Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii
Abstract
:1. Introduction
2. Results
2.1. Identification of WOX Gene Family Members in P. ostii
2.2. Physicochemical Properties Analysis of PoWOX Genes
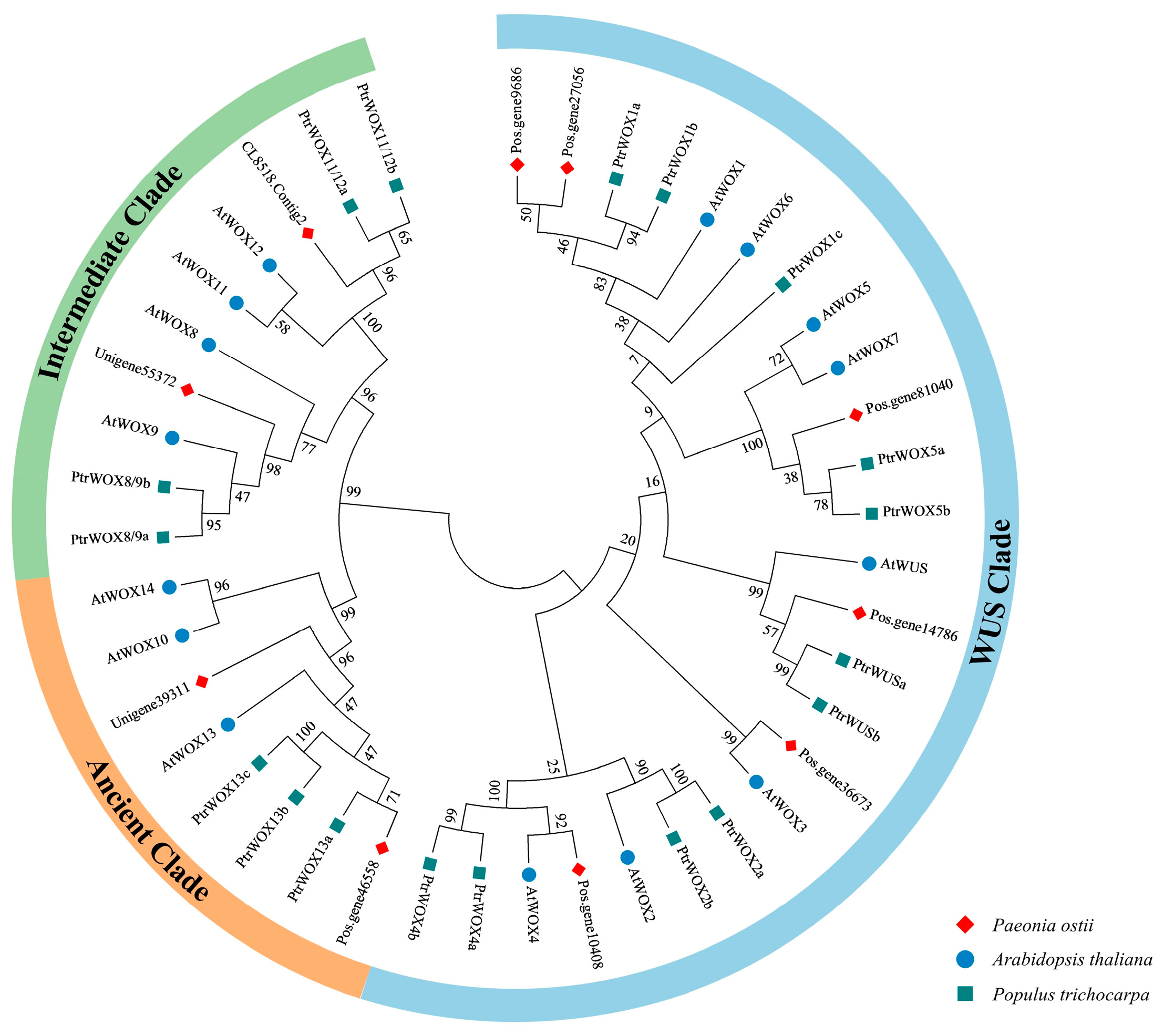
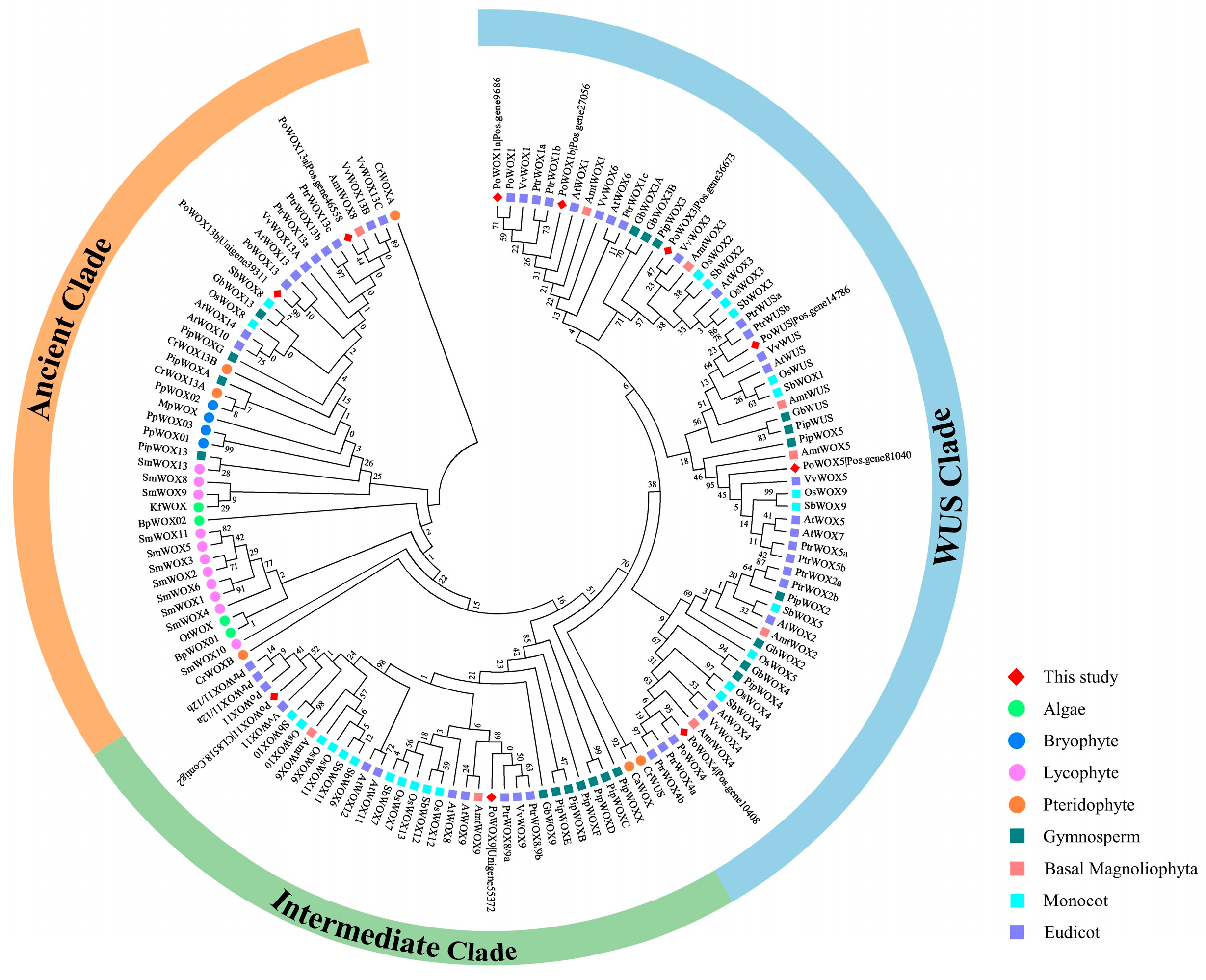
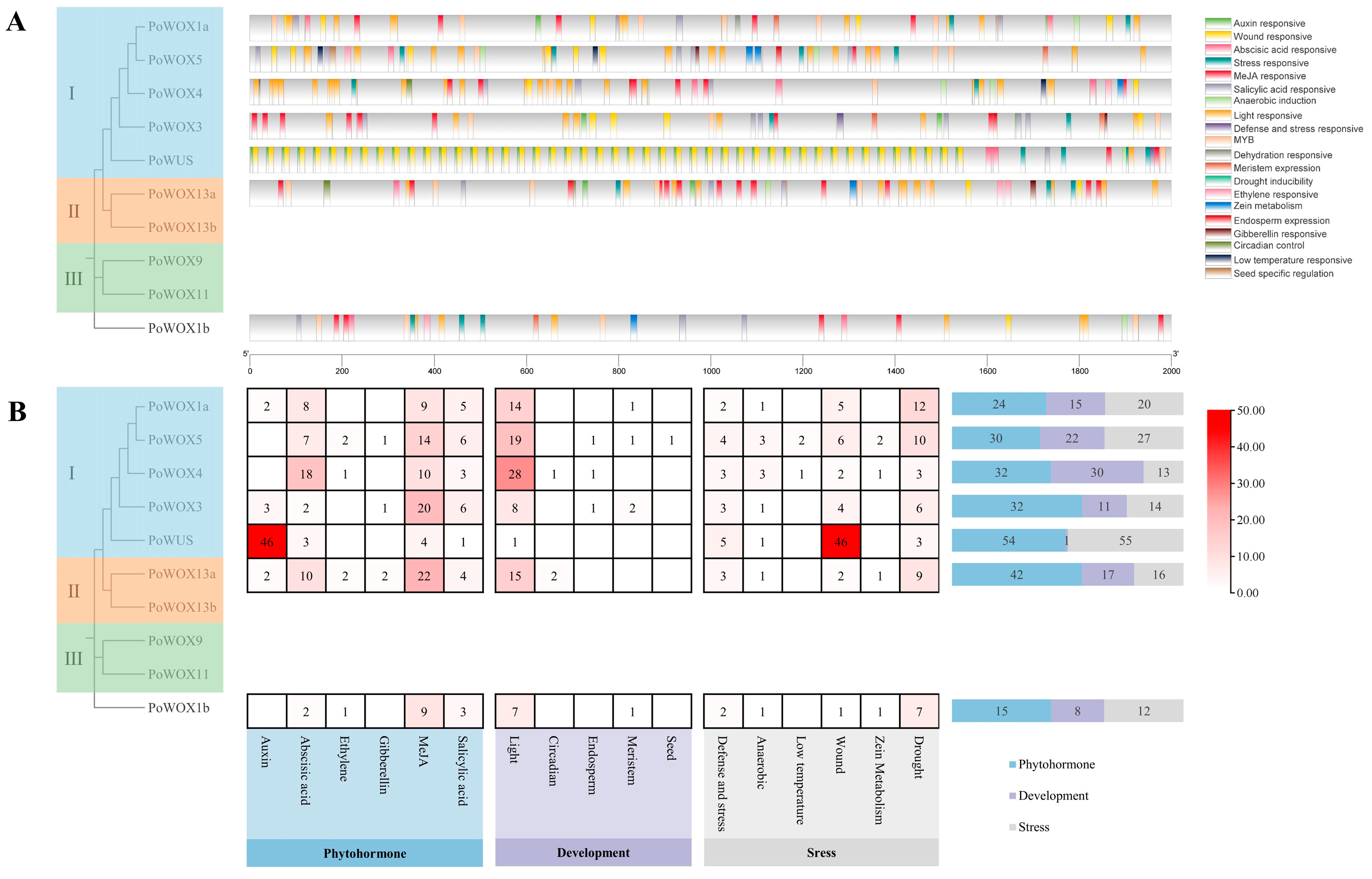
2.3. Phylogenetic Analysis of PoWOX Proteins
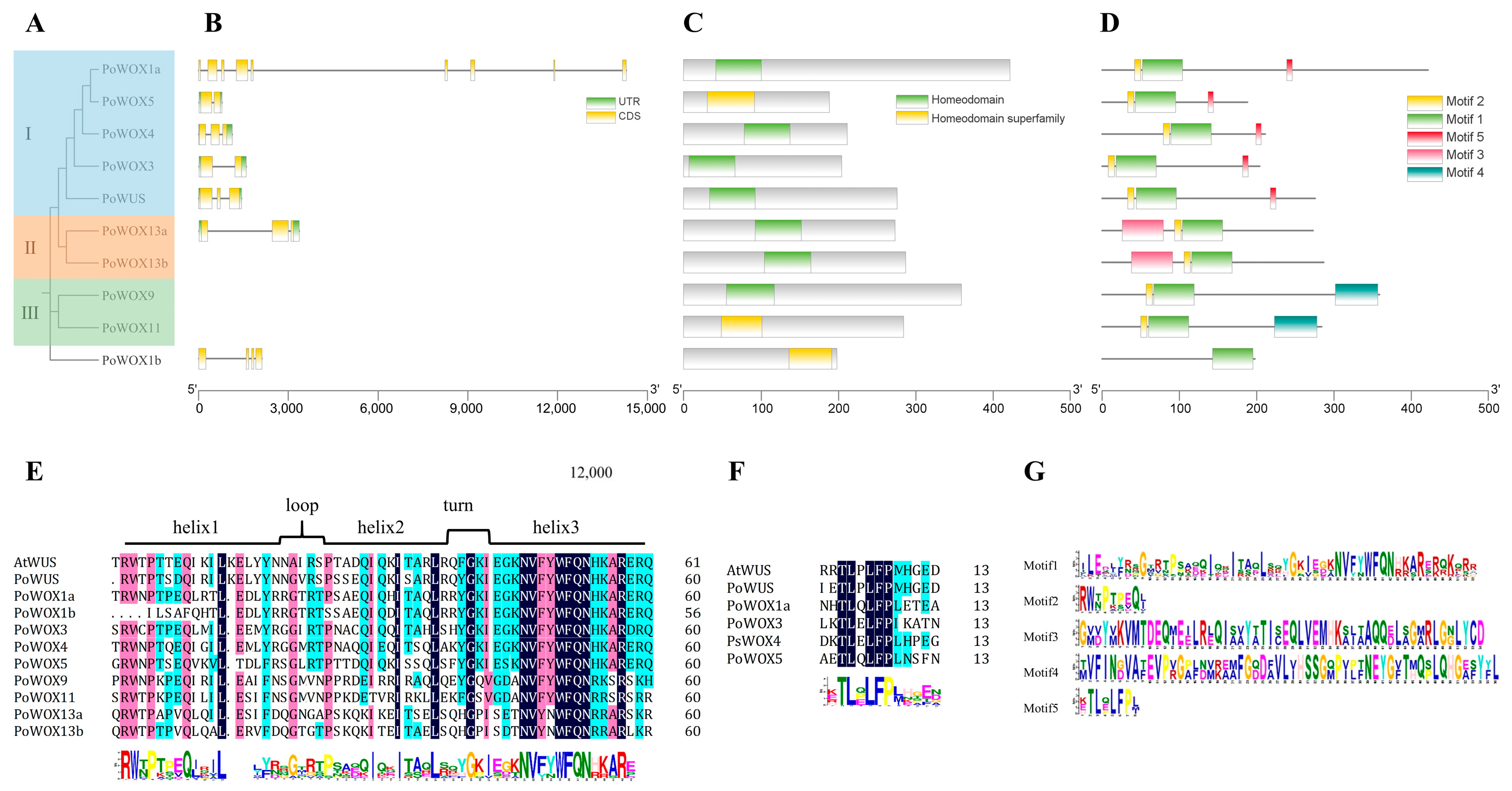
2.4. The Conserved Motifs and Structural Analysis of PoWOX Proteins
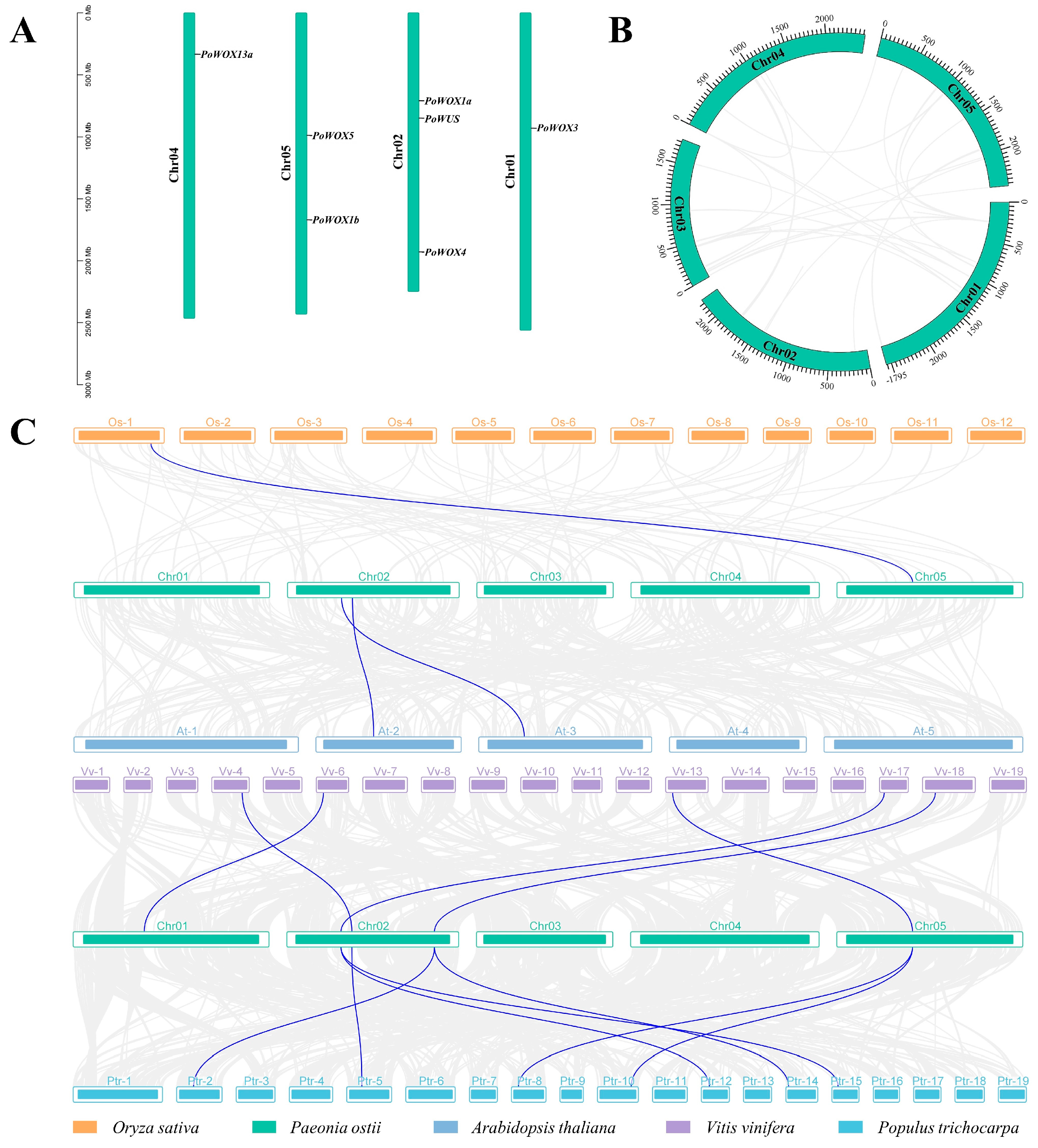
2.5. Genome Distribution and Collinearity Relationships of PoWOX Genes
2.6. The Cis-Elements Analysis in Promoter Regions of PoWOX Genes
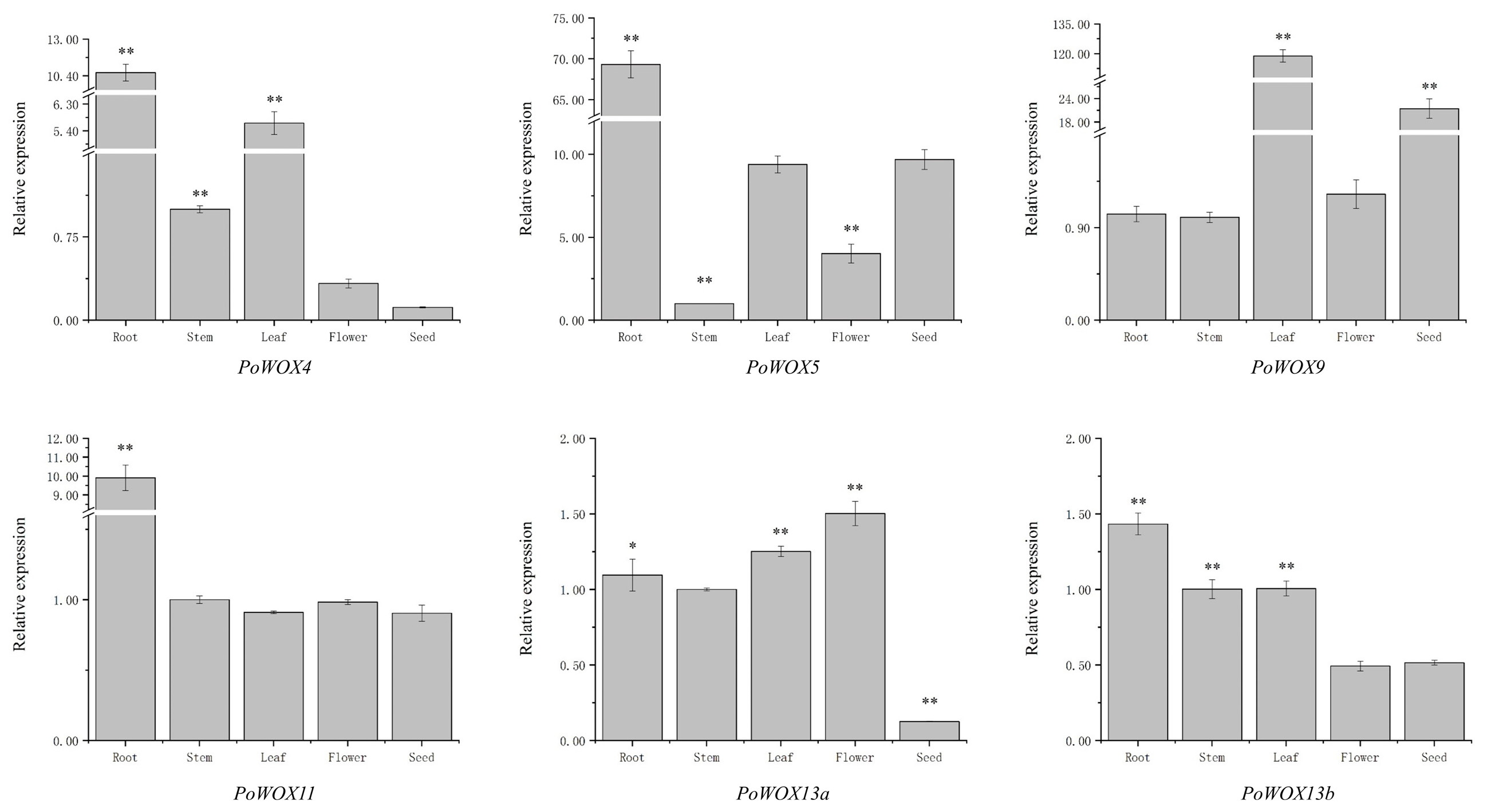
2.7. The Tissue Expression Pattern of PoWOX Genes
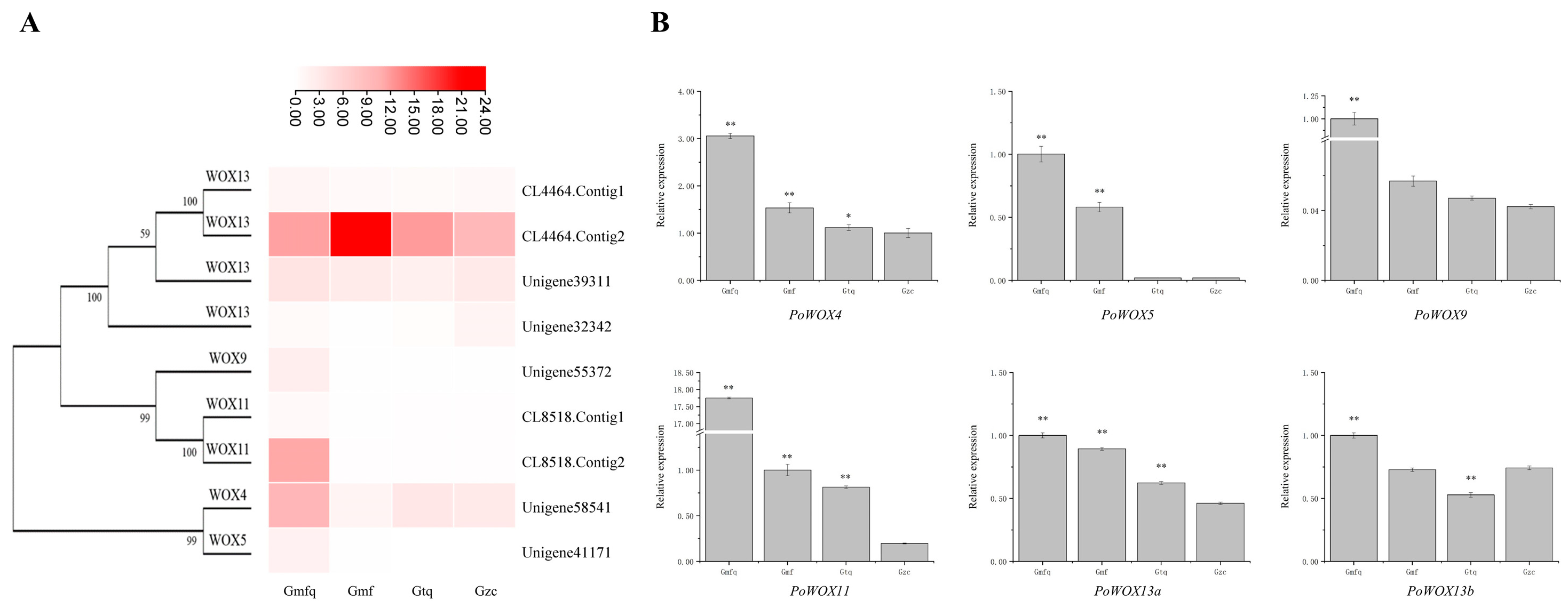
2.8. The Expression Analysis of PoWOX Genes in Roots
3. Discussion
3.1. Phylogenetic Analysis of PoWOX Proteins
3.2. Sequence Analysis of PoWOX Proteins
3.3. Expression Analysis of PoWOX Genes
3.4. The Relationship of PoWOX Genes with Adventitious Roots
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Extraction and cDNA Synthesis
4.3. Identification of WOX Family Members
4.4. Physiological and Biochemical Properties Analysis
4.5. Phylogenetic, Motif, and Structure Analysis
4.6. Chromosomal Localization, Collinearity, and Duplication Analysis
4.7. Putative Promoter Region Analysis
4.8. Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gehring, W.J.; Qian, Y.Q.; Billeter, M.; Furukubo-Tokunaga, K.; Schier, A.F. Homeodomain-DNA recognition. Cell 1994, 78, 211–223. [Google Scholar] [CrossRef]
- Haecker, A.; Groß–Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox–containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Tanaka, W.; Hirano, H.Y. Antagonistic action of TILLERS ABSENT1 and FLORAL ORGAN NUMBER2 regulates stem cell maintenance during axillary meristem development in rice. New Phytol. 2020, 225, 974–984. [Google Scholar] [CrossRef]
