Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds
Abstract
1. Introduction
2. Results and Discussion
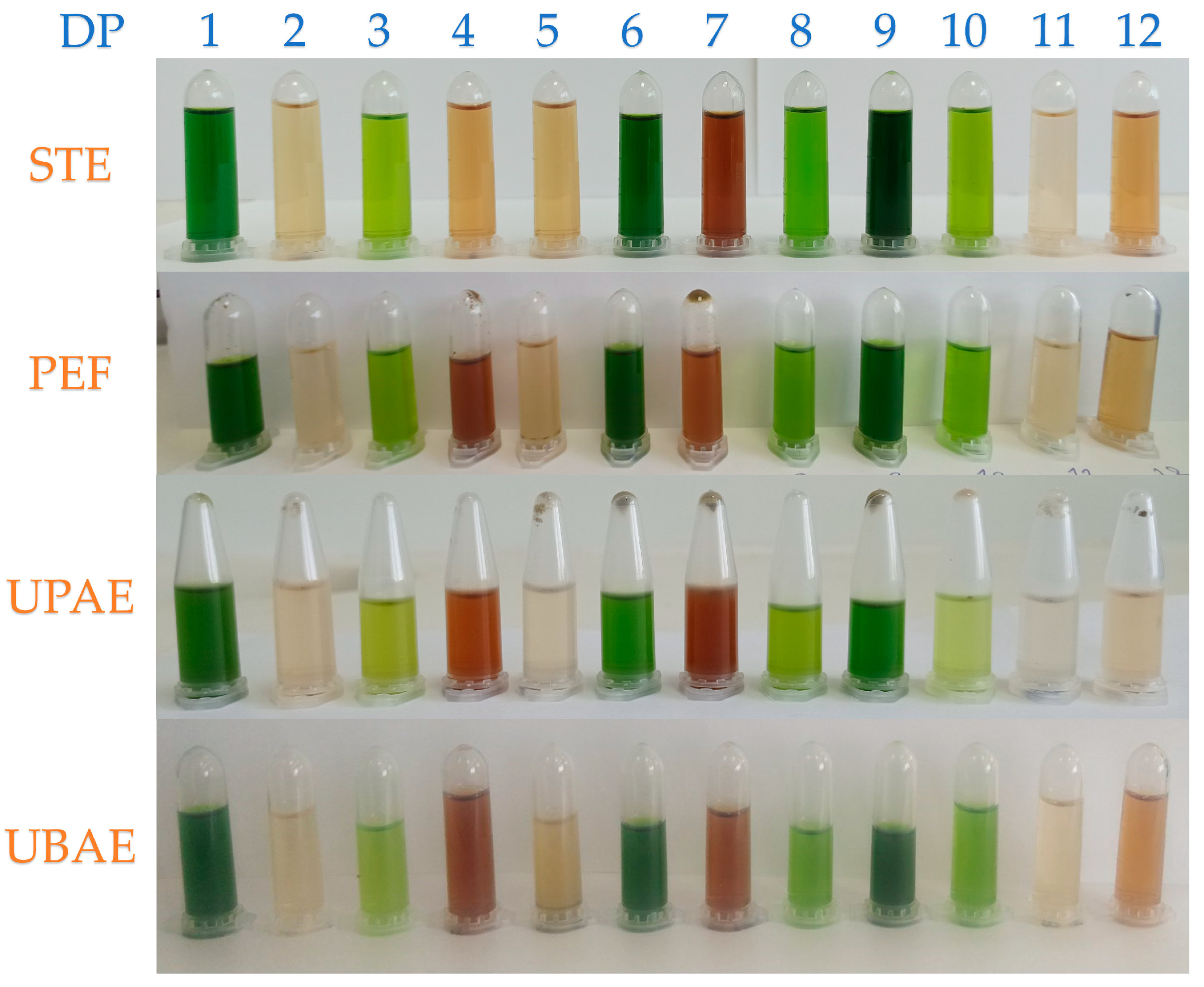
2.1. Extraction Techniques
2.1.1. Stirring Extraction (STE) Technique
2.1.2. Pulsed Electric Field (PEF)-Assisted Extraction Technique
2.1.3. Ultrasonic Probe-Assisted Extraction (UPAE) Technique
2.1.4. Ultrasonic Bath-Assisted Extraction (UBAE) Technique
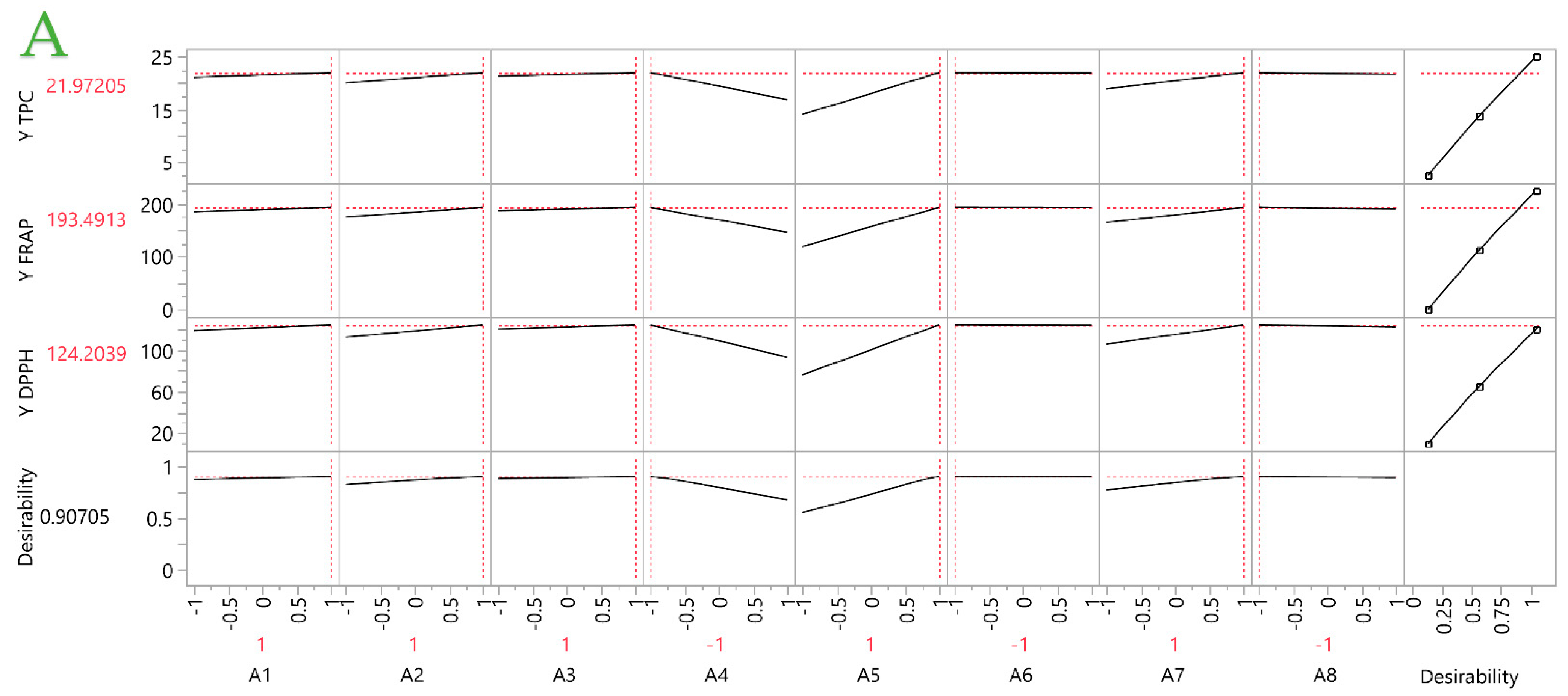
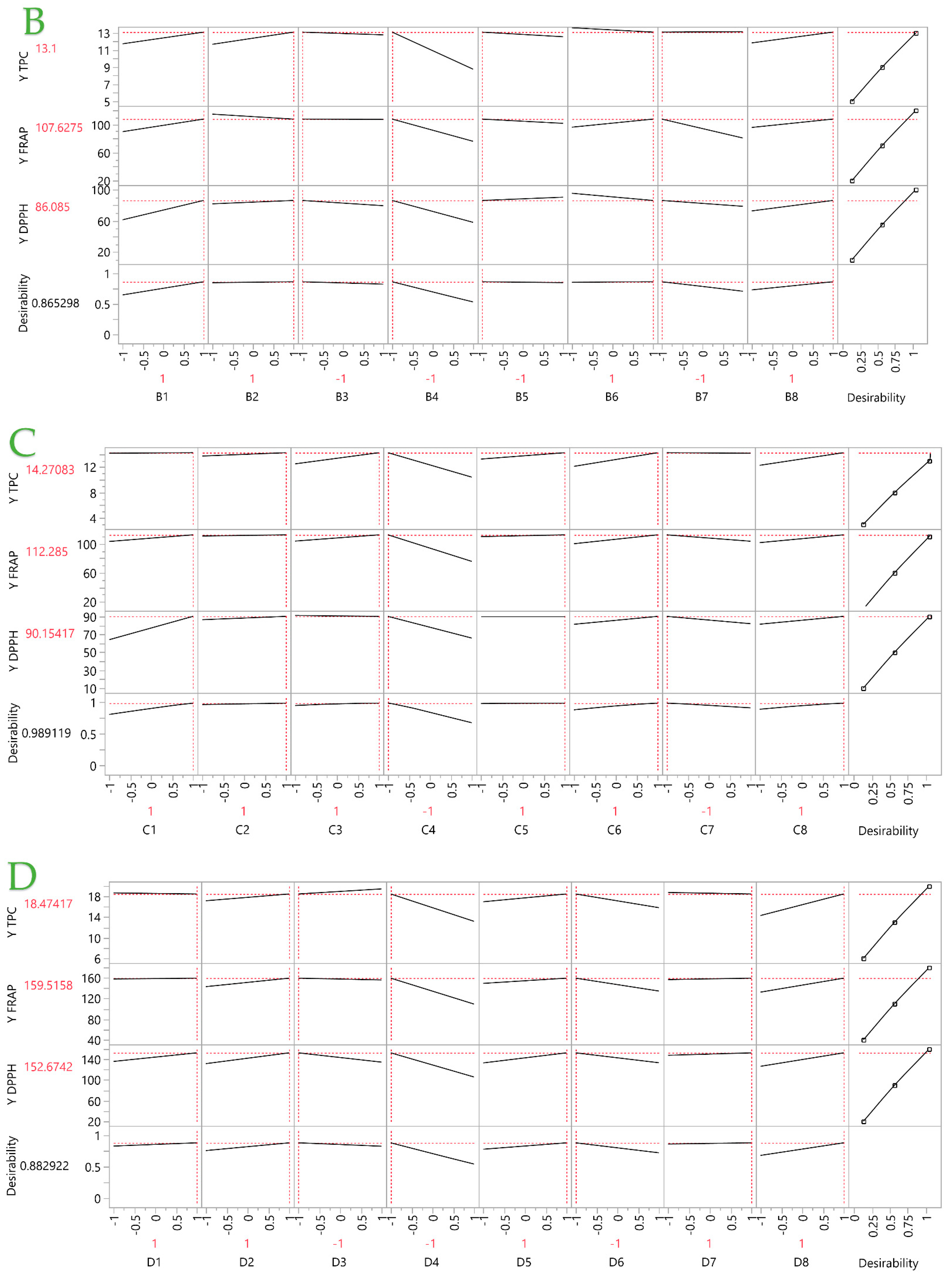
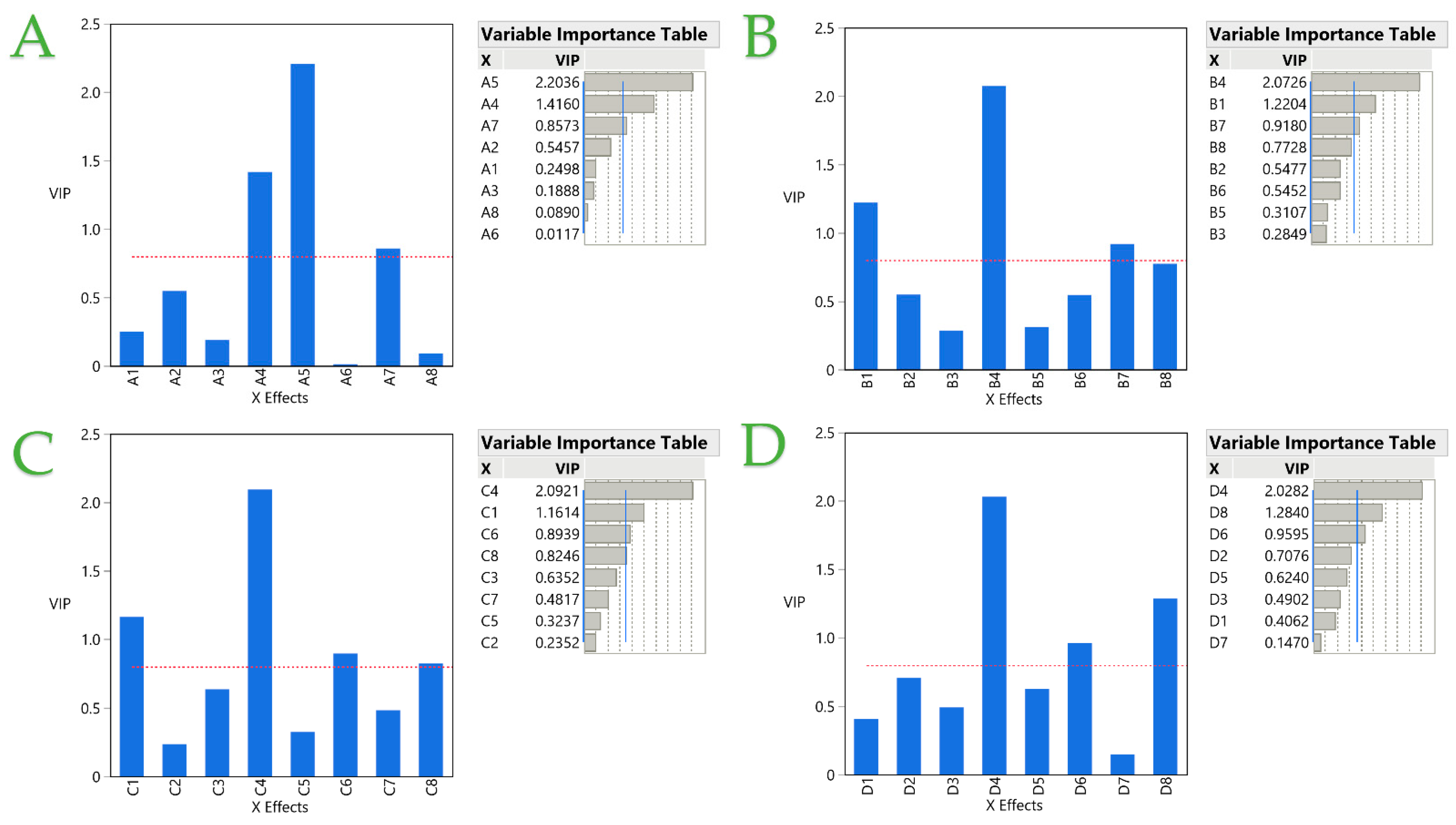
2.2. Partial Least Squares (PLS) Analysis
2.2.1. Prediction Profiler
2.2.2. Analysis of the Optimal Extracts
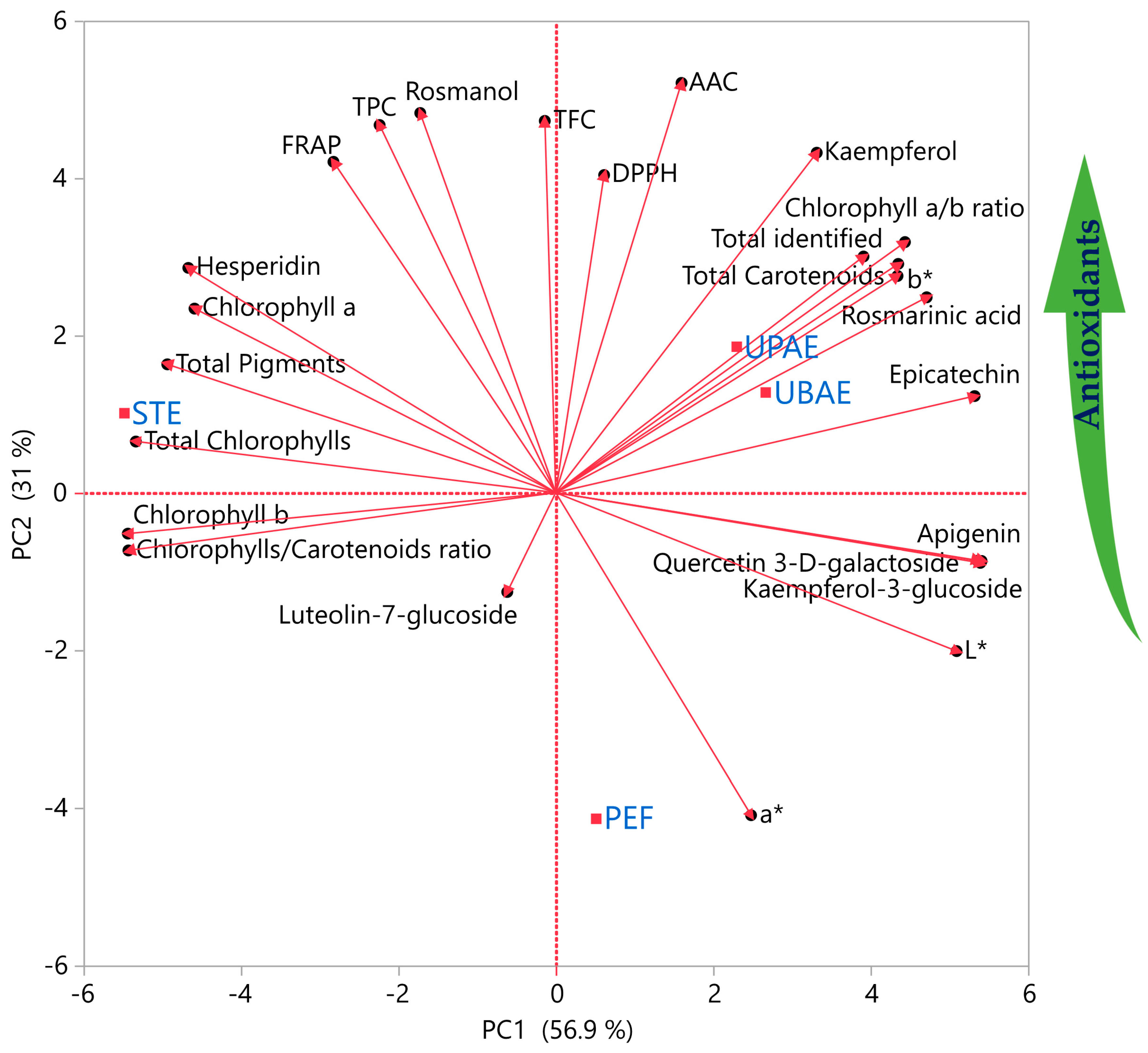
2.3. Principal Component Analysis (PCA)
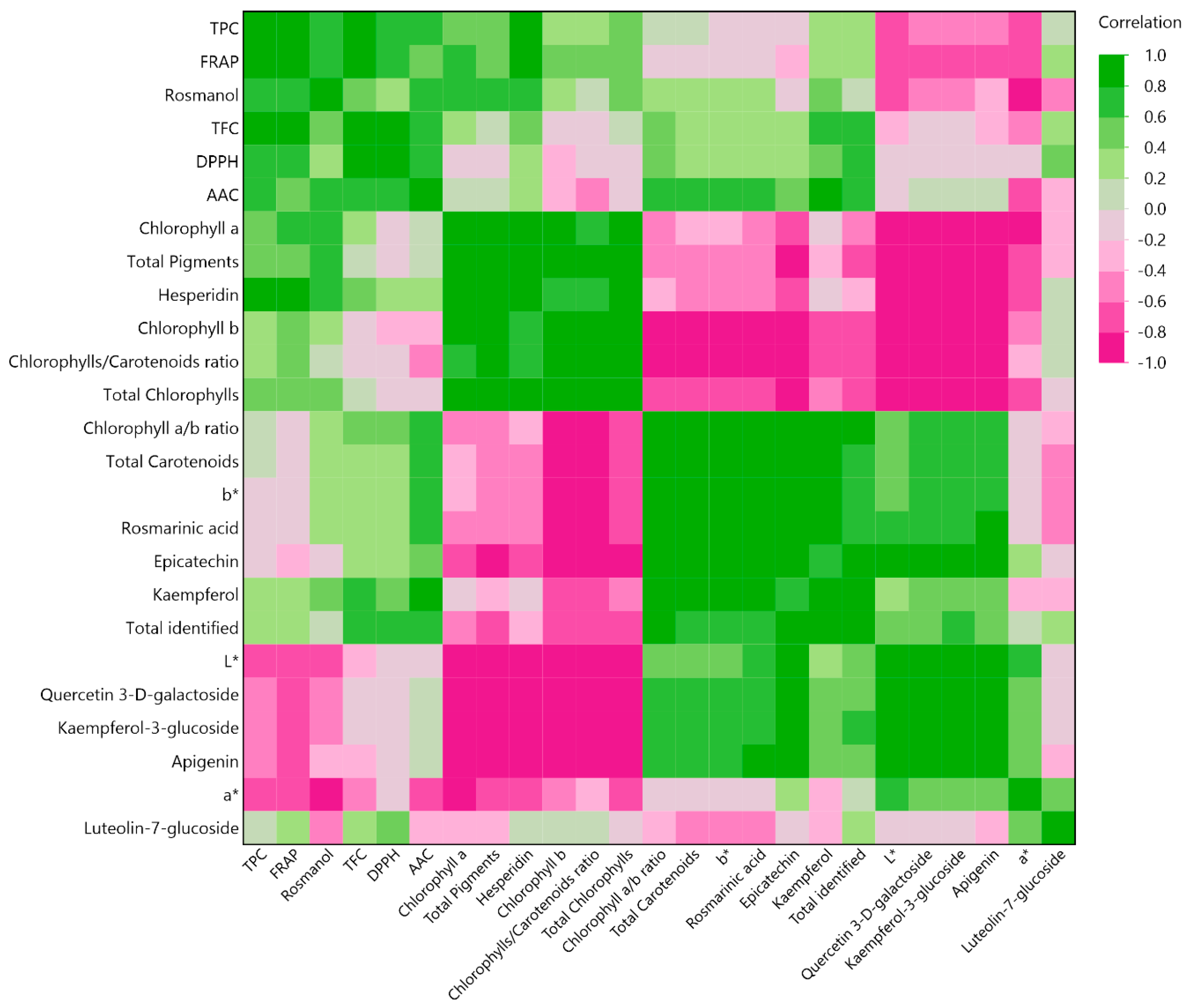
2.4. Multivariate Correlation Analysis (MCA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Collection and Handling of RO Leaves
3.4. RO Leaves Extraction Procedure through Plackett–Burman Design
3.5. Bioactive Compounds Determination
3.5.1. Total Polyphenol Content (TPC)
3.5.2. Total Flavonoid Content (TFC)
3.5.3. HPLC Quantification of Polyphenolic Compounds
3.5.4. Ascorbic Acid Content (AAC)
3.5.5. Total Pigment Concentration
3.5.6. Color Analysis of the Extracts
3.6. Antioxidant Activity of the Extracts
3.6.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.6.2. DPPH• Antiradical Activity Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Sandhya, S.; Shaffath Ali, S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Polonorum. Technol. Aliment. 2013, 12, 61–73. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780429126581. [Google Scholar]
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and Ethnopharmacology--Interdisciplinary Links with the Historical Sciences. J. Ethnopharmacol. 2006, 107, 157–160. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal Medicinal Products or Preparations for Neuropathic Pain. Cochrane Database Syst. Rev. 2019, 4, CD010528. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Refined Exposure Assessment of Extracts of Rosemary (E 392) from Its Use as Food Additive. EFSA J. 2018, 16, e05373. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Kasparavičienė, G.; Ramanauskienė, K.; Savickas, A.; Velžienė, S.; Kalvėnienė, Z.; Kazlauskienė, D.; Ragažinskienė, O.; Ivanauskas, K. Evaluation of Total Phenolic Content and Antioxidant Activity of Different Rosmarinus officinalis L. Ethanolic Extracts. Biologija 2013, 59, 39–44. [Google Scholar] [CrossRef]
