Abstract
Diabetes mellitus, as a chronic metabolic disorder, significantly impacts the pancreas and among other organs, affects duodenal function. Emerging evidence suggests that probiotics can exert beneficial effects on gut health and metabolism. In our previous research, we evaluated the probiotic Lactobacillus paraplantarum BGCG11 primarily for its protective properties against diabetic rats’ damaged liver and kidneys. In this work, we further examined the effects of probiotic strain BGCG11 on the function of the duodenum and pancreas in diabetic rats. We explored the potential mechanisms underlying the probiotic’s effects, focusing on general indicators of diabetes, the architecture and morphology of pancreatic islets, duodenal integrity (measuring the transfer of fluid and serum zonulin level), and the modulation of gut microbiota composition. Our findings reveal the protective and regulatory roles of L. paraplantarum BGCG11 in mitigating diabetes-induced pancreatic and duodenal dysfunction regardless of its application time (pre- or post-treatment), highlighting its therapeutic potential in managing diabetes-related gastrointestinal complications.
1. Introduction
Diabetes is the most common metabolic disease with multifactorial origins, including genetic and environmental factors. Diabetes is characterized by hyperglycaemia, target-tissue resistance to insulin, and insufficient insulin secretion. The pancreas plays an important role in secreting insulin and glucagon hormones, which are essential for glucose/carbohydrate utilization and the regulation of blood glucose levels. Prolonged hyperglycaemia, increased oxidative stress, and inflammation promote different diabetic complications, such as neuropathy, nephropathy, retinopathy, macro and microvascular complications, and liver damage. In addition, about 75% of patients with diabetes have significant gastrointestinal disorders [1]. Gut microbiota modulates the functions of the gut immune system, affecting the innate and the adaptive immune systems. Immunological changes in the gut can profoundly affect the pancreas, modulating the incidence of diabetes as a result of the immunological link between the gut and pancreas [2,3]. Gastrointestinal problems are manifested through altered gut microbiota and increased intestinal permeability caused by changes in the tight junction. The consequence of the reduced expression of tight-junction proteins is increased endotoxins in the blood, metabolic endotoxaemia, an altered mucosal immune response, and insulin resistance, which contributes to the development of diabetes and diabetic complications [4,5]. However, the precise mechanisms that result in disorders such as leaky gut before the development of diabetes are still controversial and are not fully understood [6].
Gut microbiota is the major source of microbial stimulation that expresses both injurious and beneficial effects on homeostasis and health. Recent in-vitro and in-vivo studies have shown that adequate consumption of probiotics inhibits the growth of pathogenic bacteria, decreases the level of circulating endotoxins, reduces inflammatory responses, and stimulates the immune system [7,8]. Bifidobacteria and Lactobacillus are the most frequently used probiotic dietary supplements. It has been reported that dahi, a traditional Indian dairy product that contains Lactobacillus acidophilus and Lactobacillus casei probiotic strains, reduced HbA1c, lipid peroxidation, and oxidative stress in streptozotocin-induced diabetic rats [9]. Treatment with the Bifidobacterium animalis subsp. lactis 420 strain can reverse bacterial translocation from the intestine to tissues, thus preventing the early onset of high-fat diet-induced hyperglycaemia and leading to improved insulin sensitivity and equilibration/physiological balance between the inflammatory and metabolic status in a mouse model [10]. Several clinical studies on diabetic patients have also been undertaken. Studies in humans have shown that the gastrointestinal tract contains, on average, 1014 microorganisms/mL of luminal content and over 1000 different bacterial species [11]. Approximately 90% of the resident bacteria belong to the Bacteroidetes and Firmicutes phyla [12]. One study has shown that daily consumption of a symbiotic shake containing Lactobacillus acidophilus, Bifidobacterium bifidum, and oligofructose improved blood glucose concentration and led to a significant increase in HDL cholesterol [8,13]. Others showed that ingestion of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 for 6 weeks reduced fasting glucose, and HbA1c levels increased erythrocyte superoxide dismutase and glutathione peroxidase activities and the total antioxidant status in diabetic patients [14].
The probiotic strain Lactobacillus paraplantarum BGCG11 generates a “ropy” exopolysaccharide (EPS) and displays anti-inflammatory and immunosuppressive effects [15]. Previously, we showed that administration of the strain Lactobacillus paraplantarum BGCG11 had an antidiabetic effect and attenuated oxidative stress, inflammation, and fibrosis in the liver and kidneys of diabetic rats [16]. The current study’s objective was to evaluate the beneficial in-vivo effects of the strain L. paraplantarum BGCG11 on pancreatic and duodenal functionality in streptozotocin-induced diabetic rats.
2. Results
2.1. Administration of Probiotic L. paraplantarum BGCG11 Influences the Biochemical Parameters of Diabetes
The non-diabetic (ND) group of rats receiving the BGCG11 strain showed no signs of diarrhea or weight loss during the probiotic treatment. The body weight of both the ND group and non-diabetic group rats treated with probiotics (ND/P) displayed an increase in body weight of 30 ± 1.2%, indicating that the probiotic was not harmful. The diabetic group (D) exhibited a loss in body weight of 16.6 ± 0.6% at the end of the study when compared with the body weight at the start. The D group treated with probiotics displayed a loss of weight of 12 ± 0.4%. Food and water intake for the ND group and the ND/P group that received the probiotic were the same throughout the whole period of treatment, while in the D group, uptake of food and water was increased 4-fold for food and 10-fold for water when compared to the ND group. In the D groups treated with probiotics, food and water intake were lower as compared to the D group (2-fold for both food and water).
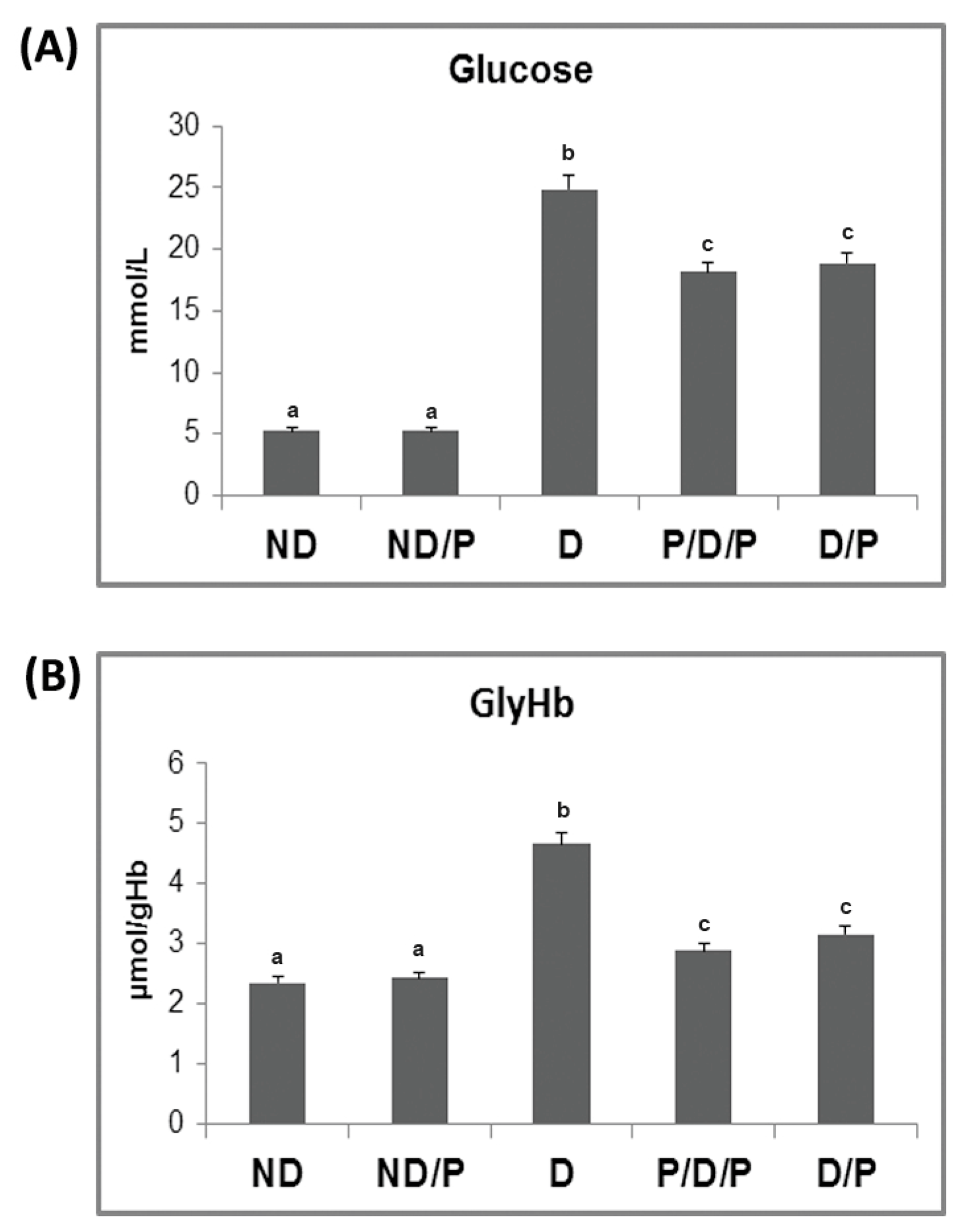
The biochemical markers of diabetes, glucose, and glycohemoglobin (GlyHb) are presented in Figure 1. While both pre-treatment (P/D/P) (p < 0.05) and treatment (D/P) (p < 0.05) of D rats with the probiotic lowered the glucose concentration 3.5-fold, the concentration of glucose in diabetic rats was 4.7-fold higher (p < 0.05) than in the untreated ND rats (Figure 1A). In D rats, the level of GlyHb was 1.9-fold higher (p < 0.05) compared to the ND control (Figure 1B). GlyHb in the P/D/P group was 1.6-fold (p < 0.05) lower, while in the D/P group, the level of GlyHb was 1.4-fold (p < 0.05) lower when compared to the D group (Figure 1B).
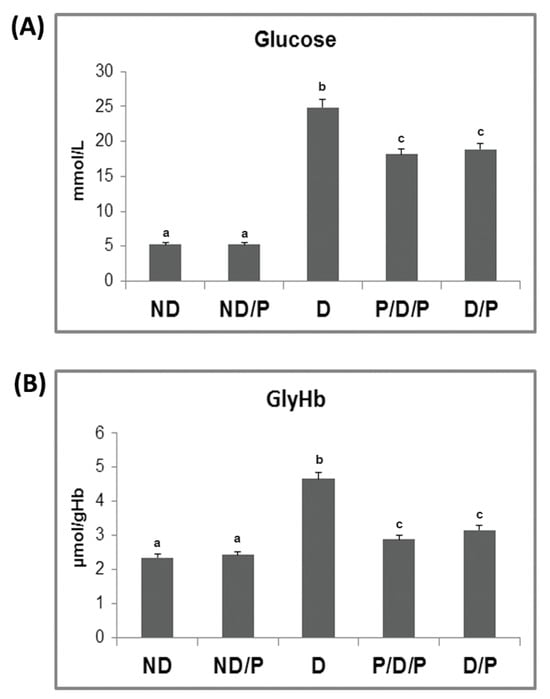
Figure 1.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on fasting blood glucose concentration (A) and level of glycated haemoglobin (B). ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11-treated non-diabetic group; D—streptozotocin (STZ)-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks. The values are means ± standard error of the mean (S.E.M.) from three separate measurements (technical replica). Groups’ means that do not have the same letter differ significantly from one another (p < 0.05).
2.2. Histological Changes, Collagen-Fibre Deposition, and Immunohistochemical Localization of Insulin and Glucagon in Pancreatic Islets Analysis after the Probiotic Administration
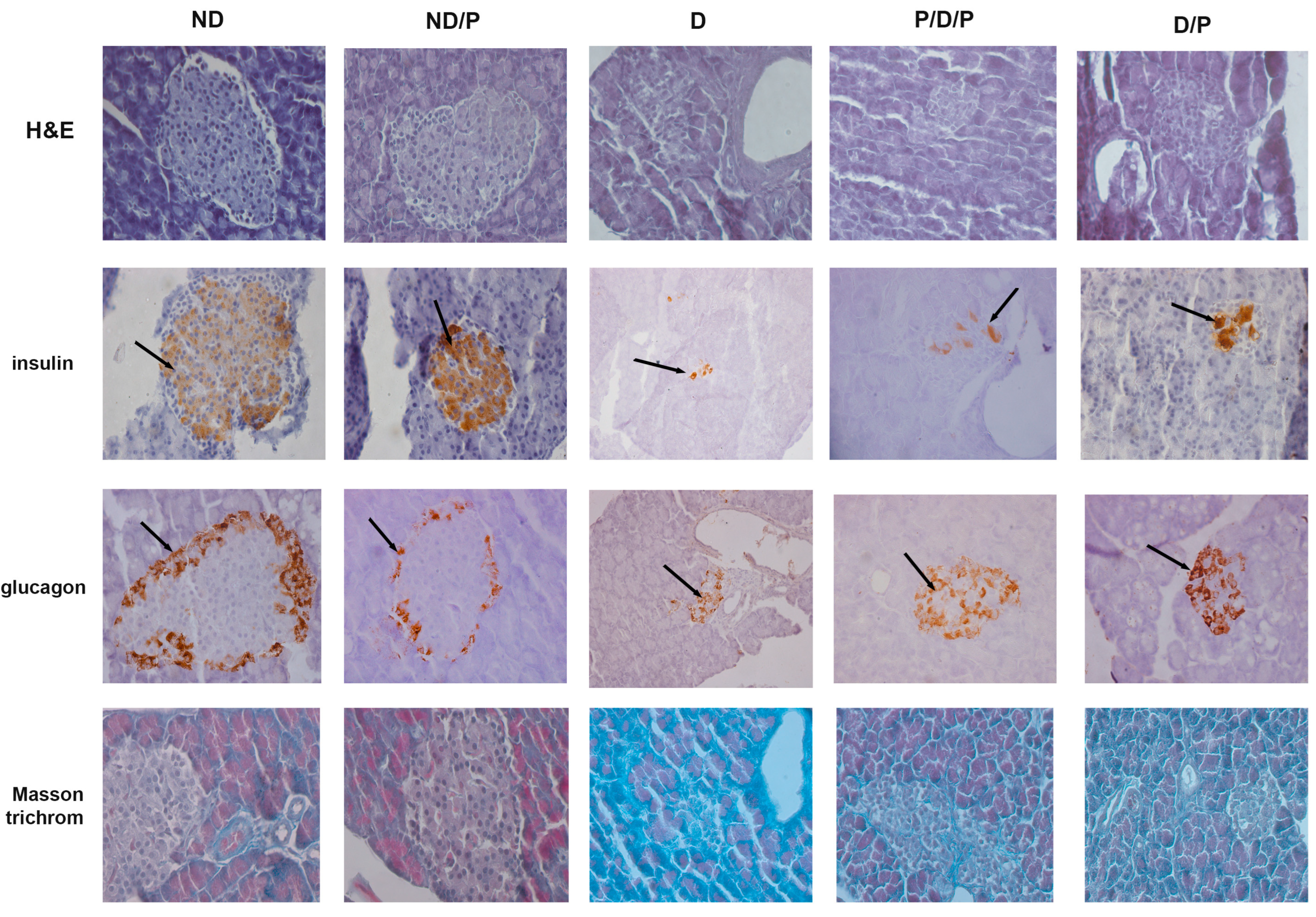
H&E-stained sections from the control group showed that the pancreas had normal histology, consisting of closely packed lobules of pancreatic acini, while the islets of Langerhans consisted of numerous and compactly arranged cells that appeared as dense cords (Figure 2, ND, H&E). In the diabetic group, pathological changes in both exocrine and endocrine parts of the pancreas were revealed, manifesting vacuolation, smaller pancreatic islets, and a marked decrease in β-cells (Figure 2, D, H&E). In both P/D/P and D/P groups, improvement of cell architecture and morphology of pancreatic islets was observed; most of the islets of Langerhans were intact with only rare vacuoles (Figure 2, P/D/P and D/P, H&E). Treatment of control rats with the probiotics showed no differences compared to the control group (Figure 2, ND/P, H&E).
