What Not to Overlook in the Management of Patients with Type 2 Diabetes Mellitus: The Nephrological and Hepatological Perspectives
Abstract
:1. Introduction
2. Diabetes, Overview of Pathophysiology and Treatment

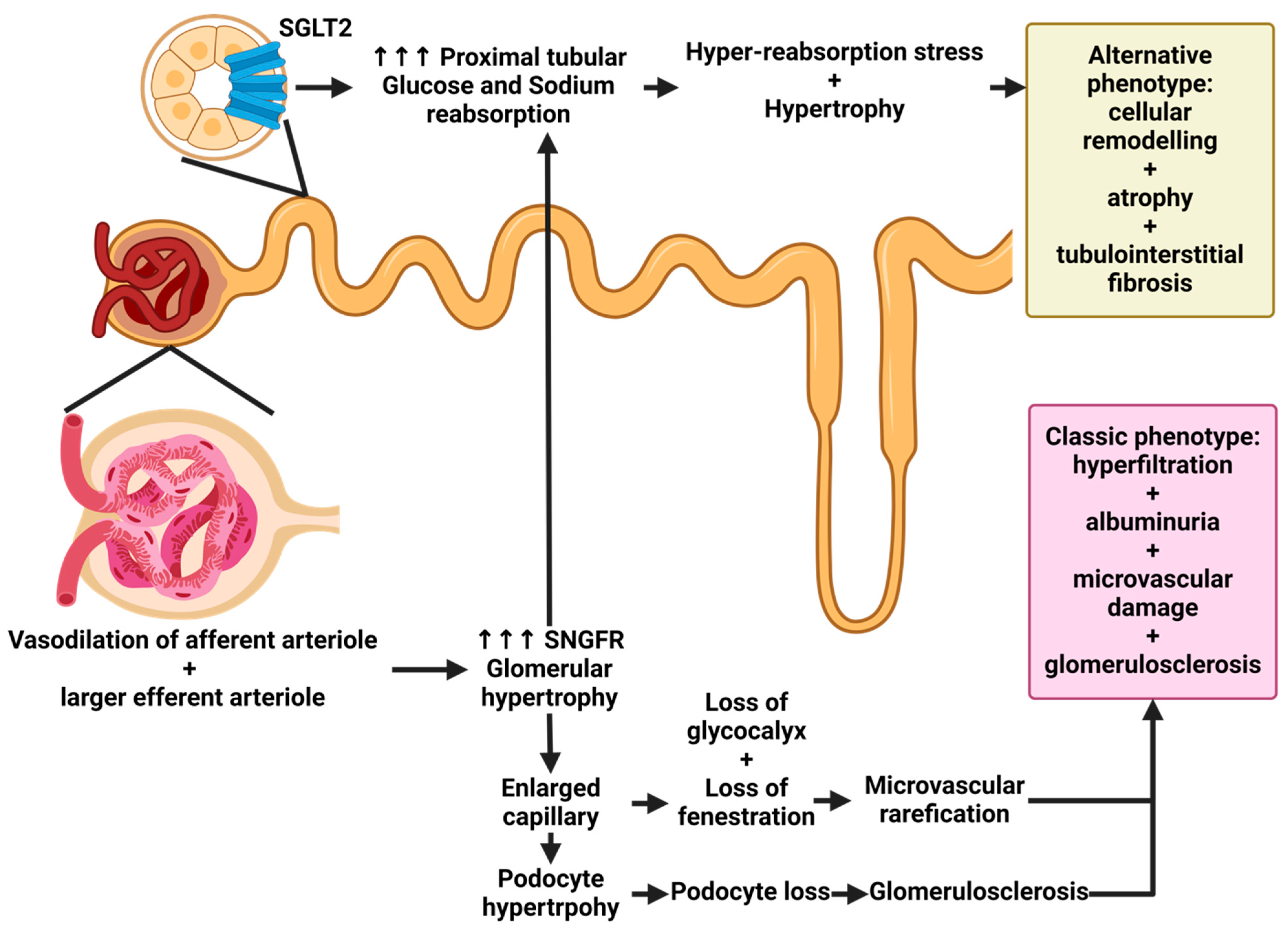
3. Renal Involvement in Patients with Diabetes Mellitus: What Not to Overlook
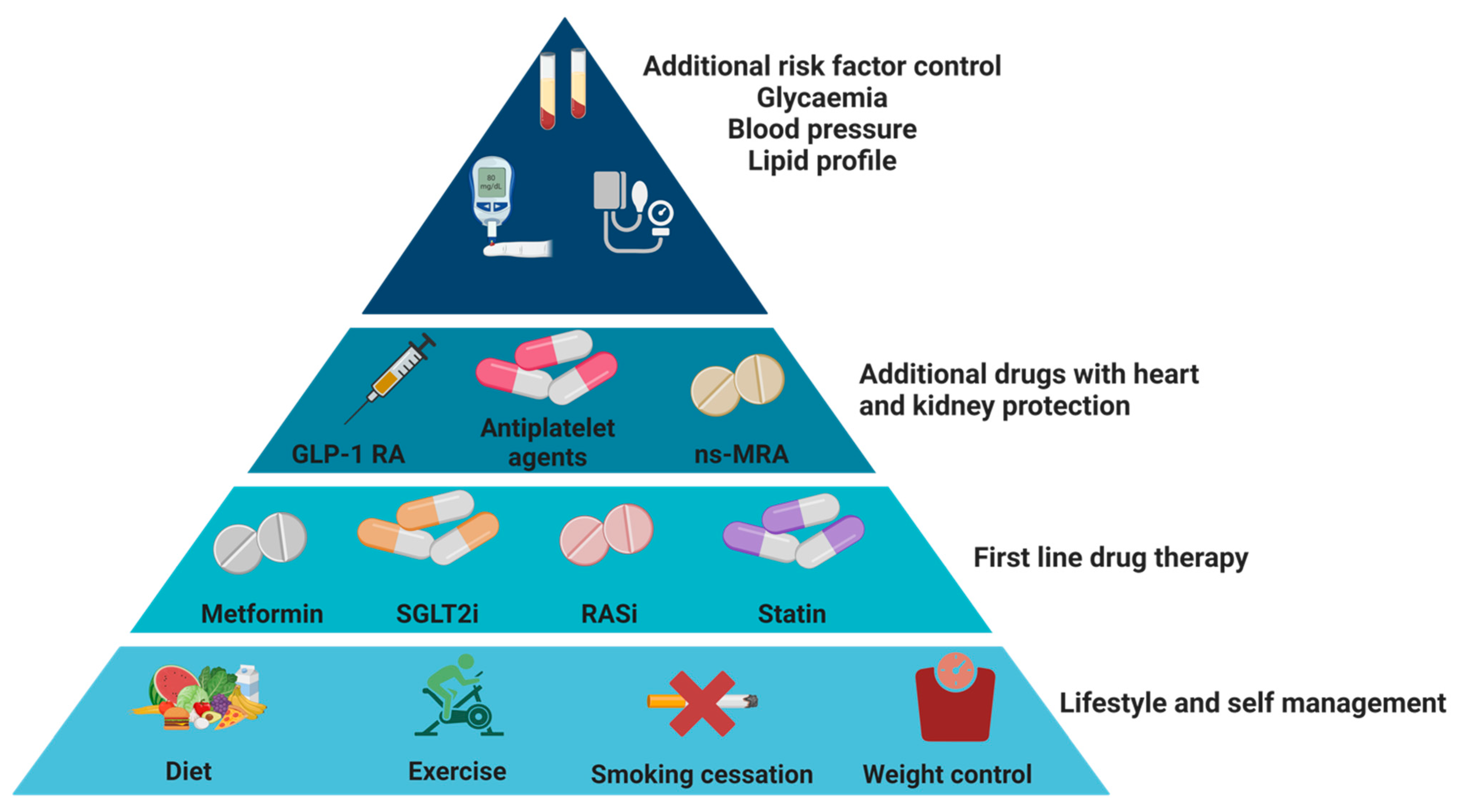
4. How to Reduce the Progression of Kidney Disease in Patients with DKD?
5. Kidney and Diabetes: Special Populations
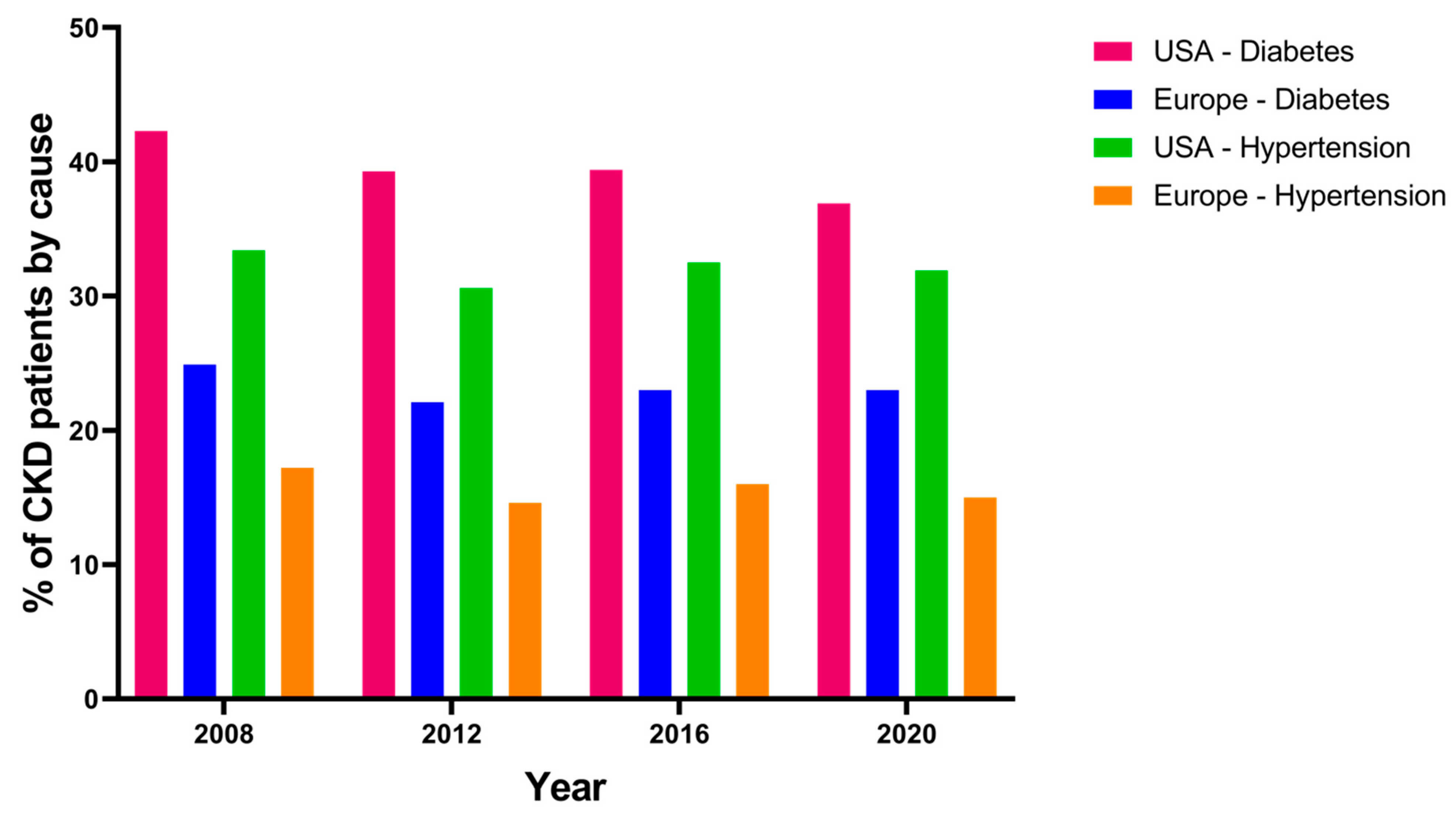