- Zeghad Nadia, M.R. Antioxidant Activity of Flavonoids Isolated from Rosmarinus officinalis L. J. Plant Sci. Res. 2016, 3, 142. [Google Scholar]
- Soltanabad, M.H.; Bagherieh-Najjar, M.B.; Mianabadi, M. Seasonal Variations in Carnosic Acid Content of Rosemary Correlates with Anthocyanins and Soluble Sugars. J. Med. Plants By-Prod.-Jmpb 2018, 7, 163–171. [Google Scholar] [CrossRef]
- Rizkiyah, D.N.; Putra, N.R.; Idham, Z.; Aziz, A.H.A.; Che Yunus, M.A.; Veza, I.; Irianto; Chinedu Mamah, S.; Qomariyah, L. Recovery of Anthocyanins from Hibiscus sabdariffa L. Using a Combination of Supercritical Carbon Dioxide Extraction and Subcritical Water Extraction. Processes 2023, 11, 751. [Google Scholar] [CrossRef]
- Putra, N.R.; Yustisia, Y.; Heryanto, R.B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review. S. Afr. J. Chem. Eng. 2023, 46, 88–98. [Google Scholar] [CrossRef]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations 2023, 10, 121. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochemistry 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Janaki, P.; Bhuvanadevi, S.; Dhananivetha, M.; Murali Arthanari, P.; Chinnusamy, C. Persistence of Quizalofop Ethyl in Soil and Safety to Ground Nut by Ultrasonic Bath Extraction and HPLC-DAD Detection. J. Res. Weed Sci. 2018, 1, 1–12. [Google Scholar]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed Electric Fields as a Green Technology for the Extraction of Bioactive Compounds from Thinned Peach By-Products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Kotsou, K.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Sustainable Valorization of Sour Cherry (Prunus cerasus) By-Products: Extraction of Antioxidant Compounds. Sustainability 2024, 16, 32. [Google Scholar] [CrossRef]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between Pressurized Liquid Extraction and Conventional Soxhlet Extraction for Rosemary Antioxidants, Yield, Composition, and Environmental Footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Process Optimization of Intermediate-Wave Infrared Drying: Screening by Plackett–Burman; Comparison of Box-Behnken and Central Composite Design and Evaluation: A Case Study. Ind. Crops Prod. 2021, 162, 113287. [Google Scholar] [CrossRef]