- Lopes, F.L.; Galvan-Ampudia, C.; Landrein, B. WUSCHEL in the shoot apical meristem: Old player, new tricks. J. Exp. Bot. 2021, 72, 1527–1535. [Google Scholar] [CrossRef]
- Brand, U.; Fletcher, J.C.; Hobe, M.; Meyerowitz, E.M.; Simon, R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 2000, 289, 617–619. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jürgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jönsson, H.; Reddy, G.V. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes. Dev. 2011, 25, 2025–2030. [Google Scholar] [CrossRef]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. CLAVATA–WUSCHEL signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Jiao, H.; Zhao, H.; Zhu, Y.X. Two-step functional innovation of the stem-cell factors WUS/WOX5 during plant evolution. Mol. Biol. Evol. 2017, 34, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, M.; Bohnert, A.; Jürgens, G.; Laux, T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001, 105, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, J.U.; Hong, R.L.; Hobe, M.; Busch, M.A.; Parcy, F.; Simon, R.; Weigel, D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 2001, 105, 793–803. [Google Scholar] [CrossRef]
- Liu, X.; Kim, Y.J.; Müller, R.; Yumul, R.E.; Liu, C.; Pan, Y.; Cao, X.; Goodrich, J.; Chen, X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of polycomb group proteins. Plant Cell 2011, 23, 3654–3670. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Looi, L.S.; Guo, S.; He, Z.; Gan, E.S.; Huang, J.; Xu, Y.; Wee, W.Y.; Ito, T. Timing mechanism dependent on cell division is invoked by Polycomb eviction in plant stem cells. Science 2014, 343, 1248559. [Google Scholar] [CrossRef]
- Nakata, M.; Matsumoto, N.; Tsugeki, R.; Rikirsch, E.; Laux, T.; Okada, K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 2012, 24, 519–535. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tanaka, S.Y.; Masumoto, Y.; Nobori, N.; Ishii, H.; Hibara, K.; Itoh, J.; Tanisaka, T.; Taketa, S. Barley NARROW LEAFED DWARF1 encoding a WUSCHEL–RELATED HOMEOBOX 3 (WOX3) regulates the marginal development of lateral organs. Breed. Sci. 2016, 66, 416–424. [Google Scholar] [CrossRef]