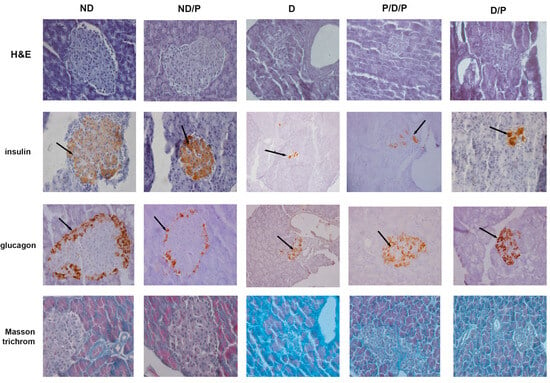
Figure 2.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the histological changes, collagen-fibres deposition, and immunohistochemical localization of insulin and glucagon in pancreatic islets. H&E—hematoxylin and eosin staining of pancreatic sections; insulin—light photomicrographs of insulin immunohistochemical staining; glucagon—light photomicrographs of glucagon immunohistochemical staining; Masson trichrome—staining of pancreatic sections within islets (magnification 40×); ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks. The black arrows indicate the insulin and glucagon accumulations.
Insulin immunostaining in the control group showed a positive reaction, observed as brown granules in the cytoplasm in a considerable number of β-cells, with a homogeneous distribution (Figure 2, ND, insulin). In the diabetic group, the immunoreactivity for anti-insulin antibodies was defined in a small part of the islet as the consequence of an evident decrease in the number of insulin-positive cells (Figure 2, D, insulin) and islet atrophy. In both P/D/P and D/P groups, an increase in areas of the Langerhans islets with an increased number of positive immunoreactions of β-cells for anti-insulin antibodies was detected (Figure 2, P/D/P and D/P, insulin). The control pancreas of rats treated with the probiotic showed a strong insulin-positive reaction as observed in the control (Figure 2, ND/P, insulin).
Glucagon immunostaining in the control group was mostly observed along the islet periphery, as can be seen in the control group treated with the probiotic (Figure 2, ND, glucagon), where the majority of pancreatic α-cells are expected to be localised. In the diabetic group, an increase in glucagon-positive cells was observed; these cells were observed not only along the periphery but within the core of the islet (Figure 2, D, glucagon). In P/D/P and D/P groups, glucagon-positive cells were also found in the core of the pancreatic islet in a number that is much higher in comparison to the diabetic group (Figure 2, P/D/P and D/P, glucagon).
Positive Masson trichrome staining of the pancreas of control rats was observed in parts around cells of the islands of Langerhans, and slight collagen-fibre deposition was noted around the blood vessels and pancreatic acini (Figure 2, ND, Masson trichrome). In the diabetic group, Masson trichrome staining of the pancreas revealed thickening of the connective tissue septa with dense collagen fibres around the acini and blood vessels and that the fibrotic process of islets was advanced (Figure 2, D, Masson trichrome). In the P/D/P and D/P groups, collagen-fibre deposition and fewer regions of dense connective tissue septa were observed (Figure 2, P/D/P and D/P, Masson trichrome).
2.3. Effects of Probiotic Administration on Transfer of Fluid through the Intestinal Mucosa and the Serum Zonulin Level
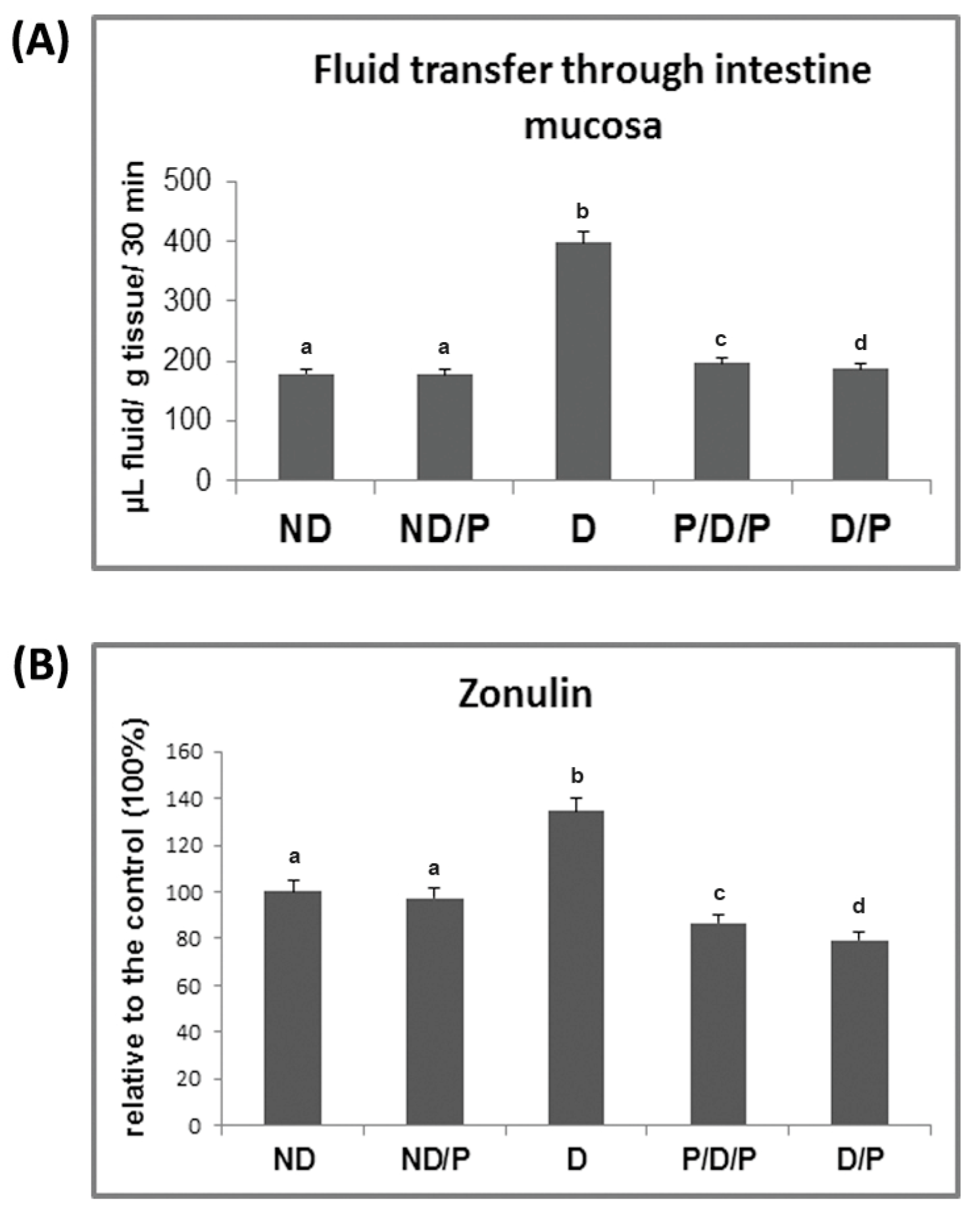
In the diabetic group, the transfer of fluid through the small intestinal mucosa is significantly more pronounced in comparison to the control animals (Figure 3A). Treatment with the probiotic in both analysed cases (P/D/P and D/P) led to a reduction in fluid transfer through the small intestinal mucosa (Figure 3A), which approached the control value.
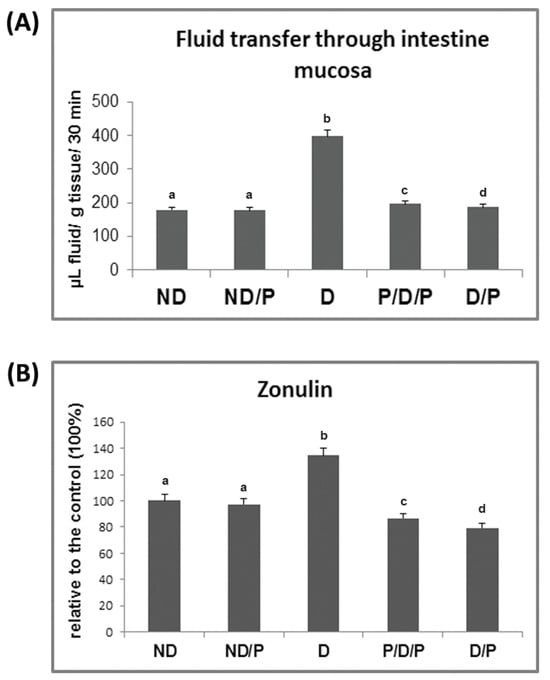
Figure 3.
The effect of probiotic L. paraplantarum BGCG11 consumption on the transfer of fluid through intestinal mucosa (A) and serum zonulin levels (B). ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks. Groups’ means that do not have the same letter differ significantly from one another (p < 0.05).
As can be seen in Figure 3B, the concentration of serum zonulin increased 1.3-fold in the diabetic group when compared to the control group, while treatment with the probiotic decreased the serum levels of zonulin in both P/D/P and D/P rats. Treatment of control rats with the probiotic showed no difference in relation to the intact control group (Figure 3B).