- Spadi, A.; Angeloni, G.; Guerrini, L.; Corti, F.; Michelozzi, M.; Cencetti, G.; Parenti, A.; Masella, P. Using a Plackett–Burman Design to Maximise Yield of Rosemary Essential Oil by Distillation. Ind. Crops Prod. 2021, 166, 113488. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. An Investigation into Crithmum maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds 2023, 3, 532–551. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. ISBN 978-0-12-813768-0. [Google Scholar]
- Athanasiadis, V.; Lakka, A.; Palaiogiannis, D.; Pappas, V.M.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field and Salvia officinalis L. Leaves: A Successful Combination for the Extraction of High Value Added Compounds. Foods 2021, 10, 2014. [Google Scholar] [CrossRef]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-Thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.-W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Process Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Ziagova, M.G.; Mavromatidou, C.; Samiotis, G.; Amanatidou, E. Total Phenolic Content and Antioxidant Capacity of Greek Medicinal and Aromatic Plant Extracts Using Pulsed Electric Field Followed by Ultrasounds Extraction Process. Food Process. Preserv. 2022, 46, e16639. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.-J. Increase the Yield of Bioactive Compounds from Elder Bark and Annatto Seeds Using Ultrasound and Microwave Assisted Extraction Technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D.; Khadka, A.; Ojha, P. Profile of Chlorophylls and Carotenoids of Wheat (Triticum aestivum L.) and Barley (Hordeum vulgare L.) Microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.-S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus Microwave as Green Processes for Extraction of Rosmarinic, Carnosic and Ursolic Acids from Rosemary. Ultrason. Sonochemistry 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Karabegović, I.T.; Stojičević, S.S.; Veličković, D.T.; Todorović, Z.B.; Nikolić, N.Č.; Lazić, M.L. The Effect of Different Extraction Techniques on the Composition and Antioxidant Activity of Cherry Laurel (Prunus laurocerasus) Leaf and Fruit Extracts. Ind. Crops Prod. 2014, 54, 142–148. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Mantiniotou, M.; Kalompatsios, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Pressurized Liquid Extraction (PLE) Parameters for Extraction of Bioactive Compounds from Moringa oleifera Leaves and Bioactivity Assessment. Int. J. Mol. Sci. 2024, 25, 4628. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research: Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, 1–6 August 1983; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 2, pp. 9–12. ISBN 978-94-017-6368-4. [Google Scholar]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]

| Design Point | Independent Variables 1 | Responses 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | TPC | FRAP | DPPH | |
| 1 | 100 (1) | 10 (−1) | 30 (−1) | 0.4 (−1) | 80 (1) | 250 (−1) | 10 (−1) | 2 (1) | 14.98 | 132.85 | 90.07 |
| 2 | 0 (−1) | 50 (1) | 30 (−1) | 1.2 (1) | 80 (1) | 500 (1) | 10 (−1) | 1 (−1) | 10.55 | 94.22 | 59.22 |
| 3 | 100 (1) | 50 (1) | 30 (−1) | 0.4 (−1) | 20 (−1) | 500 (1) | 10 (−1) | 1 (−1) | 9.76 | 95.81 | 66.54 |
| 4 | 0 (−1) | 10 (−1) | 120 (1) | 1.2 (−1) | 20 (−1) | 500 (1) | 10 (−1) | 2 (1) | 4.90 | 34.09 | 25.89 |
| 5 | 0 (−1) | 50 (1) | 30 (−1) | 1.2 (−1) | 80 (1) | 250 (−1) | 25 (1) | 2 (1) | 19.63 | 188.05 | 112.75 |
| 6 | 100 (1) | 10 (−1) | 30 (−1) | 0.4 (1) | 20 (−1) | 500 (1) | 25 (1) | 2 (1) | 7.95 | 66.19 | 30.31 |
| 7 | 0 (−1) | 10 (−1) | 120 (1) | 0.4 (−1) | 80 (1) | 500 (1) | 25 (1) | 1 (−1) | 23.42 | 202.47 | 103.57 |
| 8 | 100 (1) | 50 (1) | 120 (1) | 1.2 (1) | 80 (1) | 500 (1) | 25 (1) | 2 (1) | 14.46 | 106.49 | 94.47 |
| 9 | 100 (1) | 10 (−1) | 120 (1) | 1.2 (1) | 80 (1) | 250 (−1) | 10 (−1) | 1 (−1) | 12.70 | 111.08 | 54.92 |
| 10 | 100 (1) | 50 (1) | 120 (1) | 0.4 (−1) | 20 (−1) | 250 (−1) | 25 (1) | 1 (−1) | 13.44 | 89.91 | 77.40 |
| 11 | 0 (−1) | 50 (1) | 120 (1) | 1.2 (1) | 20 (−1) | 250 (−1) | 10 (−1) | 2 (1) | 7.71 | 54.67 | 29.79 |
| 12 | 0 (−1) | 10 (−1) | 30 (−1) | 1.2 (1) | 20 (−1) | 250 (−1) | 25 (1) | 1 (−1) | 3.95 | 22.54 | 11.86 |
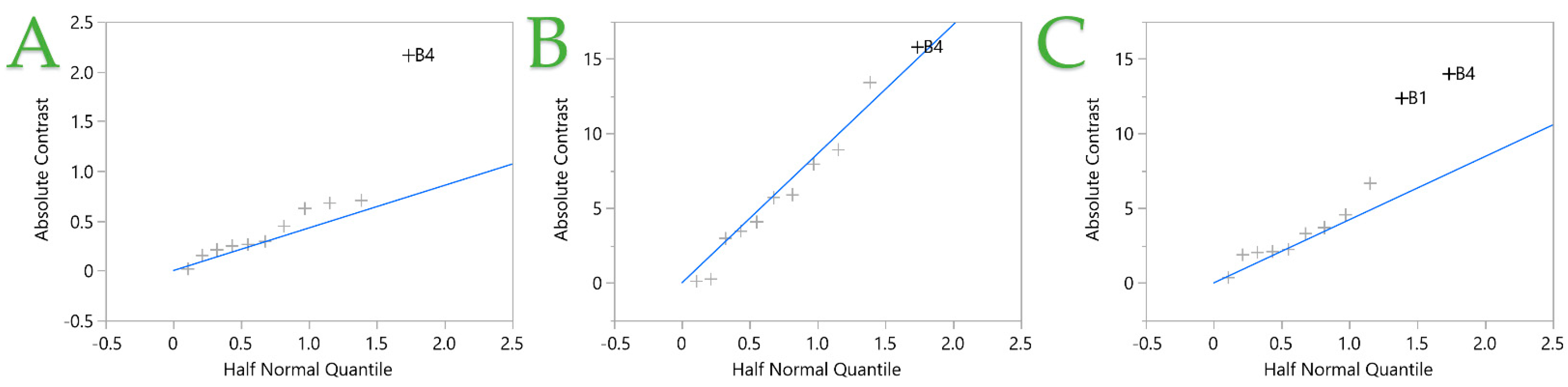
| Design Point | Independent Variables 1 | Responses 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | TPC | FRAP | DPPH | |