- Ueda, M.; Zhang, Z.; Laux, T. Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Dev. Cell 2011, 20, 264–270. [Google Scholar] [CrossRef]
- Lie, C.; Kelsom, C.; Wu, X. WOX2 and STIMPY–LIKE/WOX8 promote cotyledon boundary formation in Arabidopsis. Plant J. 2012, 72, 674–682. [Google Scholar] [CrossRef]
- Park, S.O.; Zheng, Z.; Oppenheimer, D.G.; Hauser, B.A. The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development 2005, 132, 841–849. [Google Scholar] [CrossRef]
- Ji, J.; Strable, J.; Shimizu, R.; Koenig, D.; Sinha, N.; Scanlon, M.J. WOX4 promotes procambial development. Plant Physiol. 2010, 152, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Etchells, J.P.; Provost, C.M.; Mishra, L.; Turner, S.R. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 2013, 140, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Vassileva, V.; De Rybel, B.; Levesque, M.P.; Grunewald, W.; Van Damme, D.; Van Noorden, G.; Naudts, M.; Van Isterdael, G.; De Clercq, R.; et al. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 2008, 322, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Ogawa-Ohnishi, M.; Mori, A.; Matsubayashi, Y. Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 2010, 329, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Grabowski, S.; Bleckmann, A.; Kühnemuth, R.; Weidtkamp-Peters, S.; Pinto, K.G.; Kirschner, G.K.; Schmid, J.B.; Wink, R.H.; Hülsewede, A.; et al. Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr. Biol. 2013, 23, 362–371. [Google Scholar] [CrossRef]
- Forzani, C.; Aichinger, E.; Sornay, E.; Willemsen, V.; Laux, T.; Dewitte, W.; Murray, J.A. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr. Biol. 2014, 24, 1939–1944. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Liu, Z.; Zhu, Y.X. ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nat. Commun. 2015, 6, 6003. [Google Scholar] [CrossRef]
- Wu, X.; Dabi, T.; Weigel, D. Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr. Biol. 2005, 15, 436–440. [Google Scholar] [CrossRef]
