A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress
Abstract
1. Introduction
2. Results
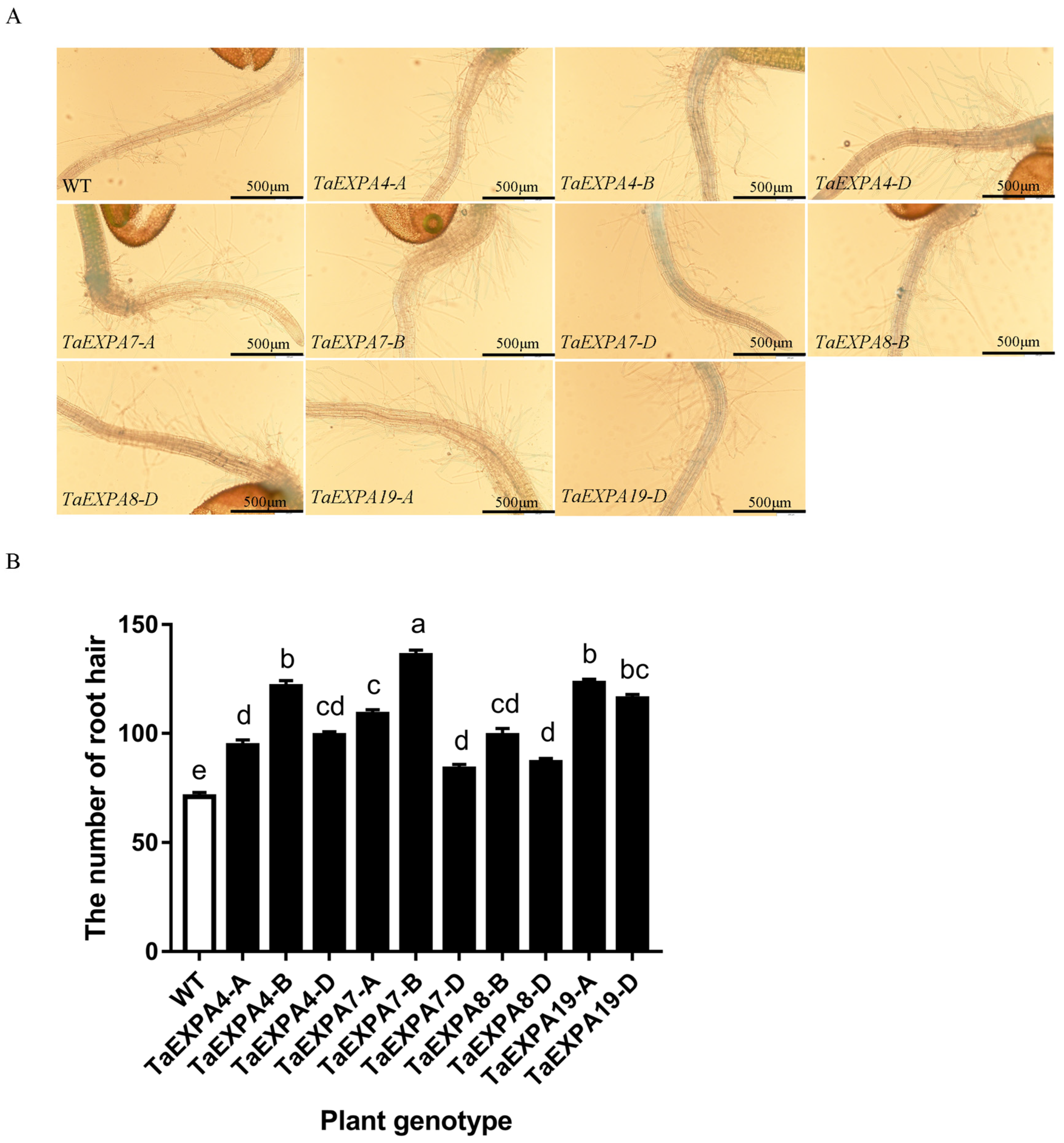
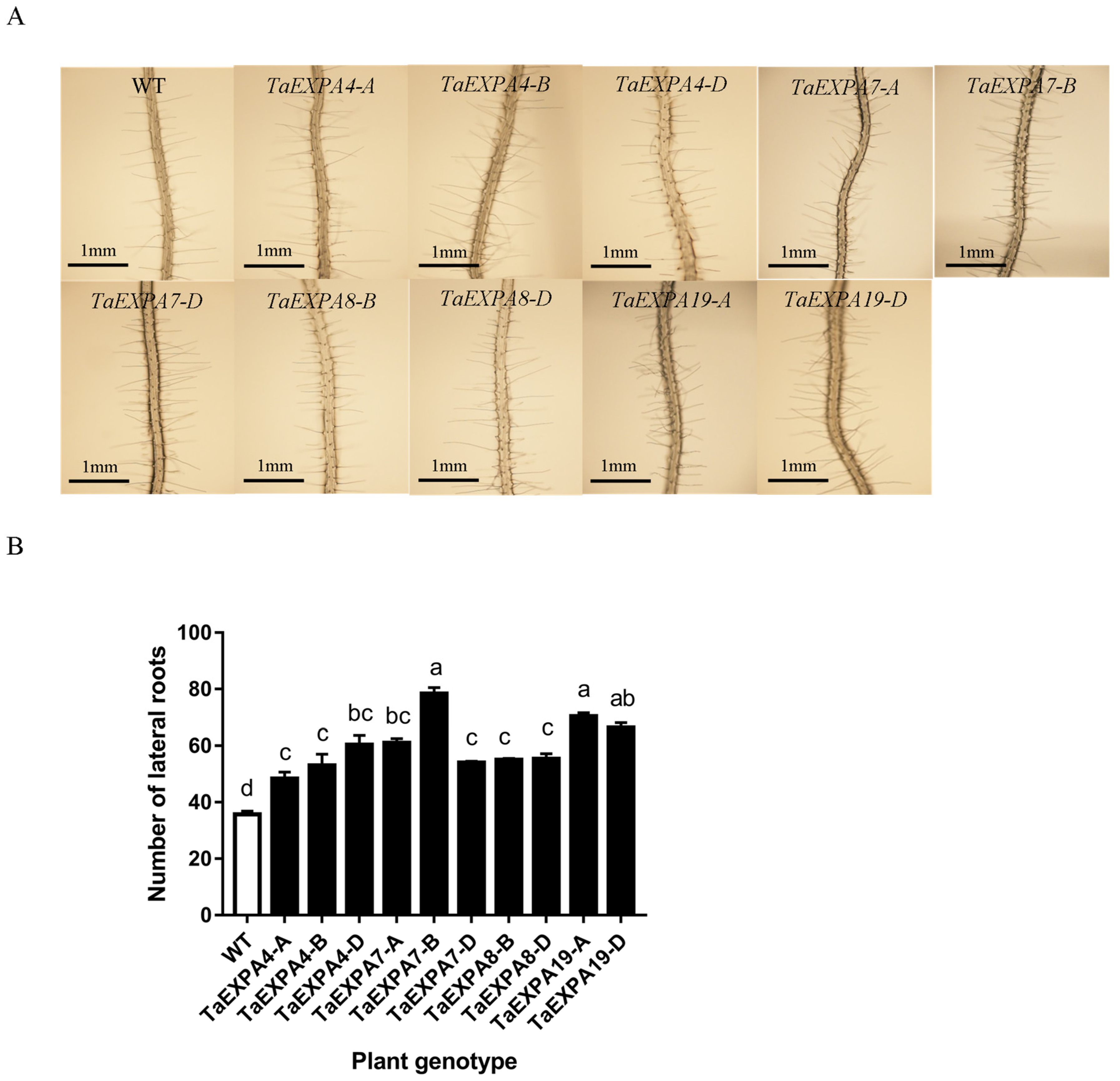
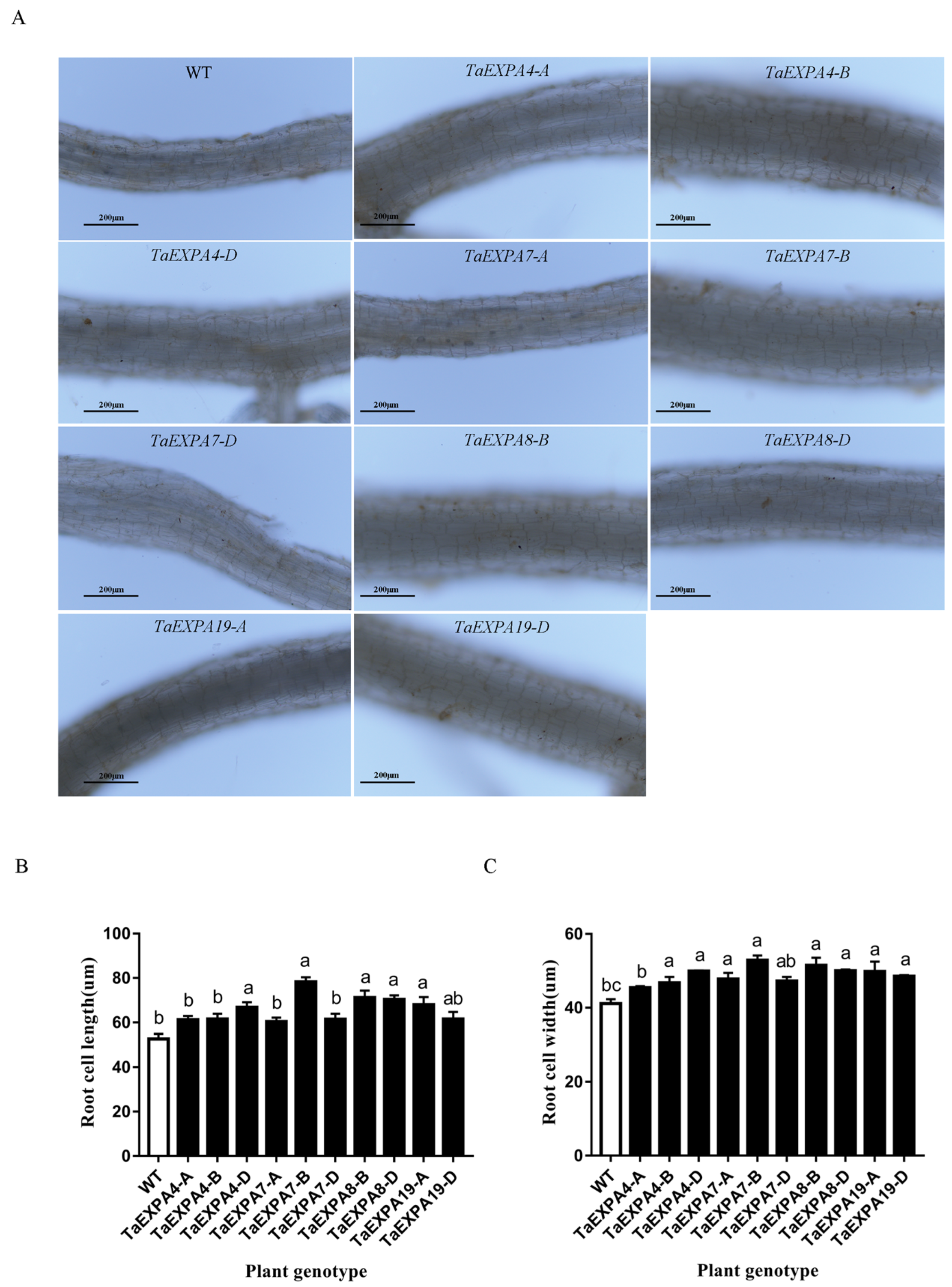
2.1. Morphological Observation of Arabidopsis Roots Overexpressing TaEXPAs
2.1.1. Analysis of Arabidopsis Root Hairs Overexpressing TaEXPAs
2.1.2. Analysis of Arabidopsis Root Length Overexpressing TaEXPAs
2.1.3. Analysis of Arabidopsis Lateral Root Overexpressing TaEXPAs
2.1.4. Analysis of Arabidopsis Root Cell Overexpressing TaEXPAs
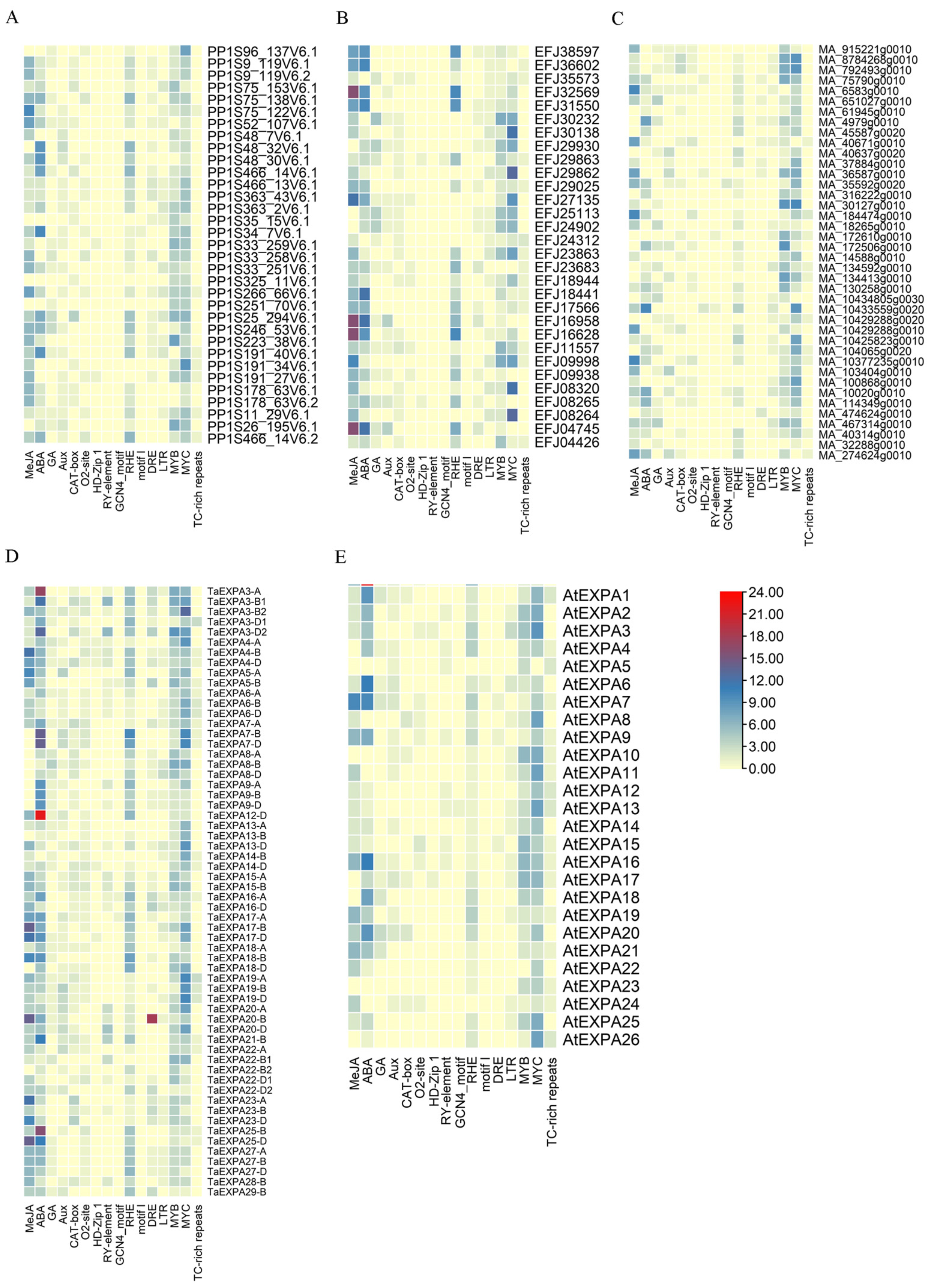
2.2. Bioinformatics Analysis
2.2.1. Phylogenetic Analysis of the EXPA Family Genes in Five Plants
2.2.2. Promoter Analysis of EXPA Family Genes in Five Plants
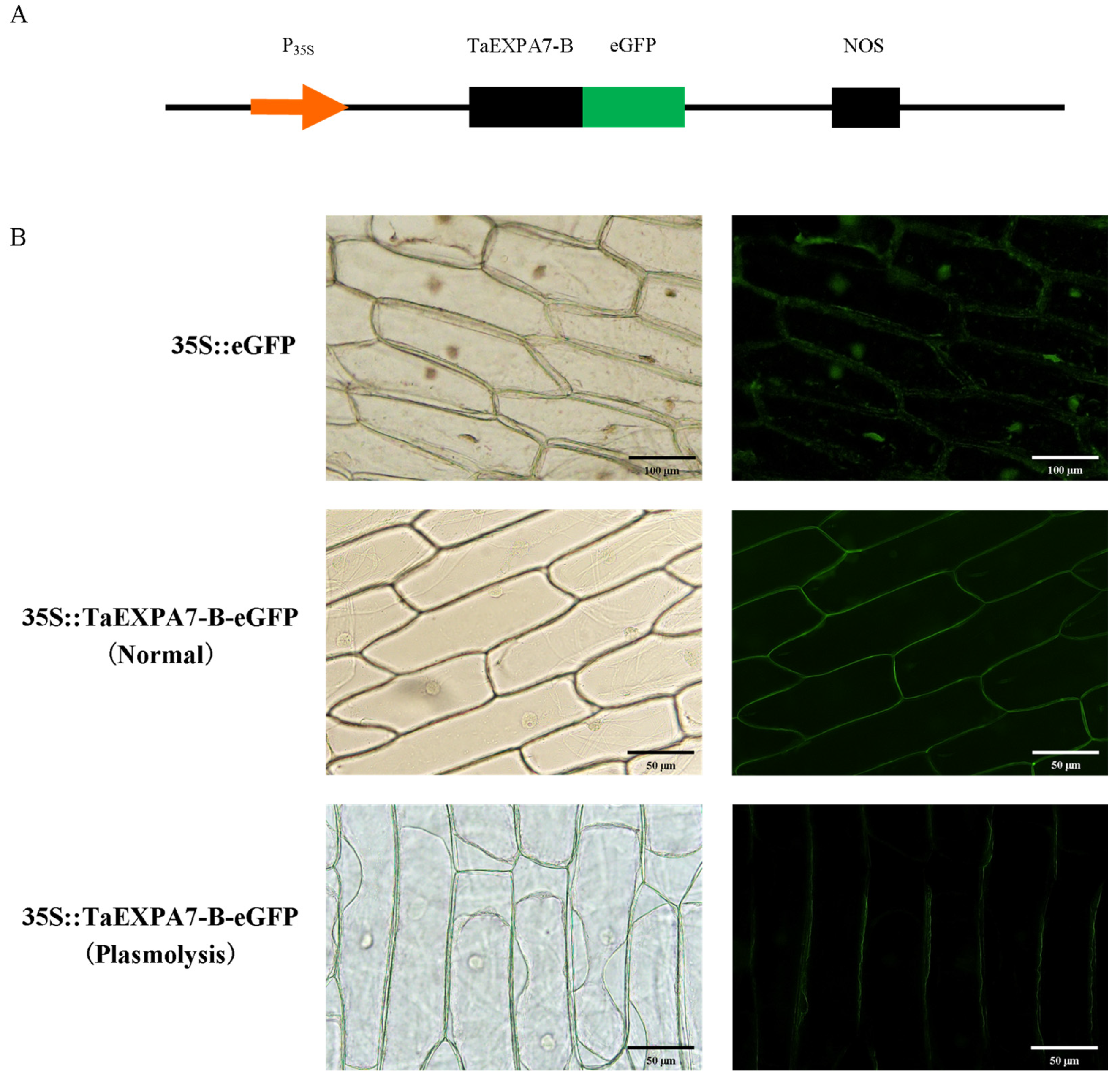
2.3. Subcellular Localization of TaEXPA7-B Protein
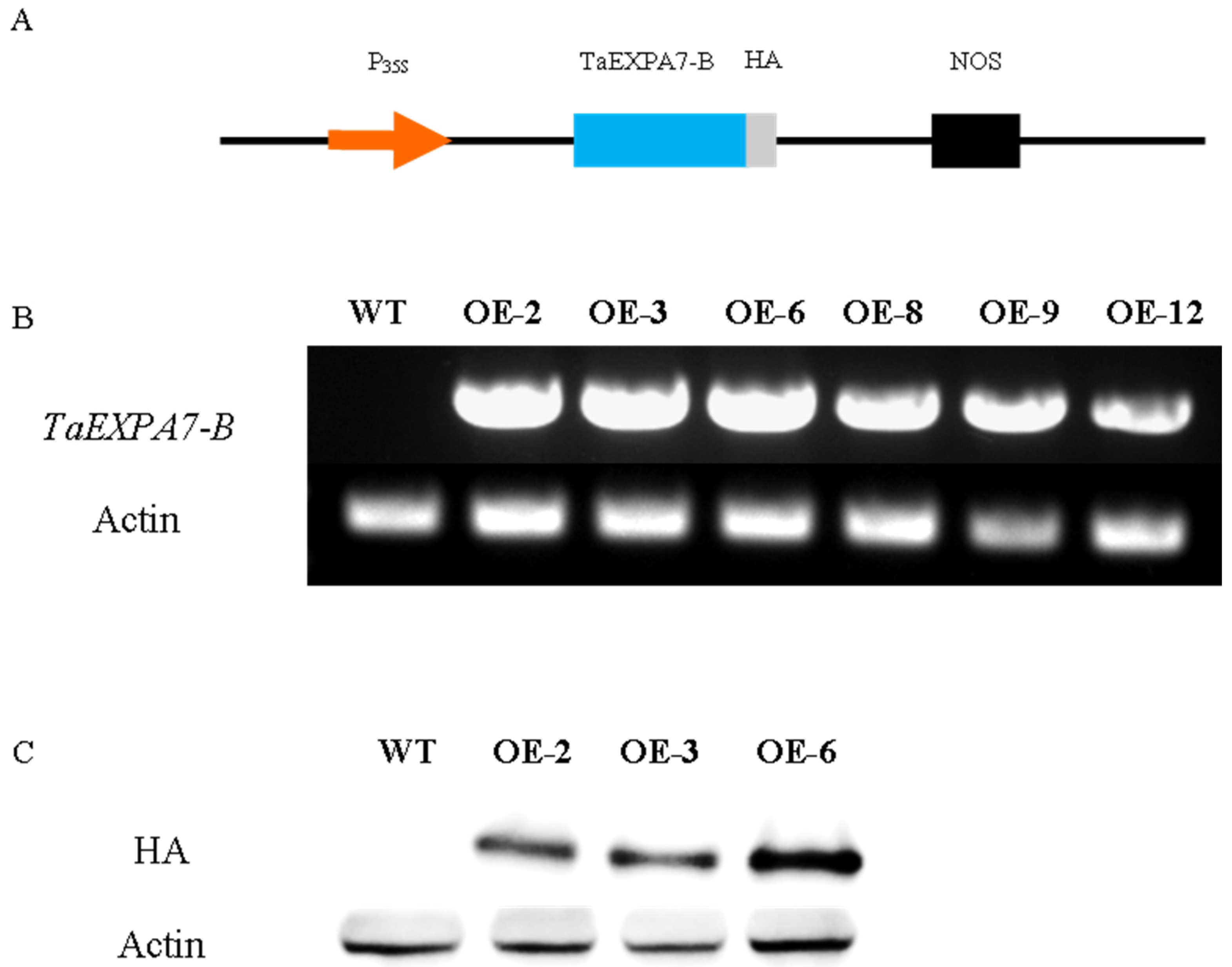
2.4. Identification of Rice Overexpressing TaEXPA7-B Gene
2.5. Phenotypic Observation of Rice Overexpressing TaEXPA7-B Gene
2.5.1. External Structure of Rice Overexpressing TaEXPA7-B Gene
2.5.2. Anatomical Structure of Rice Overexpressing TaEXPA7-B Gene
2.6. Salt Stress Tolerance of Rice Overexpressing TaEXPA7-B Gene
2.6.1. Phenotype of Rice Overexpressing TaEXPA7-B Gene under Salt Stress
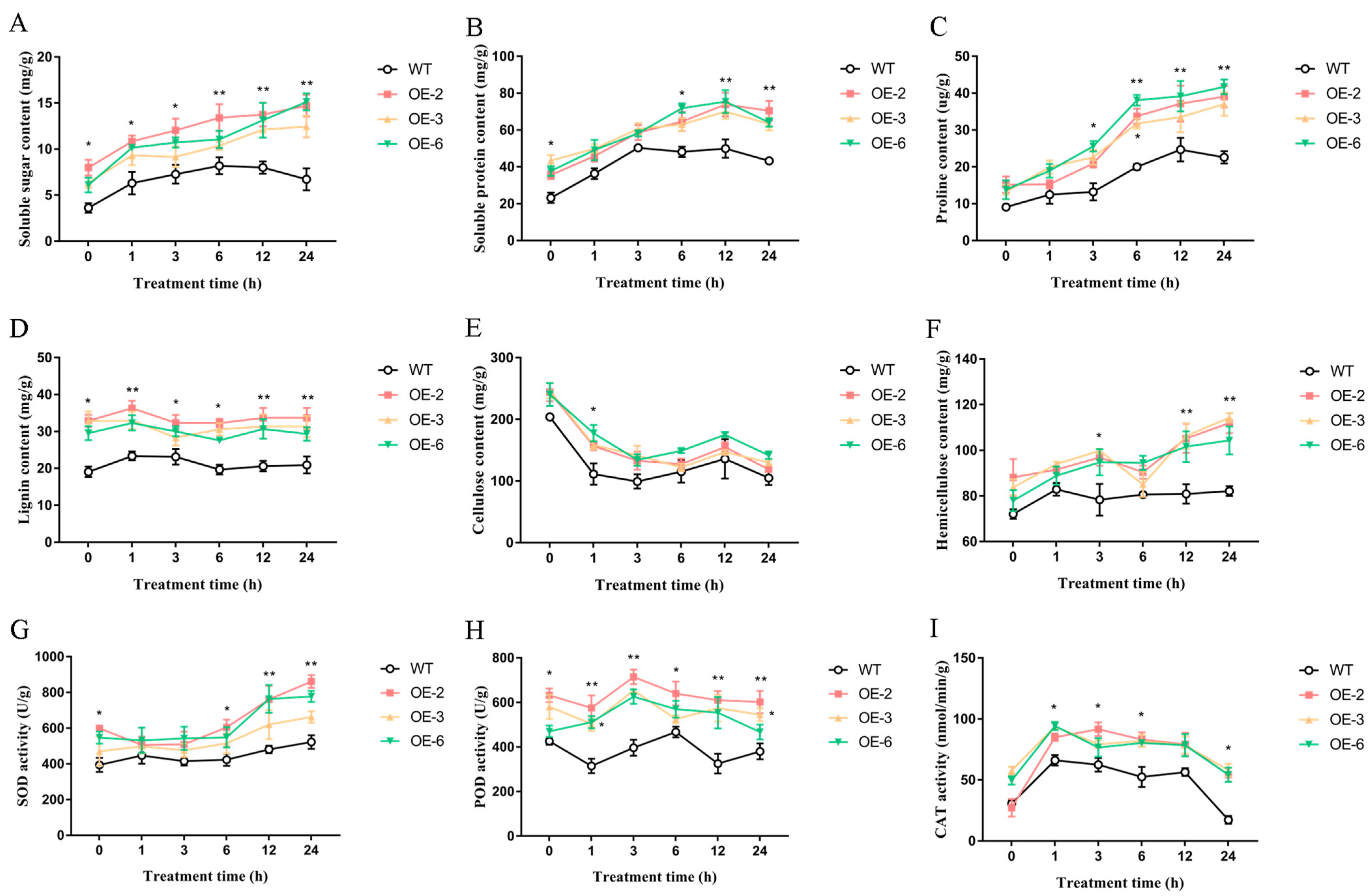
2.6.2. Osmoregulatory Substance Content of Rice Overexpressing TaEXPA7-B Gene under Salt Stress
