Sex Dependence in Control of Renal Haemodynamics and Excretion in Streptozotocin Diabetic Rats—Role of Adenosine System and Nitric Oxide
Abstract
:1. Introduction
2. Results
2.1. Chronic Study
2.1.1. Phase of the Oestrus Cycle, Body Weight (Bwt), Blood Glucose Level (BG)
2.1.2. Blood Parameters
2.1.3. Daily Water Intake and Urine Excretion
2.2. Acute Experiments
2.2.1. Effects of L-NAME4 Pretreatment in NG and DM Rats on Baseline Haemodynamics and Renal Circulation
2.2.2. Effects of L-NAME4 Pretreatment in NG and DM Rats on Baseline Renal Excretion
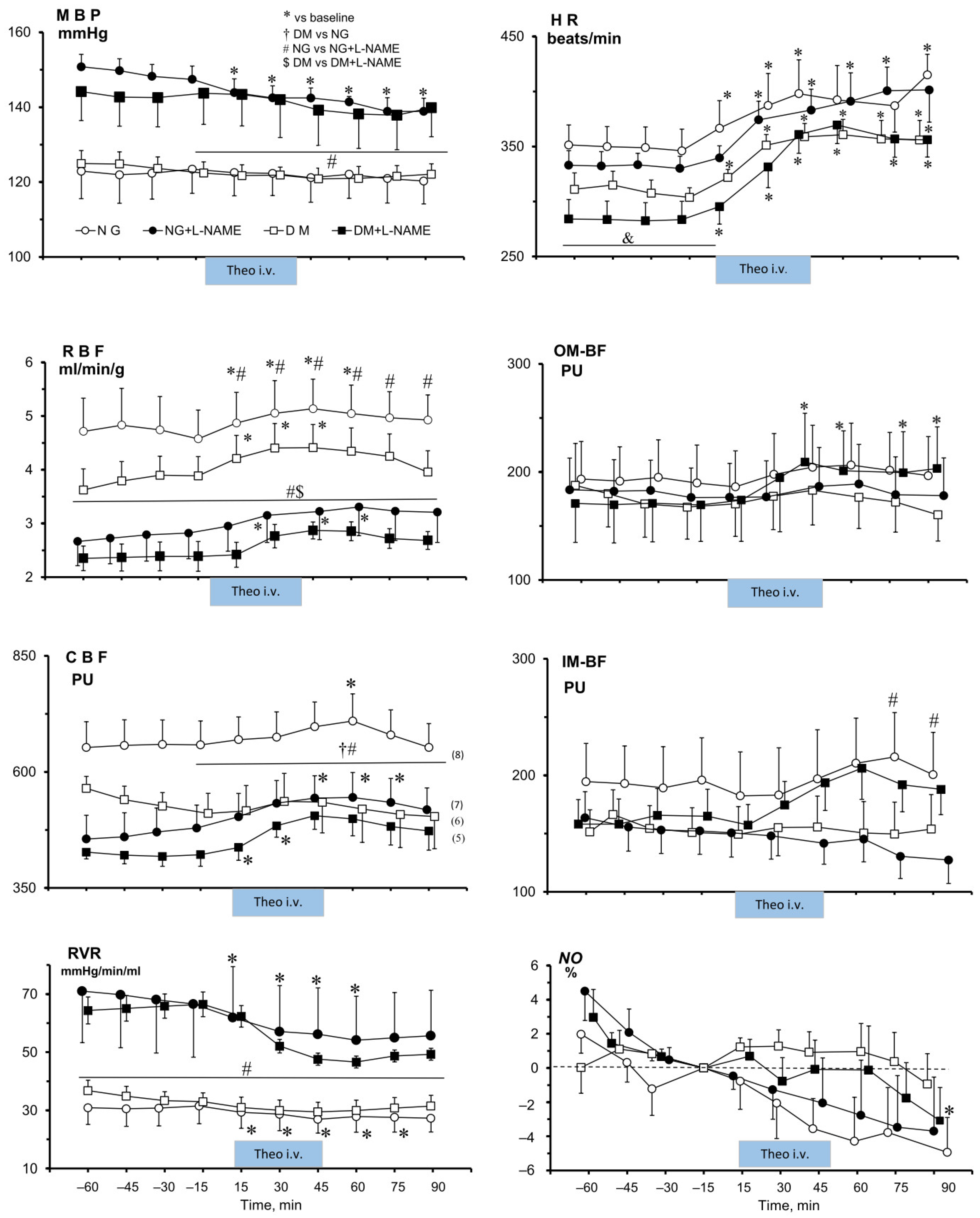
2.2.3. Effects of Theophylline on MABP, Heart Rate, and on Renal Total and Regional Perfusion in NG and DM Rats Untreated or Pre-Treated with L-NAME4
2.2.4. Effects of Theophylline (Theo) on Renal Tissue NO Signal in NG and DM Female Rats, Untreated or Pre-Treated with L-NAME4
2.2.5. Effects of Theophylline on Renal Excretion in NG and DM Female Rats, Untreated or Pre-Treated with L-NAME
3. Discussion
3.1. Effects of STZ-Induced Hyperglycaemia on Metabolic and Renal Excretion Parameters
3.2. Effects of STZ-Induced Hyperglycaemia on Systemic and Renal Haemodynamics and Renal Excretion
3.2.1. Renal Regional Blood Perfusion
3.2.2. Renal Excretion
3.3. Effects of Ado Receptor Blockade in NO-Intact Rats on Systemic and Renal Haemodynamics, and on Renal Excretion and Tissue NO
3.3.1. Renal Haemodynamics
3.3.2. Renal Excretion
3.3.3. Tissue NO
3.4. Effects of Ado Receptor Blockade in NO-Deficient Rats on Systemic and Renal Haemodynamics, and on Renal Excretion and Tissue NO
3.4.1. Unexpected Post-Theo Decrease in MABP in NG Female Rats
3.4.2. Renal Blood Perfusion
3.4.3. Renal Excretion
3.4.4. Tissue NO
4. Materials and Methods
4.1. Animals
4.2. Chronic Studies
4.2.1. Induction of Diabetes
4.2.2. L-NAME Pretreatment
4.3. Acute Experiment
4.3.1. Surgical Preparations
4.3.2. Experimental Protocols