- Hu, X.; Xu, L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Deveaux, Y.; Toffano-Nioche, C.; Claisse, G.; Thareau, V.; Morin, H.; Laufs, P.; Moreau, H.; Kreis, M.; Lecharny, A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 2008, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xue, J.; Xue, Y.; Liu, R.; Ren, X.; Wang, S.; Zhang, X. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of tree peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors. Plant Physiol. Biochem. 2020, 149, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F. Advances in the breeding of tree peonies and a cultivar system for the cultivar group. Int. J. Plant Breed. 2007, 1, 89–104. [Google Scholar]
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Shen, M.; Yu, X.N. Tissue culture and micropropagation of tree peony (Paeonia suffruticosa Andr.). J. Crop Sci. Biotech. 2012, 15, 159–168. [Google Scholar] [CrossRef]
- Wen, S.S.; Chen, L.; Tian, R.N. Micropropagation of tree peony (Paeonia sect. Moutan): A review. Plant Cell Tiss. Organ. Cult. 2020, 141, 1–14. [Google Scholar]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots, similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Huang, J.; Lin, Z.-C.; Wang, F.; Yang, S.-M.; Jiang, X.; Ahmad, S.; Zhou, Y.-Z.; Lan, S.; Liu, Z.-J.; et al. Genome-wide analysis of WUSCHEL-related homeobox gene family in sacred lotus (Nelumbo nucifera). Int. J. Mol. Sci. 2023, 24, 14216. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, Z.; Xu, T.; Yao, S.; Zhang, M.; Cheng, X.; Zhao, Y.; Ji, K. Identification and expression patterns of WOX transcription factors under abiotic stresses in Pinus massoniana. Int. J. Mol. Sci. 2024, 25, 1627. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zheng, Q.; He, X.; Zhao, X.; Zhang, M.; Huang, Y.; Cai, B.; Liu, Z. The evolution of the WUSCHEL-related homeobox gene family in Dendrobium species and its role in sex organ development in D. chrysotoxum. Int. J. Mol. Sci. 2024, 25, 5352. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, W.; Chang, Y.; Ma, Y.; Deng, Y.; Fan, K.; Zhang, X.; Jiang, Z.; Hu, T. A preliminary investigation on the functional validation and interactions of PoWOX genes in peony (Paeonia ostii). Horticulturae 2022, 8, 266. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y.; et al. Genomic basis of the giga-chromosomes and giga-genome of tree peony Paeonia ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Wang, Z.; Shi, L.; Yu, S.; Xu, Y.; Wang, G.; He, D.; Jiang, L.; Shang, W.; et al. RNA sequencing analysis and verification of Paeonia ostii ‘Fengdan’ CuZn Superoxide Dismutase (PoSOD) genes in root development. Plants 2024, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Nardmann, J.; Werr, W. The invention of WUS-like stem cell-promoting functions in plants predates leptosporangiate ferns. Plant Mol. Biol. 2012, 78, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Morris, J.L. The origin and early evolution of vascular plant shoots and leaves. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zong, J.; Liu, J.; Yin, J.; Zhang, D. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 2010, 52, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, L.; McHale, N.A.; Ohme-Takagi, M.; Mysore, K.S.; Tadege, M. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc. Natl. Acad. Sci. USA 2013, 110, 366–371. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Cai, M.; Zheng, H.; Cheng, Z.; Gao, J. Identification and evolution of the WUSCHEL–related homeobox protein family in bambusoideae. Biomole 2020, 10, 739. [Google Scholar] [CrossRef]
- Zeng, M.; Hu, B.; Li, J.; Zhang, G.; Ruan, Y.; Huang, H.; Wang, H.; Xu, L. Stem cell lineage in body layer specialization and vascular patterning of rice root and leaf. Sci. Bull. 2016, 61, 847–858. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Ding, Z.; Wang, Q.; Zhang, D.; Xu, J. Origins and evolution of WUSCHEL-related homeobox protein family in plant kingdom. Sci. World J. 2014, 2014, 534140. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 2009, 21, 3493–3505. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, N.; Yu, B.; Wu, Q.; Li, X.; Wang, G.; Huang, J. The WUSCHEL-related homeobox transcription factor OsWOX4 controls the primary root elongation by activating OsAUX1 in rice. Plant Sci. 2020, 298, 110575. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Nagasaki, H.; Morikami, A.; Sato, Y.; Matsuoka, M. Isolation and characterization of a rice WUSCHEL–type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J. 2003, 35, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Nardmann, J.; Zimmermann, R.; Durantini, D.; Kranz, E.; Werr, W. WOX gene phylogeny in Poaceae, a comparative approach addressing leaf and embryo development. Mol. Biol. Evol. 2007, 24, 2474–2484. [Google Scholar] [CrossRef] [PubMed]
- Hendelman, A.; Zebell, S.; Rodriguez-Leal, D.; Dukler, N.; Robitaille, G.; Wu, X.; Kostyun, J.; Tal, L.; Wang, P.; Bartlett, M.E.; et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell 2021, 184, 1724–1739.e16. [Google Scholar] [CrossRef]
- Wolabu, T.W.; Wang, H.; Tadesse, D.; Zhang, F.; Behzadirad, M.; Tvorogova, V.E.; Abdelmageed, H.; Liu, Y.; Chen, N.; Chen, J.; et al. WOX9 functions antagonistic to STF and LAM1 to regulate leaf blade expansion in Medicago truncatula and Nicotiana sylvestris. New Phytol. 2021, 229, 1582–1597. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot–borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Rigal, A.; Yordanov, Y.S.; Perrone, I.; Karlberg, A.; Tisserant, E.; Bellini, C.; Busov, V.B.; Martin, F.; Kohler, A.; Bhalerao, R.; et al. The AINTEGUMENTA LIKE1 homeotic transcription factor PtAIL1 controls the formation of adventitious root primordia in poplar. Plant Physiol. 2012, 160, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jürgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hofhuis, H.; Heidstra, R.; Sauer, M.; Friml, J.; Scheres, B. A molecular framework for plant regeneration. Science 2006, 311, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef]