2.6.3. Morphogenesis of Related Substance Content of Rice Overexpressing TaEXPA7-B Gene under Salt Stress
2.6.4. Antioxidant Enzyme Activity of Rice Overexpressing TaEXPA7-B Gene under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis of EXPA Family Genes
4.2. Morphological Observation of Arabidopsis Root System
4.3. Subcellular Localization of TaEXPA7-B Protein in Onion
4.4. Overexpression of TaEXPA7-B Gene in Rice
4.5. Phenotype Observation of Rice Overexpressing TaEXPA7-B Gene
4.6. Salt Treatment and Physiological Index Determination of Rice Overexpressing TaEXPA7-B Gene
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.Y.; Zhang, C.; Li, J.Y.; Yan, L.; Wang, N.; Xia, L.Q. Present and future prospects for wheat improvement through genome editing and advanced technologies. Plant Commun. 2021, 2, 100211. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Xia, X.C.; Chen, X.M.; Zhuang, Q.S. Progress of wheat breeding in China and the future perspective. Acta Agron. Sin. 2011, 37, 202–215. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.H.; Sui, X.X.; Xia, X.C.; Zhang, X.K.; Zhang, G.S. Genetic improvement of grain yield and associated traits in the northern China winter wheat region from 1960 to 2000. Crop Sci. 2007, 47, 245–253. [Google Scholar] [CrossRef]
- Kamara, M.M.; Rehan, M.; Mohamed, A.M.; El Mantawy, R.F.; Kheir, A.M.S.; Abd El-Moneim, D.; Safhi, F.A.; ALshamrani, S.M.; Hafez, E.M.; Behiry, S.I.; et al. Genetic Potential and inheritance patterns of physiological, agronomic