Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. PD-1 Expression and Function: What Do We Know?
3. PD-1/PD-L1/PD-L2 in Central and Peripheral Tolerance
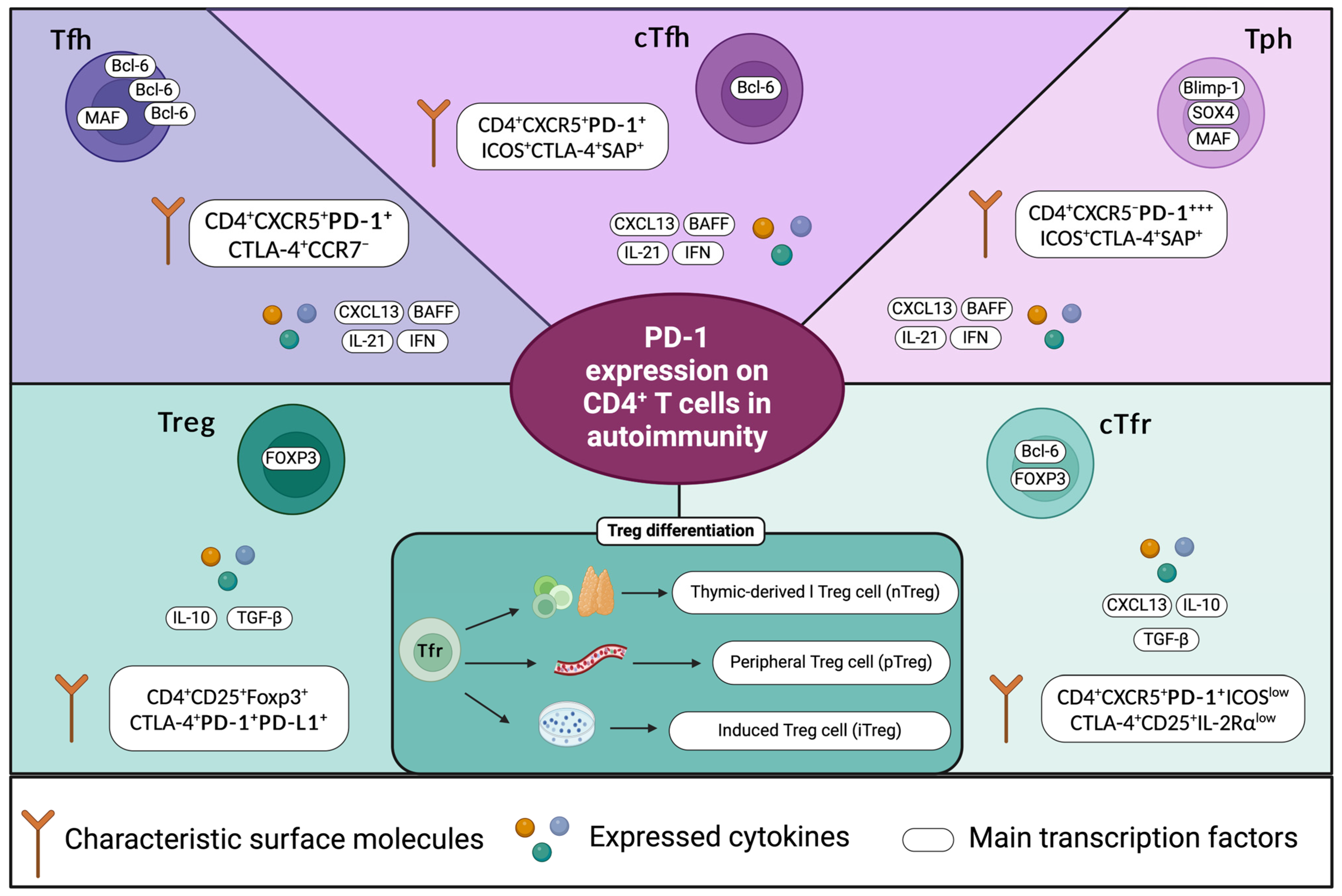
4. Regulation of PD-1 Expression and Its Relationship with the Autoimmune Response in SLE
4.1. Stimulation of PD-1 Expression
4.2. Repression of PD-1 Expression
5. PD-1 Ligands Emerged as a New and Important Focus of Study in the Field of SLE
6. Regulation of PD-L1 and PD-L2 Expression and Its Association with the Autoimmune Response
7. Is It Possible That PD-1/PD-L1/2 Has Another Important Role beyond Inhibition?
8. PD-1 as a Possible Therapeutic Target in SLE
8.1. Accessibility between PD-1 and Its Ligands
8.2. PD-1 Agonists
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fugger, L.; Jensen, L.T.; Rossjohn, J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell 2020, 181, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Reis, P.C.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Burke, K.P.; Patterson, D.G.; Liang, D.; Sharpe, A.H. Immune checkpoint receptors in autoimmunity. Curr. Opin. Immunol. 2023, 80, 102283. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, E.D.; Elpek, K.G.; Francisco, L.; Bronson, R.; Bellemare-Pelletier, A.; Sharpe, A.H.; Freeman, G.J.; Turley, S.J. Intestinal Tolerance Is Converted to Autoimmune Enteritis upon PD-1 Ligand Blockade. J. Immunol. 2009, 182, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A.; Menke, J.; Rabacal, W.A.; Schoen, F.J.; Sharpe, A.H.; Kelley, V.R. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. J. Immunol. 2008, 181, 2513–2521. [Google Scholar] [CrossRef]
- Salama, A.D.; Chitnis, T.; Imitola, J.; Ansari, M.J.I.; Akiba, H.; Tushima, F.; Azuma, M.; Yagita, H.; Sayegh, M.H.; Khoury, S.J. Critical Role of the Programmed Death-1 (PD-1) Pathway in Regulation of Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2003, 198, 71–78. [Google Scholar] [CrossRef]
- Ansari, M.J.I.; Salama, A.D.; Chitnis, T.; Smith, R.N.; Yagita, H.; Akiba, H.; Yamazaki, T.; Azuma, M.; Iwai, H.; Khoury, S.J.; et al. The Programmed Death-1 (PD-1) Pathway Regulates Autoimmune Diabetes in Nonobese Diabetic (NOD) Mice. J. Exp. Med. 2003, 198, 63–69. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Kroner, A.; Mehling, M.; Hemmer, B.; Rieckmann, P.; Toyka, K.V.; Mäurer, M.; Wiendl, H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann. Neurol. 2005, 58, 50–57. [Google Scholar] [CrossRef]
- Lee, Y.H.; Woo, J.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Association of programmed cell death 1 polymorphisms and systemic lupus erythematosus: A meta-analysis. Lupus 2009, 18, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gai, N.; Wang, L.; Liu, K.; Liu, X.-H.; Wei, L.-T.; Tian, T.; Li, S.-L.; Zheng, Y.; Deng, Y.-J.; et al. Meta-analysis of programmed cell death 1 polymorphisms with systemic lupus erythematosus risk. Oncotarget 2017, 8, 36885–36897. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C.; Kim, J.H.; Song, G.G. Meta-analysis of genetic polymorphisms in programmed cell death 1. Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z. Rheumatol. 2015, 74, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ba, X.; Han, L.; Wang, H.; Lin, W.; Chen, Z.; Tu, S. T peripheral helper cells in autoimmune diseases: What do we know? Front. Immunol. 2023, 14, 1145573. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1-deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 524474. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dermani, F.K.; Samadi, P.; Rahmani, G.; Kohlan, A.K.; Najafi, R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J. Cell. Physiol. 2019, 234, 1313–1325. [Google Scholar] [CrossRef]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Lenardo, M.J. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019, 216, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Catakovic, K.; Klieser, E.; Neureiter, D.; Geisberger, R. T cell exhaustion: From pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017, 15, 1. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Lee, Y.C.; Luh, F.; Kuo, C.N.; Chang, W.C.; Yen, Y. Development and clinical applications of cancer immunotherapy against PD-1 signaling pathway. J. Biomed. Sci. 2019, 26, 96. [Google Scholar]
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Honjo, T.; Minato, N. Facilitation of β Selection and Modification of Positive Selection in the Thymus of Pd-1–Deficient Mice. J. Exp. Med. 2000, 191, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Latchman, Y.E.; Freeman, G.J.; Sharpe, A.H. Programmed Death-1 (PD-1):PD-Ligand 1 Interactions Inhibit TCR-Mediated Positive Selection of Thymocytes. J. Immunol. 2005, 175, 7372. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Brown, I.; Marks, R.; Nishimura, H.; Honjo, T.; Gajewski, T.F. Absence of Programmed Death Receptor 1 Alters Thymic Development and Enhances Generation of CD4/CD8 Double-Negative TCR-Transgenic T Cells. J. Immunol. 2003, 171, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Policheni, A.N.; Teh, C.E.; Robbins, A.; Tuzlak, S.; Strasser, A.; Gray, D.H.D. PD-1 cooperates with AIRE-mediated tolerance to prevent lethal autoimmune disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2120149119. [Google Scholar] [CrossRef] [PubMed]