- Imin, N.; Nizamidin, M.; Wu, T.; Rolfe, B.G. Factors involved in root formation in Medicago truncatula. J. Exp. Bot. 2007, 58, 439–451. [Google Scholar] [CrossRef]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Sheng, L.; Hu, X.; Du, Y.; Zhang, G.; Huang, H.; Scheres, B.; Xu, L. Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development 2017, 144, 3126–3133. [Google Scholar] [CrossRef]
- Ge, Y.; Fang, X.; Liu, W.; Sheng, L.; Xu, L. Adventitious lateral rooting: The plasticity of root system architecture. Physiol. Plant 2019, 165, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Baesso, B.; Chiatante, D.; Terzaghi, M.; Zenga, D.; Nieminen, K.; Mahonen, A.P.; Siligato, R.; Helariutta, Y.; Scippa, G.S.; Montagnoli, A. Transcription factors PRE3 and WOX11 are involved in the formation of new lateral roots from secondary growth taproot in A. thaliana. Plant Biol. 2018, 20, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D.X. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Ogura, N.; Sasagawa, Y.; Ito, T.; Tameshige, T.; Kawai, S.; Sano, M.; Doll, Y.; Iwase, A.; Kawamura, A.; Suzuki, T.; et al. WUSCHEL-RELATED HOMEOBOX 13 suppresses de novo shoot regeneration via cell fate control of pluripotent callus. Sci. Adv. 2023, 9, eadg6983. [Google Scholar] [CrossRef]
- Chen, M.; Luo, J.; Lin, Y.; Huang, A.; Liu, G. Identification and expression profile analysis of WOX family genes in the formation of Eucalyptus adventitious root. Forests 2024, 15, 442. [Google Scholar] [CrossRef]

| Gene Name | PoWUS | PoWOX1a | PoWOX1b | PoWOX3 | PoWOX4 | PoWOX5 | PoWOX9 | PoWOX11 | PoWOX13a | PoWOX13b |
|---|---|---|---|---|---|---|---|---|---|---|
| Gene ID | Pos.gene14786 | Pos.gene9686 | Pos.gene27056 | Pos.gene36673 | Pos.gene10408 | Pos.gene81040 | Unigene55372 | CL8518.Contig2 | Pos.gene46558 | Unigene39311 |
| GenBank Accession | PP978709 | PP978710 | PP978711 | PP978712 | PP978713 | PP978714 | PP978715 | PP978716 | PP978717 | PP978718 |