and quality traits in bread wheat under normal and water deficit conditions. Plants 2022, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Guo, Y. Unraveling salt-stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Deng, X.; Liu, D.; Liu, Y.; Hu, Y.; Yan, Y. Genome-wide identification and expression analysis of expansin gene family in common wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, J. EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol. 2013, 10, 1600–1611. [Google Scholar] [CrossRef]
- Boron, A.K.; Van Loock, B.; Suslov, D.; Markakis, M.N.; Verbelen, J.-P.; Vissenberg, K. Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth. Ann. Bot. 2014, 115, 67–80. [Google Scholar] [CrossRef]
- Liu, W.M.; Xu, L.A.; Lin, H.; Cao, J. Two Expansin Genes, AtEXPA4 and AtEXPB5, are redundantly required for pollen tube growth and AtEXPA4 is involved in primary root elongation in Arabidopsis thaliana. Genes 2021, 12, 249. [Google Scholar] [CrossRef]
- Cho, H.T.; Cosgrove, D.J. Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 2002, 14, 3237–3253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.L.; Li, L.; Yang, R.R.; Yi, X.; Zhang, B. Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci. Rep. 2015, 5, 13639. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Zhang, P.; Duo, T.Q.; Wang, F.D.; Zhang, X.Z.; Yang, Z.Z.; Hu, G.F. De novo transcriptome in roots of switchgrass (Panicum virgatum L.) reveals gene expression dynamic and act network under alkaline salt stress. BMC Genom. 