6. CKD Not Related to Diabetes
7. Hepatic Involvement in Patients with Diabetes Mellitus: What Not to Overlook
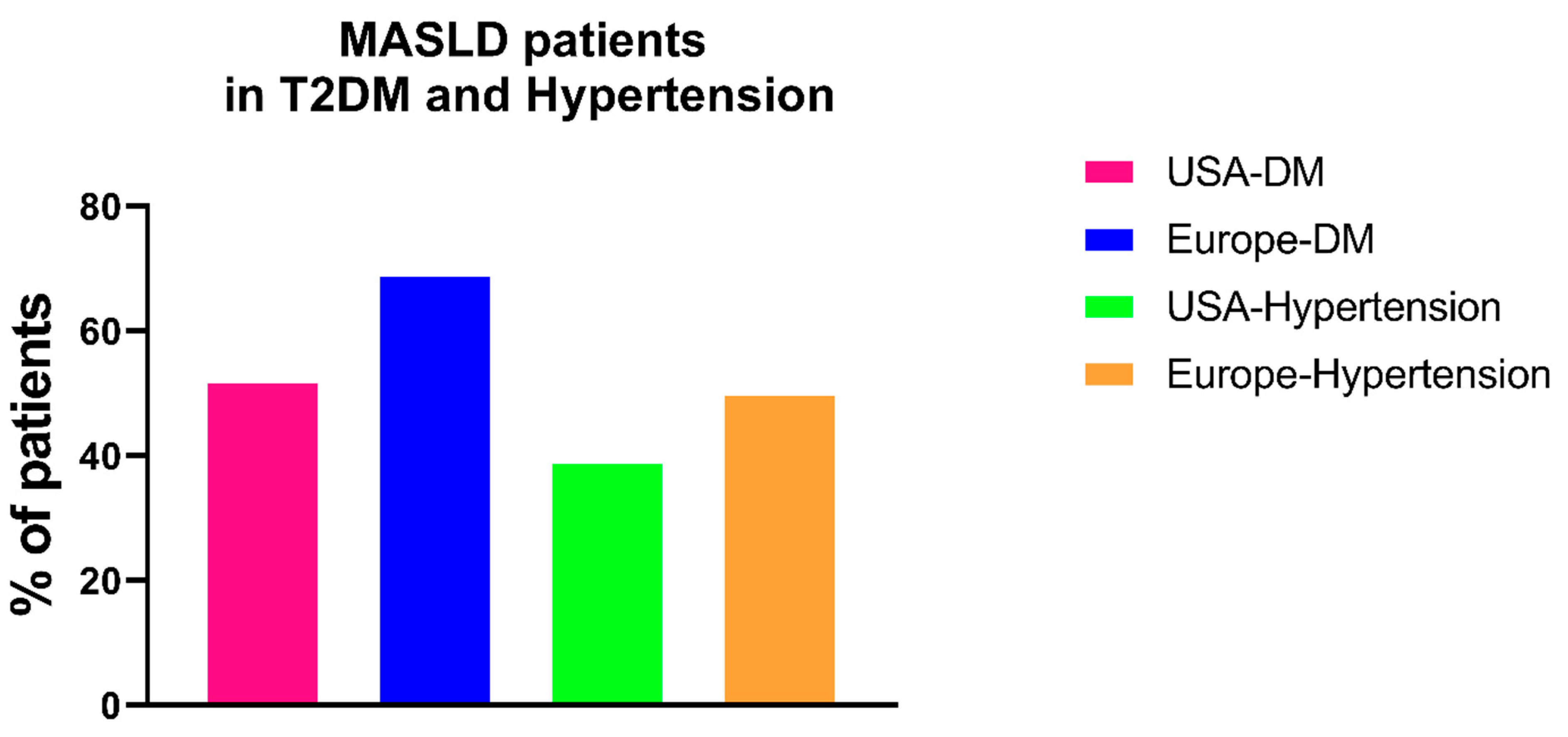
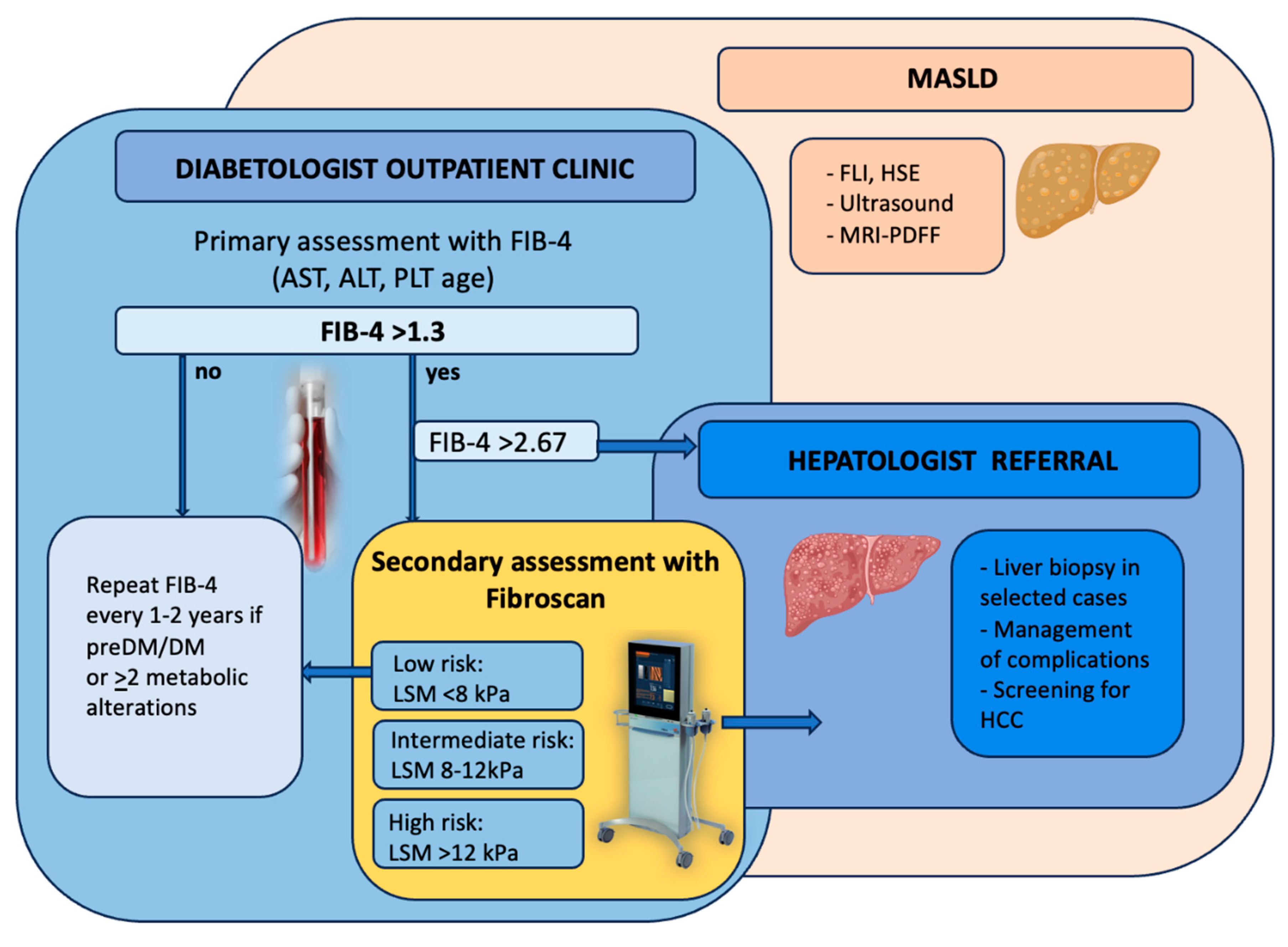
Screening Diabetic Patients for MASLD
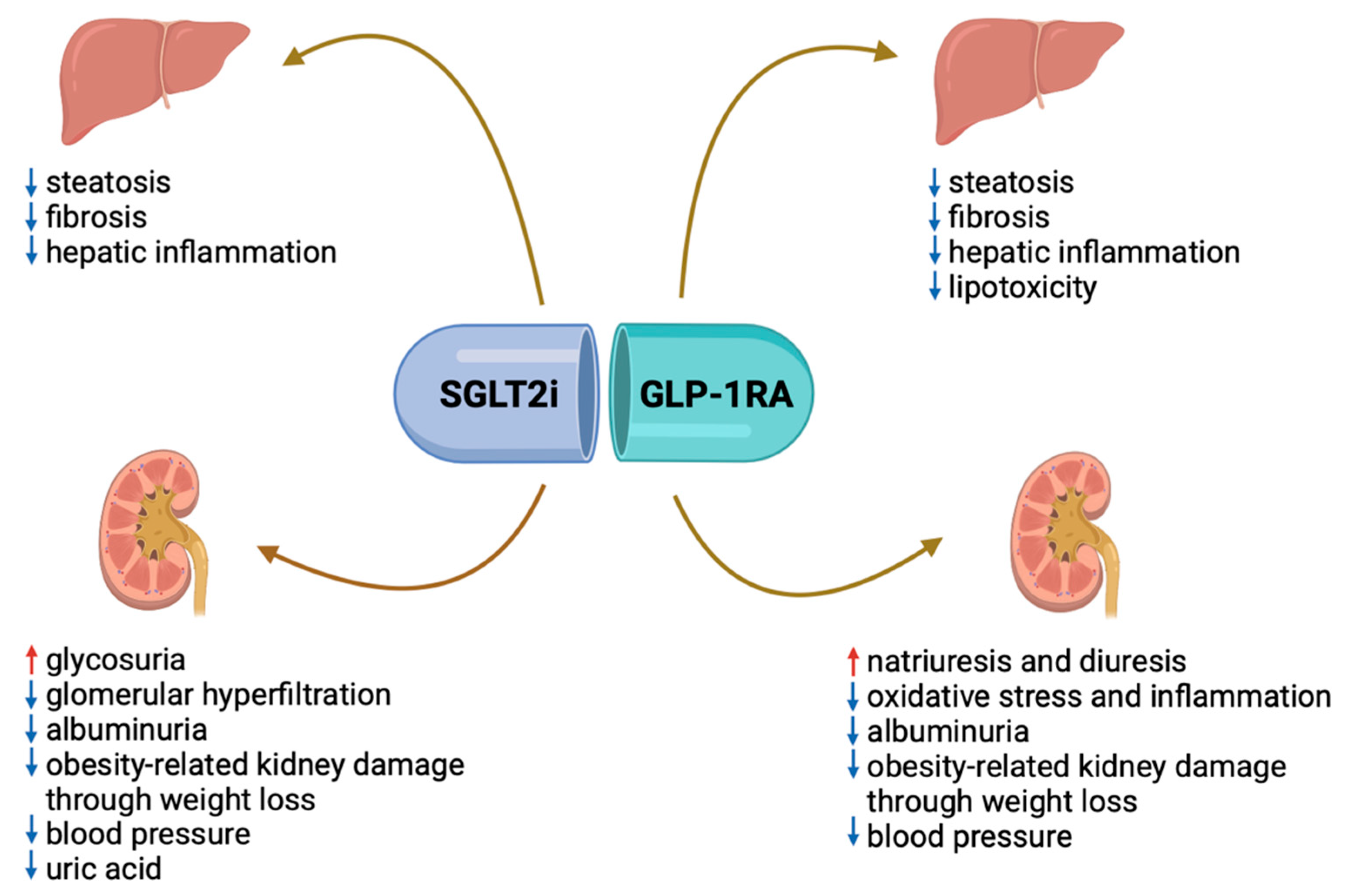
8. How to Reduce the Progression of MASLD in Patients with T2DM?
9. Liver and Diabetes: Special Populations
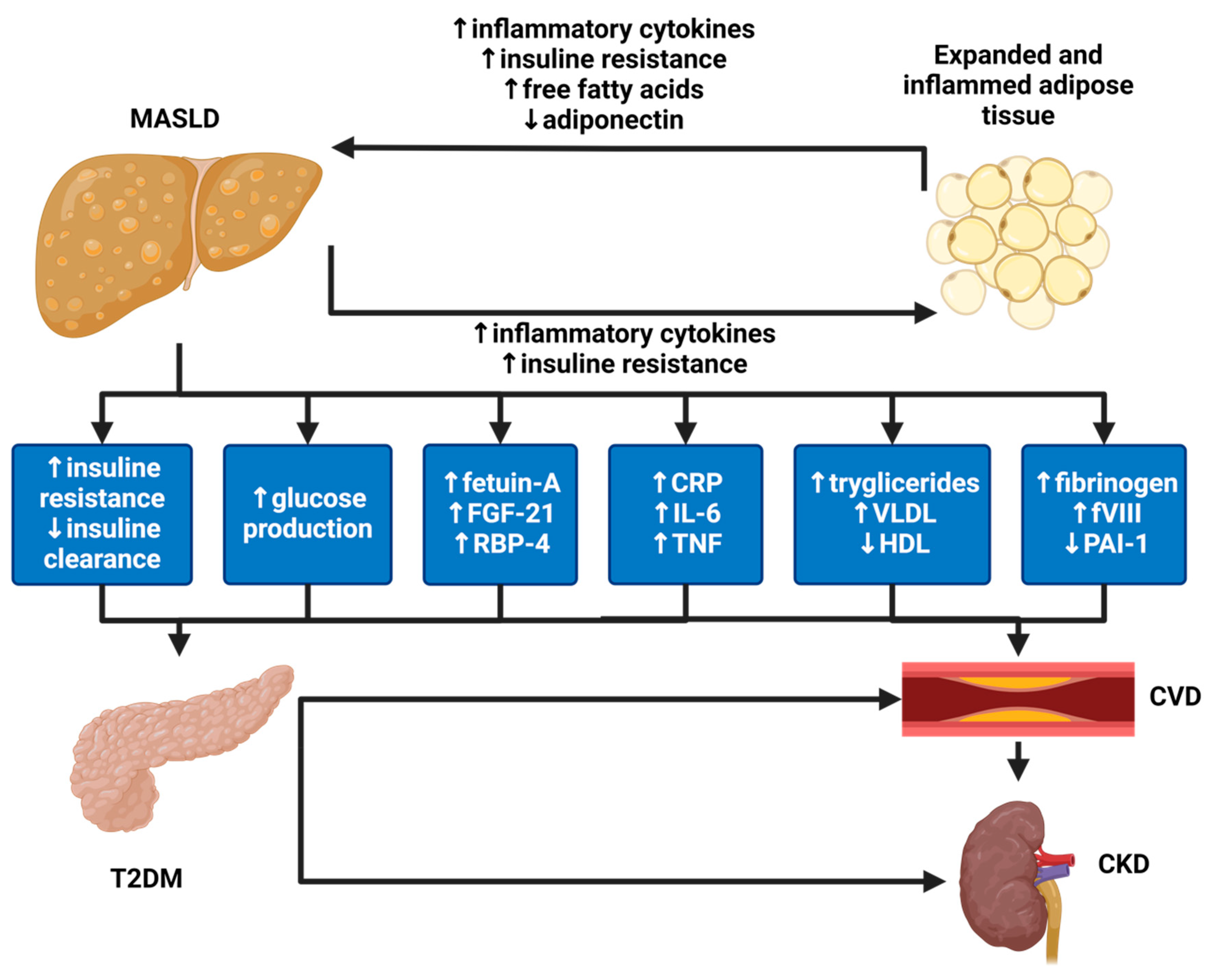
The Kidney and the Liver Disease: Two Faces of the Same Disease