| 1 | 100 (1) | 10 (−1) | 10 (−1) | 0.4 (−1) | 1 (1) | 100 (−1) | 10 (−1) | 2 (1) | 12.02 | 112.55 | 99.10 |
| 2 | 0 (−1) | 50 (1) | 10 (−1) | 1.2 (1) | 1 (1) | 1000 (1) | 10 (−1) | 1 (−1) | 5.78 | 44.70 | 28.35 |
| 3 | 100 (1) | 50 (1) | 10 (−1) | 0.4 (−1) | 0.6 (−1) | 1000 (1) | 10 (−1) | 1 (−1) | 11.66 | 91.14 | 68.30 |
| 4 | 0 (−1) | 10 (−1) | 30 (1) | 1.2 (−1) | 0.6 (−1) | 1000 (1) | 10 (−1) | 2 (1) | 10.66 | 92.69 | 49.56 |
| 5 | 0 (−1) | 50 (1) | 10 (−1) | 1.2 (−1) | 1 (1) | 100 (−1) | 100 (1) | 2 (1) | 11.38 | 29.76 | 63.20 |
| 6 | 100 (1) | 10 (−1) | 10 (−1) | 0.4 (1) | 0.6 (−1) | 1000 (1) | 100 (1) | 2 (1) | 6.53 | 48.94 | 47.30 |
| 7 | 0 (−1) | 10 (−1) | 30 (1) | 0.4 (−1) | 1 (1) | 1000 (1) | 100 (1) | 1 (−1) | 7.57 | 55.23 | 34.17 |
| 8 | 100 (1) | 50 (1) | 30 (1) | 1.2 (1) | 1 (1) | 1000 (1) | 100 (1) | 2 (1) | 8.82 | 49.79 | 46.89 |
| 9 | 100 (1) | 10 (−1) | 30 (1) | 1.2 (1) | 1 (1) | 100 (−1) | 10 (−1) | 1 (−1) | 5.36 | 37.78 | 42.99 |
| 10 | 100 (1) | 50 (1) | 30 (1) | 0.4 (−1) | 0.6 (−1) | 100 (−1) | 100 (1) | 1 (−1) | 12.26 | 61.52 | 72.04 |
| 11 | 0 (−1) | 50 (1) | 30 (1) | 1.2 (1) | 0.6 (−1) | 100 (−1) | 10 (−1) | 2 (1) | 6.92 | 49.88 | 36.34 |
| 12 | 0 (−1) | 10 (−1) | 10 (−1) | 1.2 (1) | 0.6 (−1) | 100 (−1) | 100 (1) | 1 (−1) | 6.14 | 21.87 | 16.10 |
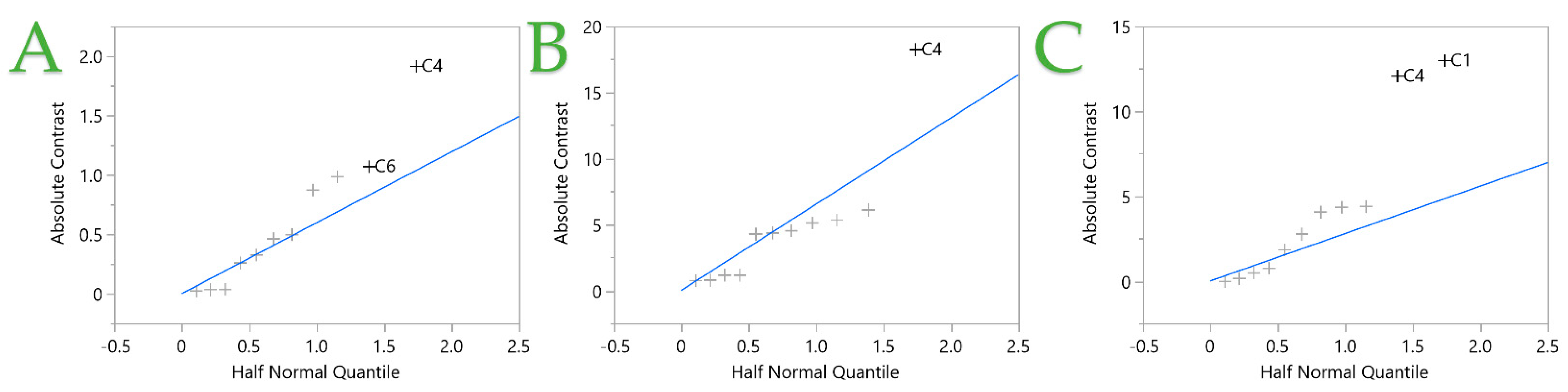
| Design Point | Independent Variables 1 | Responses 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | TPC | FRAP | DPPH | |
| 1 | 100 (1) | 10 (−1) | 5 (−1) | 0.4 (−1) | 120 (1) | 12 (−1) | 5 (−1) | 2 (1) | 10.67 | 95.17 | 83.42 |
| 2 | 0 (−1) | 50 (1) | 5 (−1) | 1.2 (1) | 120 (1) | 48 (1) | 5 (−1) | 1 (−1) | 7.19 | 56.19 | 34.08 |
| 3 | 100 (1) | 50 (1) | 5 (−1) | 0.4 (−1) | 60 (−1) | 48 (1) | 5 (−1) | 1 (−1) | 8.96 | 81.59 | 80.54 |
| 4 | 0 (−1) | 10 (−1) | 20 (1) | 1.2 (−1) | 60 (−1) | 48 (1) | 5 (−1) | 2 (1) | 12.90 | 100.78 | 57.86 |
| 5 | 0 (−1) | 50 (1) | 5 (−1) | 1.2 (−1) | 120 (1) | 12 (−1) | 15 (1) | 2 (1) | 9.38 | 68.12 | 43.29 |
| 6 | 100 (1) | 10 (−1) | 5 (−1) | 0.4 (1) | 60 (−1) | 48 (1) | 15 (1) | 2 (1) | 6.58 | 56.23 | 54.71 |
| 7 | 0 (−1) | 10 (−1) | 20 (1) | 0.4 (−1) | 120 (1) | 48 (1) | 15 (1) | 1 (−1) | 11.37 | 80.37 | 46.04 |
| 8 | 100 (1) | 50 (1) | 20 (1) | 1.2 (1) | 120 (1) | 48 (1) | 15 (1) | 2 (1) | 10.84 | 65.01 | 58.15 |