- Probst, H.C.; McCoy, K.; Okazaki, T.; Honjo, T.; Van Den Broek, M. Resting dendritic cells induce peripheral CD8+ T cell tolerance in vivo through PD-1 and CTLA-4. Nat. Immunol. 2005, 6, 280–286. [Google Scholar] [CrossRef]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 2023, 134, 102976. [Google Scholar] [CrossRef]
- Mesas-Fernández, A.; Bodner, E.; Hilke, F.J.; Meier, K.; Ghoreschi, K.; Solimani, F. Interleukin-21 in autoimmune and inflammatory skin diseases. Eur. J. Immunol. 2023, 53, e2250075. [Google Scholar] [CrossRef]
- Vogelzang, A.; McGuire, H.M.; Yu, D.; Sprent, J.; Mackay, C.R.; King, C. A Fundamental Role for Interleukin-21 in the Generation of T Follicular Helper Cells. Immunity 2008, 29, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.H.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, B.; Huang, N.; Mo, X.; Li, W.; Zhang, B.; Wei, B.; Gao, M.; Wang, Y.; Liu, X.; et al. Aberrant T cell subsets and cytokines expression profile in systemic lupus erythematosus. Clin. Rheumatol. 2018, 37, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, X.; Yu, Y.; Wan, W.; Lv, L.; Zou, H. Associations of circulating CXCR3–PD-1+CD4+T cells with disease activity of systemic lupus erythematosus. Mod. Rheumatol. 2019, 29, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lindwall, E.; Gauthier, C.; Lyman, J.; Spencer, N.; Alarakhia, A.; Fraser, A.; Ing, S.; Chen, M.; Webb-Detiege, T.; et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 2015, 24, 909–917. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ho, J.H.E.; Pasoto, S.G.; Bunin, V.; Kim, S.T.; Carrasco, S.; Borba, E.F.; Gonçalves, C.R.; Costa, P.R.; Kallas, E.G.; et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis Rheumatol. 2015, 67, 988–999. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, P.; Ma, L.; Shan, Y.; Jiang, Z.; Wang, J.; Jiang, Y. Increased Interleukin 21 and Follicular Helper T-like Cells and Reduced Interleukin 10+ B cells in Patients with New-onset Systemic Lupus Erythematosus. J. Rheumatol. 2014, 41, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Chi, S.; Wang, X.; Sun, J.; Zhang, Y. CD4+CXCR5+PD-1+ T Follicular Helper Cells Play a Pivotal Role in the Development of Rheumatoid Arthritis. Med. Sci. Monit. 2019, 25, 3032–3040. [Google Scholar] [CrossRef]
- Liu, R.; Wu, Q.; Su, D.; Che, N.; Chen, H.; Geng, L.; Chen, J.; Chen, W.; Li, X.; Sun, L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R255. [Google Scholar]
- Wang, X.; Yang, C.; Xu, F.; Qi, L.; Wang, J.; Yang, P. Imbalance of circulating Tfr/Tfh ratio in patients with rheumatoid arthritis. Clin. Exp. Med. 2019, 19, 55–64. [Google Scholar] [CrossRef]
- Fonseca, V.R.; Romão, V.C.; Agua-Doce, A.; Santos, M.; López-Presa, D.; Ferreira, A.C.; Fonseca, J.E.; Graca, L. The Ratio of Blood T Follicular Regulatory Cells to T Follicular Helper Cells Marks Ectopic Lymphoid Structure Formation While Activated Follicular Helper T Cells Indicate Disease Activity in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 774–784. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, J.; Hong, S.-M.; Lee, J.; Cho, M.-L.; Park, S.-H. Circulating CCR7loPD-1hi Follicular Helper T Cells Indicate Disease Activity and Glandular Inflammation in Patients with Primary Sjögren’s Syndrome. Immune Netw. 2019, 19, e26. [Google Scholar] [CrossRef]
- Pontarini, E.; Murray-Brown, W.J.; Croia, C.; Lucchesi, D.; Conway, J.; Rivellese, F.; Fossati-Jimack, L.; Astorri, E.; Prediletto, E.; Corsiero, E.; et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centres and MALT lymphoma. Ann. Rheum. Dis. 2020, 79, 1588–1599. [Google Scholar] [CrossRef]
- Szabó, K.; Papp, G.; Szántó, A.; Tarr, T.; Zeher, M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin. Exp. Immunol. 2015, 183, 76–89. [Google Scholar] [CrossRef]
- Szabó, K.; Jámbor, I.; Szántó, A.; Horváth, I.F.; Tarr, T.; Nakken, B.; Szodoray, P.; Papp, G. The Imbalance of Circulating Follicular T Helper Cell Subsets in Primary Sjögren’s Syndrome Associates With Serological Alterations and Abnormal B-Cell Distribution. Front. Immunol. 2021, 12, 639975. [Google Scholar] [CrossRef]