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ewing, G.W.; Parvez, S.H. The multisystemic nature of diabetes mellitus: Genotype or phenotype? N. Am. J. Med. Sci. 2010, 2, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Tomlinson, J.W. Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013, 169, R27–R37. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Gaelle, L.; Roxane, D.; Perrine, W.; Marion, A.; Fleur, B.; Zoé, L.; Aurélie, L.; Solen, D.; Patricia, D.; et al. Efficiency of a multidisciplinary team care approach through a short hospitalization of patients with poorly controlled diabetes mellitus: A 12 months prospective monocentric study. Pan Afr. Med. J. 2022, 41, 192. [Google Scholar] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014, 311, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Allebeck, P.; Hallqvist, J.; Moradi, T.; Sidorchuk, A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 804–818. [Google Scholar] [CrossRef]
- Basu, A.; Dalla Man, C.; Basu, R.; Toffolo, G.; Cobelli, C.; Rizza, R.A. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care. 2009, 32, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.K.; Bolgiano, D.C.; McKnight, B.; Halter, J.B.; Porte, D., Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1984, 74, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyoty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Veijola, R.; Virtanen, S.M.; Hyoty, H.; Vaarala, O.; Akerblom, H.K. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005, 54 (Suppl. S2), S125–S136. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, M.; Klingel, K. Virus infections and type 1 diabetes risk. Curr. Diab. Rep. 2010, 10, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Van der Zijl, N.J.; Goossens, G.H.; Moors, C.C.; van Raalte, D.H.; Muskiet, M.H.; Pouwels, P.J.; Blaak, E.E.; Diamant, M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: Impact on beta-cell function in individuals with impaired glucose metabolism. J. Clin. Endocrinol. Metab. 2011, 96, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Araneta, M.R.G.; Kanaya, A.M.; Hsu, W.C.; Chang, H.K.; Grandinetti, A.; Boyko, E.J.; Hayashi, T.; Kahn, S.E.; Leonetti, D.L.; McNeely, M.J.; et al. Optimum BMI cut points to screen asian americans for type 2 diabetes. Diabetes Care 2015, 38, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M.; Varney, M.D.; Carlson, J.A.; Moonsamy, P.; Fear, A.L.; Lane, J.A.; Lavant, E.; Rappner, R.; Louey, A.; et al. HLA class I and genetic susceptibility to type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010, 59, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Howson, J.M.M.; Smyth, D.; Walker, N.M.; Stevens, H.; Yang, J.H.M.; She, J.-X.; Eisenbarth, G.S.; Rewers, M.; Todd, J.A.; et al. Confirmation of novel type 1 diabetes risk loci in families. Diabetologia 2012, 55, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, K.J.; Ferreira, T.; Lee, Y.; Raimondo, A.; Magi, R.; Reschen, M.E.; Mahajan, A.; Locke, A.; Rayner, N.W.; Robertson, N.; et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat. Genet. 2015, 47, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.E.; Mackay, D.J.; