Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod
Abstract
1. Introduction
2. Results
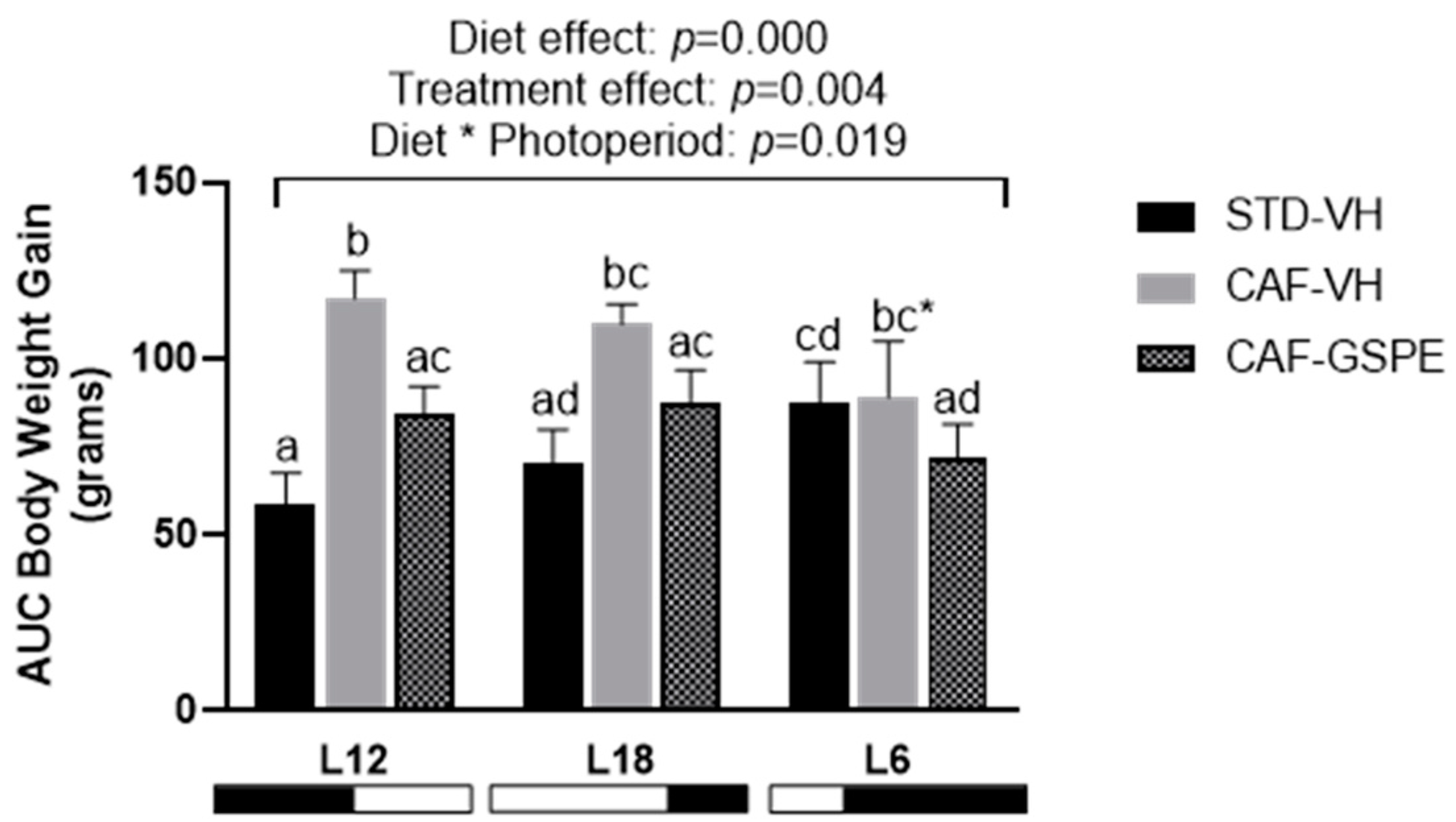
2.1. The Effect of GSPE on Body Weight Gain in CAF-fed Rats Differs according to the Photoperiod
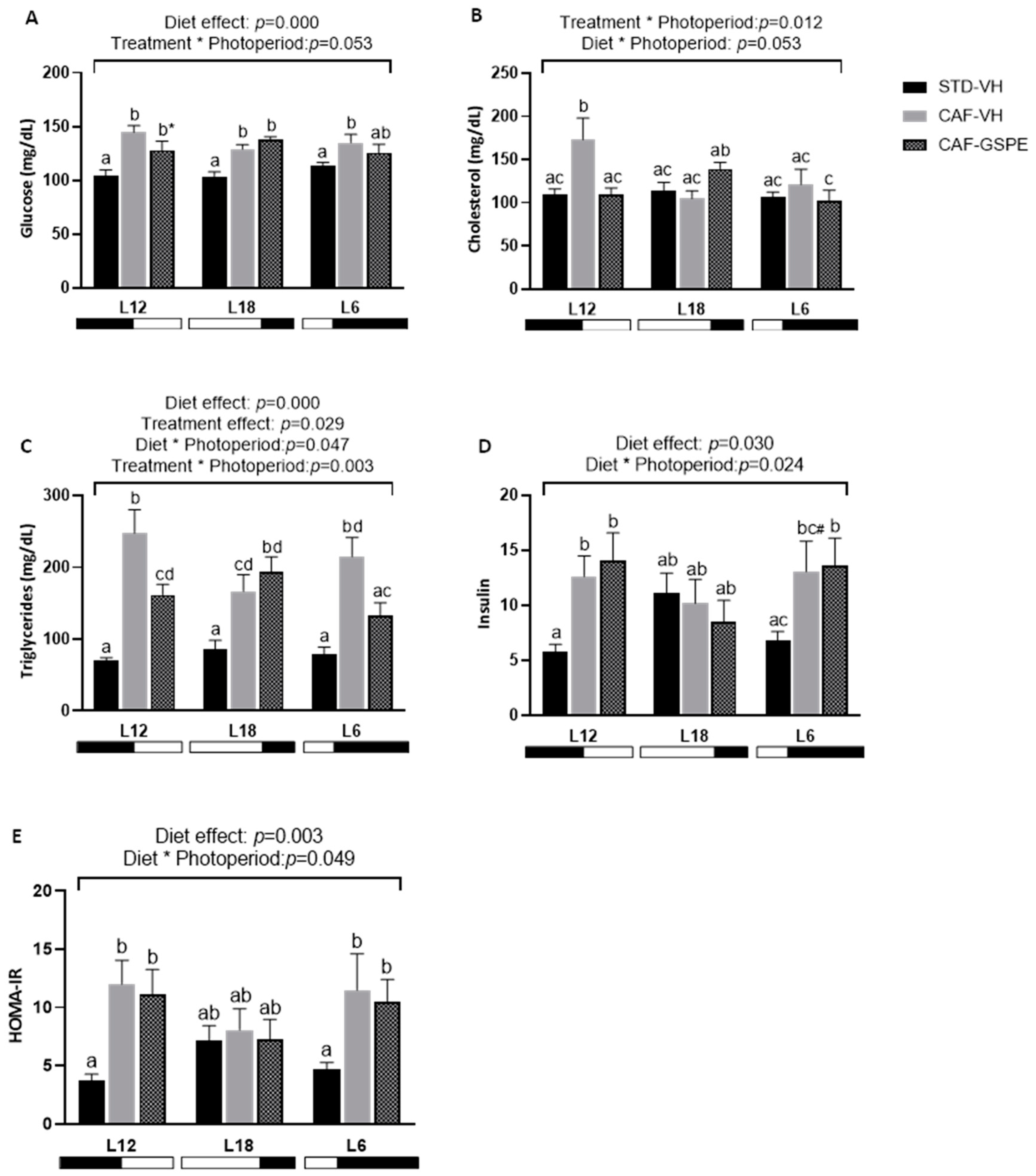
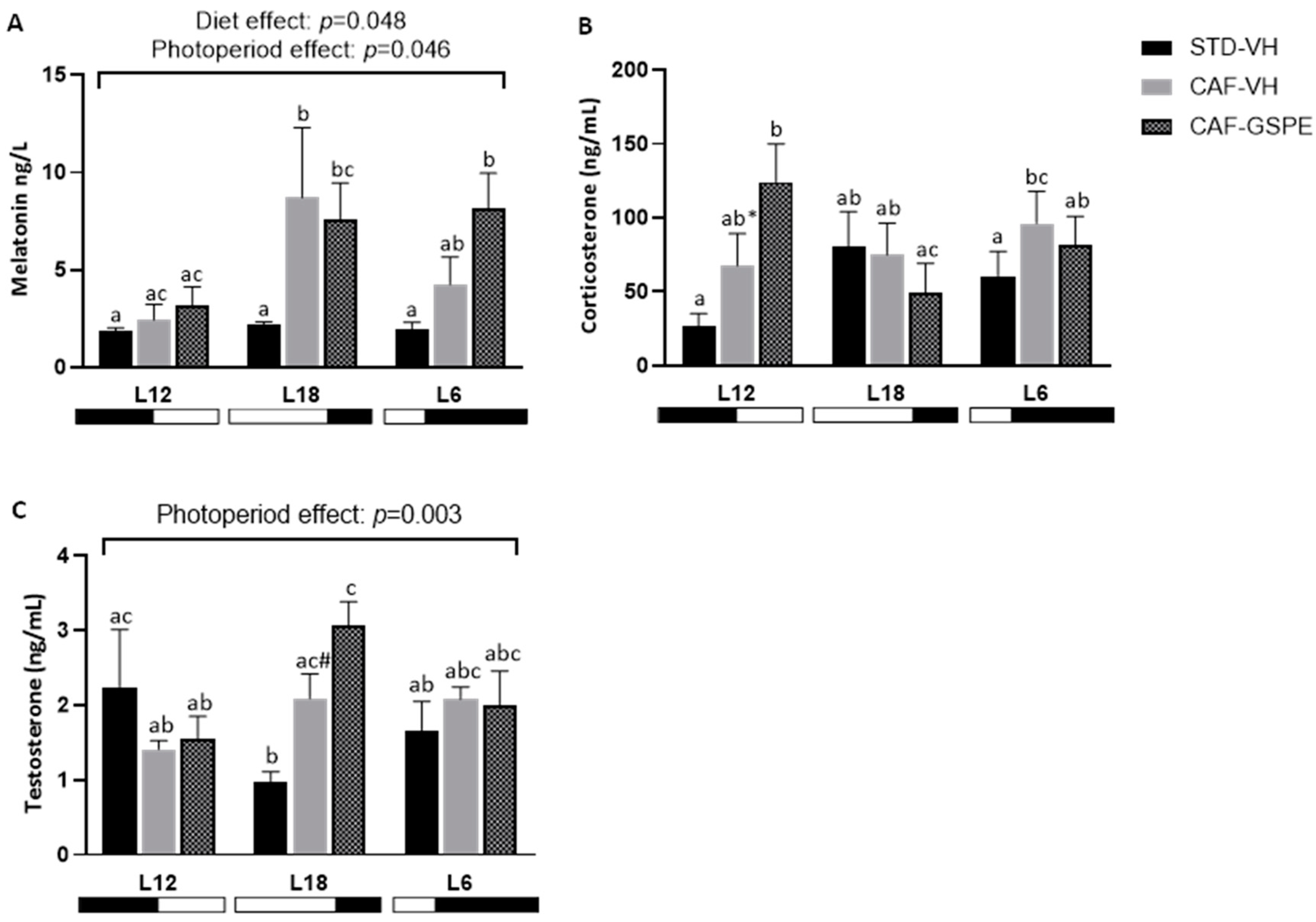
2.2. The Impact of GSPE on Serum Parameters Is Influenced by the Photoperiod in CAF-fed Rats
2.3. Photoperiod Significantly Influences Lipid Levels in the Livers of Obese Rats
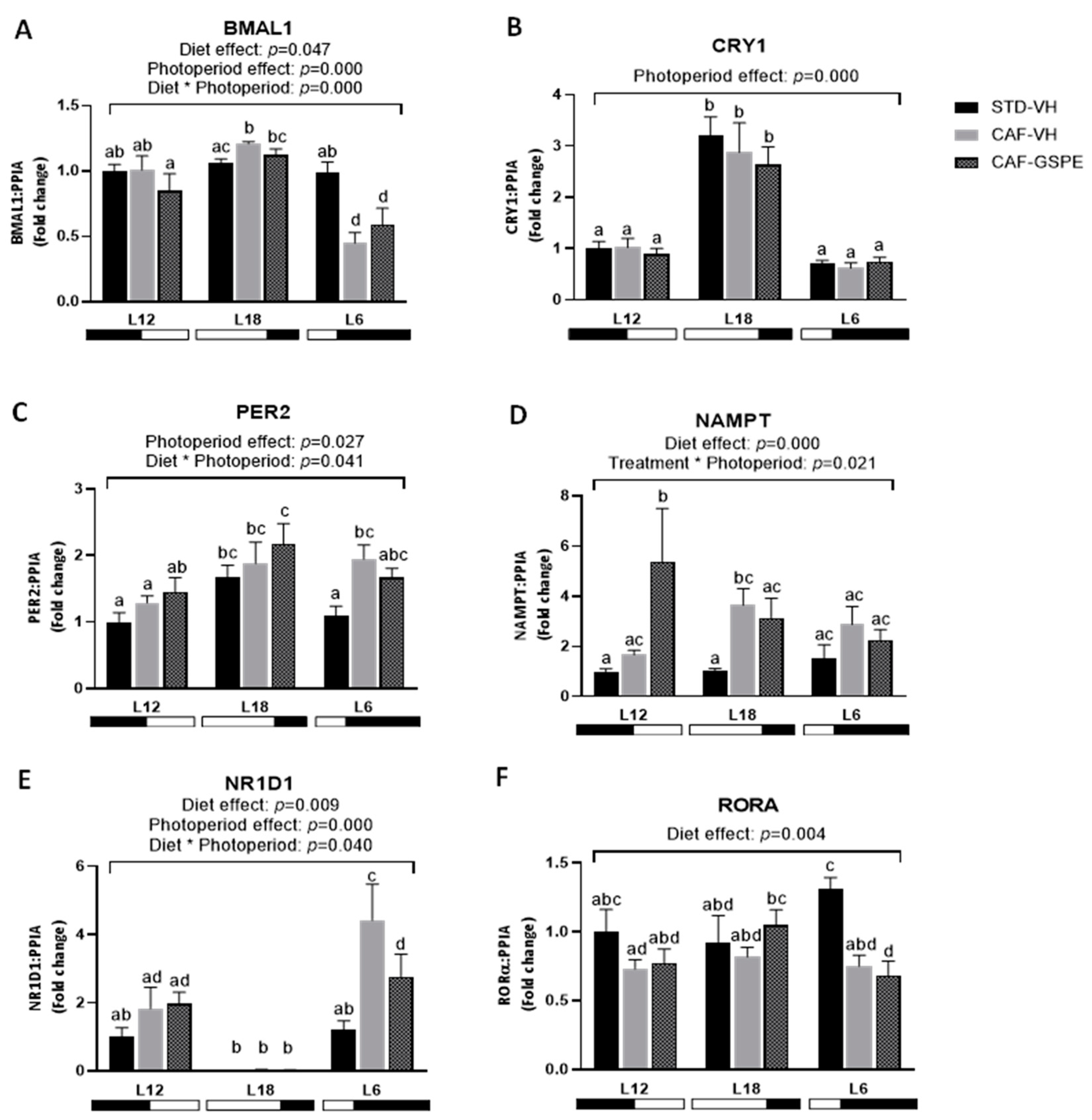
2.4. Exposure to Either Long or Short Photoperiods Alters the Expression of Clock Genes in the Livers of Obese Rats
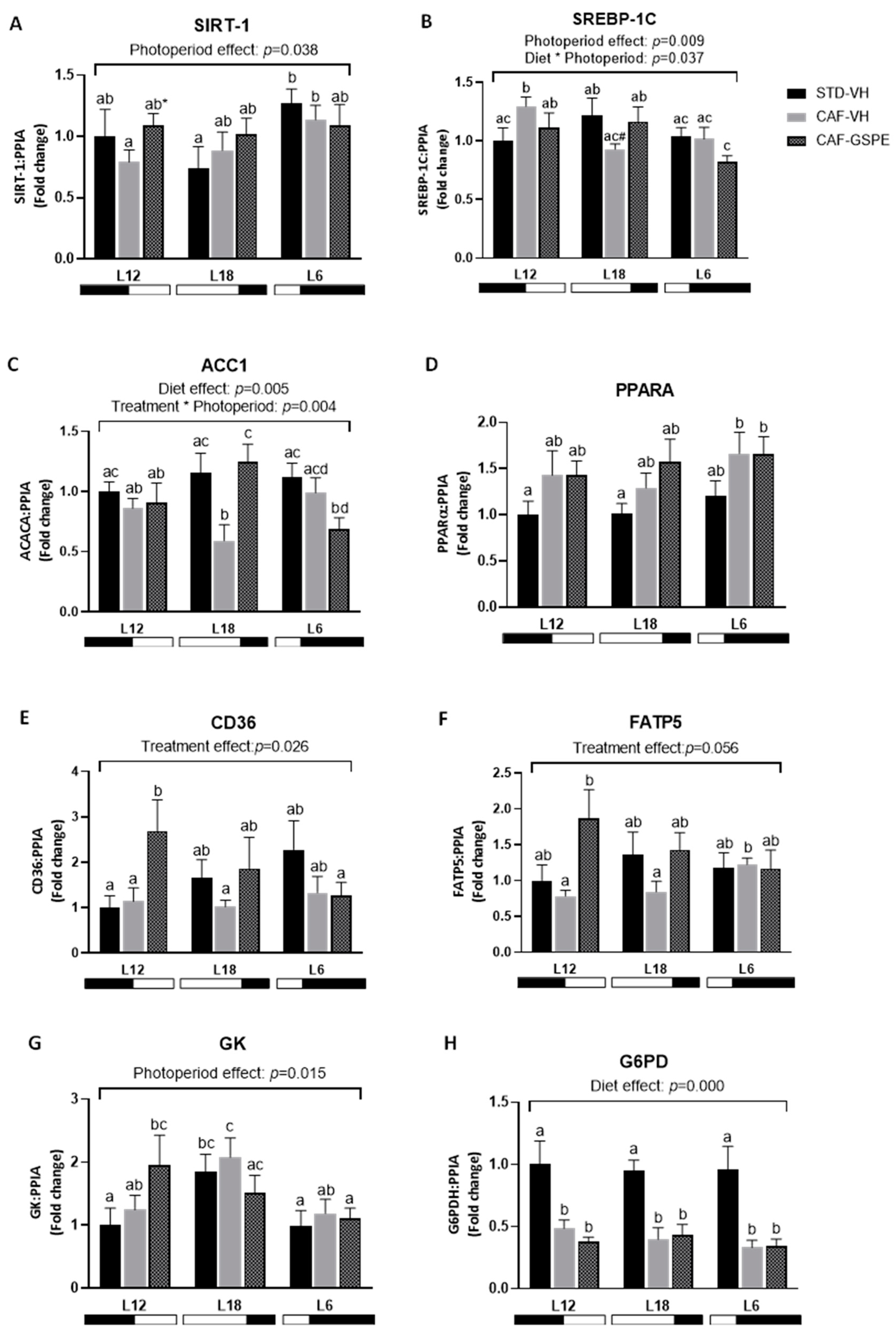
2.5. The Effect of GSPE on the Expression of Lipid- and Glucose-Related Genes in the Liver Is Influenced by CAF Dietary Intake and Chronic Exposure to Different Photoperiods
3. Discussion
4. Materials and Methods
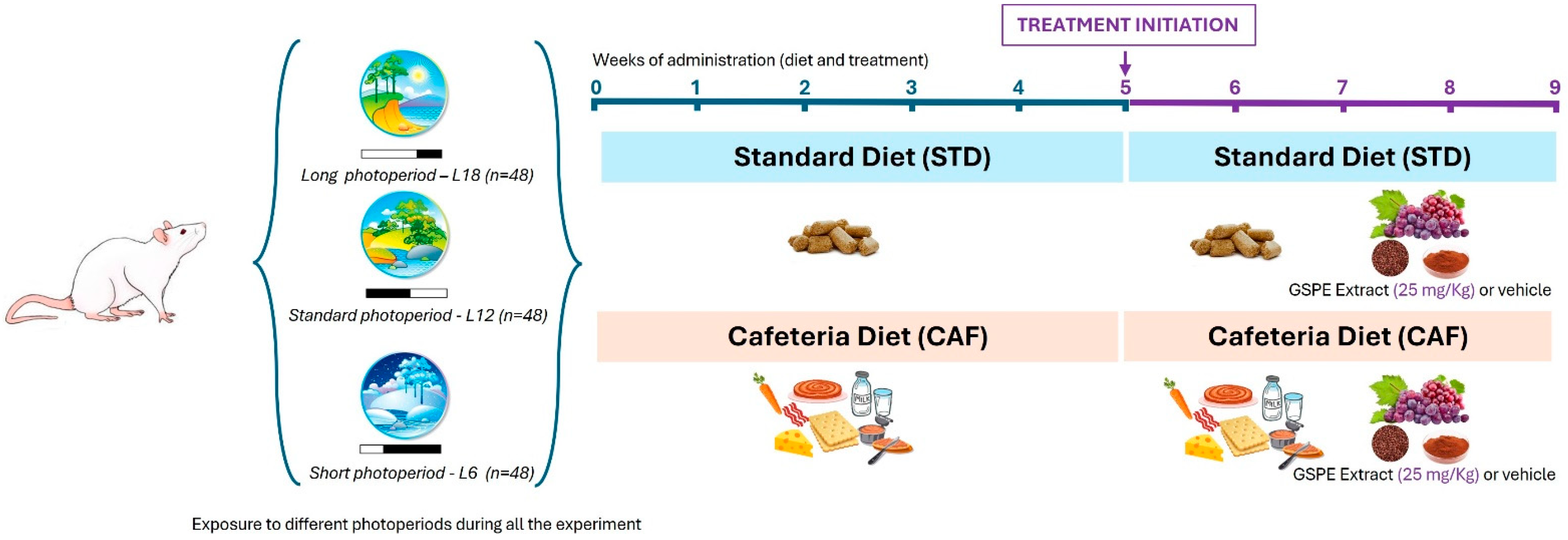
4.1. Animal Handling
4.2. Serum Analysis
4.3. Gene Expression Analysis
4.4. Liver Lipid Profile
4.5. Serum Hormones Quantification
4.6. Metabolome Analysis by GC-MS in Rat Serum and Liver Samples
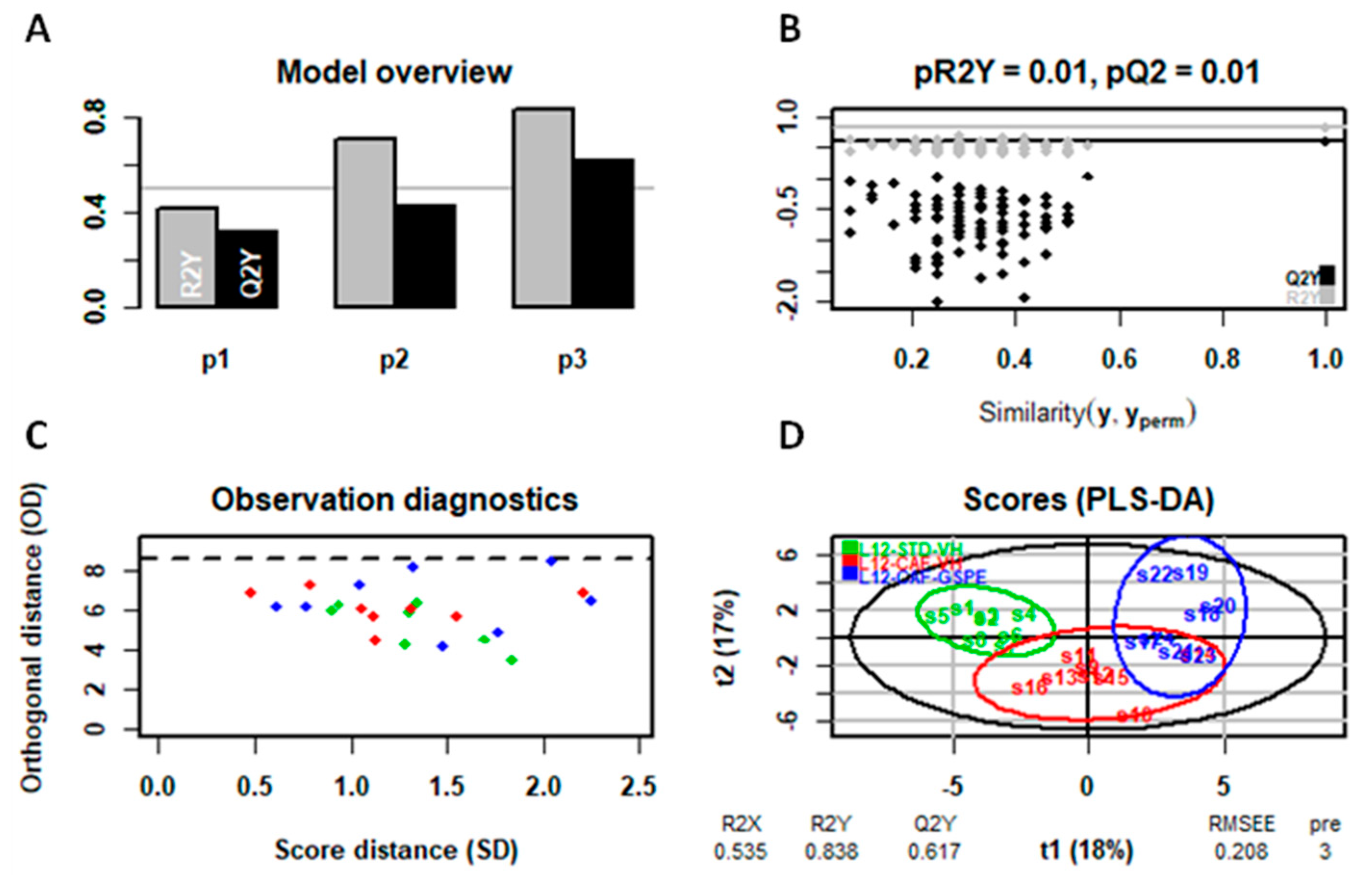
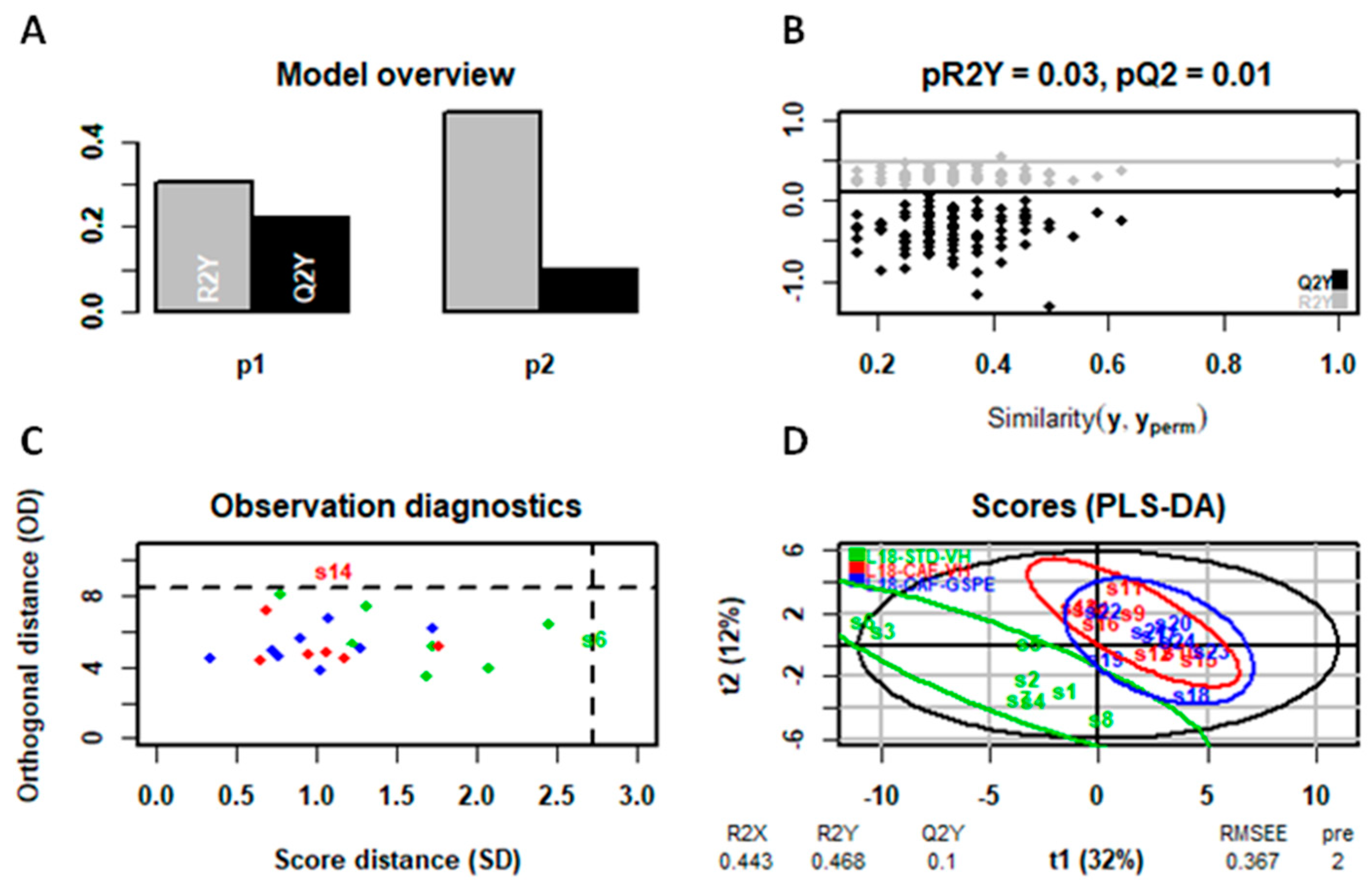
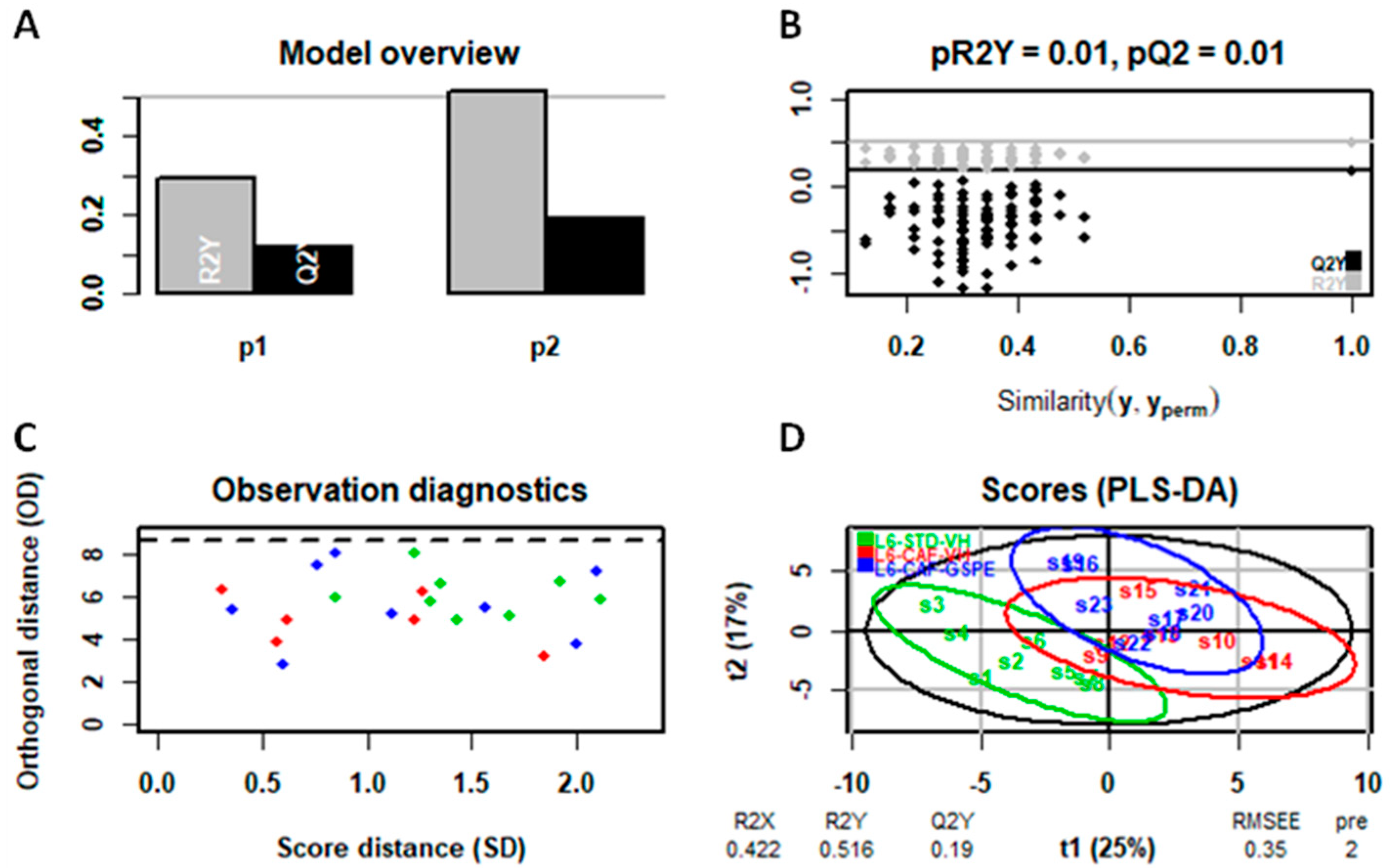
4.7. Statistical Analysis of Serum and Liver Metabolomic Assays
4.8. General Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cretenet, G.; Le Clech, M.; Gachon, F. Circadian Clock-Coordinated 12 Hr Period Rhythmic Activation of the IRE1α Pathway Controls Lipid Metabolism in Mouse Liver. Cell Metab. 2010, 11, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; Bhagat, S.; Bloss, E.B.; Morrison, J.H.; McEwen, B.S. Disruption of Circadian Clocks Has Ramifications for Metabolism, Brain, and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Ayyar, V.S.; Sukumaran, S. Circadian Rhythms: Influence on Physiology, Pharmacology, and Therapeutic Interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mishra, I. Circannual Rhythms. Encycl. Reprod. 2018, 1, 442–450. [Google Scholar] [CrossRef]
- Challet, E. Circadian Clocks, Food Intake, and Metabolism. Prog. Mol. Biol. Transl. Sci. 2013, 119, 105–135. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B. The Central Circadian Timing System. Curr. Opin. Neurobiol. 2013, 23, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, M.; Wang, X.; Jiang, S.H.; Hu, L.P.; Zhang, X.L.; Zhang, Z.G. Signalling Entrains the Peripheral Circadian Clock. Cell Signal 2020, 69, 109433. [Google Scholar] [CrossRef]
- Grosjean, E.; Simonneaux, V.; Challet, E. Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology 2023, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of Altered Photoperiod on Circadian Clock and Lipid Metabolism in Rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. Intake of an Obesogenic Cafeteria Diet Affects Body Weight, Feeding Behavior, and Glucose and Lipid Metabolism in a Photoperiod-Dependent Manner in F344 Rats. Front. Physiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. The Exposure to Different Photoperiods Strongly Modulates the Glucose and Lipid Metabolisms of Normoweight Fischer 344 Rats. Front. Physiol. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.; Tsai, Y.C.; Hwang, K.; Huang, Y.W.; Tzeng, J.E. Repeated Light-Dark Shifts Speed up Body Weight Gain in Male F344 Rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Abeliansky, A.L.; Strulik, H. Season of Birth, Health and Aging. Econ. Hum. Biol. 2020, 36, 100812. [Google Scholar] [CrossRef] [PubMed]
- Turtinen, M.; Härkönen, T.; Ilonen, J.; Parkkola, A.; Knip, M.; Knip, M.; Groop, P.H.; Ilonen, J.; Otonkoski, T.; Veijola, R.; et al. Seasonality in the Manifestation of Type 1 Diabetes Varies According to Age at Diagnosis in Finnish Children. Acta Paediatr. 2022, 111, 1061–1069. [Google Scholar] [CrossRef]
- Kuzmenko, N.V.; Shchegolev, B.F. Dependence of Seasonal Dynamics in Healthy People’s Circulating Lipids and Carbohydrates on Regional Climate: Meta-Analysis. Indian J. Clin. Biochem. 2022, 37, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hoshide, S.; Kario, K. Seasonal Variation in Blood Pressure: Current Evidence and Recommendations for Hypertension Management. Hypertens. Res. 2021, 44, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Lazar, M.A. Interconnections between Circadian Clocks and Metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016, 7, 399. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-Alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of Circadian Disruption on Glucose Metabolism: Implications for Type 2 Diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.V.M.; Demir, M.; Oster, H. The Role of the Circadian Clock in the Development, Progression, and Treatment of Non-Alcoholic Fatty Liver Disease. Acta Physiol. 2023, 237, e13915. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Chronic Consumption of Dietary Proanthocyanidins Modulates Peripheral Clocks in Healthy and Obese Rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Modulate Melatonin Levels in Plasma and the Expression Pattern of Clock Genes in the Hypothalamus of Rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic Compounds and Biological Rhythms: Who Takes the Lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Soliz-Rueda, J.R.; López-Fernández-Sobrino, R.; Bravo, F.I.; Aragonès, G.; Suarez, M.; Muguerza, B. Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats. Nutrients 2022, 14, 1834. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.M.; Colom-Pellicer, M.; Blanco, J.; Calvo, E.; Aragonès, G.; Mulero, M. Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats. Biomolecules 2022, 12, 839. [Google Scholar] [CrossRef]
- Sato, T.; Sato, S. Circadian Regulation of Metabolism: Commitment to Health and Diseases. Endocrinology 2023, 164, bqad086. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Crescenti, A.; Salvadó, M.J. Consumption of Cherry out of Season Changes White Adipose Tissue Gene Expression and Morphology to a Phenotype Prone to Fat Accumulation. Nutrients 2018, 10, 1102. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone: A Metabolic Hormone in Health and Disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Maria Pérez-Vendrell, A.; Teresa Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. A Specific Dose of Grape Seed-Derived Proanthocyanidins to Inhibit Body Weight Gain Limits Food Intake and Increases Energy Expenditure in Rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape Seed Proanthocyanidin Supplementation Reduces Adipocyte Size and Increases Adipocyte Number in Obese Rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Bladé, C.; Suárez, M.; Arola-Arnal, A. Grape Seed Proanthocyanidins Improve White Adipose Tissue Expansion during Diet-Induced Obesity Development in Rats. Int. J. Mol. Sci. 2018, 19, 2632. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic Administration of Grape-Seed Polyphenols Attenuates the Development of Hypertension and Improves Other Cardiometabolic Risk Factors Associated with the Metabolic Syndrome in Cafeteria Diet-Fed Rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef]
- Chao, H.W.; Chao, S.W.; Lin, H.; Ku, H.C.; Cheng, C.F. Homeostasis of Glucose and Lipid in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2019, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.W.; Russell, L.; Helfer, G.; Thomson, L.M.; Dalby, M.J.; Morgan, P.J. Photoperiod Regulates Lean Mass Accretion, but Not Adiposity, in Growing F344 Rats Fed a High Fat Diet. PLoS ONE 2015, 10, e0119763. [Google Scholar] [CrossRef] [PubMed]
- Mody, A.; Whitec, D.; Kanwal, F.; Garcia, J.M. Relevance of Low Testosterone to Non-Alcoholic Fatty Liver Disease. Cardiovasc. Endocrinol. 2015, 4, 83–89. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, H.; Park, J.H.; Cho, B.; Kim, D.; Oh, S.W.; Lee, C.M.; Choi, H.C. A Low Level of Serum Total Testosterone Is Independently Associated with Nonalcoholic Fatty Liver Disease. BMC Gastroenterol. 2012, 12, 69. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Franczyk, M.P.; Chondronikola, M.; Qi, N.; Gunawardana, S.C.; Stromsdorfer, K.L.; Porter, L.C.; Wozniak, D.F.; Sasaki, Y.; Rensing, N.; et al. Adipose Tissue NAD+ Biosynthesis Is Required for Regulating Adaptive Thermogenesis and Whole-Body Energy Homeostasis in Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 23822–23828. [Google Scholar] [CrossRef] [PubMed]
- Li, X. SIRT1 and Energy Metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in Cellular Functions: Role of Polyphenols. Arch. Biochem. Biophys. 2010, 501, 79. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Boost Hepatic NAD + Metabolism and SIRT1 Expression and Activity in a Dose-Dependent Manner in Healthy Rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Buonvicino, D.; Ranieri, G.; Pittelli, M.; Lapucci, A.; Bragliola, S.; Chiarugi, A. SIRT1-Dependent Restoration of NAD+ Homeostasis after Increased Extracellular NAD+ Exposure. J. Biol. Chem. 2021, 297, 100855–100856. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. Targeting Sirtuin 1 to Improve Metabolism: All You Need Is NAD+? Pharmacol. Rev. 2012, 64, 166–187. [Google Scholar] [CrossRef]
- Carlson, L.A.; ROSENHAMER, G. Reduction of Mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by Combined Treatment with Clofibrate and Nicotinic Acid. Acta Med. Scand. 1988, 223, 405–418. [Google Scholar] [CrossRef]
- Taylor, A.J.; Sullenberger, L.E.; Lee, H.J.; Lee, J.K.; Grace, K.A. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: A Double-Blind, Placebo-Controlled Study of Extended-Release Niacin on Atherosclerosis Progression in Secondary Prevention Patients Treated with Statins. Circulation 2004, 110, 3512–3517. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, C.; Hu, K.; Greenberg, A.; Wu, D.; Ausman, L.M.; McBurney, M.W.; Wang, X.D. Ablation of Systemic SIRT1 Activity Promotes Nonalcoholic Fatty Liver Disease by Affecting Liver-Mesenteric Adipose Tissue Fatty Acid Mobilization. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, W.G.; Lu, P.; Miller, T.M.; Poloyac, S.M.; Eyre, N.S.; Mayrhofer, G.; Xu, M.; Ren, S.; Xie, W. Hepatic Overexpression of CD36 Improves Glycogen Homeostasis and Attenuates High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance. Mol. Cell Biol. 2016, 36, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Enooku, K.; Tsutsumi, T.; Kondo, M.; Fujiwara, N.; Sasako, T.; Shibahara, J.; Kado, A.; Okushin, K.; Fujinaga, H.; Nakagomi, R.; et al. Hepatic FATP5 Expression Is Associated with Histological Progression and Loss of Hepatic Fat in NAFLD Patients. J. Gastroenterol. 2020, 55, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Gerhart-Hines, Z.; Lazar, M.A. Rev-Erbα and the Circadian Transcriptional Regulation of Metabolism. Diabetes Obes. Metab. 2015, 17, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-Driven and Oscillator-Dependent Circadian Transcription in Mice with a Conditionally Active Liver Clock. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Raspé, E.; Duez, H.; Mansén, A.; Fontaine, C.; Fiévet, C.; Fruchart, J.C.; Vennström, B.; Staels, B. Identification of Rev-Erbα as a Physiological Repressor of ApoC-III Gene Transcription. J. Lipid Res. 2002, 43, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, X.; Arthurs, B.; Guha, A.; Rui, L.; Kamath, A.; Inoki, K.; Yin, L. Liver Clock Protein BMAL1 Promotes de Novo Lipogenesis through Insulin-MTORC2-AKT Signaling. J. Biol. Chem. 2014, 289, 25925–25935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, X.; Nelson, B.B.; Jin, E.; Sit, J.; Charney, N.; Yang, M.; Omary, M.B.; Yin, L. The Hepatic BMAL1/AKT/Lipogenesis Axis Protects against Alcoholic Liver Disease in Mice via Promoting PPARα Pathway. Hepatology 2018, 68, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Eckel-Mahan, K.L. Circadian Metabolomics in Time and Space. Front. Neurosci. 2017, 11, 280159. [Google Scholar] [CrossRef]
- Ch, R.; Chevallier, O.; Elliott, C.T. Metabolomics Reveal Circadian Control of Cellular Metabolism. TrAC Trends Anal. Chem. 2020, 130, 115986. [Google Scholar] [CrossRef]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of Circadian- and Behavior-Driven Metabolite Rhythms in Humans Provides a Window on Peripheral Oscillators and Metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef] [PubMed]
- Colom-Pellicer, M.; Rodríguez, R.M.; Soliz-Rueda, J.R.; Vinícius, L.; De Assis, M.; Navarro-Masip, È.; Quesada-Vázquez, S.; Escoté, X.; Oster, H.; Mulero, M.; et al. Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption. Nutrients 2022, 14, 2246. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, A.; Filser, J.; Lenz, R.; Bertrand, C.; Charrier, M. Adjustment of Metabolite Composition in the Haemolymph to Seasonal Variations in the Land Snail Helix Pomatia. J. Comp. Physiol. B 2011, 181, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Vesala, L.; Salminen, T.S.; Kostál, V.; Zahradníĉková, H.; Hoikkala, A. Myo-Inositol as a Main Metabolite in Overwintering Flies: Seasonal Metabolomic Profiles and Cold Stress Tolerance in a Northern Drosophilid Fly. J. Exp. Biol. 2012, 215, 2891–2897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- do Nascimento, T.G.; dos Santos Arruda, R.E.; da Cruz Almeida, E.T.; dos Santos Oliveira, J.M.; Basílio-Júnior, I.D.; Celerino de Moraes Porto, I.C.; Rodrigues Sabino, A.; Tonholo, J.; Gray, A.; Ebel, R.A.E.; et al. Comprehensive Multivariate Correlations between Climatic Effect, Metabolite-Profile, Antioxidant Capacity and Antibacterial Activity of Brazilian Red Propolis Metabolites during Seasonal Study. Sci. Rep. 2019, 9, 18293. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythm. 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Jordan, H.; Martín-González, M.Z.; Suárez, M.; Aragonès, G.; Mugureza, B.; Rodríguez, M.A.; Bladé, C. The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats. Nutrients 2020, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and Hydroxyproline Metabolism: Implications for Animal and Human Nutrition. Amino Acids 2011, 40, 1053. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Melnikova, A.K.; Saso, L.; Schmalhausen, E.V. Influence of Oxidative Stress on Catalytic and Non-Glycolytic Functions of Glyceraldehyde-3-Phosphate Dehydrogenase. Curr. Med. Chem. 2018, 27, 2040–2058. [Google Scholar] [CrossRef]
- Gil, A.; Siegel, D.; Permentier, H.; Reijngoud, D.J.; Dekker, F.; Bischoff, R. Stability of Energy Metabolites—An Often Overlooked Issue in Metabolomics Studies: A Review. Electrophoresis 2015, 36, 2156–2169. [Google Scholar] [CrossRef]
- Kleczkowski, L.A.; Igamberdiev, A.U. Multiple Roles of Glycerate Kinase—From Photorespiration to Gluconeogenesis, C4 Metabolism, and Plant Immunity. Int. J. Mol. Sci. 2024, 25, 3258. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Leong, J.; Jialal, I. Amino Acid Levels in Nascent Metabolic Syndrome: A Contributor to the pro-Inflammatory Burden. J. Diabetes Complicat. 2018, 32, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Römisch-Margl, W.; et al. Childhood Obesity Is Associated with Changes in the Serum Metabolite Profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hong, Y.; Gu, Y.; Ye, S.; Hu, K.; Yao, J.; Ding, K.; Zhao, A.; Jia, W.; Li, H. Functional Metabolomics Reveals That Astragalus Polysaccharides Improve Lipids Metabolism through Microbial Metabolite 2-Hydroxybutyric Acid in Obese Mice. Engineering 2022, 9, 111–122. [Google Scholar] [CrossRef]
- Stojanovic, V.; Ihle, S. Role of Beta-Hydroxybutyric Acid in Diabetic Ketoacidosis: A Review. Can. Vet. J. 2011, 52, 426. [Google Scholar] [PubMed]
- Arora, S.; Menchine, M. The Role of Point-of-Care β-Hydroxybutyrate Testing in the Diagnosis of Diabetic Ketoacidosis: A Review. Hosp. Pract. 2012, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Vice, E.; Privette, J.D.; Hickner, R.C.; Barakat, H.A. Ketone Body Metabolism in Lean and Obese Women. Metabolism 2005, 54, 1542–1545. [Google Scholar] [CrossRef]
- Liu, T.T.; He, X.R.; Xu, R.X.; Wu, X.B.; Qi, Y.X.; Huang, J.Z.; Chen, Q.H.; Chen, Q.X. Inhibitory Mechanism and Molecular Analysis of Furoic Acid and Oxalic Acid on Lipase. Int. J. Biol. Macromol. 2018, 120, 1925–1934. [Google Scholar] [CrossRef]
- Annandale, M.; Daniels, L.J.; Li, X.; Neale, J.P.H.; Chau, A.H.L.; Ambalawanar, H.A.; James, S.L.; Koutsifeli, P.; Delbridge, L.M.D.; Mellor, K.M. Fructose Metabolism and Cardiac Metabolic Stress. Front. Pharmacol. 2021, 12, 695486. [Google Scholar] [CrossRef]
- Kawasaki, T.; Akanuma, H.; Yamanouchi, T. Increased Fructose Concentrations in Blood and Urine in Patients with Diabetes. Diabetes Care 2002, 25, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; MacI, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of Procyanidin Dimers and Trimers and Matrix Food Effects in in Vitro and in Vivo Models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.M.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Feillet-Coudray, C.; Casas, F.; Colom-Pellicer, M.; Aragonès, G.; Avila-Román, J.; Muguerza, B.; Mulero, M.; et al. Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats. Nutrients 2022, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F.; Thomasen, T.K. Evaluation of the Bligh & Dyer Lipid Determination Method. Mar. Pollut. Bull. 1996, 32, 681–688. [Google Scholar] [CrossRef]
- Llorach-Asunción, R.; Jauregui, O.; Urpi-Sarda, M.; Andres-Lacueva, C. Methodological Aspects for Metabolome Visualization and Characterization: A Metabolomic Evaluation of the 24 h Evolution of Human Urine after Cocoa Powder Consumption. J. Pharm. Biomed. Anal. 2010, 51, 373–381. [Google Scholar] [CrossRef]
| Parameters | L12 | L18 | L6 | 2wA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| STD-VH | CAF-VH | CAF-GSPE | STD-VH | CAF-VH | CAF-GSPE | STD-VH | CAF-VH | CAF-GSPE | ||
| Cholesterol (mg/g) | 1.19 ± 0.1 a | 1.63 ± 0.1 b | 1.84 ± 0.1 bc | 1.04 ± 0.1 a | 2.05 ± 0.1 c | 2.05 ± 0.2 c | 1.14 ± 0.1 a | 1.78 ± 0.1 bc | 2.01 ± 0.3 bc | D. D*P |
| Triglycerides (mg/g) | 2.62 ± 0.2 a | 5.18 ± 0.4 b | 4.84 ± 0.4 b | 3.44 ± 0.3 a | 6.75 ± 0.4 c | 6.93 ± 0.4 c | 3.68 ± 0.3 a | 6.90 ± 0.5 c | 6.92 ± 0.7 c | D. P |
| Phospholipids (mg/g) | 6.82 ± 0.3 ab | 5.94 ± 0.3 a | 6.36 ± 0.4 a | 6.30 ± 0.3 a | 7.06 ± 0.4 ab* | 7.06 ± 0.3 ab | 6.92 ± 0.3 ab | 7.16 ± 0.7 ab# | 7.89 ± 0.6 b | P |
| Liver weight (g) | 14.78 ± 0.5 a | 20.3 ± 1.4 bc | 18.64 ± 0.6 bc | 15.21 ± 0.7 a | 20.01 ± 0.5 c | 17.53 ± 0.7 b | 15.60 ± 0.5 ad | 19.23 ± 1.5 bc | 17.91 ± 1.3 bcd | D |
| Metabolite | STD-VH | CAF-VH | CAF-GSPE | p-Value | STD-VH vs. CAF-VH | STD-VH vs. CAF-GSPE | CAF-VH vs. CAF-GSPE | VIP |
|---|---|---|---|---|---|---|---|---|
| 2-Hydroxyisobutyric acid | 1.83 ± 0.11 | 2.49 ± 0.08 | 3.09 ± 0.17 | <0.001 | 0.016 | <0.001 | 0.039 | 1.78 |
| Oleic acid | 2.2 ± 0.32 | 9.61 ± 1.13 | 6.9 ± 0.67 | <0.001 | <0.001 | 0.005 | 0.129 | 1.76 |
| Indole-3-propanoic acid | 1.7 ± 0.17 | 0.76 ± 0.18 | 0.33 ± 0.09 | 0.001 | 0.012 | <0.001 | 0.074 | 1.68 |
| Dodecanoic acid | 0.16 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.03 | 0.001 | 0.001 | 0.001 | 0.375 | 1.59 |
| Glycine | 5.32 ± 0.13 | 4.75 ± 0.09 | 4.6 ± 0.11 | 0.001 | 0.006 | 0.001 | 0.170 | 1.41 |
| Serine | 10.01 ± 0.32 | 12.89 ± 0.36 | 12.63 ± 0.42 | 0.001 | 0.001 | 0.002 | 0.458 | 1.57 |
| 2-HydroxyButyric acid | 0.6 ± 0.04 | 1.34 ± 0.36 | 2.08 ± 0.42 | 0.001 | 0.013 | 0.001 | 0.115 | 1.34 |
| Hippuric acid | 0.43 ± 0.09 | 0.09 ± 0.02 | 0.1 ± 0.03 | 0.002 | 0.002 | 0.003 | 0.500 | 1.43 |
| Pipecolic acid | 0.11 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.003 | 0.005 | 0.003 | 0.349 | 1.45 |
| Heptanoic | 0.23 ± 0.01 | 0.28 ± 0.01 | 0.32 ± 0.02 | 0.004 | 0.023 | 0.002 | 0.137 | 1.37 |
| Glycolic acid | 0.1 ± 0.01 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.006 | 0.013 | 0.004 | 0.251 | 1.38 |
| Threonic acid | 0.82 ± 0.09 | 1.27 ± 0.13 | 1.39 ± 0.13 | 0.009 | 0.010 | 0.007 | 0.362 | 1.20 |