2021, 22, 82. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.-K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.; Zhang, M.; Zhou, S.; Kong, X.; Wang, W. Overexpression of the wheat expansin gene TaEXPA2 improved seed production and drought tolerance in transgenic tobacco plants. PLoS ONE 2016, 11, e0153494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Han, Y.Y.; Kong, X.Z.; Kang, H.; Ren, Y.; Wang, W. Ectopic expression of wheat expansin gene TaEXPA2 improved the salt tolerance of transgenic tobacco by regulating Na+/K+ and antioxidant competence. Physiol. Plant. 2017, 159, 161–177. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, G.Q.; An, J.; Li, Q.; Chen, Y.; Zhao, X.; Wu, J.; Wang, Y.; Hao, Q.; Wang, W.; et al. Expansin gene TaEXPA2 positively regulates drought tolerance in transgenic wheat (Triticum aestivum L.). Plant Sci. 2020, 298, 110596. [Google Scholar] [CrossRef]
- Jadamba, C.; Kang, K.; Paek, N.-C.; Lee, S.I.; Yoo, S.-C. Overexpression of Rice Expansin7 (Osexpa7) Confers Enhanced Tolerance to Salt Stress in Rice. Int. J. Mol. Sci. 2020, 21, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Xu, Y.Q.; Dong, J.M.; Peng, L.-N.; Feng, X.; Wang, X.; Li, F.; Miao, Y.; Yao, S.-K.; Zhao, Q.-Q.; et al. Genome-wide identification of wheat (Triticum aestivum) expansins and expansin expression analysis in cold-tolerant and cold-sensitive wheat cultivars. PLoS ONE 2018, 13, e0195138. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, Y.; Wang, X.; Feng, X.; Zhao, Q.; Feng, S.; Zhao, Z.; Hu, B.; Li, F.; Li, F. Overexpression of paralogues of the wheat expansin gene TaEXPA8 improves low-temperature tolerance in Arabidopsis. Plant Biol. 2019, 21, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Xu, Y.Q.; Dong, J.M.; Wu, J.W.; Peng, L.N.; Feng, X.; Feng, S.S.; Hu, B.Z.; Li, F.L. Genetic transformation and functional analysis of TaEXPA7 homologous gene of winter wheat in cold region. J. Triticeae Crops 2020, 40, 401–407. (In Chinese) [Google Scholar]
- Feng, X.; Xu, Y.Q.; Peng, L.N.; Yu, X.; Zhao, Q.; Feng, S.; Zhao, Z.; Li, F.; Hu, B. TaEXPB7-B, a β-expansin gene involved in low-temperature stress and abscisic acid responses, promotes growth and cold resistance in Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153004. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.F.; Liebe, S.; Thiel, H.; Lennefors, B.; Kraft, T.; Gilmer, D.; Maiss, E.; Varrelmann, M.; Savenkov, E.I. Massive up-regulation of LBD transcription factors and EXPANSINs highlights the regulatory programs of rhizomania disease. Mol. Plant Pathol. 2018, 19, 2333–2348. [Google Scholar]
- Wei, W.; Yang, C.; Luo, J.; Lu, C.; Wu, Y.; Yuan, S. Synergism between cucumber α-expansin, fungal endoglucanase and pectinlyase. J. Plant Physiol. 