of the results of a 15 year citizen science project recording ancient, veteran and notable trees across the UK. Biodivers. Conserv. 2020, 29, 3103–3129. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part II: Reinvigoration, rejuvenation and juvenility maintenance. New For. 2014, 45, 473–486. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Li, Y.; Han, J.; Si, H.; Mi, Y.; Wang, S.; Wei, X.; Yang, H.; Sun, Y.; et al. Effects of the vegetative propagation method on juvenility in Robinia pseudoacacia L. For. Res. 2022, 2, 17. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Z.; Zhang, X.; Lai, Q.; Li, Y.; Zhao, F.; Zhao, Z. Tree age did not affect the leaf anatomical structure or ultrastructure of Platycladus orientalis L. (Cupressaceae). PeerJ 2019, 7, e7938. [Google Scholar] [CrossRef]
- Raihan, T.; Geneve, R.L.; Perry, S.E.; Rodriguez Lopez, C.M. The regulation of plant vegetative phase transition and rejuvenation: miRNAs, a key regulator. Epigenomes 2021, 5, 24. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Song, X.; Ma, Q.; Zhang, J.; Pei, D. A novel rejuvenation approach to induce endohormones and improve rhizogenesis in mature Juglans tree. Plant Methods 2018, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Shi, Y.; Luo, D.; Qian, W.; Shen, J.; Ding, S.; Ding, Z.; Wang, Y. Comparative transcriptome and hormone analysis of mature leaves and new shoots in tea cuttings (Camellia sinensis) among three cultivars with different rooting abilities. J. Plant Growth Regul. 2022, 41, 2833–2845. [Google Scholar] [CrossRef]
- Negishi, N.; Nakahama, K.; Urata, N.; Kojima, M.; Sakakibara, H.; Kawaoka, A. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New For. 2014, 45, 577–587. [Google Scholar] [CrossRef]