- Diabetic rats (0.9%NaCl i.v.), DM (0.9%NaCl);
- Diabetic rats (Theo i.v.), DM (Theo);
- Normoglycaemic rats (0.9%NaCl i.v.), NG (0.9%NaCl);
- Normoglycaemic rats (Theo i.v.), NG (Theo);
- Diabetic rats, L-NAME pre-treated (0.9%NaCl i.v.), DM+L-NAME (0.9%NaCl);
- Diabetic rats, L-NAME pre-treated (Theo i.v.), DM+L-NAME (Theo);
- Normoglycaemic rats, L-NAME pre-treated (0.9%NaCl i.v.), NG+L-NAME (0.9%NaCl);
- Normoglycaemic rats, L-NAME pre-treated (Theo i.v.), NG+L-NAME (Theo).
4.4. Analytical Procedures and Calculations
4.5. Statistics
5. Conclusions
- In both male and female rats with intact NO synthesis, no tonic influence of the Ado system on the resistance of the peripheral vasculature was seen. However, in the kidneys of female rats, unlike in males, the vasoactive influence of the Ado system was not altered by hyperglycaemia.
- In female rats, blockade of NO synthesis caused a slightly greater increase in MABP and a decrease in renal haemodynamics in NG compared with DM animals, indicating enhanced vasodilator influence of NO in diabetic females, but this was not found in an earlier study with diabetic males.
- In NO-deficient NG and DM female rats, Ado receptor blockade induced comparable renal vasodilatation, suggesting a comparable vasoconstrictor influence of the Ado system in this sex, independent of the glycaemia level. However, the novel finding in NG female rats was an associated decrease in arterial pressure, of unclear origin.
- Another novel and unexpected finding was that in female rats, both with intact or deficient NO synthesis, Ado receptor blockade had no or only a very slight impact on kidney tissue NO, in contrast to a distinct increase reported in males. Thus, in females only, Theo might somehow weaken rather than stimulate NO synthesis. The mechanism might be, in both sexes, the abolishment of the NO-inhibitory action of P1 receptors, presumably A1.