2010, 167, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Monti, F.; Dell’Anna, R.; Sanson, A.; Fasoli, M.; Pezzotti, M.; Zenoni, S. A multivariate statistical analysis approach to highlight molecular processes in plant cell walls through ATR FT-IR microspectroscopy: The role of the α-expansin PhEXPA1 in Petuniahybrida. Vib. Spectrosc. 2013, 65, 36–43. [Google Scholar] [CrossRef]
- Cho, H.T.; Kende, H. Expression of expansin gene is correlated with growth in deepwater rice. Plant Cell 1997, 9, 1661–1671. [Google Scholar]
- Shin, J.-H.; Jeong, D.-H.; Park, M.C.; An, G. Characterization and transcriptional expression of the α-expansin gene family in rice. Mol. Cells 2005, 20, 210–218. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, B.; He, X.; Lv, S.; Bai, Y.; Ding, W.; Chen, M.; Cho, H.-T.; Wu, P. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011, 66, 725–734. [Google Scholar]
- Ma, N.; Wang, Y.; Qiu, S.; Kang, Z.; Che, S.; Wang, G.; Huang, J. Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 2013, 8, e75997. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Choi, H.S.; Cho, H.T. Root hair-specific EXPANSIN A7 is required for root hair elongation in Arabidopsis. Mol. Cells 2011, 31, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, M.; Kim, N.Y.; Lee, S.H.; Kim, J. LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 2013, 73, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ma, B.; Shen, J.J.; Zhao, S.; Ma, X.; Wang, Z.; Fan, Y.; Tang, Q.; Wei, D. The evolution of the expansin gene family in Brassica species. Plant Physiol. Biochem. 