2.4. Probiotic Administration Influences Histological Changes, Collagen-Fibre Deposition and Immunohistochemical Localization of Alpha-Smooth Muscle, Fibronectin and E-cadherin in the Duodenum Section
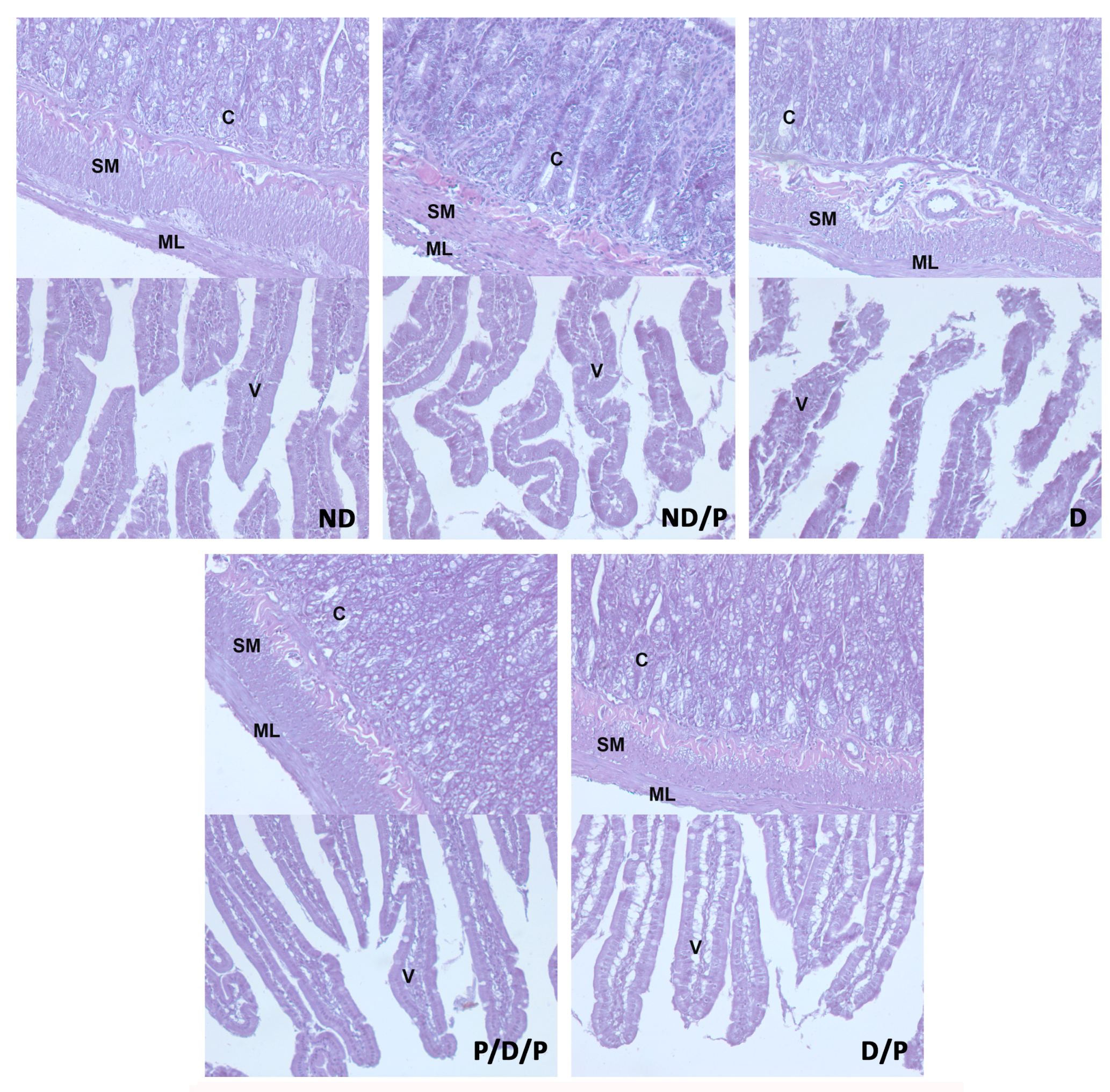
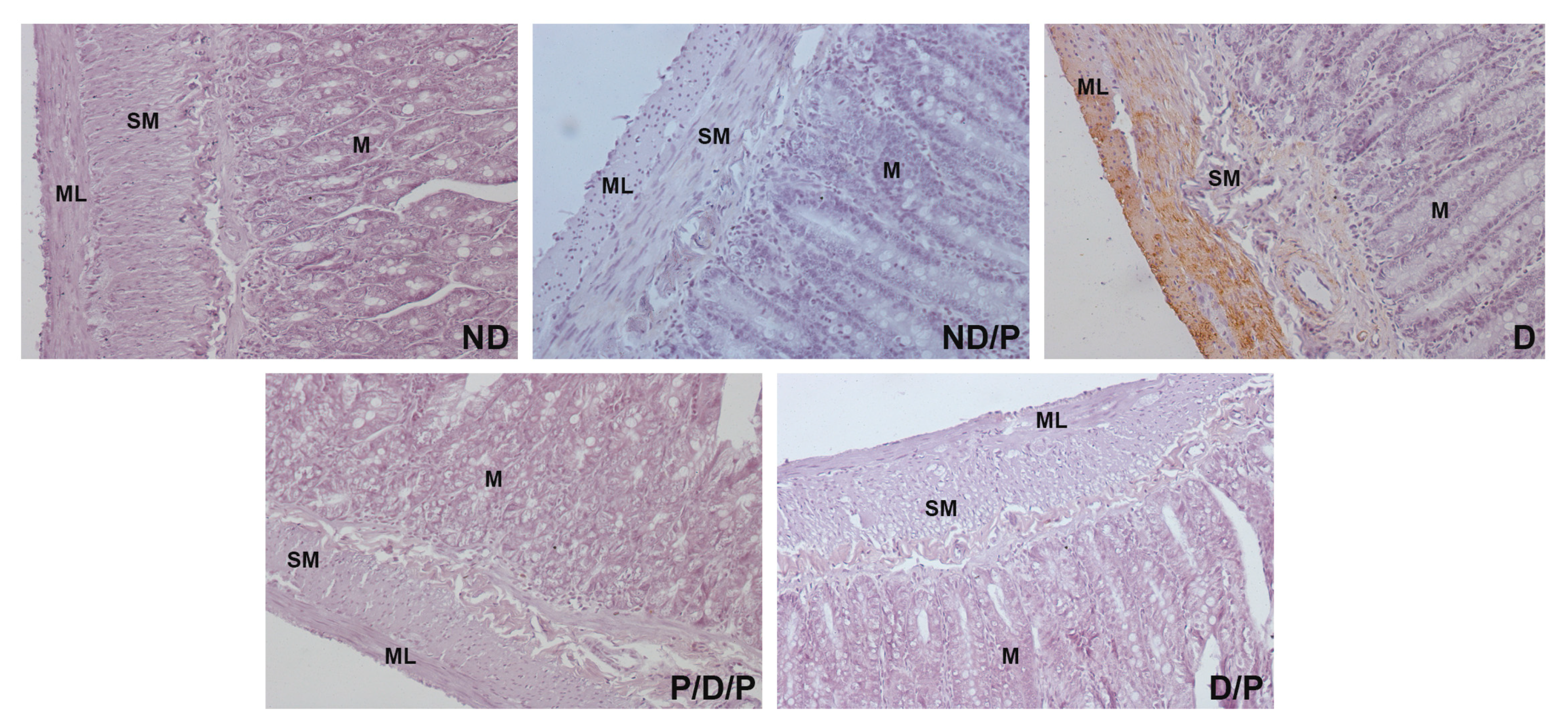
Histological examination of the duodenum in the control group revealed well-shaped villi with brush borders and crypts with clear lumen (Figure 4, ND). In the diabetic group, disorganisation and deformation of villi and intestinal glands were observed (Figure 4, D). In both P/D/P and D/P groups, decreased tissue damage and preservation of villi and intestinal glands were observed (Figure 4, P/D/P and D/P). Histological organisation in the ND/P group was similar to the control group (Figure 4, ND/P).
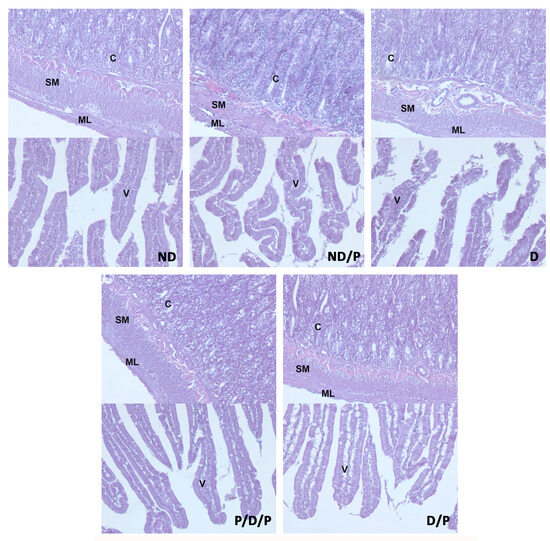
Figure 4.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the histological changes of the intestine (duodenum section). H&E staining of duodenum section; V—villi (magnification 20×); SM—submucosa (magnification 40×); ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks; C—crypts; SM—submucosa; ML—muscular layer; V—villi.
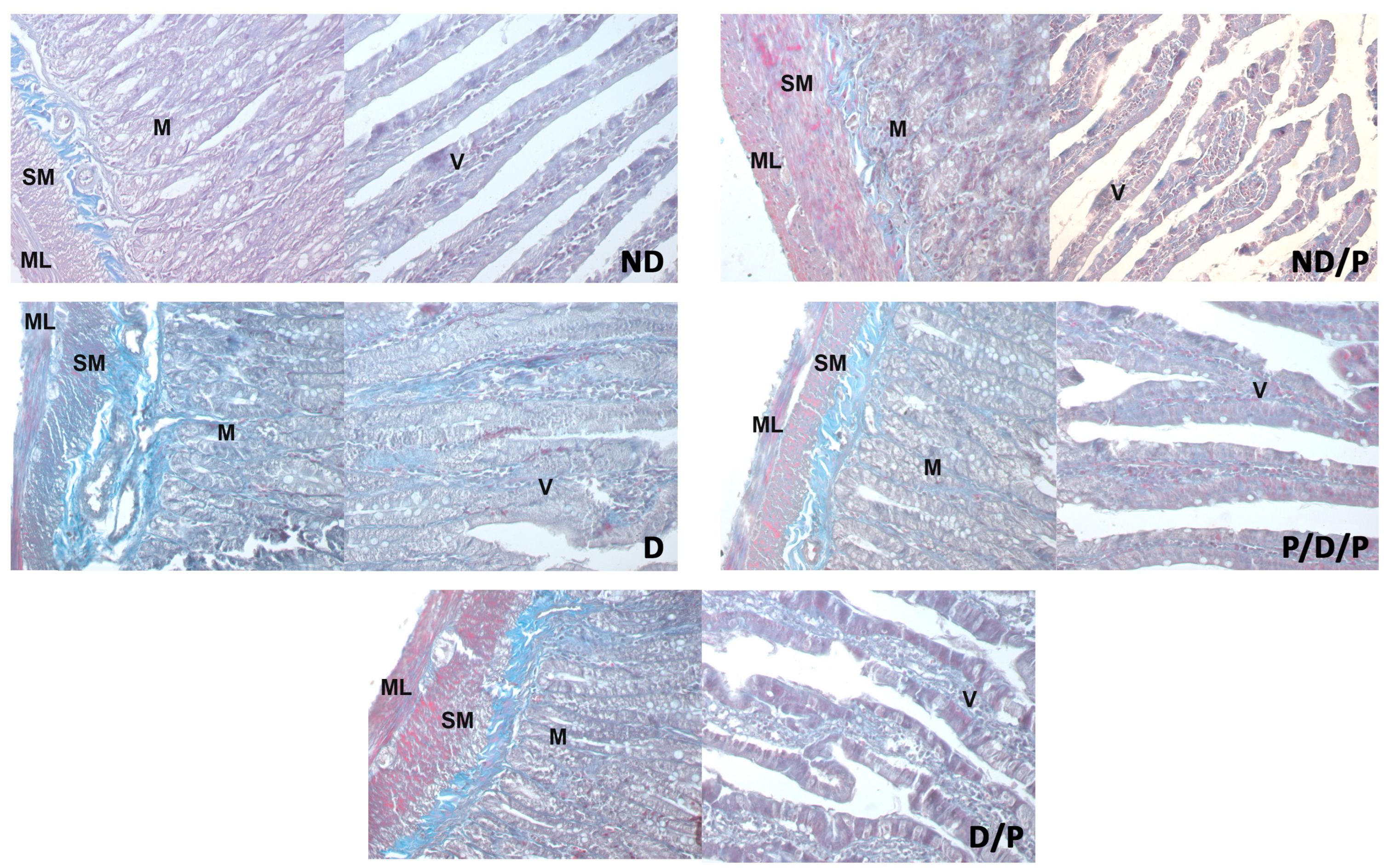
Duodenal sections stained with Masson trichrome are shown in Figure 5. Sections from the control group showed delicate collagen fibres in the submucosa (Figure 5, ND), whereas in the diabetic group, an apparent increase in collagen fibres was observed throughout the muscular layer, the submucosa, and in the middle of the villus (Figure 5, D). Diminished collagen-fibre deposition was detected in sections from the duodenum of the P/D/P and D/P groups (Figure 5, P/D/P and D/P). In the duodenum section of the ND/P group, Masson trichrome staining showed similar collagen deposition as was observed in the control group (Figure 5, ND/P).
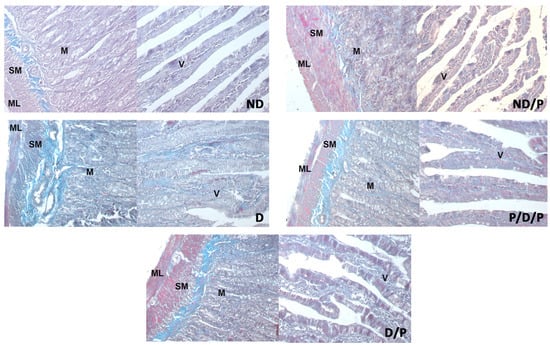
Figure 5.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the collagen fibres deposition of the intestine (duodenum section). Masson trichrome staining of duodenum section; V—villi (magnification 20×); SM—submucosa (magnification 40×); ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks; C—crypts; SM—submucosa; ML—muscular layer; V—villi.
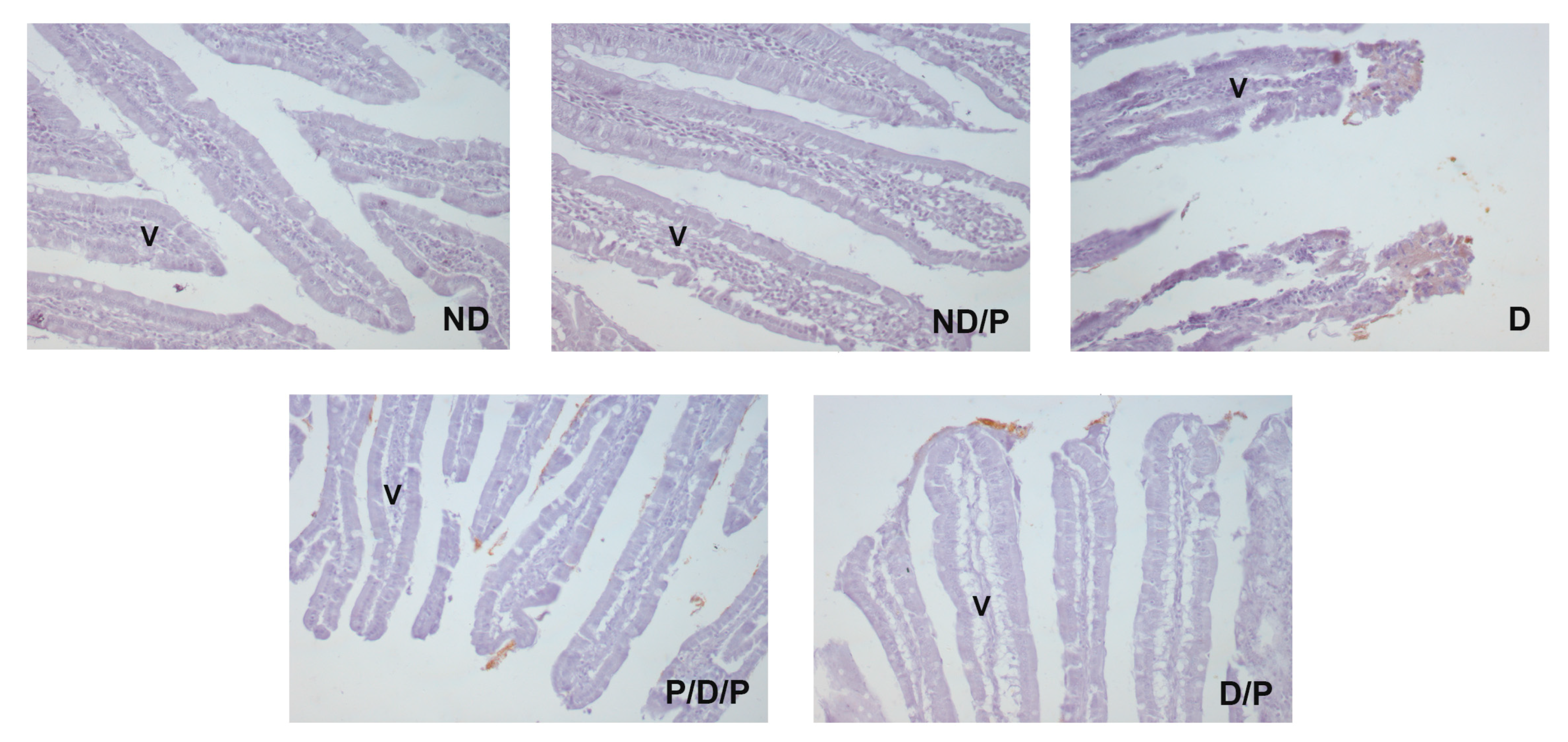
Alpha-smooth muscle actin (alpha-SMA) immunostaining was observed only in the muscular layer and submucosa of the duodenum section of the diabetic group (Figure 6, D), while in other experimental groups, no alpha-SMA-positive staining was detected (Figure 6, ND, ND/P, P/D/P, and D/P).