- Lowered baseline renal excretion in female DM suggested stimulation of renal tubular fluid reabsorption, possibly due to the prevalence of antinatriuretic A1 over natriuretic A2 receptors. Remarkably, an opposite balance pattern between individual P1 receptor types emerged from the studies with males.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Pin-Lan Li Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Hennington, B.S.; Moore, A.G.; Blanchard, E.J.; Cameron, J. Gender differences in the renal nitric oxide (NO) system: Dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am. J. Hypertens. 1998, 11, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Endothelial Dysfunction in Experimental Models of Arterial Hypertension: Cause or Consequence? Biomed. Res. Int. 2014, 2014, 598271. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Semprun-Prieto, L.; Boesen, E.I.; Pollock, D.M.; Pollock, J.S. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1573–R1579. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Pardieck, J.L.; Hyndman, K.A.; Pollock, J.S. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R61–R69. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Garvin, J.L. Effects of Reactive Oxygen Species on Tubular Transport along the Nephron. Antioxidants 2017, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Nakamura, M.; Suzuki, A.; Tsukada, H.; Horita, S.; Suzuki, M.; Moriya, K.; Seki, G. Effects of Nitric Oxide on Renal Proximal Tubular Na+ Transport. Biomed. Res. Int. 2017, 2017, 6871081. [Google Scholar] [CrossRef]
- Hansen, P.B.; Schnermann, J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am. J. Physiol. Ren. Physiol. 2003, 285, F590–F599. [Google Scholar] [CrossRef] [PubMed]
- Pflueger, A.C.; Osswald, H.; Knox, F.G. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: Role of nitric oxide. Am. J. Physiol. 1999, 276, F340–F346. [Google Scholar] [CrossRef]
- Palm, F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Hansell, P.; Palm, F. Reduced adenosine A2a receptor–mediated efferent arteriolar vasodilation contributes to diabetes-induced glomerular hyperfiltration. Kidney Int. 2015, 87, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.B.; Diniz, C. The Adenosinergic System as a Therapeutic Target in the Vasculature: New Ligands and Challenges. Molecules 2017, 22, 752. [Google Scholar] [CrossRef] [PubMed]
- Vitzthum, H.; Weiss, B.; Bachleitner, W.; Krämer, B.K.; Kurtz, A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004, 65, 1180–1190. [Google Scholar] [CrossRef]
- Burnstock, G.; Novak, I. Purinergic signalling and diabetes. Purinergic Signal. 2013, 9, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Csóka, B.; Pacher, P.; Haskó, G. Adenosine signalling in diabetes mellitus-pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2015, 11, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Sanni, O.; Terre’ Blanche, G. Dual A1 and A2A adenosine receptor antagonists, methoxy substituted 2-benzylidene-1-indanone, suppresses intestinal postprandial glucose and attenuates hyperglycaemia in fructose-streptozotocin diabetic rats. BMC Endocr. Disord. 2023, 23, 97. [Google Scholar] [CrossRef]
- Kuczeriszka, M.; Sitek, J.D.; Walkowska, A.; Sadowski, J.; Dobrowolski, L. Interplay of the adenosine system and NO in control of renal haemodynamics and excretion: Comparison of normoglycaemic and streptozotocin diabetic rats. Nitric Oxide 2020, 104–105, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mannon, E.C.; Ray, S.C.; Ryan, M.J.; Sullivan, J.C. Does sex matter? An update on the implementation of sex as a biological variable in research. Am. J. Physiol. Renal Physiol. 2020, 318, F329–F333. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.R.; Stanford, S.C.; Panattieri, R.A.; Alexander, S.P.H.; Cirino, G.; George, C.H.; Hoyer, D.; Izzo, A.A.; Ji, Y.; Lilley, E.; et al. Sex: A change in our guideleines to authors to ensure that this no longer an ignored experimental variable. Br. J. Pharmacol. 2019, 176, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. No- menclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Osswald, H.; Schnermann, J. Methylxanthines and the kidney. Handb. Exp. Pharmacol. 2011, 200, 391–412. [Google Scholar] [CrossRef]