2021, 167, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Narayan, J.A.; Dharshini, S.; Manoj, V.M.; Padmanabhan, T.S.S.; Kadirvelu, K.; Suresha, G.S.; Subramonian, N.; Ram, B.; Premachandran, M.N.; Appunu, C. Isolation and characterization of water-deficit stress-responsive α-expansin 1 (EXPA1) gene from Saccharum complex. 3 Biotech 2019, 9, 186. [Google Scholar] [CrossRef]
- He, X.Y.; Zeng, J.B.; Cao, F.B.; Ahmed, I.M.; Zhang, G.; Vincze, E.; Wu, F. HvEXPB7, a novel β-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J. Exp. Bot. 2015, 66, 7405–7419. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, G.; Zheng, X.; Zhong, Q.; Zhang, M.; Wu, Z.; Wen, C.; Liu, M. Comprehensive transcriptome reveals an opposite regulatory effect of plant growth retardants in controlling seedling overgrowth between roots and shoots. Int. J. Mol. Sci. 2019, 20, 3307. [Google Scholar] [CrossRef] [PubMed]
- Hasseb, N.M.; Sallam, A.; Karam, M.A.; Gao, L.; Wang, R.R.C.; Moursi, Y.S. High-LD SNP markers exhibiting pleiotropic effects on salt tolerance at germination and seedlings stages in spring wheat. Plant Mol. Biol. 2022, 108, 585–603. [Google Scholar] [CrossRef]
- Jones, L.; Mcqueen-Mason, S. A role for expansins in dehydration and rehydration of the resurrection plant Craterostigma plantagineum. FEBS Lett. 2004, 559, 61–65. [Google Scholar] [CrossRef]