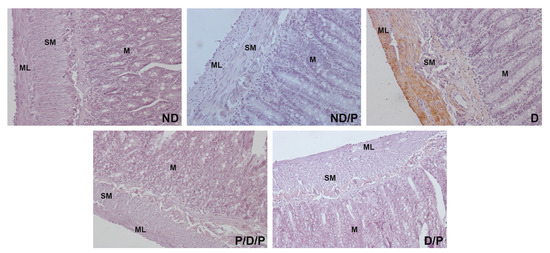
Figure 6.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the immunohistochemical localization of alpha-smooth muscle actin in the intestine (duodenum section). Light photomicrographs of alpha-smooth muscle actin immunohistochemical staining of duodenum section (magnification 40×). ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks; C—crypts; SM—submucosa; ML—muscular layer.
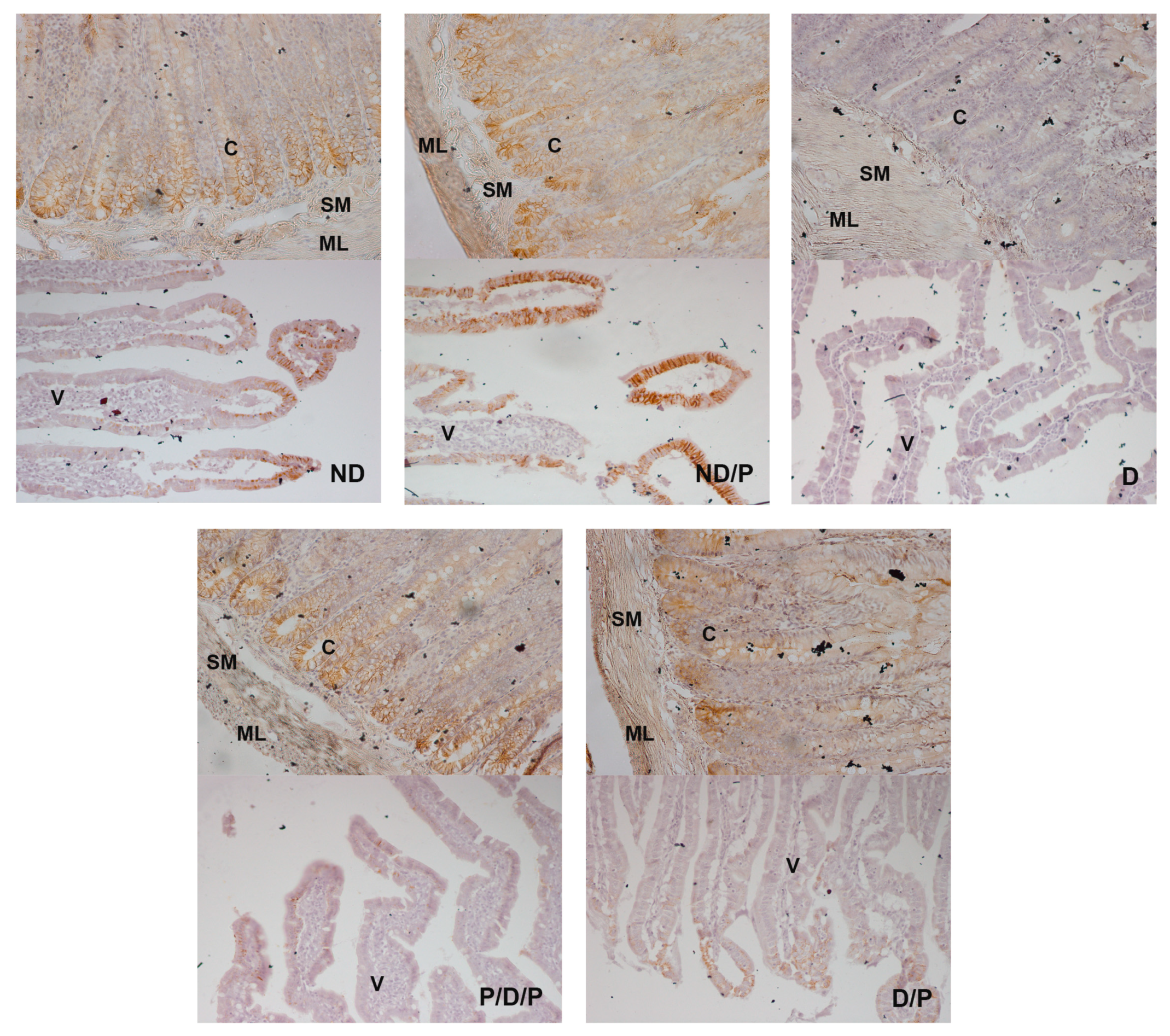
The marker of fibrosis, fibronectin-positive immunostaining, was observed in the upper part of the villi in the diabetic group (Figure 7, D), while in the P/D/P and D/P groups, decreased fibronectin-positive staining in the villi was observed (Figure 7, P/D/P and D/P). In the ND and ND/P groups, no fibronectin-positive staining was observed (Figure 7, ND and ND/P).
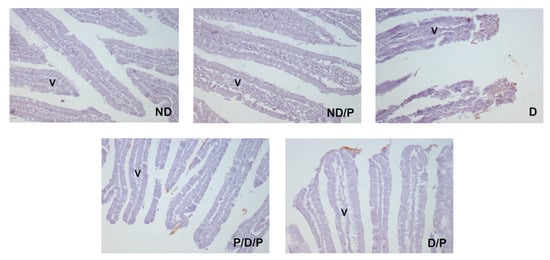
Figure 7.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the immunohistochemical localization of fibronectin in the intestine (duodenum section). Light photomicrographs of fibronectin immunohistochemical staining of duodenum section (magnification 20×). ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks; V—villi.
E-cadherin (a prominent epithelial marker)-immunostaining was observed in the epithelium of the villi, crypts, the submucosa, and muscular layers in the duodenum sections in the ND and ND/P groups (Figure 8, ND and ND/P). In the diabetic group, no E-cadherin-positive staining was detected (Figure 8, D). In the P/D/P and D/P groups, E-cadherin immunostaining was observed in the same regions as in the control group but with a slightly weaker signal, especially in the villi part (Figure 8, P/D/P and D/P).
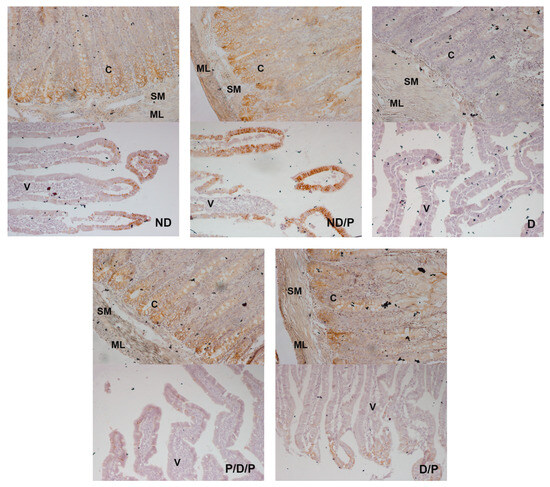
Figure 8.
The effect of probiotic Lactobacillus paraplantarum BGCG11 administration on the immunohistochemical localization of E-cadherin in the intestine (duodenum section). Light photomicrographs of fibronectin immunohistochemical staining of duodenum section V—villi (magnification 20×); SM—submucosa (magnification 40×); ND—non-diabetic control group; ND/P—probiotic Lactobacillus paraplantarum BGCG11 treated non-diabetic group; D—STZ-induced diabetic group; P/D/P—one week pre-treatment and four weeks post-treatment with probiotic Lactobacillus paraplantarum BGCG11 of STZ-induced diabetic group; D/P—STZ-induced diabetic rats treated with probiotic Lactobacillus paraplantarum BGCG11 for four weeks; C—crypts; SM—submucosa; ML—muscular layer; V—villi.
2.5. Effects of Probiotic BGCG11 Administration on Changes in Gut Microbiota Composition
We profiled the gut microbiota composition in each experimental group and identified several associations between bacterial taxa and metabolic pathways. We compared the final effect of the treatment of diabetic rats with probiotic BGCG11 on changes in gut microbiota in the case when the disease was induced at the same time as the initiation of probiotics in the D/P group and rats in which diabetes was induced during the administration of probiotics in the P/D/P group with the diabetic group of rats. There were no significant changes in the alpha diversity during the whole experiment (the beginning, induction, and the end of treatment) in the D group or in the P/D/P group, as well as in treatment (D/P in induction and at the end), and also no significant changes were observed at the end of the experiment for all groups (Figure S1).
Regarding beta diversity, notable different clustering of groups was observed in the D group and the P/D/P group during the experiment (the beginning, induction, and the end of treatment), while in the treatment (D/P induction and D/P end) and at the end for all groups, no such clustering of groups was observed (Figure S2).
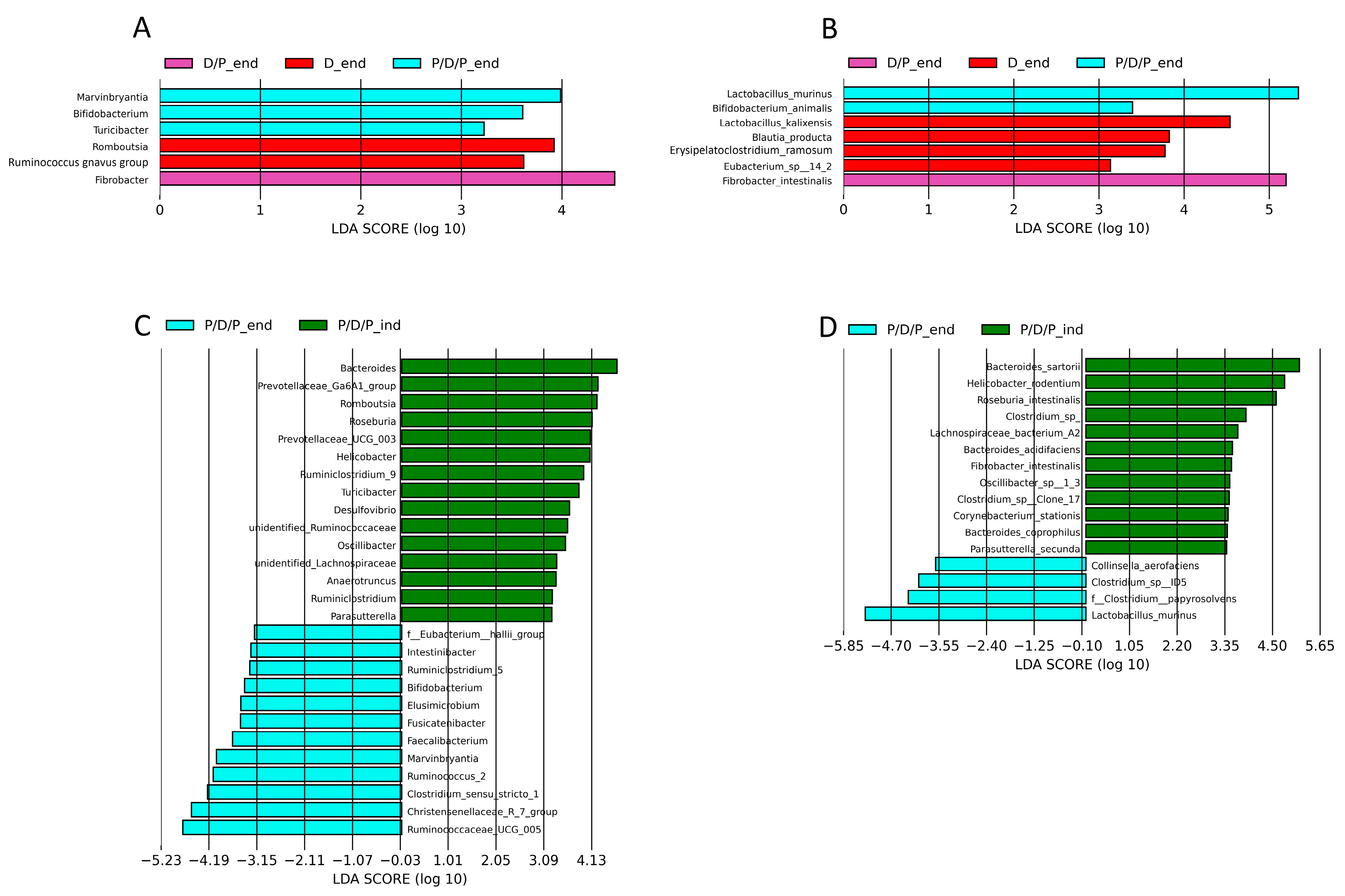
To compare the relative abundances between the groups, we conducted a linear discriminant analysis effect size (LEfSe), as shown in Figure 9. Genera Marvinbryantia, Bifidobacterium, Lactococcus, and Turicibacter, as well as species Bifidobacterium animalis and Lactobacillus murinus, were shown to be microbial markers of the P/D/P group, whereas Fibrobacter intestinalis were enriched in the D/P group, and genera Romboutsia and Ruminococcus gnavus, as well as species Lactobacillus kalixensis, Blautia producta, Erysipelatoclostridium ramosum, and Eubacterium sp. were enriched in the D group (Figure 9A,B).