- Haque, R.; Kim, Y.; Park, K.; Jang, H.; Kim, S.Y.; Lee, H.; Kim, H.J. Altered distributions in circulating follicular helper and follicular regulatory T cells accountable for imbalanced cytokine production in multiple sclerosis. Clin. Exp. Immunol. 2021, 205, 75–88. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, C.; Wu, F.; Tao, L.; Zhang, C.; Zhao, D.; Yang, S.; Jiang, D.; Wang, J.; Sun, Y.; et al. T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Front. Immunol. 2018, 9, 348539. [Google Scholar] [CrossRef]
- Lin, J.; Yu, Y.; Ma, J.; Ren, C.; Chen, W. PD-1+CXCR5−CD4+T cells are correlated with the severity of systemic lupus erythematosus. Rheumatology 2019, 58, 2188–2192. [Google Scholar] [CrossRef]
- Makiyama, A.; Chiba, A.; Noto, D.; Murayama, G.; Yamaji, K.; Tamura, N.; Miyake, S. Expanded circulating peripheral helper T cells in systemic lupus erythematosus: Association with disease activity and B cell differentiation. Rheumatology 2019, 58, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Sagrero-Fabela, N.; Ortíz-Lazareno, P.C.; Salazar-Camarena, D.C.; Cruz, A.; Cerpa-Cruz, S.; Muñoz-Valle, J.F.; Marín-Rosales, M.; Alvarez-Gómez, J.A.; Palafox-Sánchez, C.A. BAFFR expression in circulating T follicular helper (CD4+CXCR5+PD-1+) and T peripheral helper (CD4+CXCR5−PD-1+) cells in systemic lupus erythematosus. Lupus 2023, 32, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Fu, P.; Guo, Y.; Fu, B.; Guo, Y.; Huang, Q.; Huang, Z.; Li, J. Increased TIGIT+PD-1+CXCR5-CD4+T cells are associated with disease activity in rheumatoid arthritis. Exp. Ther. Med. 2022, 24, 642. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Zeng, X.; Xia, C.; Xu, L.; Xu, Q.; Song, Y.; Liu, C. Circulating CD4+FoxP3–CXCR5–CXCR3+PD-1hi cells are elevated in active rheumatoid arthritis and reflect the severity of the disease. Int. J. Rheum. Dis. 2021, 24, 1032–1039. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Goodman, V.P.B.S.M.; Pernis, V.P.B.S.M.G.A.B.; Marshall, J.L.; Ivashkiv, L.T.D.A.B.P.L.B.; Karlson, E.W.; Nigrovic, L.A.H.P.A.; Filer, A.; Buckley, J.L.M.A.F.C.D.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Sakuragi, T.; Yamada, H.; Haraguchi, A.; Kai, K.; Fukushi, J.-I.; Ikemura, S.; Akasaki, Y.; Fujiwara, T.; Tsushima, H.; Tsutsui, T.; et al. Autoreactivity of Peripheral Helper T Cells in the Joints of Rheumatoid Arthritis. J. Immunol. 2021, 206, 2045–2051. [Google Scholar] [CrossRef]
- Chen, W.; Yang, F.; Lin, J. Tph Cells Expanded in Primary Sjögren’s Syndrome. Front. Med. 2022, 9, 900349. [Google Scholar] [CrossRef]
- Dupré, A.; Pascaud, J.; Rivière, E.; Paoletti, A.; Ly, B.; Mingueneau, M.; Mariette, X.; Nocturne, G. Association between T follicular helper cells and T peripheral helper cells with B-cell biomarkers and disease activity in primary Sjögren syndrome. RMD Open 2021, 7, e001442. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Way, S.S.; Abbas, A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2015, 16, 90–101. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human T H1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, X.; Zhou, X.; Wang, H.; Gu, L.; Ke, Y.; Zhang, M.; Ji, X.; Yang, X. Low expressions of PD-L1 and CTLA-4 by induced CD4+CD25+ Foxp3+ Tregs in patients with SLE and their correlation with the disease activity. Cytokine 2020, 133, 155119. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Castro Dopico, X.; Oliveira, J.J.; Rainbow, D.B.; Yang, J.H.; Trzupek, D.; Todd, S.A.; McNeill, M.; Steri, M.; Orrù, V.; et al. Chronic Immune Activation in Systemic Lupus Erythematosus and the Autoimmune PTPN22 Trp620 Risk Allele Drive the Expansion of FOXP3+ Regulatory T Cells and PD-1 Expression. Front. Immunol. 2019, 10, 2606. [Google Scholar] [CrossRef]
- Oestreich, K.J.; Yoon, H.; Ahmed, R.; Boss, J.M. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 2008, 181, 4832–4839. [Google Scholar] [CrossRef]
- Austin, J.W.; Lu, P.; Majumder, P.; Ahmed, R.; Boss, J.M. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J. Immunol. 2014, 192, 4876–4886. [Google Scholar] [CrossRef]
- Wang, G.; Tajima, M.; Honjo, T.; Ohta, A. STAT5 interferes with PD-1 transcriptional activation and affects CD8+ T-cell sensitivity to PD-1-dependent immunoregulation. Int. Immunol. 2021, 33, 563–572. [Google Scholar] [CrossRef]
- Terawaki, S.; Chikuma, S.; Shibayama, S.; Hayashi, T.; Yoshida, T.; Okazaki, T.; Honjo, T. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J. Immunol. 2011, 186, 2772–2779. [Google Scholar] [CrossRef]
- Xiao, G.; Deng, A.; Liu, H.; Ge, G.; Liu, X. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15419–15424. [Google Scholar] [CrossRef]
- Staron, M.M.; Gray, S.M.; Marshall, H.D.; Parish, I.A.; Chen, J.H.; Perry, C.J.; Cui, G.; Li, M.O.; Kaech, S.M. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 2014, 41, 802–814. [Google Scholar] [CrossRef]
- Chi, Z.; Lu, Y.; Yang, Y.; Li, B.; Lu, P. Transcriptional and epigenetic regulation of PD-1 expression. Cell. Mol. Life Sci. 2021, 78, 3239–3246. [Google Scholar] [CrossRef]