- Rieg, T.; Steigele, H.; Schnermann, J.; Richter, K.; Osswald, H.; Vallon, V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J. Pharmacol. Exp. Ther. 2005, 313, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sitek, J.D.; Kuczeriszka, M.; Walkowska, A.; Kompanowska-Jezierska, E.; Dobrowolski, L. Nonselective and A2a-Selective Inhibition of Adenosine Receptors Modulates Renal Perfusion and Excretion Depending on the Duration of Streptozotocin-Induced Diabetes in Rats. Pharmaceuticals 2023, 16, 732. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.N.B.; Dember, L.M.; Ingelfinger, J.R.; Vinson, A.; Neugarten, J.; Sandberg, K.L.; Sullivan, J.C.; Maric-Bilkan, C.; Rankin, T.L.; Kimmel, P.L.; et al. Sex and the kidneys: Current understanding and research opportunities. Nat. Rev. Nephrol. 2019, 15, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Soler, M.J.; Stevens, K.I.; Torra, R. Why do we keep ignoring sex in kidney disease? Clin. Kidney J. 2023, 16, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; Bellehumeur, T.G. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension 1996, 28, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Role of macula densa NOS in tubuloglomerular feedback. Curr. Opin. Nephrol. Hypertens. 1998, 7, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Grzelec-Mojzesowicz, M.; Sadowski, J. Renal tissue NO and intrarenal haemodynamics during experimental variations of NO content in anaesthetised rats. J. Physiol. Pharmacol. 2007, 58, 149–163. [Google Scholar] [PubMed]
- Kuczeriszka, M.; Olszynski, K.H.; Gąsiorowska, A.; Sadowski, J.; Kompanowska-Jezierska, E. Interaction of nitric oxide and the cytochrome P-450 system on blood pressure and renal function in the rat: Dependence on sodium intake. Acta Physiol. 2011, 201, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R13–R27. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, L.; Kuczeriszka, M.; Castillo, A.; Majid, D.S.; Navar, L.G. Role of atrial natriuretic peptide in mediating the blood pressure-independent natriuresis elicited by systemic inhibition of nitric oxide. Pflugers Arch. 2015, 467, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Osswald, H. Adenosine receptors and the kidney. Handb. Exp. Pharmacol. 2009, 193, 443–470. [Google Scholar] [CrossRef]
- Yap, S.C.; Lee, H.T. Adenosine and protection from acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2012, 21, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine receptors and the heart:role in regulation of coronary blood flow and cardiac electrophysiology. Handb. Exp. Pharmacol. 2009, 193, 161–188. [Google Scholar] [CrossRef]
- Nassi, A.; Malorgio, F.; Tedesco, S.; Cignarella, A.; Gaion, R.M. Upregulation of inducible NO synthase by exogenous adenosine in vascular smooth muscle cells activated by inflammatory stimuli in experimental diabetes. Cardiovasc. Diabetol. 2016, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, J.; Bądzyńska, B. Altered renal medullary blood flow: A key factor or a parallel event in control of sodium excretion and blood pressure? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.J.; Droppleman, D.A. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. Am. J. Physiol. Renal Fluid. Electrolyte Physiol. 1993, 265, F651–F659. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Sarrado, C.; Zhou, Y.; Salgues, V.; Parent, M.; Giummelly, P.; Lartaud, I.; Gaucher, C. S-Nitrosothiols as potential therapeutics to induce a mobilizable vascular store of nitric oxide to counteract endothelial dysfunction. Biochem. Pharmacol. 2020, 173, 113686. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S. Pathogenic role of nitric oxide alterations in diabetic nephropathy. Curr. Diabetes Rep. 2005, 5, 449–454. [Google Scholar] [CrossRef] [PubMed]