| 9 | 100 (1) | 10 (−1) | 20 (1) | 1.2 (1) | 120 (1) | 12 (−1) | 5 (−1) | 1 (−1) | 4.95 | 45.82 | 39.80 |
| 10 | 100 (1) | 50 (1) | 20 (1) | 0.4 (−1) | 60 (−1) | 12 (−1) | 15 (1) | 1 (−1) | 9.63 | 87.01 | 66.30 |
| 11 | 0 (−1) | 50 (1) | 20 (1) | 1.2 (1) | 60 (−1) | 12 (−1) | 5 (−1) | 2 (1) | 6.97 | 50.29 | 33.65 |
| 12 | 0 (−1) | 10 (−1) | 5 (−1) | 1.2 (1) | 60 (−1) | 12 (−1) | 15 (1) | 1 (−1) | 3.35 | 20.26 | 11.83 |
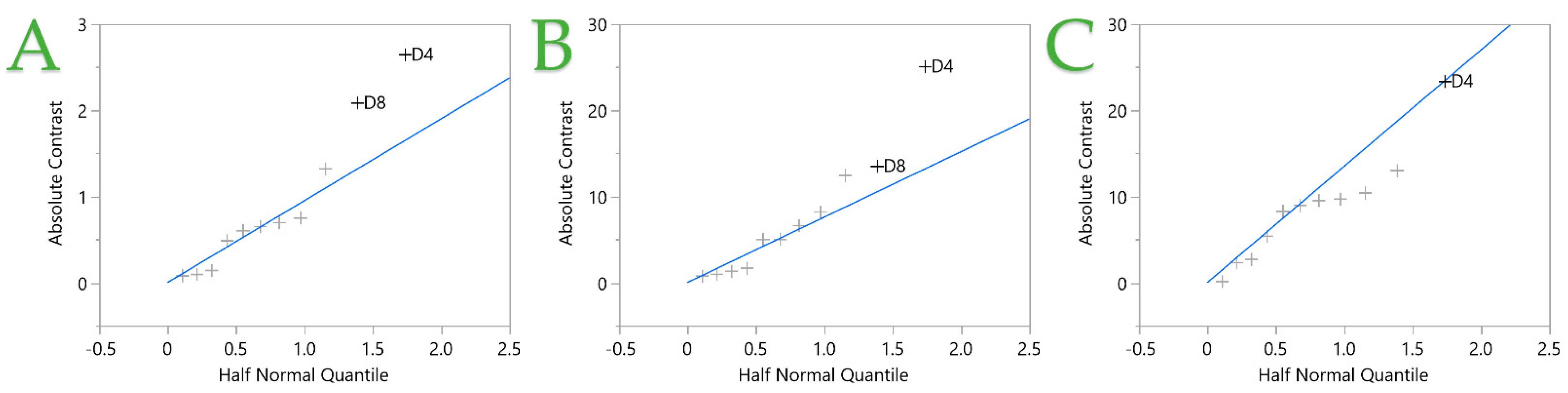
| Design Point | Independent Variables 1 | Responses 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | TPC | FRAP | DPPH | |
| 1 | 100 (1) | 10 (−1) | 5 (−1) | 0.4 (−1) | 220 (1) | 37 (−1) | 1 (−1) | 2 (1) | 17.42 | 128.76 | 123.58 |
| 2 | 0 (−1) | 50 (1) | 5 (−1) | 1.2 (1) | 220 (1) | 80 (1) | 1 (−1) | 1 (−1) | 8.24 | 57.44 | 46.11 |
| 3 | 100 (1) | 50 (1) | 5 (−1) | 0.4 (−1) | 110 (−1) | 80 (1) | 1 (−1) | 1 (−1) | 9.02 | 89.96 | 76.28 |
| 4 | 0 (−1) | 10 (−1) | 20 (1) | 1.2 (−1) | 110 (−1) | 80 (1) | 1 (−1) | 2 (1) | 15.69 | 108.86 | 63.12 |
| 5 | 0 (−1) | 50 (1) | 5 (−1) | 1.2 (−1) | 220 (1) | 37 (−1) | 2 (1) | 2 (1) | 18.71 | 169.40 | 139.46 |
| 6 | 100 (1) | 10 (−1) | 5 (−1) | 0.4 (1) | 110 (−1) | 80 (1) | 2 (1) | 2 (1) | 7.91 | 65.08 | 47.17 |
| 7 | 0 (−1) | 10 (−1) | 20 (1) | 0.4 (−1) | 220 (1) | 80 (1) | 2 (1) | 1 (−1) | 10.30 | 77.11 | 42.39 |
| 8 | 100 (1) | 50 (1) | 20 (1) | 1.2 (1) | 220 (1) | 80 (1) | 2 (1) | 2 (1) | 11.29 | 73.50 | 67.82 |
| 9 | 100 (1) | 10 (−1) | 20 (1) | 1.2 (1) | 220 (1) | 37 (−1) | 1 (−1) | 1 (−1) | 8.99 | 71.00 | 39.60 |
| 10 | 100 (1) | 50 (1) | 20 (1) | 0.4 (−1) | 110 (−1) | 37 (−1) | 2 (1) | 1 (−1) | 15.17 | 123.43 | 95.96 |
| 11 | 0 (−1) | 50 (1) | 20 (1) | 1.2 (1) | 110 (−1) | 37 (−1) | 1 (−1) | 2 (1) | 11.93 | 82.52 | 37.54 |
| 12 | 0 (−1) | 10 (−1) | 5 (−1) | 1.2 (1) | 110 (−1) | 37 (−1) | 2 (1) | 1 (−1) | 6.16 | 46.59 | 21.82 |
| Technique | Independent Variables 1 | PLS Model Values 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | TPC | FRAP | DPPH | |
| STE | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | 21.97 | 193.49 | 124.20 |
| PEF | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 13.10 | 107.63 | 86.09 |
| UPAE | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | 14.27 | 112.29 | 90.15 |
| UBAE | 1 | 1 | −1 | −1 | 1 | −1 | 1 | 1 | 18.47 | 159.52 | 152.67 |