- Okada, M.; Chikuma, S.; Kondo, T.; Hibino, S.; Machiyama, H.; Yokosuka, T.; Nakano, M.; Yoshimura, A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017, 20, 1017–1028. [Google Scholar] [CrossRef]
- Pentcheva-Hoang, T.; Chen, L.; Pardoll, D.M.; Allison, J.P. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc. Natl. Acad. Sci. USA 2007, 104, 17765–17770. [Google Scholar] [CrossRef]
- Mathieu, M.; Cotta-Grand, N.; Daudelin, J.F.; Thébault, P.; Labrecque, N. Notch signaling regulates PD-1 expression during CD8+ T-cell activation. Immunol. Cell Biol. 2013, 91, 82–88. [Google Scholar] [CrossRef]
- Lu, P.; Youngblood, B.A.; Austin, J.W.; Mohammed, A.U.R.; Butler, R.; Ahmed, R.; Boss, J.M. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J. Exp. Med. 2014, 211, 515–527. [Google Scholar] [CrossRef]
- Kao, C.; Oestreich, K.J.; A Paley, M.; Crawford, A.; Angelosanto, J.M.; A Ali, M.-A.; Intlekofer, A.M.; Boss, J.M.; Reiner, S.L.; Weinmann, A.S.; et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011, 12, 663–671. [Google Scholar] [CrossRef]
- Shin, H.; Blackburn, S.D.; Intlekofer, A.M.; Kao, C.; Angelosanto, J.M.; Reiner, S.L.; Wherry, E.J. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 2009, 31, 309–320. [Google Scholar] [CrossRef]
- Shankar, E.M.; Che, K.F.; Messmer, D.; Lifson, J.D.; Larsson, M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol. Med. 2011, 17, 229–240. [Google Scholar] [CrossRef]
- Che, K.F.; Shankar, E.M.; Muthu, S.; Zandi, S.; Sigvardsson, M.; Hinkula, J.; Messmer, D.; Larsson, M. p38 mitogen-activated protein kinase/signal transducer and activator of transcription-3 pathway signaling regulates expression of inhibitory molecules in T Cells activated by HIV-1-exposed dendritic cells. Mol. Med. 2012, 18, 1169–1182. [Google Scholar] [CrossRef]
- Philips, E.A.; Garcia-España, A.; Tocheva, A.S.; Ahearn, I.M.; Adam, K.R.; Pan, R.; Mor, A.; Kong, X.-P. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. J. Biol. Chem. 2020, 295, 4372–4380. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef]
- Ishida, M.; Iwai, Y.; Tanaka, Y.; Okazaki, T.; Freeman, G.J.; Minato, N.; Honjo, T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 2002, 84, 57–62. [Google Scholar] [CrossRef]
- Ibañez-Vega, J.; Vilchez, C.; Jimenez, K.; Guevara, C.; Burgos, P.I.; Naves, R. Cellular and molecular regulation of the programmed death-1/programmed death ligand system and its role in multiple sclerosis and other autoimmune diseases. J. Autoimmun. 2021, 123, 102702. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Nie, X.; Chen, W.; Zhu, Y.; Huang, B.; Yu, W.; Wu, Z.; Guo, S.; Zhu, Y.; Luo, L.; Wang, S.; et al. B7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMb. Cell. Mol. Immunol. 2018, 15, 888–897. [Google Scholar] [CrossRef]
- Muraro, E.; Romanò, R.; Fanetti, G.; Vaccher, E.; Turturici, I.; Lupato, V.; La Torre, F.B.; Polesel, J.; Fratta, E.; Giacomarra, V.; et al. Tissue and circulating PD-L2: Moving from health and immune-mediated diseases to head and neck oncology. Crit. Rev. Oncol. Hematol. 2022, 175, 103707. [Google Scholar] [CrossRef]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef]
- Van Der Merwe, P.A.; Bodian, D.L.; Daenke, S.; Linsley, P.; Davis, S.J. CD80 (B7-1) Binds Both CD28 and CTLA-4 with a Low Affinity and Very Fast Kinetics. J. Exp. Med. 1997, 185, 393–403. [Google Scholar] [CrossRef]
- Butte, M.J.; Peña-Cruz, V.; Kim, M.J.; Freeman, G.J.; Sharpe, A.H. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008, 45, 3567–3572. [Google Scholar] [CrossRef]
- Sugiura, D.; Maruhashi, T.; Okazaki, I.M.; Shimizu, K.; Maeda, T.K.; Takemoto, T.; Okazaki, T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science 2019, 364, 558–566. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, C.K.; Lin, C.-H.; Gassen, R.B.; Xu, X.; Huang, Z.; Xiao, C.; Bonorino, C.; Lu, L.-F.; Bui, J.D.; et al. Article PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. Immunity 2019, 51, 1059–1073.e9. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Beyer, T.; Nirschl, L.; Farrenkopf, D.; Lößlein, L.; Vandrey, O.; Peter, A.; Tsaktanis, T.; Kebir, H.; Laplaud, D.; et al. PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Nat. Commun. 2023, 14, 5555. [Google Scholar] [CrossRef]
- Raptopoulou, A.P.; Bertsias, G.; Makrygiannakis, D.; Verginis, P.; Kritikos, I.; Tzardi, M.; Klareskog, L.; Catrina, A.I.; Sidiropoulos, P.; Boumpas, D.T. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010, 62, 1870–1880. [Google Scholar] [CrossRef]
- Sugiura, D.; Okazaki, I.-M.; Maeda, T.K.; Maruhashi, T.; Shimizu, K.; Arakaki, R.; Takemoto, T.; Ishimaru, N.; Okazaki, T. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat. Immunol. 2022, 23, 399–410. [Google Scholar] [CrossRef]
- Nielsen, C.; Ohm-Laursen, L.; Barington, T.; Husby, S.; Lillevang, S.T. Alternative splice variants of the human PD-1 gene. Cell. Immunol. 2005, 235, 109–116. [Google Scholar] [CrossRef]