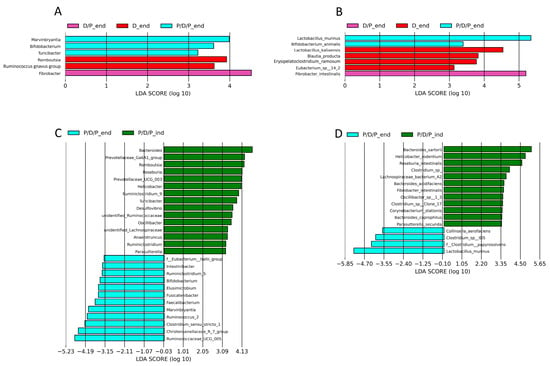
Figure 9.
Graphical display of genus and species among groups given by Linear discriminant analysis effect size (LEfSe) of gut microbiota at genus level at the end of the experiment in all groups (A) and for the same groups at the species level (B), and between induction of diabetes and the end of the experiment for the P/D/P group at the genus level genus (C) and at the species level (D).
During the moment of diabetes induction, LEfSe analysis revealed that 15 bacterial genera (Bacteroides, Prevotellaceae_Ga6A1_group, Romboutsia, Roseburia, Prevotellaceae_UCG_003, Helicobacter, Ruminiclostridium_9, Turicibacter, Desulfovibrio, Unidentified_Ruminococcaceae, Oscillibacter, Unidentified_Lachnospiraceae, Anaerotruncus, Ruminiclostridium, and Parasutterella (Figure 9C) and 12 bacterial species (Bacteroides sartorii, Helicobacter rodentium, Roseburia intestinalis, Clostridium sp., Lachnospiraceae bacterium A2, Bacteroides acidifaciens, Fibrobacter intestinalis, Oscillibacter sp. 1_3, Clostridium sp. Clone 17, Corynebacterium stationis, Bacteroides coprophilus, and Parasutterella secunda) were enriched in the P/D/P group (Figure 9D). After four weeks of treatment with BGCG11, the enrichment pattern changed. Twelve bacterial genera (F_Eubacterium hallii_group, Intestinibacter, Ruminiclostridium_9, Bifidobacterium, Elusimicrobium, Fusicatenibacter, Faecalibacterium, Marvinbryantia, Ruminococcus_2 Clostridium sensu stricto 1, Christensenellaceae_R7_group, and Ruminococcaceae UCG 005) and four bacterial species (Collinsella aerofaciens, Clostridium sp. ID5, Clostridium papyrosolvens, and Lactobacillus murinus) were enriched at the end of the treatment period (Figure 9C,D).
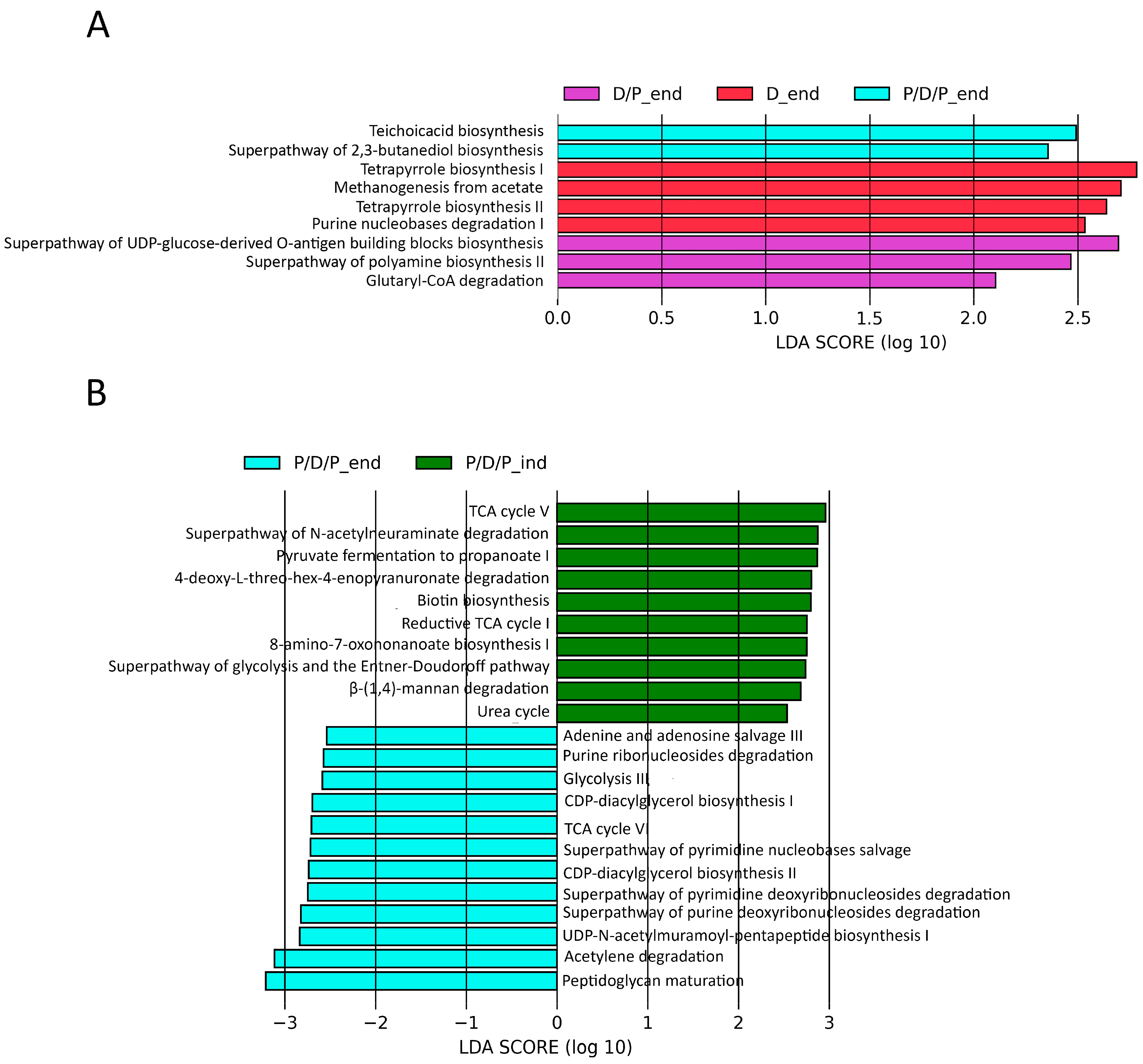
Furthermore, we used the PICRUSt2 pipeline with default options to compare projected microbial metabolic functions between the groups in order to evaluate probable microbial functional composition, presenting only pathways that reached an LDA significance threshold of >2.5. At the end of the treatment, these analyses revealed several pathways involved in teichoic acid biosynthesis (TEICHOICACID-PWY) and butanediol biosynthesis (PWY-6396) as potential markers of the P/D/P group. The D/P group was characterized by pathways involved in lipopolysaccharide (PWY-7328) and polyamines (POLYAMINSYN3-PWY) biosynthesis and carboxylate degradation (PWY-5177). According to the findings, the D group was distinguished by an enrichment of the pathways involved in methanogenesis from acetate (METH-ACETATE-PWY), tetrapyrrole biosynthesis (PWY-5188, PWY-5189), and purine nucleotide degradation (P164-PWY) (Figure 10A).
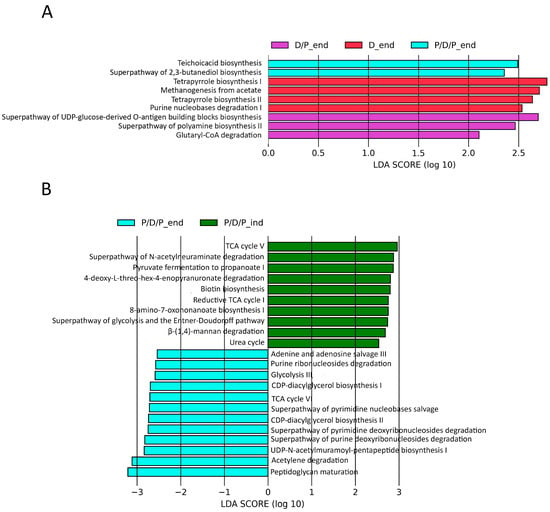
Figure 10.
Differentially abundant predicted metabolic pathways generated using the PICRUSt2 pipeline with default options, showing discriminative predicted pathways among the groups at the end of the experiment (A) and for the P/D/P group at the moment of diabetes induction and at the end of the experiment (B).
We also compared which metabolic pathways were enriched during the continuous administration of probiotics at the end of the treatment, P/D/P_end, with those that were enriched during the time of diabetes induction, P/D/P_ind. The analyses indicated that at the time of disease induction, the P/D/P group was marked by enrichment pathways involved in biotin (BIOTIN-BIOSYNTHESIS-PWY, PWY-6519) biosynthesis, precursor metabolites, and energy generation (PWY-6969, GLYCOLYSIS-E-D, P108-PWY), CO2 fixation reductive (P23-PWY), carboxylate degradation (P441-PWY), carbohydrate degradation (PWY-6507, PWY-7456), and urea cycle (PWY-4984). At the end of the treatment, the P/D/P group displayed the greatest quantity of enriched pathways implicated in purine and pyrimidine nucleotide salvage (PWY-6609, PWY-7208) and degradation (PWY0-1296, PWY0-1297, PWY0-1298), generation of precursor metabolites and energy (ANAGLYCOLYSIS-PWY, PWY-6969, P161-PWY), phospholipid biosynthesis (PWY-5667, PWY0-1319), and peptidoglycan biosynthesis (PWY-6387, PWY0-1586) (Figure 10B).
3. Discussion
Intestinal dysfunction has been intensively studied in the aetiology of diabetes [17,18,19]. It has been shown that disturbed resident gut microbiota is associated with chronic inflammation and that it contributes to diabetes onset [20]. Altered gut microbiota and inflammation lead to increased intestinal permeability and metabolic endotoxaemia [2,4]. A connection between the microbiome and pathogenesis of type-2 diabetes (T2D) and impaired glucose tolerance has been reported, as well as in the pathogenesis of type-1 diabetes (T1D) and disturbed glycaemic response [21]. Altered gut microbiota and inflammation also lead to disturbed normal glucose tolerance, insulin resistance, modulation of gut peptide YY, and glucagon-like peptide-1 secretion [22]. A review by Kobyliak et al. elaborated on the efficacy of probiotics for the protection of pancreatic beta cell damage and explained the mechanism of probiotic action in patients with T2D showing amelioration of diabetes [23]. Probiotics are live microorganisms naturally occurring in fermented food products with multiple health benefits, including in diabetes and diabetic complications [24,25]. Bifidobacterium sp. and Lactobacillus acidophilus are most commonly used by humans and have been investigated for their potential effect on human health [26]. L. paraplantarum BGCG11 was isolated from soft, white, homemade cheese. It produces EPS as a heteropolysaccharide, showing a “ropy” phenotype, denoted by the formation of a long filament in the culture, which is due to the synthesis of EPS. The biopolymer is a heteropolysaccharide mainly composed of glucose (76%) and rhamnose (21%) with some traces of galactose [27]. In previous articles, we have shown that L. paraplantarum BGCG11 was able to survive simulated gastrointestinal transit (GIT) and was able to elicit different in-vitro immune responses upon peripheral blood mononuclear cells; it has an anti-inflammatory or immunosuppressor profile, thus could be included in the diet of patients suffering diseases associated with an increased inflammatory status [15]. In addition, purified EPS-CG11 synthesized by the strain L. paraplantarum BGCG11 was able to partially counteract the HT29-MTX cellular damage (release of LDH) caused by some pathogens [28].
In that regard, L. paraplantarum BGCG11 was chosen for evaluation of its probiotic potential in diabetic rats. We showed that both pre- and post-treatment with the probiotic BGCG11 showed an antidiabetic effect in STZ-induced diabetic rats. We observed that administration of this probiotic improved the disturbance in redox homeostasis, inflammation, and fibrosis in the liver and kidneys of diabetic rats, and as a result of the improvement of the antioxidant system, an increase in E-cadherin and decrease in α-smooth muscle actin and fibronectin [16]. In this paper, we have further analysed its protective mechanism in diabetes. The study’s findings demonstrate that giving diabetic rats the BGCG11 strain orally both before and after treatments enhanced the histology of the pancreatic islets, the areas that produce insulin, and decreased collagen-fibre deposition as a marker of fibrosis. After both treatments, glucagon-positive cells were found in the core of the pancreatic islet, which was obviously a reflection of the diabetes-related destruction of pancreatic morphology linked to impaired glucagon signalling that leads to a marked increase in alpha cell mass [29]. Although a different animal model was used in comparison to our research, it is interesting that in several studies, different lactobacilli exhibited protective effects on diabetic animals. For example, Lactobacillus paracasei strain NL41 was able to prevent induced diabetes (HFD/STZ-T2DM) in rats by reducing insulin resistance and oxidative-stress status and protecting beta-cell function [30]. In HFD and type-2 diabetic models, Lactobacillus fermentum MCC2759 and MCC2760 showed beneficial effects through increased GLUT4, GLP1, and adiponectin expression, improved intestinal barrier function, and decreased pro-inflammatory cytokines in the liver, intestine, MAT, and muscle tissue [31]. L. plantarum HAC01 could alleviate hyperglycaemia and T2DM by regulating glucose metabolism in the liver, protecting the islet β-cell mass, and restoring the gut microbiota and SCFAs in mice [32]. Moreover, in mice, L. plantarum Pro1 and a combination of L. rhamnosus Pro2 and L. plantarum Pro1 reduced the harmful effects of high levels of fructose in the blood, repaired histopathological changes in the pancreas, and altered the expression of GH, IGF1, and GLP-1 genes to normal levels, which are important for a balance between pancreatic islet cell proliferation and apoptosis [33]. Regarding the effect of our BGCG11 strain, its treatment decreased the disturbance in intestine permeability (decrease in zonulin secretion, improvement of fluid transfer through the intestinal mucosa), improved intestinal histology, and decreased the fibrotic process (lowered collagen-fibre deposition, cytoskeletal marker alpha-SMA and extracellular protein fibronectin, and increased cell-surface marker, E cadherin).