| Days after Buffer or STZ Injection | ||||
|---|---|---|---|---|
| Parameter | 0 | 10 | 14 | |
| Body weight | NG | 239 ± 4 | 251 ± 4 * | 258 ± 3 * |
| (g) | DM | 237 ± 3 | 233 ± 5 # | 233 ± 8 # |
| Glycaemia | NG | 175 ± 2 | 171 ± 8 | 165 ± 13 |
| (mg/dL) | DM | 180 ± 3 | 493 ± 24 *# | 526 ± 25 *# |
| Haematocrit | NG | 44 ± 0 | 46 ± 1 * | 46 ± 1 * |
| (%) | DM | 44 ± 0 | 44 ± 1 | 45 ± 1 * |
| Plasma osmolality | NG | 299 ± 6 | 299 ± 9 | 319 ± 4 *$ |
| (mosmol/kg H2O) | DM | 300 ± 3 | 311 ± 2 * | 324 ± 5 *$ |
| Plasma sodium concentration | NG | 135 ± 1 | 131 ± 2 | 130 ± 2 |
| (mmol/L) | DM | 132 ± 2 | 127 ± 1 *# | 126 ± 2 * |
| Plasma potassium concentration | NG | 5.4 ± 0.4 | 5.8 ± 0.5 | 5.4 ± 0.7 |
| (mmol/L) | DM | 4.9 ± 0.3 | 5.2 ± 0.4 | 5.9 ± 0.5 |
| Water intake | NG | 31±1 | 30 ± 3 | 50 ± 26 |
| (ml/24 h) | DM | 25±2 # | 95 ± 11 *# | 126 ± 14 *# |
| Urine flow | NG | 13±1 | 14 ± 2 | 15 ± 2 |
| (ml/24 h) | DM | 11±1 | 82 ± 10 *# | 104 ± 10 *# |
| Urine osmolality | NG | 1700±110 | 1550 ± 160 | 1400 ± 85 |
| (mosmol/kg H2O) | DM | 1730±250 | 1095 ± 55 *# | 1095 ± 50 * |
| Total solute excretion | NG | 27 ± 1 | 20 ± 1 * | 25 ± 2 |
| (mosmol/24 h) | DM | 22 ± 1 | 113 ± 6 *# | 126 ± 10 *# |
| Urine sodium excretion | NG | 2.0 ± 0.1 | 1.4 ± 0.2 * | 1.9±0.2 |
| (mmol/24 h) | DM | 1.6 ± 0.2 | 3.0 ± 0.3 *# | 3.2±0.4 *# |
| Urine potassium excretion | NG | 7.2 ± 0.5 | 5.2 ± 0.3 * | 6.1 ± 0.6 |
| (mmol/24 h) | DM | 5.6 ± 0.8 | 4.3 ± 1.5 | 4.1 ± 1.9 |
| Parameter | Pretreatment | NG | DM |
|---|---|---|---|
| MAP | Untreated | 123 ± 3 | 124 ± 2 |
| (mmHg) | L-NAME | 149 ± 2 * | 143 ± 4 * |
| HR | Untreated | 349 ± 9 | 309 ± 6 † |
| (beat/min) | L-NAME | 332 ± 6 | 283 ± 8 *† |
| RBF | Untreated | 4.7 ± 0.3 | 3.8 ± 0.2 † |
| (ml/min/g of kidney weight) | L-NAME | 2.7 ± 0.2 * | 2.4 ± 0.1 * |
| RVR | Untreated | 31 ± 3 | 34 ± 2 |
| (mmHg min/ml) | L-NAME | 69 ± 8 * | 65 ± 2 * |
| CBF | Untreated | 655 ± 25 | 535 ± 15† |
| (perfusion units) | L-NAME | 480 ± 50 * | 422 ± 9 * |
| V | Untreated | 10.8 ± 1.4 | 7.0 ± 0.8 † |
| (µl/min/g of kidney weight) | L-NAME | 7.3 ± 0.8 * | 8.5 ± 3.2 |
| UosmV | Untreated | 7.3 ± 1.3 | 7.8 ± 1.3 |
| (µosmol/min/g of kidney weight) | L-NAME | 5.3 ± 0.6 | 9.6 ± 1.2 † |
| UNaV | Untreated | 1.4 ± 0.4 | 0.5 ± 0.0 † |
| (µmol/min/g of kidney weight) | L-NAME | 0.7 ± 0.1* | 1.7 ± 0.3 *† |
| UKV | Untreated | 1.0 ± 0.1 | 0.3 ± 0.0 † |
| (µmol/min/g of kidney weight) | L-NAME | 0.6 ± 0.1 * | 0.6 ± 0.1 * |
| DM vs. NG | |||
|---|---|---|---|
| NO Status | Females | Males | |
| MABP | NO-intact | ↔ | ↔ |
| NO-deficient | (−) * | ↔ | |
| V | NO-intact | (−) * | (+) * |
| NO-deficient | (−) * | ↔ | |
| UosmV | NO-intact | ↔ | (+) * |
| NO-deficient | (+) * | (+) * | |
| UNaV | NO-intact | (−) * | (+) * |
| NO-deficient | (+) * | ↔ | |
| UKV | NO-intact | (−) * | (−) * |
| NO-deficient | ↔ | No data | |
| Uosm | NO-intact | (+) * | ↔ |
| NO-deficient | (+) * | ↔ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczeriszka, M.; Dobrowolski, L. Sex Dependence in Control of Renal Haemodynamics and Excretion in Streptozotocin Diabetic Rats—Role of Adenosine System and Nitric Oxide. Int. J. Mol. Sci. 2024, 25, 7699. https://doi.org/10.3390/ijms25147699
Kuczeriszka M, Dobrowolski L. Sex Dependence in Control of Renal Haemodynamics and Excretion in Streptozotocin Diabetic Rats—Role of Adenosine System and Nitric Oxide. International Journal of Molecular Sciences. 2024; 25(14):7699. https://doi.org/10.3390/ijms25147699
Chicago/Turabian StyleKuczeriszka, Marta, and Leszek Dobrowolski. 2024. "Sex Dependence in Control of Renal Haemodynamics and Excretion in Streptozotocin Diabetic Rats—Role of Adenosine System and Nitric Oxide" International Journal of Molecular Sciences 25, no. 14: 7699. https://doi.org/10.3390/ijms25147699