- Gu, D.; Ao, X.; Yang, Y.; Chen, Z.; Xu, X. Soluble immune checkpoints in cancer: Production, function and biological significance. J. Immunother. Cancer 2018, 6, 132. [Google Scholar] [CrossRef]
- Király, Z.; Nagy, E.; Bokor, L.; Kovács, A.; Marschalkó, M.; Hidvégi, B. The Possible Clinical Significance of a Decreased Serum Level of Soluble PD-L1 in Discoid Lupus Erythematosus, but Not in Subacute Cutaneous Lupus Erythematosus—A Pilot Study. J. Clin. Med. 2023, 12, 5648. [Google Scholar] [CrossRef]
- Hirahara, S.; Katsumata, Y.; Kawasumi, H.; Kawaguchi, Y.; Harigai, M. Serum levels of soluble programmed cell death protein 1 and soluble programmed cell death protein ligand 2 are increased in systemic lupus erythematosus and associated with the disease activity. Lupus 2020, 29, 686–696. [Google Scholar] [CrossRef]
- Du, Y.; Nie, L.; Xu, L.; Wu, X.; Zhang, S.; Xue, J. Serum levels of soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1(sPD-L1) in systemic lupus erythematosus: Association with activity and severity. Scand. J. Immunol. 2020, 92, e12884. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, L.; Nie, L.; Zhang, S.; Liu, L.; Du, Y.; Xue, J. Soluble programmed death molecule 1 (sPD-1) as a predictor of interstitial lung disease in rheumatoid arthritis. BMC Immunol. 2021, 22, 69. [Google Scholar] [CrossRef]
- Greisen, S.; Rasmussen, T.; Stengaard-Pedersen, K.; Hetland, M.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Gao, L.; Wang, X.; Hu, X.; Wu, M.; Wu, J.; Xu, T.; Shi, Q.; Zhang, X. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res. Ther. 2015, 17, 340. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, L.; Zhu, W.; Xu, C.; Xu, M.; Guo, L.; Hu, W.; Xu, D.; Jing, R.; et al. Influence of total glucosides of paeony on PD-1/PD-L1 expression in primary Sjögren’s syndrome. Int. J. Rheum. Dis. 2019, 22, 200–206. [Google Scholar] [CrossRef]
- Qian, S.; Xu, J.; Zhao, S.; Yang, P.; Yang, C. CMTM6: Increased circulating level and up-regulated expression in labial salivary glands in patients with primary Sjogren’s syndrome. Clin. Exp. Immunol. 2022, 207, 65–71. [Google Scholar] [CrossRef]
- Yoon, T.; Ahn, S.S.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum soluble programmed cell death protein 1 could predict the current activity and severity of antineutrophil cytoplasmic antibody-associated vasculitis: A monocentric prospective study. Clin. Exp. Rheumatol. 2019, 37, 116–121. [Google Scholar]
- Bailly, C.; Thuru, X.; Quesnel, B. Soluble programmed death ligand-1 (Spd-l1): A pool of circulating proteins implicated in health and diseases. Cancers 2021, 13, 3034. [Google Scholar] [CrossRef]
- He, X.H.; Xu, L.H.; Liu, Y. Identification of a novel splice variant of human PD-L1 mRNA encoding an isoform-lacking Igv-like domain. Acta Pharmacol. Sin. 2005, 26, 462–468. [Google Scholar] [CrossRef]
- He, X.H.; Liu, Y.; Xu, L.H.; Zeng, Y.Y. Cloning and Identification of Two Novel Splice Variants of Human PD-L2. Acta Biochim. Biophys. Sin. 2004, 36, 284–289. [Google Scholar] [CrossRef]
- Niu, M.; Liu, Y.; Yi, M.; Jiao, D.; Wu, K. Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer. Front. Immunol. 2022, 13, 827921. [Google Scholar] [CrossRef]
- Bertsias, G.K.; Nakou, M.; Choulaki, C.; Raptopoulou, A.; Papadimitraki, E.; Goulielmos, G.; Kritikos, H.; Sidiropoulos, P.; Tzardi, M.; Kardassis, D.; et al. Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 207–218. [Google Scholar] [CrossRef]
- Liu, M.F.; Weng, C.T.; Weng, M.Y. Variable increased expression of program death-1 and program death-1 ligands on peripheral mononuclear cells is not impaired in patients with systemic lupus erythematosus. J. Biomed. Biotechnol. 2009, 2009, 406136. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Z.; Ye, J.; Deng, Y.; Fang, L.; Li, X.; Guo, Y.; Jiang, H.; Ju, B.; Huang, Q.; et al. PD-L1-expressing neutrophils as a novel indicator to assess disease activity and severity of systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 47. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Zhu, Q.-q.; Wang, Y.-Y.; Lu, Y.; Li, Z.-J.; Li, B.-Q.; Tang, J.; Wang, H.-T.; Song, C.-W.; Xie, C.-H.; et al. The role and clinical significance of programmed cell death- ligand 1 expressed on CD19+B-cells and subsets in systemic lupus erythematosus. Clin. Immunol. 2019, 198, 89–99. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Zhang, L.; Wang, M.; Chen, Z.; Shi, J.; Li, J.; Li, B.; Li, Z.; Wang, Y.; et al. Patients with systemic lupus erythematosus show increased proportions of CD19+CD20− B cells and secretion of related autoantibodies. Clin. Rheumatol. 2021, 40, 151–165. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Wiedemann, A.; Stefanski, A.-L.; Lettau, M.; Szelinski, F.; Fuchs, S.; Frei, A.P.; Steinberg, M.; Kam-Thong, T.; Hatje, K.; et al. Deep Phenotyping of CD11c+ B Cells in Systemic Autoimmunity and Controls. Front. Immunol. 2021, 12, 635615. [Google Scholar] [CrossRef]