Endotoxaemia in T1D induces oxidative stress in the pancreas, destroys β-cells, and impairs insulin production [8]. The results of this study revealed the destruction of pancreatic islets in diabetic rats, whereas the islets were preserved after both pre- and posttreatment with the probiotic. In accordance with this result, we also observed collagen-fibre deposition in the islets of diabetic rats, which was noticeably reduced in the pancreatic islet in both probiotic-treated groups. Preserved structure and reduced fibrosis in pancreatic tissue in the probiotic-treated groups were followed by increased functionality, reflected in increased areas of the pancreatic islets that produce insulin. The possible mechanism underlying this positive effect of the probiotic on the pancreas could be the result of the attenuation of inflammation and endotoxaemia observed in diabetes. In diabetes, altered proportions of bacterial phyla in the intestine and consequent endotoxaemia increase the absorption of lipopolysaccharide, which spreads in circulation, initiating activation of inflammatory pathways and impairment of insulin signalling [34]. For example, pancreatic immunity and disease pathogenesis are significantly influenced by the gut–pancreatic axis, which is regulated by the microbiome. There is a growing amount of evidence supporting the role of the intestinal microbiota in pancreatic disease diagnosis and treatment. Faecal microbiota transplantation (FMT) is therefore becoming recognized as a potentially effective treatment option for a number of pancreatic conditions, such as cancer, pancreatitis, and type-1 diabetes (T1D) [35]. Recent evidence indicates that transformed gut microbiota contribute to the different changes observed in diabetes and are thus of great interest as potential treatments for obesity and diabetes. Histological evidence for pancreatic islet destruction in BioBreeding diabetic-prone rats indicates that increased intestinal permeability occurs at least one month before manifest diabetes [36]. Some intestinal aberrations in human autoimmune diabetes have been also reported. In patients with T1D, increased intestinal permeability, ultrastructural changes, and increased circulating zonulin have been observed [37]. Increased permeability of the intestinal epithelium could enable contact with food antigens, enteroviruses, live bacteria, and bacterial products with the mucosal immune system leading to intestinal inflammation, and it could also trigger T1D [38]. Blockage of the cannabinoid receptor-1 (CB1) using CB1 antagonists lowered gut permeability in obese mice through normalizing occludin and zonulin expression [39]. In accordance with the literature data, the results of this study show the ameliorating effect of the probiotic Lactobacillus paraplantarum BGCG11 on the reduction in fluid transfer through the small intestinal mucosa. In patients with diabetes, as in animals with experimental-induced diabetes, disorders occur in a thin permeability hose at different levels, including the transfer of fluid and glucose through the intestinal mucosa. Potential mechanisms that underlie the verified effects of probiotics (including the effects of L. paraplantarum BGCG11) on diabetes may include changes in transport capacity, passive permeability, and changes in the mucous membrane of the small intestine. All mentioned diverse effects require more detailed investigation and experimental proof. The integrity of the intestinal epithelium is primarily preserved by intercellular junctional complexes such as tight junctions (TJs). Circulating zonulin, a pre-haptoglobin molecule that has, up to now, been only regarded as the inactive precursor of haptoglobin, is set as a marker of intestinal permeability in autoimmune diseases and diabetes [40,41]. By modulating intercellular tight junctions, zonulin can influence intestinal permeability [42]. Recently, zonulin was detected in an uncleaved form in human serum, and a major biological effect of zonulin in influencing the integrity of intercellular TJ was proposed [43]. Several potential intestinal luminal stimuli can trigger zonulin release, and when intestinal permeability is disrupted, serum zonulin levels are increased. It has been reported that abnormal serum zonulin levels are associated with increased intestinal permeability in a subgroup of T1D patients [42]. In BioBreeding diabetes-prone rats, the zonulin-dependent increase in intestinal permeability was recorded 2–3 weeks before the onset of T1D [44]. Multi-strain probiotic formulation (L. rhamnosus, B. lactis, and B. longum) modulates the expression of TJ proteins and prevents inflammatory damage [45]. The results reported in this study show that the probiotic strain L. paraplantarum BGCG11 significantly reduced serum zonulin levels after pre- and post-treatment of diabetic rats.
The observed decrease in fluid transfer through small intestine mucosa and serum zonulin levels in diabetic rats treated with the probiotic correlates with other results obtained in this study. Increased collagen-fibre deposition observed in the duodenal section through the muscular layer, the submucosa, and in the middle of the villus was significantly decreased after treatment with the probiotic. In line with collagen deposition, the diabetic state was also accompanied by the increased presence of two fibrotic marker proteins. Tissue fibrosis is characterized by the deposition of fibronectin, the extracellular matrix glycoprotein, which serves as a scaffold for collagen and other extracellular matrix proteins [46]. Also, the de novo appearance of alpha-SMA in response to tissue injury and its increased expression is frequently used as a marker of fibrosis [47]. The levels of both fibrotic markers were markedly decreased in diabetic rats treated with the probiotic when compared to untreated diabetic animals. The antifibrotic effects of the probiotic were additionally confirmed by the finding that the expression of the epithelial cell marker E-cadherin remained elevated in probiotic-treated rats, while complete loss of its expression was observed in diabetic rats as another hallmark of fibrosis development.
The full mechanism of probiotic action in diabetes is still unclear. Higher excretion of glucagon-like peptide 1 (GLP-1) from intestinal L-cells, which raises insulin sensitivity, is one of the recognized mechanisms of probiotic activity [9,48,49]. For example, the genetically engineered strain Lactobacillus plantarum (L. plantarum)-pMG36e-GLP-1 regulated the intestinal microbiota, the apoptosis of pancreatic islet cells was inhibited, islet β-cell proliferation and insulin secretion were all promoted, and it showed its ameliorative effect on T2DM in artificially induced mice and transgenic mice [50]. Clinical trials in humans revealed, using the HOMA2 calculator, that probiotic therapies have a slight positive effect on the function of β-cells in individuals with T2D [51]. Other points of action of probiotics in diabetes are linked with an improvement in intestinal integrity, diminishment of endotoxaemia, and immunomodulatory, anti-inflammatory, and antioxidant effects [2,16,52]. Recently it was shown that Lacticaseibacillus paracasei SD1 and Lacticaseibacillus rhamnosus SD11 attenuate inflammation and β-cell death in streptozotocin-induced type-1 diabetic mice, preserve islet integrity and increased β-cell mass, upregulate anti-apoptotic Bcl2, as well as prevent infiltration of macrophages, CD4+, and CD8+ T cells into the islets [53]. Another study has shown that for T2DM mice, supplementing with the Bifidobacterium longum NBM7-1 and Lactiplantibacillus rhamnosus NBM17-4 alleviates glucose intolerance through the upregulation of IL-22, which enhances insulin sensitivity and pancreatic functions while reducing liver steatosis [54]. In light of the findings presented herein, we can conclude that enhancement of the epithelial barrier and a decrease in intestinal permeability are essential elements of the L. paraplantarum BGCG11 probiotic’s mode of action, as well as the lowering of the fibrotic process, which leads to a reduction in endotoxin concentrations in circulation and its adverse consequences.
It was observed that unlike the use of probiotic BGCG11 strain after the onset of diabetes, continuous application of the probiotic leads to a statistically significantly increased abundance of genera Marvinbryantia, Bifidobacterium, and Turicibacter, as well as species Lactobacillus murinus and Bifidobacterium animalis, taxa associated with enhanced insulin sensitivity, and the production of short-chain fatty acids (SCFA). The literature data indicate that butyric acid enhances insulin sensitivity, promotes pancreatic insulin production, and modifies insulin signaling [55]. It has significant functions, such as providing anti-obesity effects, reducing metabolic stress, and inhibiting inflammatory reactions [56]. Marvinbryantia butyrate-producing bacteria may reduce the incidences of insulin resistance, and these genera were more prevalent in healthy controls than in T2D [57,58]. Several studies have found that Bifidobacterium sp. can improve insulin sensitivity in people with T2D. In high-fat diet mice, administration of B. animalis ssp. lactis increased insulin sensitivity, decreased inflammatory cytokine levels, and ameliorated T2DM liver injuries [59,60]. Likewise, a combination of the two strains Bifdobacterium BL21 and Lacticaseibacillus LRa05 can ameliorate T2DM in mice by regulating the intestinal flora and reducing inflammation and oxidative stress [61]. Turicibacter has also been associated with butyric acid production in the intestine [62]. Diabetes is known to be a risk factor for various complications, including the development of mesenteric ischemia, and one study has demonstrated that Lactobacillus murinus can help mitigate the negative effects of intestinal ischemia/reperfusion injury [63,64].
Analysis of the obtained results revealed that continuous probiotic treatment had a better effect on the microbiota of diabetic rats, so we decided to compare the changes in the microbiota between the end of treatment in the P/D/P group (P/D/P_end) and the time of disease induction (P/D/P_ind). During a maintained application of the probiotic in the P/D/P group, we saw numerous shifts in relative abundances at various taxonomic levels. At the end of treatment, a reduction in potentially harmful bacteria was observed. A high relative abundance of bacterial taxa, negatively correlated with diabetic complications, was observed during disease induction in the P/D/P_ind group but not at the end of the treatment for the P/D/P_end group, such as genera Helicobacter, Ruminococcaceae, Oscillibacter Lachnospiraceae, and Parasutterella, and species Lachnospiraceae bacterium, and Corynebacterium stationis. This is consistent with prior data revealing that Ruminococcaceae was the predominant family in T1D and that Oscillibacter, an opportunistic pathogen, was associated with T1D, T2D, and obesity in humans and mice [65,66]. Additionally, research indicates that intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice [67]. Furthermore, Parasutterella was positively associated with BMI and T2D, and Corynebacterium stationis bacteria correlated with diabetic foot infections [68,69].
The composition of the gut microbiota continued to evolve under the influence of the probiotic treatment, and LEfSe analysis has shown that the intestinally beneficial bacteria were significantly more prevalent at the end of treatment. Among the beneficial bacteria, we identified the presence of bacterial taxa that negatively correlate with diabetes, such as Intestinibacter, Bifidobacterium, Elusimicrobium, Fusicatenibacter, Faecalibacterium, Marvinbryantia, Clostridium sensu stricto 1, Christensenellaceae_R7_group, and Ruminococcaceae UCG 005. One study showed that a higher abundance of Christensenellaceae, Clostridiaceae 1, Peptostreptococcaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG005, Ruminococcaceae UCG008, Ruminococcaceae UCG010, Ruminococcaceae NK4A214 group, C sensu stricto 1, Intestinibacter, and Romboutsia may benefit the risk of insulin resistance and T2D [57]. Probiotic L. paracasei NL41 induced changes in the microbiota structure; for example, Bacteroides, Clostridia (specifically, Ruminococcus torques), and Parasutterella were significantly reduced, while some beneficial microorganisms (Bacteroidales_S24-7_group and the families Lachnospiraceae and Ruminococcaceae) were enriched by NL41 [70]. Other studies revealed that the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia were negatively associated with T2D. Also, bacterial genera Faecalibacterium, Fusobacterium, Dialister, Elusimicrobium, Anaerostipes, Coprococcus, Fusicatenibacter, Lachnospira, Marvinbryantia, Roseburia, Faecalibacterium, Ruminococcus, Subdoligranulum, UCG-002, Agathobacter, Butyricicoccus, Alistipes, Clostridium sensu stricto 1, and all Lachnospiraceae were identified in significantly higher abundance in healthy individuals than in people with diabetes [71,72,73].
The literature data revealed that a Ruminococcaceae UCG-005 abundance positively correlated with SCFA concentration in the faeces of piglets, and Intestinibacter bartlettii produces the SCFAs isobutyrate and isovalerate, while Marvinbryantia and Clostridium sensu stricto 1 were butyrate-producing bacteria [74,75,76,77]. Furthermore, Bifidobacterium species have been proven to lower serum glucose levels, increase insulin-signaling protein expression, and improve adipokine profiles in diabetic mice [78]. It is a very significant finding that under diabetic conditions, Faecalibacterium prausnitzii anti-inflammatory compounds could restore the gut barrier and zonula occludens-1 expression, possibly through the tight junction pathway [79]. Also, Mancabelli et al. reported Christensenellaceae as one of five taxa considered a signature of a healthy gut [80].
Because 2, 3-butanediol has been found to be effective in the treatment of conditions such as obesity, insulin resistance, and diabetes, the enrichment of the pathway leading to butanediol biosynthesis (PWY-6396) was identified as a potential marker pathway in the P/D/P_end group (WO2021038253A1WIPO (PCT)) (https://patents.google.com/patent/WO2021038253A1/en (accessed on 14 July 2023)). On the other hand, the super pathway of polyamine biosynthesis II (POLYAMINSYN3-PWY) could be selected as a potential marker pathway in the D/P_end group. Polyamines, such as spermine, may have a role in maintaining mitochondrial energetics in a high-glucose environment. Moreover, supplementation of spermine improves glucose uptake, reduces myocardial cell death and oxidative stress, and attenuates cardiac dysfunction in the streptozotocin-induced T1D hearts [81]. Our findings suggest that a putative marker pathway in the D_end group could be a process involved in methanogenesis (METH-ACETATE-PWY). A recent study revealed that changes in intestinal methane production play a role in diabetes glycemic management [82]. In diabetic individuals, intestinal methane production is additionally related to reduced glucose tolerance and may be linked to altered glycemic control [83].