| Parameters | STE | PEF | UPAE | UBAE |
|---|---|---|---|---|
| TPC (mg GAE/g dw) | 18.57 ± 1.21 a | 11.94 ± 0.82 c | 15.68 ± 1.08 b | 17.34 ± 1.20 a,b |
| TFC (mg RtE/g dw) | 5.62 ± 0.15 b | 3.21 ± 0.24 d | 5.01 ± 0.12 c | 6.46 ± 0.14 a |
| FRAP (μmol AAE/g dw) | 161.95 ± 3.56 a | 101.65 ± 3.05 d | 127.69 ± 6.51 c | 146.36 ± 7.46 b |
| DPPH (μmol AAE/g dw) | 110.1 ± 6.61 b | 69.86 ± 3.63 c | 97.96 ± 3.33 b | 142.65 ± 5.71 a |
| AAC (mg/g dw) | 18.32 ± 1.15 a,b | 16.23 ± 0.81 b | 20.01 ± 0.86 a | 19.3 ± 0.68 a |
| Chlorophyll a (μg/g dw) | 849.31 ± 56.05 a | 608.98 ± 17.66 c | 713.13 ± 33.52 b | 595.46 ± 18.46 c |
| Chlorophyll b (μg/g dw) | 744.64 ± 45.42 a | 500.31 ± 12.51 b | 396.97 ± 10.32 c | 347.09 ± 15.97 c |
| Chlorophyll a/b ratio | 1.14 ± 0.01 d | 1.22 ± 0.00 c | 1.8 ± 0.04 a | 1.72 ± 0.03 b |
| Total chlorophylls (μg/g dw) | 1593.95 ± 66.95 a | 1109.29 ± 43.26 b | 1110.1 ± 62.17 b | 942.55 ± 33.93 c |
| Total carotenoids (μg/g dw) | 215.39 ± 7.32 c | 240.2 ± 18.01 c | 372.36 ± 23.83 a | 317.89 ± 21.3 b |
| Chlorophylls/carotenoids ratio | 7.4 ± 0.06 a | 4.62 ± 0.17 b | 2.98 ± 0.02 c | 2.96 ± 0.09 c |
| Total Pigments (μg/g dw) | 1809.34 ± 74.27 a | 1349.49 ± 61.28 b,c | 1482.46 ± 86 b | 1260.44 ± 55.23 c |
| L* | 57 ± 0.6 c | 73 ± 0.4 a | 70.7 ± 0.8 b | 71.2 ± 0.6 b |
| a* | −19.7 ± 0.5 c | −13.2 ± 0.5 a | −19.5 ± 0.5 c | −14.9 ± 0.4 b |
| b* | 41.9 ± 0.8 c | 43 ± 0.5 c | 47.9 ± 0.4 a | 45.6 ± 0.8 b |
| Color 1 |
| Polyphenolic Compound | STE | PEF | UPAE | UBAE |
|---|---|---|---|---|
| Epicatechin | n.d. * | 0.05 ± 0.00 b | 0.08 ± 0.00 a | 0.09 ± 0.01 a |
| Quercetin 3-D-galactoside | n.d. | 1.03 ± 0.07 a | 1.13 ± 0.08 a | 1.11 ± 0.03 a |
| Luteolin-7-glucoside | 0.57 ± 0.03 b | 0.58 ± 0.03 b | 0.07 ± 0.00 c | 0.85 ± 0.02 a |
| Kaempferol-3-glucoside | 1.12 ± 0.07 a | 1.24 ± 0.08 a | 1.25 ± 0.09 a | 1.26 ± 0.07 a |
| Hesperidin | 2.24 ± 0.10 a | 1.26 ± 0.04 c | 1.54 ± 0.04 b | 1.51 ± 0.11 b |
| Rosmarinic acid | 2.85 ± 0.08 b | 2.92 ± 0.11 a,b | 3.11 ± 0.07 a | 3.04 ± 0.06 a,b |
| Apigenin | 0.76 ± 0.03 a | 0.82 ± 0.04 a | 0.83 ± 0.03 a | 0.82 ± 0.04 a |
| Kaempferol | 0.97 ± 0.03 a | 0.95 ± 0.05 a | 1.01 ± 0.04 a | 1.00 ± 0.04 a |
| Rosmanol | 3.79 ± 0.27 a | 3.47 ± 0.11 a | 3.80 ± 0.17 a | 3.63 ± 0.10 a |
| Total identified | 12.31 ± 0.61 a | 12.32 ± 0.53 a | 12.82 ± 0.53 a | 13.31 ± 0.47 a |