- Shi, H.; Ye, J.; Teng, J.; Yin, Y.; Hu, Q.; Wu, X.; Liu, H.; Cheng, X.; Su, Y.; Liu, M.; et al. Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients. Arthritis Res. Ther. 2017, 19, 52. [Google Scholar] [CrossRef]
- Tong, M.; Fang, X.; Yang, J.; Wu, P.; Guo, Y.; Sun, J. Abnormal membrane-bound and soluble programmed death ligand 2 (PD-L2) expression in systemic lupus erythematosus is associated with disease activity. Immunol. Lett. 2020, 227, 96–101. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, J.; Sun, Y.; Chen, Y.; Guo, Y.; Liu, C.; Sun, J. Dysregulated PD-L2 is correlated with disease activity and inflammation in rheumatoid arthritis. Immunogenetics 2023, 75, 425–431. [Google Scholar] [CrossRef]
- Guo, Y.; Walsh, A.M.; Canavan, M.; Wechalekar, M.D.; Cole, S.; Yin, X.; Scott, B.; Loza, M.; Orr, C.; McGarry, T.; et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS ONE 2018, 13, e0192704. [Google Scholar] [CrossRef]
- Matsuda, K.; Miyoshi, H.; Hiraoka, K.; Hamada, T.; Yoshida, S.; Ishibashi, Y.; Haraguchi, T.; Shiba, N.; Ohshima, K. Clinicopathological value of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) expression in synovium of patients with rheumatoid arthritis. Clin. Exp. Med. 2018, 18, 487–494. [Google Scholar] [CrossRef]
- Moret, F.M.; van der Wurff-Jacobs, K.M.G.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; van Roon, J.A.G. Synovial T cell hyporesponsiveness to myeloid dendritic cells is reversed by preventing PD-1/PD-L1 interactions. Arthritis Res. Ther. 2014, 16, 497. [Google Scholar] [CrossRef]
- Wan, B.; Nie, H.; Liu, A.; Feng, G.; He, D.; Xu, R.; Zhang, Q.; Dong, C.; Zhang, J.Z. Aberrant Regulation of Synovial T Cell Activation by Soluble Costimulatory Molecules in Rheumatoid Arthritis. J. Immunol. 2006, 177, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Bommarito, D.; Hall, C.; Taams, L.S.; Corrigall, V.M. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin. Exp. Immunol. 2017, 188, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawano, S.; Hatachi, S.; Kurimoto, C.; Okazaki, T.; Iwai, Y.; Honjo, T.; Tanaka, Y.; Minato, N.; Komori, T.; et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjögren’s syndrome. J. Rheumatol. 2005, 32, 2156–2163. [Google Scholar] [PubMed]
- Loureiro-Amig, J.; Franco-Jarav, C.; Perurena-Priet, J.; Palacio, C.; Martínez-Vall, F.; Soláns-Laqu, R. Serum CXCL13, BAFF, IL-21 and IL-22 levels are related to disease activity and lymphocyte profile in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, A.; Suzuki, K.; Kassai, Y.; Gotou, Y.; Takiguchi, M.; Miyazaki, T.; Yoshimoto, K.; Yasuoka, H.; Yamaoka, K.; Morita, R.; et al. Identification of definitive serum biomarkers associated with disease activity in primary Sjögren’s syndrome. Arthritis Res. Ther. 2016, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Trabattoni, D.; Saresella, M.; Pacei, M.; Marventano, I.; Mendozzi, L.; Rovaris, M.; Caputo, D.; Borelli, M.; Clerici, M. Costimulatory Pathways in Multiple Sclerosis: Distinctive Expression of PD-1 and PD-L1 in Patients with Different Patterns of Disease. J. Immunol. 2009, 183, 4984–4993. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Horita, H.; Law, A.; Hong, S.; Middleton, K. Identifying Regulatory Posttranslational Modifications of PD-L1: A Focus on Monoubiquitinaton. Neoplasia 2017, 19, 346–353. [Google Scholar] [CrossRef]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef]
- Yao, H.; Lan, J.; Li, C.; Shi, H.; Brosseau, J.P.; Wang, H.; Lu, H.; Fang, C.; Zhang, Y.; Liang, L.; et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 2019, 3, 306–317. [Google Scholar] [CrossRef]
- Linder, M.E.; Deschenes, R.J. Palmitoylation: Policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, W.; Young, K.H.; Li, Y. Posttranslational Modifications in PD-L1 Turnover and Function: From Cradle to Grave. Biomedicines 2021, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Sparbier, C.E.; Chan, Y.C.; Williamson, J.C.; Woods, K.; Beavis, P.A.; Lam, E.Y.N.; Henderson, M.A.; Bell, C.C.; Stolzenburg, S.; et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017, 549, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mezzadra, R.; Sun, C.; Jae, L.T.; Gomez-Eerland, R.; De Vries, E.; Wu, W.; Logtenberg, M.E.W.; Slagter, M.; Rozeman, E.A.; Hofland, I.; et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017, 549, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.A.; Lareau, C.A.; White, B.C.; Pandey, A.; Wiley, G.; Montgomery, C.G.; Gaffney, P.M.; McKinney, B.A. Encore: Genetic Association Interaction Network Centrality Pipeline and Application to SLE Exome Data. Genet. Epidemiol. 