| Glycerol | 0.9 ± 0.16 | 1.52 ± 0.08 | 1.44 ± 0.15 | 0.014 | 0.009 | 0.019 | 0.298 | 1.28 |
| 3-Hydroxyisovaleric acid | 0.37 ± 0.02 | 0.44 ± 0.03 | 0.49 ± 0.03 | 0.016 | 0.050 | 0.007 | 0.161 | 1.27 |
| Proline | 23.86 ± 3.30 | 33.98 ± 2.12 | 26.32 ± 1.58 | 0.019 | 0.018 | 0.444 | 0.013 | 1.18 |
| 3-hydroxybutyric acid | 8.78 ± 0.69 | 13.41 ± 1.17 | 15.42 ± 2.37 | 0.019 | 0.011 | 0.022 | 0.500 | 1.10 |
| 3-Phosphoglyceric acid | 0.02 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.024 | 0.011 | 0.179 | 0.050 | 1.36 |
| Valine | 10.23 ± 0.35 | 9.71 ± 0.31 | 9 ± 0.30 | 0.039 | 0.122 | 0.016 | 0.126 | 1.14 |
| Glucose | 29.87 ± 1.18 | 31.52 ± 1.4 | 34.74 ± 1.18 | 0.044 | 0.179 | 0.020 | 0.090 | 1.14 |
| Metabolite | STD-VH | CAF-VH | CAF-GSPE | p-Value | STD-VH vs. CAF-VH | STD-VH vs. CAF-GSPE | CAF-VH vs. CAF-GSPE | VIP |
|---|---|---|---|---|---|---|---|---|
| 2-HydroxyButyric acid | 0.52 ± 0.02 | 2.07 ± 0.28 | 1.23 ± 0.15 | <0.001 | <0.001 | 0.006 | 0.069 | 1.73 |
| Glycolic acid | 0.09 ± 0.00 | 0.13 ± 0.01 | 0.12 ± 0.01 | <0.001 | <0.001 | 0.003 | 0.161 | 1.51 |
| d-Sucrose | 0.04 ± 0.01 | 0.37 ± 0.11 | 0.66 ± 0.17 | 0.001 | 0.005 | <0.001 | 0.153 | 1.31 |
| Dodecanoic acid | 0.17 ± 0.00 | 0.29 ± 0.04 | 0.35 ± 0.04 | 0.001 | 0.003 | 0.001 | 0.274 | 1.23 |
| Oleic acid | 3.15 ± 0.35 | 6.92 ± 1.06 | 8.91 ± 0.87 | 0.001 | 0.007 | <0.001 | 0.137 | 1.44 |
| Glycerol | 1.07 ± 0.08 | 1.67 ± 0.11 | 1.64 ± 0.11 | 0.001 | 0.001 | 0.002 | 0.336 | 1.34 |
| Threonic acid | 0.68 ± 0.06 | 1.47 ± 0.24 | 1.53 ± 0.19 | 0.001 | 0.002 | 0.001 | 0.336 | 1.22 |
| Serine | 10.17 ± 0.23 | 13.34 ± 0.70 | 13.9 ± 0.33 | 0.001 | 0.007 | 0.001 | 0.161 | 1.47 |
| 2-Hydroxyisobutyric acid | 1.78 ± 0.16 | 3.05 ± 0.19 | 2.65 ± 0.25 | 0.002 | 0.001 | 0.010 | 0.161 | 1.39 |
| Hippuric acid | 0.53 ± 0.09 | 0.1 ± 0.02 | 0.12 ± 0.03 | 0.002 | 0.003 | 0.003 | 0.402 | 1.45 |
| Indole-3-propanoic acid | 1.49 ± 0.25 | 0.38 ± 0.08 | 0.71 ± 0.31 | 0.005 | 0.004 | 0.007 | 0.323 | 1.15 |
| Pyruvic acid | 17.86 ± 1.19 | 24.24 ± 0.80 | 23.07 ± 1.65 | 0.006 | 0.003 | 0.021 | 0.179 | 1.22 |
| Fumaric acid | 1.19 ± 0.11 | 2.22 ± 0.35 | 2.86 ± 0.53 | 0.007 | 0.016 | 0.004 | 0.240 | 1.17 |
| Pipecolic acid | 0.11 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.010 | 0.015 | 0.006 | 0.298 | 1.23 |
| Leucine | 7.82 ± 0.14 | 6.94 ± 0.26 | 8.18 ± 0.30 | 0.011 | 0.054 | 0.122 | 0.005 | 1.66 |
| 3-Hydroxyisovaleric acid | 0.34 ± 0.02 | 0.42 ± 0.01 | 0.41 ± 0.02 | 0.013 | 0.006 | 0.033 | 0.198 | 1.11 |
| Methionine | 3.73 ± 0.09 | 3.32 ± 0.10 | 3.61 ± 0.05 | 0.013 | 0.006 | 0.198 | 0.033 | 1.48 |
| Malic acid | 0.98 ± 0.11 | 1.49 ± 0.22 | 2.02 ± 0.33 | 0.014 | 0.058 | 0.006 | 0.129 | 1.18 |
| 4-hydroxyPhenyllactic acid | 23.56 ± 5.77 | 38.09 ± 5.54 | 52.31 ± 7.62 | 0.014 | 0.058 | 0.006 | 0.129 | 1.22 |
| Valine | 9.65 ± 0.22 | 8.46 ± 0.26 | 9.67 ± 0.42 | 0.016 | 0.010 | 0.486 | 0.018 | 1.41 |
| Oxoproline | 122.6 ± 4.81 | 138.36 ± 4.8 | 147.59 ± 7.23 | 0.024 | 0.042 | 0.012 | 0.229 | 1.10 |
| 3-hydroxybutyric acid | 10.25 ± 0.86 | 18.3 ± 2.32 | 13.99 ± 2.11 | 0.027 | 0.011 | 0.108 | 0.110 | 1.18 |
| Isoleucine | 5.12 ± 0.13 | 4.72 ± 0.22 | 5.6 ± 0.24 | 0.035 | 0.129 | 0.110 | 0.015 | 1.48 |
| Phenylalanine | 4.25 ± 0.09 | 3.75 ± 0.12 | 4.02 ± 0.10 | 0.038 | 0.016 | 0.145 | 0.103 | 1.31 |