Different TCA and glycolysis pathways, as well as pathways involved in biotin biosynthesis on one side, pathways involved in purine and pyrimidine nucleotide biosynthesis and degradation, pathways involved in the synthesis of acetate and lactate (glycolysis III and pyruvate fermentation to acetate and lactate II) and phospholipid and peptidoglycan biosynthesis were shown as potentially dominant microbial marker pathways in the P/D/P_ind and P/D/P_end groups, respectively. In T1D, the TCA cycle is known to be significantly altered, which contributes to the development of cardiovascular autonomic neuropathy [84]. Similar findings, including an inefficient mitochondrial TCA cycle influx and reduced energy yield in skeletal muscle, were observed in T2D [85]. Also, using biotin as an adjuvant to the insulin regimen has been shown to improve glycemic control and lower plasma lipid concentrations in poorly managed T1D patients [86]. Also, it has been demonstrated that, in addition to altering insulin secretion in pancreatic cells, phospholipids can alter the effect of insulin in a variety of physiological processes [87].
In summary, diabetes-related gut health is a complex and interconnected system involving the gut microbiota, gut barrier integrity, inflammation, and metabolic regulation. The strain BGCG11 was able to assist in the reparation and restoration of both the pancreas and intestines of diabetic rats while assembling the beneficial microbiota. Ongoing research continues to unravel the intricate mechanisms underlying these relationships, paving the way for innovative therapeutic approaches to improve gut health and overall metabolic outcomes in individuals with diabetes.
4. Materials and Methods
4.1. Preparation of Bacteria
The study used Lactobacillus paraplantarum BGCG11, a naturally occurring isolate from soft, white, handmade cheese from Serbia. Bacteria were cultivated in De Man-Rogosa-Sharpe (MRS) medium (Merck GmbH, Darmstadt, Germany) at 30 °C. For the animal treatment, 10 mL of overnight culture (containing in total 2 × 108 to 3 × 108 CFU/mL) was pelleted, and the bacterial precipitate was washed in saline and resuspended in 1 mL of 11% sterile skimmed milk (AD Mlekara Subotica, Subotica, Serbia), as described by Geier et al. [88].
4.2. Animals
Experiments were performed on 2.5-month-old adult albino Wistar rats weighing 220–250 g. All animal procedures complied with Directive 2010/63/EU on the protection of animals used for experimental and other scientific purposes. The procedures were approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research “Siniša Stanković”, University of Belgrade, Serbia, No. 02-12/14.
4.3. Experimental Protocol
The animals were fasted overnight and sacrificed by euthanasia using a guillotine, according to all recommendations of the guidelines for euthanasia of animals. Death comes quickly without any suffering for the animals. Euthanasia was performed on animals one by one (in the block separated from the experimental room where the animals were held in order to avoid stress to the animals) by well-trained staff. Immediately before sacrifice, blood was collected from the tail vein of the animal for glucose measurement and for the preparation of serum, which was stored at −20 °C until use. After blood collection and sacrifice, an abdominal incision was made, and the tail part of the pancreas was removed and fixed in 10%-buffered formalin for histological examinations. The pancreas and duodenum from all experimental groups were removed and fixed in 10%-buffered formalin for histological and immunohistological examinations.
STZ (MLDS)-induced diabetes was employed as the experimental model. Male albino Wistar rats were given an intraperitoneal injection of STZ (40 mg/kg/day) (MP Biomedicals, Solon, OH, USA) for five days in order to induce diabetes. Prior to the injection, STZ was dissolved in a sodium citrate buffer (0.1 M, pH 4.5). Twenty-four hours following the last STZ injection, blood glucose was evaluated. Rats that had fasted for the whole night had blood samples taken from their tail veins, and a blood glucose meter (Accu-Check Active, Roche Diagnostics Scientific Office, Cairo, Egypt) was used to determine the glucose levels. When the blood glucose level in rats was more than 20 mmol/L while fasting, the rats were diagnosed as diabetic. The rats were split into the following five groups at random: (i) ND—the non-diabetic group (n = 8), or the negative control, that were given injections of citrate buffer equivalent to those of STZ for five days in a row; (ii) ND/P—the non-diabetic group, also referred to as the positive control (n = 8), that was orally administered L. paraplantarum BGCG11 for four weeks (10 mL; 108 CFU/mL); (iii) D—the diabetic group (n = 8), that was injected with STZ (40 mg/kg; i.p.) for five consecutive days and left untreated throughout the four-week period; (iv) P/D/P—the pretreated diabetic group (n = 6), that received the BGCG11 strain at the same dose one week prior to the first STZ injection and kept receiving the BGCG11 strain for four weeks following the STZ treatment; (v) D/P—the post-treatment diabetic group (n = 6), which began on the last day of STZ administration and received the same daily dose of the BGCG11 strain for four weeks. Blood serum was obtained from the rats after an overnight fast following four weeks of diabetes.
4.4. Determination of General Indicators of Diabetes
After blood coagulation and centrifugation at 2000× g for 10 min, the serum was extracted. Blood glucose levels were measured in all experimental groups using a commercial kit (Gluco-quant Glucose/HK; Boehringer Mannheim, Mannheim, Germany) based on the hexokinase/G6P-DH enzymatic method at the start of the experiments, 24 h after the last dose of STZ was administered, and four weeks after the treatment. Hemoglobin (Hb) was determined according to Drabkin and Austin [89]. The colorimetric method was used to measure glycosylated hemoglobin (GlyHb), according to Parker et al. [90]. Serum zonulin level was determined by immunoblot analysis (goat anti-Human Haptoglobin H5015 (1:1000), Sigma-Aldrich, St. Louis, MO, USA). Immunoreactive bands were identified with a chemiluminescence detection system (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and quantified using TotalLab electrophoresis software, ver. 1.10 (Phoretix, Newcastle-upon-Tyne, UK).
4.5. Histological Analysis and Immunostaining
All experimental groups’ pancreas and duodenum were removed, and they were all fixed in 10%-buffered formalin for histology and immunohistology analyses. All examined groups’ tissues were sectioned at a thickness of 5 µm after being blocked in paraffin for histological and immunohistochemical analysis (Leica DMLB, Wetzlar, Germany). Tissue sections for histological examination were coloured with hematoxylin and eosin (H&E) and observed under a light microscope. Sections of the pancreas and duodenum were stained with Masson trichrome stain for collagen-fibre detection [91]. For immunohistochemical analysis, deparaffinised sections were passed through xylene and rehydrated in sequentially graduated ethyl alcohol. Slides were incubated in 0.3%-hydrogen peroxide/methanol for 20 min to reduce nonspecific background staining due to endogenous peroxidase. After washing in PBS, the sections were treated with 0.01 M sodium citrate buffer at 98 °C for antigen retrieval. The cooled tissues were washed four times in PBS prior to application of the blocking serum for 5 min (0.05% Tween 20, 5% bovine serum albumin). The primary antibody was applied overnight at room temperature (RT). Primary polyclonal antibodies against insulin, glucagon, E-cadherin, fibronectin, and alpha-smooth muscle actin (alpha-SMA) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were diluted 1:50 in PBS with 2% dry skimmed milk. After washing in PBS, sections were incubated at room temperature for 1 h with secondary antibody horseradish peroxidase (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Tissues were incubated for 20 min at room temperature in a solution of 3,3′-diaminobenzidine (DAB). After washing with PBS, the tissues were counter-stained with haematoxylin and washed in water, and mounting media was applied to the coverslips. For the negative control, the primary antibody was not added to the sections.
4.6. Mucosal Fluid Transfer
Mucosal fluid transport was measured by the everted intestinal sac technique as described by Obembe et al. [92]. Briefly, the small intestine was removed, thoroughly washed in PBS, and cut into 10 cm segments for sac-making. Each segment was tied at one end and inverted, exposing the highly active mucosa. After blotting, segments with ligatures were weighed, filled with 1 mL of Krebs bicarbonate solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose) as the serosal fluid, and the free end was then tied. The filled sacs were weighed again, and the increase in weight was taken as the initial serosal volume (ISV). Krebs bicarbonate solution representing mucosal fluid (40 mL) was placed in an incubating flask, and the sacs were immersed and incubated for 30 min. The final serosal volume (FSV) in the sac was determined by weighing the blotted sac before and after draining its contents. The difference between the final weight of the empty sac and the initial weight of the empty sac represents gut fluid uptake (GFU). Mucosal fluid transfer (MFT) was then determined as FSV − ISV + GFU and expressed as µL/g sac/30 min.
4.7. DNA Extraction from Faeces
Faecal material from different diabetic rat groups (D (n = 3), P/D/P (n = 4), D/P (n = 4)) was collected at the beginning, at the moment of diabetes induction, and at the end of the experiments. It was kept at −80 °C, and metagenomic DNA was extracted from rat faeces using a commercially available kit, ZR Tissue DNA MiniPrep™ Kit (Zymo Research Corp., Irvine, CA, USA). The concentration of isolated DNA was measured on a BioSpec-nano spectrophotometer (Shimadzu, Columbia, MD, USA) and kept at −20 °C. All samples had a necessary concentration (≥12 ng/μL) and, in a final volume of 30 μL, were sent to Novogene Company (Beijing, China). The library was constructed, and the V3-V4 hypervariable region of the 16S rRNA amplicon was sequenced using an Illumina NovaSeq paired-end platform (Novogene Co., Ltd., Beijing, China). Quality control was performed at each step of the procedure. Each sample had a flattened refraction curve, which indicates sufficient depth during sequencing.
4.8. Data Display and Statistical Analysis
FLASH (V1.2.7 http://ccb.jhu.edu/software/FLASH/ (accessed on 18 September 2019)) was used to merge paired-end reads. Under particular filtering criteria according to the QIIME, quality filtering on the raw tags was carried out (V1.7.0 http://qiime.org/scripts/split_libraries_fastq.html (accessed on 18 September 2019)) in order to obtain high-quality clean tags. For species annotation of each representative sequence at each taxonomic rank, QIIME was performed (Version 1.7.0 http://qiime.org/scripts/assign_taxonomy.html (accessed on 18 September 2019)) using the Mothur method against the SSUrRNA database of SILVA Database. Alpha and beta diversity analyses were carried out using normalized OTU sets of data. Alpha diversity was expressed via four indices, including Shannon, Simpson, Observed-species, and Chao1. Beta diversity was visualized using Principal coordinates analysis (PCoA) calculated in QIIME (Version 1.7.0), displayed with R software (Version 1.4.1717), and expressed via Anosim and Adonis.
A one-way analysis of variance (ANOVA) followed by Tukey’s tests was used for multiple comparisons. Data subjected to statistical analysis were expressed as the means ± S.E.M. (standard error of the mean). Each experimental group consisted of from 6 to 8 rats. Statistical analysis and preparation of graphs were done in GraphPad Prism 8.4.3 software. Different letters indicate significant differences between treatments (p < 0.05) except for the presentation of relative taxa abundance. Data are presented as mean values ± the standard deviation from different experiments, except for Alpha diversity analysis and relative abundance of OTUs, which are expressed as median ± the standard deviation.
By utilizing the PICRUSt2 [93] pipeline, which is based on marker gene surveys and the MetaCyc pathways database with default settings, predictive metagenome functions at the peak of the disease were generated. Using the Galaxy framework, PICRUSt2 output was analyzed with LEfSe to reveal discriminative predicted paths between these groups; paths that met an LDA-significance threshold of >2 were then displayed.
5. Conclusions
Our results unequivocally showed the protective antidiabetic effects of L. paraplantrum BGCG11 in STZ-induced diabetic rats. Both the pre- and post-treatment of diabetic rats with the L. paraplantrum BGCG11 preserved the pancreatic β-cells and improved insulin production in the pancreas, preserved the integrity of the intestinal epithelium, reduced intestinal permeability, and decreased fibrotic process, impacting the diminishment of endotoxaemia, and positively affecting the microbiota composition. This study has shed light on the intricate effects of administering a particular strain of BGCG11 on the repair and function of damaged rats’ organs, which may help us understand how BGCG11 may benefit individuals with diabetes. Hence, in accordance with FAO/WHO criteria and EFSA recommendations, the next step will be to prove the safety and efficacy of L. paraplantrum BGCG11 strain as a potential probiotic in human clinical trials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25147697/s1.
Author Contributions
Conceptualization, M.V., M.M., M.T. and M.Ž.; methodology, J.A.J., N.G. (Nevena Grdović), J.R., M.Đ. and N.G. (Nataša Golić); validation, A.U., S.D., J.R., M.T. and M.Ž.; formal analysis, J.R., M.Đ., S.S.B., E.B. and M.T.; investigation, N.G. (Nevena Grdović), J.A.J., S.S.B. and E.B.; writing—original draft preparation, M.V., M.M., M.Đ., S.S.B., E.B., M.T. and M.Ž.; writing—review and editing, A.U., S.D., J.A.J. and N.G. (Nataša Golić); visualization, S.S.B. and E.B.; supervision, S.D., N.G. (Nevena Grdović), A.U. and N.G. (Nataša Golić); project administration, M.V. and M.Ž.; funding acquisition, M.M., M.V. and N.G. (Nataša Golić). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science Technological Development and Innovation of the Republic of Serbia (registration No. 451-03-66/2024-03/200007 and 451-03-66/2024-03/200042).