| Variables | SΤE | PEF | UPAE | UBAE |
|---|---|---|---|---|
| X1 | Solvent choice (0–100%, CEtOH) | Solvent choice (0–100%, CEtOH) | Solvent choice (0–100%, CEtOH) | Solvent choice (0–100%, CEtOH) |
| X2 | Solvent-to-solid ratio (10–50 mL/g) | Solvent-to-solid ratio (10–50 mL/g) | Solvent-to-solid ratio (10–50 mL/g) | Solvent-to-solid ratio (10–50 mL/g) |
| X3 | Extraction time (30–120 min) | Extraction time (10–30 min) | Extraction time (5–20 min) | Extraction time (5–20 min) |
| X4 | Particle size (0.4–1.2 mm) | Particle size (0.4–1.2 mm) | Particle size (0.4–1.2 mm) | Particle size (0.4–1.2 mm) |
| X5 | Temperature (20–80 °C) | Electric field strength (0.6–1 kV/cm) | Ultrasonic power (60–120 W) | Ultrasonic power (110–220 W) |
| X6 | Stirring speed (250–500 rpm) | Pulse period (100–1000 μs) | Pulsation (12–48 P/min) | Ultrasonic frequency (37–80 kHz) |
| X7 | Magnetic stir bar (10–25 mm) | Pulse duration (10–100 μs) | Probe length position (5–15 mm) | Ultrasonic mode (sweep–pulse) |
| X8 | Number of cycles (1–2 cycles) | Number of cycles (1–2 cycles) | Number of cycles (1–2 cycles) | Number of cycles (1–2 cycles) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Kotsou, K.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds. Int. J. Mol. Sci. 2024, 25, 7708. https://doi.org/10.3390/ijms25147708
Athanasiadis V, Chatzimitakos T, Mantiniotou M, Kalompatsios D, Kotsou K, Makrygiannis I, Bozinou E, Lalas SI. Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds. International Journal of Molecular Sciences. 2024; 25(14):7708. https://doi.org/10.3390/ijms25147708
Chicago/Turabian StyleAthanasiadis, Vassilis, Theodoros Chatzimitakos, Martha Mantiniotou, Dimitrios Kalompatsios, Konstantina Kotsou, Ioannis Makrygiannis, Eleni Bozinou, and Stavros I. Lalas. 2024. "Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds" International Journal of Molecular Sciences 25, no. 14: 7708. https://doi.org/10.3390/ijms25147708
APA StyleAthanasiadis, V., Chatzimitakos, T., Mantiniotou, M., Kalompatsios, D., Kotsou, K., Makrygiannis, I., Bozinou, E., & Lalas, S. I. (2024). Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds. International Journal of Molecular Sciences, 25(14), 7708. https://doi.org/10.3390/ijms25147708