2013, 37, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Zeisbrich, M.; Chevalier, N.; Sehnert, B.; Rizzi, M.; Venhoff, N.; Thiel, J.; Voll, R.E. CMTM6-Deficient Monocytes in ANCA-Associated Vasculitis Fail to Present the Immune Checkpoint PD-L1. Front. Immunol. 2021, 12, 673912. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.R.; Aslani, S.; Salmaninejad, A.; Javan, M.R.; Rezaei, N. PD-1/PD-L and autoimmunity: A growing relationship. Cell. Immunol. 2016, 310, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Tocheva, A.S.; Peled, M.; Strazza, M.; Adam, K.R.; Lerrer, S.; Nayak, S.; Azoulay-Alfaguter, I.; Foster, C.J.R.; Philips, E.A.; Neel, B.G.; et al. Quantitative phosphoproteomic analysis reveals involvement of PD-1 in multiple T cell functions. J. Biol. Chem. 2020, 295, 18036–18050. [Google Scholar] [CrossRef]
- Strazza, M.; Bukhari, S.M.; Tocheva, A.S.; Mor, A. PD-1-induced proliferating T cells exhibit a distinct transcriptional signature. Immunology 2021, 164, 555–568. [Google Scholar] [CrossRef]
- Lerrer, S.; Tocheva, A.S.; Bukhari, S.; Adam, K.; Mor, A. PD-1-stimulated T cell subsets are transcriptionally and functionally distinct. iScience 2021, 24, 103020. [Google Scholar] [CrossRef] [PubMed]
- Haynes, N.M.; Allen, C.D.C.; Lesley, R.; Ansel, K.M.; Killeen, N.; Cyster, J.G. Role of CXCR5 and CCR7 in Follicular Th Cell Positioning and Appearance of a Programmed Cell Death Gene-1High Germinal Center-Associated Subpopulation. J. Immunol. 2007, 179, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- Good-Jacobson, K.L.; Szumilas, C.G.; Chen, L.; Sharpe, A.H.; Tomayko, M.M.; Shlomchik, M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010, 11, 535–542. [Google Scholar] [CrossRef]
- Ding, B.B.; Bi, E.; Chen, H.; Yu, J.J.; Ye, B.H. IL-21 and CD40L Synergistically Promote Plasma Cell Differentiation through Upregulation of Blimp-1 in Human B Cells. J. Immunol. 2013, 190, 1827–1836. [Google Scholar] [CrossRef]
- Kawamoto, S.; Tran, T.H.; Maruya, M.; Suzuki, K.; Doi, Y.; Tsutsui, Y.; Kato, L.M.; Fagarasan, S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012, 336, 485–489. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function Article PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kageyama, R.; Eto, D.; Escobar, T.C.; Johnston, R.J.; Monticelli, L.; Lao, C.; Crotty, S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity 2011, 34, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’Shea, M.A.; Fauci, A.S. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. J. Immunol. 2008, 181, 6738–6746. [Google Scholar] [CrossRef]
- Haymaker, C.; Wu, R.; Bernatchez, C.; Radvanyi, L. PD-1 and BTLA and CD8+ T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology 2012, 1, 735. [Google Scholar] [CrossRef]
- Dolff, S.; Abdulahad, W.H.; Westra, J.; der Meer, B.D.-V.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, R157. [Google Scholar] [CrossRef]
- Paluch, C.; Santos, A.M.; Anzilotti, C.; Cornall, R.J.; Davis, S.J. Immune checkpoints as therapeutic targets in autoimmunity. Front. Immunol. 2018, 9, 2306. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zheng, H.; Wu, S.; Zhang, Y.; Wang, W.; Zhang, Z.; Zhou, C.; Wu, H.; Min, J. The Systemic Activation of Programmed Death 1-PD-L1 Axis Protects Systemic Lupus Erythematosus Model from Nephritis. Am. J. Nephrol. 2017, 46, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.M.; Rocklin, G.J.; Bick, M.J.; Ford, A.; Majri-Morrison, S.; Kroll, A.V.; Miller, C.J.; Carter, L.; Goreshnik, I.; Kang, A.; et al. Computational design of a synthetic PD-1 agonist. Proc. Natl. Acad. Sci. USA 2021, 118, e2102164118. [Google Scholar] [CrossRef] [PubMed]
- Curnock, A.P.; Bossi, G.; Kumaran, J.; Bawden, L.J.; Figueiredo, R.; Tawar, R.; Wiseman, K.; Henderson, E.; Hoong, S.J.; Gonzalez, V.; et al. Cell-targeted PD-1 agonists that mimic PD-L1 are potent T cell inhibitors. JCI Insight 2021, 6, e152468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, C.; Wu, Z.; Zhang, W.; Wang, L.; Yu, J.; Li, L.; Zhang, J.; Duan, Y. Engineered PD-1/TIGIT dual-activating cell-membrane nanoparticles with dexamethasone act synergistically to shape the effector T cell/Treg balance and alleviate systemic lupus erythematosus. Biomaterials 2022, 285, 121517. [Google Scholar] [CrossRef]
- Khramova, T.; Beduleva, L.; Sidorov, A.; Terentiev, A.; Menshikov, I. Regulatory Rheumatoid Factor is Specific to PD-1 and Uses PD-1 Pathway to Control CD4 T Lymphocytes. Immunol. Investig. 2023, 52, 897–908. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Liu, X.; Zhou, S.; Xu, T.; Wu, M. The effect of combining PD-1 agonist and low-dose Interleukin-2 on treating systemic lupus erythematosus. Front. Immunol. 2023, 14, 1111005. [Google Scholar] [CrossRef]



| Disease | Evaluation | Finding | Reference |
|---|---|---|---|