- Perrin, Y.; Doumas, P.; Lardets, L.; Carrons, M.P. Endogenous cytokinins as biochemical markers of rubber-tree (Hevea brasiliensis) clone rejuvenation. Plant Cell Tissue Organ Cult. 1997, 47, 239–245. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Y.; Mao, X.; Yu, W.; Lu, J.; Wang, L. Integration of morphological, physiological, cytological, metabolome and transcriptome analyses reveal age inhibited accumulation of flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2022, 187, 115405. [Google Scholar] [CrossRef]
- Ye, B.B.; Zhang, K.; Wang, J.W. The role of miR156 in rejuvenation in Arabidopsis thaliana. J. Integr. Plant Biol. 2020, 62, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yan, Y.; Zhou, W.; Feng, R.; Shuai, Y.; Yang, L.; Liu, M.; He, X.; Wei, Q. Transcriptome and metabolome reveal the accumulation of secondary metabolites in different varieties of Cinnamomum longepaniculatum. BMC Plant Biol. 2022, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Ahmed, U.; Ahmed, M.H.; Duan, M.; Wang, J.; Wang, Y.; Wang, L. Comparison and quantification of metabolites and their antioxidant activities in young and mature leaves of sugarcane. ACS Food Sci. Technol. 2021, 1, 362–373. [Google Scholar] [CrossRef]
- Batubara, R.; Hanum, T.I.; Affandi, O.; Wahyuni, H.S. Chemical compounds contained in young and mature leaves of agarwood species Wikstroemia tenuiramis and its antioxidant properties. Biodiversitas 2020, 21, 4616–4622. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Z.; Zhang, X.; Zhang, T.; Zhu, H.; Cui, B.; Li, Y.; Zhao, F.; Zhao, Z. Leaf anatomy and ultrastructure in senescing ancient tree, Platycladus orientalis L. (Cupressaceae). PeerJ 2019, 7, e6766. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Zhang, M.; Li, W.; Luo, K.; Lu, Z.; Zhang, C.; Jin, B. Identification and characterization of microRNA expression in Ginkgo biloba L. leaves. Tree Genet. Genomes 2015, 11, 76. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, J.; Jin, B.; Zhao, J.; Xu, H.; Lu, Z.; Li, W.; Li, X.; Li, L.; Liang, E.; et al. Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. USA 2020, 117, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, W.; Xiao, W.; Liu, J.; Jia, Z.; Zhao, X.; Jiang, Z.; Chang, E. Effects of different donor ages on the growth of cutting seedlings propagated from ancient Platycladus orientalis. Plants 2023, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Gong, X.; Luan, X.; Wu, Z.; Li, H.; Liu, Q.; Xu, M.; Yu, F. Transcriptome and metabolome analyses reveal a key role of the anthocyanin biosynthetic pathway cascade in the pigmentation of a Cinnamomum camphora red bark mutant (‘Gantong 1’). Ind. Crops Prod. 2022, 175, 114236. [Google Scholar] [CrossRef]


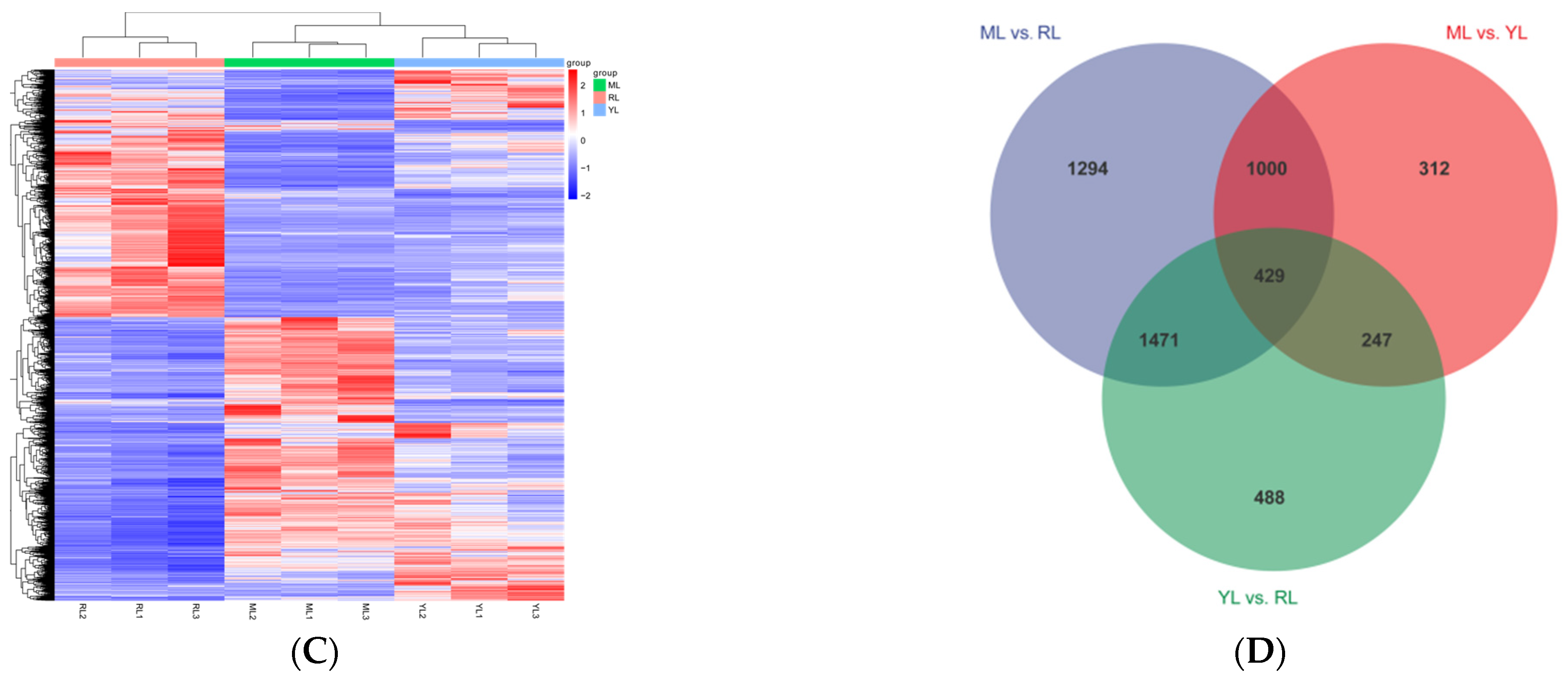
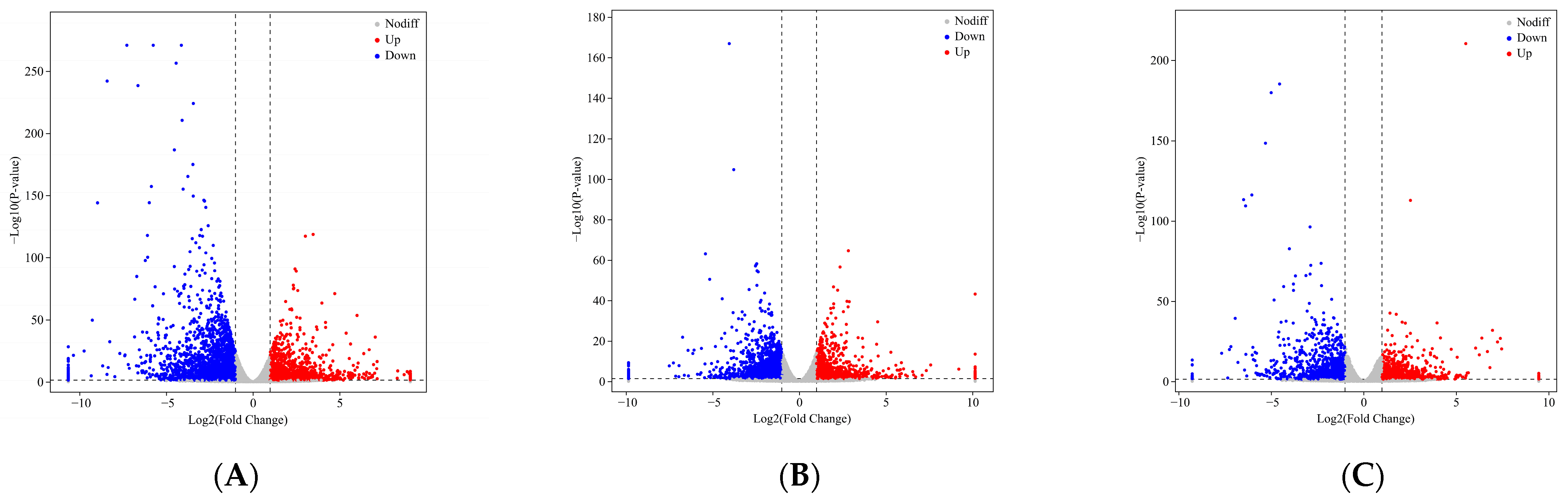

| Group Name | All Significant Difference | Down-Regulated | Up-Regulated |
|---|---|---|---|
| ML vs. RL | 235 | 89 | 146 |
| YL vs. RL | 111 | 62 | 49 |
| ML vs. YL | 92 | 38 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yang, A.; Liu, T.; Liu, S.; Hu, P.; Chen, C.; Zhou, H.; Wu, J.; Yu, F. Transcriptome and Metabolome Analyses of Leaves from Cutting Rejuvenation of Ancient Cinnamomum camphora. Int. J. Mol. Sci. 2024, 25, 7664. https://doi.org/10.3390/ijms25147664
Liu L, Yang A, Liu T, Liu S, Hu P, Chen C, Zhou H, Wu J, Yu F. Transcriptome and Metabolome Analyses of Leaves from Cutting Rejuvenation of Ancient Cinnamomum camphora. International Journal of Molecular Sciences. 2024; 25(14):7664. https://doi.org/10.3390/ijms25147664
Chicago/Turabian StyleLiu, Lipan, Aihong Yang, Tengyun Liu, Shujuan Liu, Ping Hu, Caihui Chen, Hua Zhou, Jingfang Wu, and Faxin Yu. 2024. "Transcriptome and Metabolome Analyses of Leaves from Cutting Rejuvenation of Ancient Cinnamomum camphora" International Journal of Molecular Sciences 25, no. 14: 7664. https://doi.org/10.3390/ijms25147664
APA StyleLiu, L., Yang, A., Liu, T., Liu, S., Hu, P., Chen, C., Zhou, H., Wu, J., & Yu, F. (2024). Transcriptome and Metabolome Analyses of Leaves from Cutting Rejuvenation of Ancient Cinnamomum camphora. International Journal of Molecular Sciences, 25(14), 7664. https://doi.org/10.3390/ijms25147664