| CDS length (bp) | 831 | 1269 | 597 | 615 | 636 | 567 | 1077 | 852 | 822 | 861 |
| Number of amino acids (aa) | 276 | 422 | 198 | 204 | 211 | 188 | 358 | 283 | 273 | 286 |
| Molecular weight (Da) | 31,221.97 | 47,264.16 | 22,286.59 | 23,788.08 | 24,314.62 | 21,324.07 | 39,703.43 | 31,540.35 | 31,031.63 | 32,542.32 |
| Theoretical pI | 6.32 | 6.45 | 5.55 | 9.08 | 9.45 | 8.74 | 8.60 | 6.14 | 5.29 | 4.97 |
| Asp + Glu | 30 | 55 | 28 | 17 | 26 | 24 | 26 | 26 | 36 | 45 |
| Arg + Lys | 28 | 51 | 25 | 22 | 34 | 27 | 29 | 22 | 27 | 31 |
| Instability index | 62.4 | 53.37 | 41.51 | 70.98 | 49.13 | 55.31 | 54.93 | 64.41 | 73.35 | 62.97 |
| Aliphatic index | 42.1 | 62.39 | 83.74 | 57.89 | 60.52 | 69.41 | 66.15 | 66.43 | 64.65 | 69.27 |
| GRAVY | −1.124 | −0.828 | −0.313 | −0.853 | −0.915 | −0.619 | −0.506 | −0.287 | −0.844 | −0.8 |
| Transmembrane helices | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Signal peptide | No | No | No | No | No | No | No | Yes | No | No |
| Localization prediction | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, X.; Wang, J.; Wang, G.; He, D.; Shang, W.; Song, Y.; Wang, Z.; He, S. Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii. Int. J. Mol. Sci. 2024, 25, 7668. https://doi.org/10.3390/ijms25147668
Lou X, Wang J, Wang G, He D, Shang W, Song Y, Wang Z, He S. Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii. International Journal of Molecular Sciences. 2024; 25(14):7668. https://doi.org/10.3390/ijms25147668
Chicago/Turabian StyleLou, Xueyuan, Jiange Wang, Guiqing Wang, Dan He, Wenqian Shang, Yinglong Song, Zheng Wang, and Songlin He. 2024. "Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii" International Journal of Molecular Sciences 25, no. 14: 7668. https://doi.org/10.3390/ijms25147668
APA StyleLou, X., Wang, J., Wang, G., He, D., Shang, W., Song, Y., Wang, Z., & He, S. (2024). Genome-Wide Analysis of the WOX Family and Its Expression Pattern in Root Development of Paeonia ostii. International Journal of Molecular Sciences, 25(14), 7668. https://doi.org/10.3390/ijms25147668