Institutional Review Board Statement
The study was conducted in accordance with Directive 2010/63/EU on the protection of animals used for experimental and other scientific purposes. The animal study protocol was approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research “Siniša Stanković”, University of Belgrade, Serbia, No. 02-12/14.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “Perfect Storm” for Type 1 Diabetes: The Complex Interplay Between Intestinal Microbiota, Gut Permeability, and Mucosal Immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Bitar, S.; Youssef, N.; Azar, S.; Eid, A.A. Butyrate modulates diabetes-linked gut dysbiosis: Epigenetic and mechanistic modifications. J. Mol. Endocrinol. 2020, 64, 29–42. [Google Scholar] [CrossRef]
- Li, X.; Atkinson, M.A. The role for gut permeability in the pathogenesis of type 1 diabetes—A solid or leaky concept? Pediatr. Diabetes 2015, 16, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-H.; Chen, Y.-P.; Chen, M.-J. Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; López, P.; Strahinic, I.; Suárez, A.; Kojic, M.; Fernández-García, M.; Topisirovic, L.; Golic, N.; Ruas-Madiedo, P. Characterisation of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics. Int. J. Food Microbiol. 2012, 158, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Živković, M.; Arambašić Jovanović, J.; Tolinački, M.; Sinadinović, M.; Rajić, J.; Uskoković, A.; Dinić, S.; Grdović, N.; Golić, N.; et al. Oral administration of probiotic Lactobacillus paraplantarum BGCG11 attenuates diabetes-induced liver and kidney damage in rats. J. Funct. Foods 2017, 38, 427–437. [Google Scholar] [CrossRef]
- Ordög, T.; Hayashi, Y.; Gibbons, S.J. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol. Dietol. 2009, 55, 315–343. [Google Scholar] [PubMed]
- Chandrasekharan, B.; Anitha, M.; Blatt, R.; Shahnavaz, N.; Kooby, D.; Staley, C.; Mwangi, S.; Jones, D.P.; Sitaraman, S.V.; Srinivasan, S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol. Motil. 2011, 23, 131–138.e26. [Google Scholar] [CrossRef] [PubMed]
- Maleki, D.; Locke, G.R., 3rd; Camilleri, M.; Zinsmeister, A.R.; Yawn, B.P.; Leibson, C.; Melton, L.J., 3rd. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch. Intern. Med. 2000, 160, 2808–2816. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H. Microbiota associated with type 2 diabetes and its related complications. Food Sci. Hum. Wellness 2013, 2, 167–172. [Google Scholar] [CrossRef]
- Suez, J.; Shapiro, H.; Elinav, E. Role of the microbiome in the normal and aberrant glycemic response. Clin. Nutr. Exp. 2016, 6, 59–73. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Khomenko, M.; Falalyeyeva, T.; Fedchenko, A.; Savchuk, O.; Tseyslyer, Y.; Ostapchenko, L. Probiotics for pancreatic β-cell function: From possible mechanism of action to assessment of effectiveness. Crit. Rev. Microbiol. 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Altenhoefer, A.; Oswald, S.; Sonnenborn, U.; Enders, C.; Schulze, J.; Hacker, J.; Oelschlaeger, T.A. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 2004, 40, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Schmid, S.; Huber, D.; Hummel, M.; Bonifacio, E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003, 290, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Cerning, J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol. Rev. 1990, 7, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, M.; Hidalgo-Cantabrana, C.; Kojic, M.; Gueimonde, M.; Golic, N.; Ruas-Madiedo, P. Capability of exopolysaccharide-producing Lactobacillus paraplantarum BGCG11 and its non-producing isogenic strain NB1, to counteract the effect of enteropathogens upon the epithelial cell line HT29-MTX. Food Res. Int. 2015, 74, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, V.; Habener, J.F. Evolving function and potential of pancreatic alpha cells. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 859–871. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins. 2021, 13, 1068–1080. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- ALSuhaymi, N.; Darwish, A.M.; Khattab, A.E. Protective and therapeutic effects of two novel strains of Lactobacilli on diabetes-associated disorders induced by a high level of fructose. Mol. Biol. Rep. 2021, 48, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Radilla-Vazquez, R.B.; Parra-Rojas, I.; Martínez-Hernández, N.E.; Márquez-Sandoval, Y.F.; Illades-Aguiar, B.; Castro-Alarcón, N. Gut Microbiota and Metabolic Endotoxemia in Young Obese Mexican Subjects. Obes. Facts 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J.B.; Jarand, J.; Urbanski, S.J.; Hardin, J.; Gall, D.G. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am. J. Physiol. 1999, 276, G951–G957. [Google Scholar] [CrossRef] [PubMed]
- Bosi, E.; Molteni, L.; Radaelli, M.G.; Folini, L.; Fermo, I.; Bazzigaluppi, E.; Piemonti, L.; Pastore, M.R.; Paroni, R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006, 49, 2824–2827. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.; Saukkonen, T.; Ilonen, J.; Akerblom, H.K.; Savilahti, E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity 2002, 35, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; Cavin, J.B.; Keenan, C.M.; Wallace, L.E.; Vemuri, K.; Makriyannis, A.; MacNaughton, W.K.; Sharkey, K.A. Role of CB(1) receptors in the acute regulation of small intestinal permeability: Effects of high-fat diet. Am. J. Physiol. Liver Physiol. 2022, 323, G219–G238. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernández-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef]
- Leech, B.; Schloss, J.; Steel, A. Association between increased intestinal permeability and disease: A systematic review. Adv. Integr. Med. 2019, 6, 23–34. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.; Berti, I.; Sapone, A.; Gerarduzzi, T.; Not, T.; Zielke, R.; Fasano, A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc. Natl. Acad. Sci. USA 2005, 102, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- di Vito, R.; Conte, C.; Traina, G. A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, R.X.; Hou, F.; Cui, L.J.; Li, C.Y.; Chi, C.; Yi, E.; Wen, Y.; Yin, C.H. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol. Med. Rep. 2016, 14, 3669–3675. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepatobiliary Pancreat. Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy. Res. 2008, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- Hu, H.; Luo, J.; Liu, Y.; Li, H.; Jin, R.; Li, S.; Wei, J.; Wei, H.; Chen, T. Improvement effect of a next-generation probiotic L. plantarum-pMG36e-GLP-1 on type 2 diabetes mellitus via the gut-pancreas-liver axis. Food Funct. 2023, 14, 3179–3195. [Google Scholar] [CrossRef]
- Savytska, M.; Kyriienko, D.; Komisarenko, I.; Kovalchuk, O.; Falalyeyeva, T.; Kobyliak, N. Probiotic for Pancreatic β-Cell Function in Type 2 Diabetes: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Diabetes Ther. 2023, 14, 1915–1931. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef]
- Nopparat, J.; Khuituan, P.; Peerakietkhajorn, S.; Teanpaisan, R. Probiotics of Lacticaseibacillus paracasei SD1 and Lacticaseibacillus rhamnosus SD11 attenuate inflammation and β-cell death in streptozotocin-induced type 1 diabetic mice. PLoS ONE 2023, 18, e0284303. [Google Scholar] [CrossRef]
- Kim, W.J.; Ryu, R.; Doo, E.H.; Choi, Y.; Kim, K.; Kim, B.K.; Kim, H.; Kim, M.; Huh, C.S. Supplementation with the Probiotic Strains Bifidobacterium longum and Lactiplantibacillus rhamnosus Alleviates Glucose Intolerance by Restoring the IL-22 Response and Pancreatic Beta Cell Dysfunction in Type 2 Diabetic Mice. Probiotics Antimicrob. Proteins 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Chen, X.; Li, M.Q. Butyrate alleviates metabolic impairments and protects pancreatic beta cell function in pregnant mice with obesity. Int. J. Clin. Exp. Pathol. 2013, 6, 1574–1584. [Google Scholar]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Kang, J.; Li, C.; Gao, X.; Liu, Z.; Chen, C.; Luo, D. Metformin inhibits tumor growth and affects intestinal flora in diabetic tumor-bearing mice. Eur. J. Pharmacol. 2021, 912, 174605. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Li, C.; Gao, X.; Liu, Z.; Chen, C.; Luo, D. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Zeng, Z.; Qin, Y.; Shen, Q.; Li, P. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J. Funct. Foods 2020, 67, 103843. [Google Scholar] [CrossRef]
- Gai, Z.; Liao, W.; Huang, Y.; Dong, Y.; Feng, H.; Han, M. Effects of Bifidobacterium BL21 and Lacticaseibacillus LRa05 on gut microbiota in type 2 diabetes mellitus mice. AMB Express 2023, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Nyman, M.; Fak, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.W.; Wu, C.S.; Chen, P.C.; Wei, Y.C.; Hsu, L.Y.; Wang, S.H. Risk of acute mesenteric ischemia in patients with diabetes: A population-based cohort study in Taiwan. Atherosclerosis 2020, 296, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef]
- van Heck, J.I.P.; Gacesa, R.; Stienstra, R.; Fu, J.; Zhernakova, A.; Harmsen, H.J.M.; Weersma, R.K.; Joosten, L.A.B.; Tack, C.J. The Gut Microbiome Composition Is Altered in Long-standing Type 1 Diabetes and Associates With Glycemic Control and Disease-Related Complications. Diabetes Care 2022, 45, 2084–2094. [Google Scholar] [CrossRef]
- Jung, M.J.; Lee, J.; Shin, N.R.; Kim, M.S.; Hyun, D.W.; Yun, J.H.; Kim, P.S.; Whon, T.W.; Bae, J.W. Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Henneke, L.; Schlicht, K.; Andreani, N.A.; Hollstein, T.; Demetrowitsch, T.; Knappe, C.; Hartmann, K.; Jensen-Kroll, J.; Rohmann, N.; Pohlschneider, D.; et al. A dietary carbohydrate—gut Parasutterella–human fatty acid biosynthesis metabolic axis in obesity and type 2 diabetes. Gut Microbes 2022, 14, 2057778. [Google Scholar] [CrossRef] [PubMed]
- Henciya, S.; Vengateshwaran, T.D.; Gokul, M.S.; Dahms, H.U.; James, R.A. Antibacterial Activity of Halophilic Bacteria Against Drug-Resistant Microbes Associated with Diabetic Foot Infections. Curr. Microbiol. 2020, 77, 3711–3723. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Almugadam, B.S.; Liu, Y.; Chen, S.M.; Wang, C.H.; Shao, C.Y.; Ren, B.W.; Tang, L. Alterations of Gut Microbiota in Type 2 Diabetes Individuals and the Confounding Effect of Antidiabetic Agents. J. Diabetes Res. 2020, 2020, 7253978. [Google Scholar] [CrossRef]
- Siptroth, J.; Moskalenko, O.; Krumbiegel, C.; Ackermann, J.; Koch, I.; Pospisil, H. Variation of butyrate production in the gut microbiome in type 2 diabetes patients. Int. Microbiol. 2023, 26, 601–610. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, C.X.; McTeague, M.; Summanen, P.; Finegold, S.M. Clostridium bartlettii sp. nov., isolated from human faeces. Anaerobe 2004, 10, 179–184. [Google Scholar] [CrossRef]
- Jiang, Q.; He, X.; Zou, Y.; Ding, Y.; Li, H.; Chen, H. Altered gut microbiome promotes proteinuria in mice induced by Adriamycin. AMB Express 2018, 8, 31. [Google Scholar] [CrossRef]
- Appert, O.; Garcia, A.R.; Frei, R.; Roduit, C.; Constancias, F.; Neuzil-Bunesova, V.; Ferstl, R.; Zhang, J.; Akdis, C.; Lauener, R.; et al. Initial butyrate producers during infant gut microbiota development are endospore formers. Environ. Microbiol. 2020, 22, 3909–3921. [Google Scholar] [CrossRef]
- Le, T.K.; Hosaka, T.; Nguyen, T.T.; Kassu, A.; Dang, T.O.; Tran, H.B.; Pham, T.P.; Tran, Q.B.; Le, T.H.; Pham, X.D. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed. Res. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Cocconi, D.; van Sinderen, D.; Ventura, M. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. 2017, 93, fix153. [Google Scholar] [CrossRef]
- Wang, Y.; Milani, C.; Lugli, G.A.; Turroni, F.; Cocconi, D.; van Sinderen, D.; Ventura, M. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol. 2020, 32, 101514. [Google Scholar] [CrossRef] [PubMed]
- Cesario, V.; Di Rienzo, T.A.; Campanale, M.; D’angelo, G.; Barbaro, F.; Gigante, G.; Vitale, G.; Scavone, G.; Pitocco, D.; Gasbarrini, A.; et al. Methane intestinal production and poor metabolic control in type I diabetes complicated by autonomic neuropathy. Minerva Endocrinol. 2014, 39, 201–207. [Google Scholar] [PubMed]
- Mathur, R.; Goyal, D.; Kim, G.; Barlow, G.M.; Chua, K.S.; Pimentel, M. Methane-producing human subjects have higher serum glucose levels during oral glucose challenge than non-methane producers: A pilot study of the effects of enteric methanogens on glycemic regulation. Res. J. Endoc Metab. 2014, 2, 2. [Google Scholar] [CrossRef]
- Mathew, A.V.; Jaiswal, M.; Ang, L.; Michailidis, G.; Pennathur, S.; Pop-Busui, R. Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes. Diabetes 2019, 68, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Hesselink, M.K. Reduced tricarboxylic acid cycle flux in type 2 diabetes mellitus? Diabetologia 2008, 51, 1694–1697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemmati, M.; Babaei, H.; Abdolsalehei, M. Survey of the effect of biotin on glycemic control and plasma lipid concentrations in type 1 diabetic patients in kermanshah in iran (2008–2009). Oman. Med. J. 2013, 28, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Hatch, G.M.; Wang, Y.; Yu, F.; Wang, M. The relationship between phospholipids and insulin resistance: From clinical to experimental studies. J. Cell Mol. Med. 2019, 23, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.S.; Butler, R.N.; Giffard, P.M.; Howarth, G.S. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int. J. Food Microbiol. 2007, 114, 267–274. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric Studies: II. Preparations from Washed Blood Cells; Nitric Oxide Hemoglobin and Sulfhemoglobin. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Parker, K.M.; England, J.D.; Da Costa, J.; Hess, R.L.; Goldstein, D.E. Improved colorimetric assay for glycosylated hemoglobin. Clin. Chem. 1981, 27, 669–672. [Google Scholar] [CrossRef]
- Drury, R.A.; Wallington, E.A. Carleton’s Histological Technique, 5th ed.; Oxford University Press: Oxford, UK, 1980; p. 520. [Google Scholar]
- Obembe, A.O.; Owu, D.U.; Okwari, O.O.; Antai, A.B.; Osim, E.E. Intestinal Fluid and Glucose Transport in Wistar Rats following Chronic Consumption of Fresh or Oxidised Palm Oil Diet. ISRN Gastroenterol. 2011, 2011, 972838. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).