| SLE | Tissue expression | PD-1 and PD-L1 were identified in the kidney of lupus nephritis patients. | Bertsias, George K et al., 2009 [119] |
| Cell expression | Increased percentages of PD-1-expressing CD3+ T cells and PD-1-expressing CD19+ B cells in SLE. High expression of PD-L1-expressing CD19+ B cells and PD-L2-expressing CD14+ monocytes. | Liu, Ming-Fei et al., 2009 [120] | |
| Elevated frequency of PD-L1-expressing neutrophils in SLE. This percentage decreased after receiving a 15-day treatment with corticosteroids and immunosuppressive drugs. | Luo, Qing et al., 2016 [121] | ||
| PD-L1 was significantly higher in CD19+ cells of SLE patients with active disease and LN. The expression of PD-L1 was increased in double-negative B cells (DN) and plasma cells (PC). The percentage of CD19+PD-L1+ cells was correlated with the disease activity index and the number of T follicular helper cells (Tfh). | Jia, Xiao-Yun et al., 2019 [122] | ||
| Higher expression of PD-1, PD-L1, PD-L2, and CD86 in CD19+CD20− B cells than in CD19+CD20+ B cells. | Zhu, Qingqing et al., 2021 [123] | ||
| Higher expression of PD-1 and PD-L1 in CD11c+ B cells. | Rincon-Arevalo, Hector et al., 2021 [124] | ||
| Soluble levels | Increased serum levels of anti-PD-1 IgG in new-onset SLE patients. | Shi, Hui et al., 2017 [125] | |
| Higher serum levels of sPD-1 and sPD-L1. | Du, Yan et al., 2020 [108] | ||
| Higher serum levels of sPD-1 and sPD-L2 in SLE patients with high disease activity. sPD-L1 was not elevated in SLE patients. | Hirahara, Shinya et al., 2020 [107] | ||
| Higher expression of membrane-bound PD-L2 on monocytes. Lower serum levels of sPD-L2. | Tong, Min et al., 2020 [126] | ||
| RA | Tissue expression | PD-L2 was highly expressed on macrophages in synovial tissue. | Xiong, Jian et al., 2023 [127] |
| Low PD-L1 expression in RA synovial tissue. | Guo, Yanxia et al., 2018 [128] | ||
| PD-1 expression on synovium-infiltrating lymphocytes. PD-L1 expression on cells lining synovium. | Matsuda, Kotaro et al., 2018 [129] | ||
| Cell expression | Increased percentages of PD-L2-expressing CD14+ monocytes. | Xiong, Jian et al., 2023 [127] | |
| Increased PD-L1 expression on mDCs in synovial fluid compared with mDCs in peripheral blood. | Moret, Frederique M et al., 2014 [130] | ||
| Soluble levels | Lower sPD-L2 in the serum of RA patients. | Xiong, Jian et al., 2023 [127] | |
| sPD-1 levels were increased in ACPA+ but not ACPA− early RA. | Guo, Yanxia et al., 2018 [128] | ||
| Elevated sPD-1 and sPD-L1 levels in serum and synovial fluid of RA patients. | Wan, Bing et al., 2006 [131] | ||
| Higher sPD-1 expression in early and chronic RA. | Greisen, S R et al., 2014 [110] | ||
| Elevated sPD-1 expression in serum of RA patients. | Bommarito, D et al., 2017 [132] | ||
| pSS | Tissue expression | PD-1 expression on infiltrating lymphocytes in salivary glands from 52% of SS patients. PD-L1 expression on ductal and acinar epithelial cells from 68% of SS patients. | Kobayashi, Masaya et al., 2005 [133] |
| PD-1 and PD-L1 expression in labial glands were higher than in non-pSS controls. | Qian, Sirui et al., 2022 [113] | ||
| Cell expression | Increased expression of PD-L1 in CD11c+ B cells of pSS patients. | Rincon-Arevalo, Hector et al., 2021 [124] | |
| Serum levels | Lower serum levels of sPD-L2 in pSS patients. | Loureiro-Amigo, Jose et al., 2021 [134] | |
| Elevated serum levels of sPD-L2 in pSS patients. | Nishikawa, Ayumi et al., 2016 [135] | ||
| Elevated serum levels of sPD-1 and sPD-L1 in pSS patients. | Qian, Sirui et al., 2022 [113] | ||
| MS | Tissue expression | Astrocytes in white matter lesions from MS patients upregulated PD-L1 in response to aryl hydrocarbon receptor and interferon signaling. | Linnerbauer, Mathias et al., 2023 [101] |
| Cell expression | Frequencies of PD-L1-expressing CD19+ B cells and PD-L1+/IL-10+CD14+ monocytes were higher in stable multiple sclerosis patients compared to acute MS (AMS) patients. | Trabattoni, Daria et al., 2009 [136] | |
| Serum levels | No information. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagrero-Fabela, N.; Chávez-Mireles, R.; Salazar-Camarena, D.C.; Palafox-Sánchez, C.A. Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 7726. https://doi.org/10.3390/ijms25147726
Sagrero-Fabela N, Chávez-Mireles R, Salazar-Camarena DC, Palafox-Sánchez CA. Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2024; 25(14):7726. https://doi.org/10.3390/ijms25147726
Chicago/Turabian StyleSagrero-Fabela, Nefertari, Ramón Chávez-Mireles, Diana Celeste Salazar-Camarena, and Claudia Azucena Palafox-Sánchez. 2024. "Exploring the Role of PD-1 in the Autoimmune Response: Insights into Its Implication in Systemic Lupus Erythematosus" International Journal of Molecular Sciences 25, no. 14: 7726. https://doi.org/10.3390/ijms25147726