| Metabolite | STD-VH | CAF-VH | CAF-GSPE | p-Value | STD-VH vs. CAF-VH | STD-VH vs. CAF-GSPE | CAF-VH vs. CAF-GSPE | VIP |
|---|---|---|---|---|---|---|---|---|
| Oleic acid | 2.34 ± 0.38 | 7.92 ± 0.90 | 5.5 ± 0.47 | 0.001 | <0.001 | 0.005 | 0.116 | 1.77 |
| 2-HydroxyButyric acid | 0.59 ± 0.04 | 1.32 ± 0.23 | 2.16 ± 0.28 | 0.001 | 0.297 | <0.001 | 0.046 | 1.57 |
| Glycolic acid | 0.1 ± 0.00 | 0.11 ± 0.00 | 0.13 ± 0.00 | 0.001 | 0.042 | <0.001 | 0.037 | 1.64 |
| d-Sucrose | 0.02 ± 0 | 0.4 ± 0.14 | 0.41 ± 0.17 | 0.001 | 0.002 | 0.001 | 0.406 | 1.09 |
| d-Fructose | 0.05 ± 0.00 | 0.13 ± 0.05 | 0.05 ± 0.00 | 0.002 | 0.001 | 0.290 | 0.003 | 1.21 |
| Dodecanoic acid | 0.16 ± 0.01 | 0.27 ± 0.02 | 0.25 ± 0.01 | 0.002 | 0.001 | 0.005 | 0.204 | 1.48 |
| Hippuric acid | 0.42 ± 0.08 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.002 | 0.050 | 0.001 | 0.045 | 1.61 |
| Serine | 9.87 ± 0.34 | 13.27 ± 0.69 | 13.4 ± 0.8 | 0.002 | 0.002 | 0.003 | 0.487 | 1.43 |
| Pipecolic acid | 0.1 ± 0.01 | 0.07 ± 0.00 | 0.05 ± 0.01 | 0.003 | 0.013 | 0.002 | 0.208 | 1.52 |
| Glycerol | 0.81 ± 0.07 | 1.58 ± 0.16 | 1.37 ± 0.14 | 0.006 | 0.004 | 0.011 | 0.243 | 1.43 |
| Glutamine | 0.18 ± 0.02 | 0.31 ± 0.07 | 0.39 ± 0.04 | 0.011 | 0.096 | 0.004 | 0.076 | 1.20 |
| 3-Hydroxyisovaleric acid | 0.4 ± 0.03 | 0.41 ± 0.03 | 0.51 ± 0.02 | 0.011 | 0.484 | 0.014 | 0.008 | 1.40 |
| Indole-3-propanoic acid | 1.33 ± 0.25 | 0.76 ± 0.15 | 0.44 ± 0.14 | 0.013 | 0.063 | 0.005 | 0.125 | 1.28 |
| Glucose | 29.83 ± 1.11 | 34.28 ± 0.95 | 36.23 ± 2.74 | 0.014 | 0.016 | 0.008 | 0.464 | 1.01 |
| Heptanoic | 0.24 ± 0.01 | 0.29 ± 0.02 | 0.33 ± 0.02 | 0.016 | 0.033 | 0.008 | 0.238 | 1.23 |
| 3-hydroxybutyric acid | 9.75 ± 0.97 | 11.73 ± 3.25 | 17.06 ± 1.50 | 0.020 | 0.467 | 0.022 | 0.014 | 1.27 |
| Threonine | 8.23 ± 0.26 | 10.06 ± 0.56 | 9.66 ± 0.55 | 0.026 | 0.016 | 0.028 | 0.289 | 1.13 |
| Lactic acid | 64.93 ± 2.27 | 74.75 ± 2.12 | 81.25 ± 6.33 | 0.036 | 0.027 | 0.027 | 0.420 | 1.06 |
| Methionine | 3.67 ± 0.10 | 3.52 ± 0.06 | 3.34 ± 0.07 | 0.038 | 0.183 | 0.018 | 0.086 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.M.; Colom-Pellicer, M.; Hernández-Baixauli, J.; Calvo, E.; Suárez, M.; Arola-Arnal, A.; Torres-Fuentes, C.; Aragonès, G.; Mulero, M. Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod. Int. J. Mol. Sci. 2024, 25, 7713. https://doi.org/10.3390/ijms25147713
Rodríguez RM, Colom-Pellicer M, Hernández-Baixauli J, Calvo E, Suárez M, Arola-Arnal A, Torres-Fuentes C, Aragonès G, Mulero M. Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod. International Journal of Molecular Sciences. 2024; 25(14):7713. https://doi.org/10.3390/ijms25147713
Chicago/Turabian StyleRodríguez, Romina M., Marina Colom-Pellicer, Julia Hernández-Baixauli, Enrique Calvo, Manuel Suárez, Anna Arola-Arnal, Cristina Torres-Fuentes, Gerard Aragonès, and Miquel Mulero. 2024. "Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod" International Journal of Molecular Sciences 25, no. 14: 7713. https://doi.org/10.3390/ijms25147713
APA StyleRodríguez, R. M., Colom-Pellicer, M., Hernández-Baixauli, J., Calvo, E., Suárez, M., Arola-Arnal, A., Torres-Fuentes, C., Aragonès, G., & Mulero, M. (2024). Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod. International Journal of Molecular Sciences, 25(14), 7713. https://doi.org/10.3390/ijms25147713