- Challabathula, D.; Analin, B.; Mohanan, A.; Bakka, K. Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J. Plant Physiol. 2022, 268, 153583. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Sui, Y. Efficient and Fast Production of Transgenic Rice Plants by Agrobacterium-Mediated Transformation. Methods Mol. Biol. 2019, 1864, 95–103. [Google Scholar] [PubMed]
- Chen, Y.S.; Tang, Y.; Mo, W.Y.; Zhao, Y.M.; Lu, Y.L. Study on paraffin section method of Zanthoxylum bungeanum. Sci. Technol. Vis. 2020, 16, 116. (In Chinese) [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ma, J.; He, F.; Wang, L.; Zhang, T.; Liu, D.; Xu, Y.; Li, F.; Feng, X. A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress. Int. J. Mol. Sci. 2024, 25, 7707. https://doi.org/10.3390/ijms25147707
Wang X, Ma J, He F, Wang L, Zhang T, Liu D, Xu Y, Li F, Feng X. A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress. International Journal of Molecular Sciences. 2024; 25(14):7707. https://doi.org/10.3390/ijms25147707
Chicago/Turabian StyleWang, Xue, Jing Ma, Fumeng He, Linlin Wang, Tong Zhang, Dan Liu, Yongqing Xu, Fenglan Li, and Xu Feng. 2024. "A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress" International Journal of Molecular Sciences 25, no. 14: 7707. https://doi.org/10.3390/ijms25147707
APA StyleWang, X., Ma, J., He, F., Wang, L., Zhang, T., Liu, D., Xu, Y., Li, F., & Feng, X. (2024). A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress. International Journal of Molecular Sciences, 25(14), 7707. https://doi.org/10.3390/ijms25147707





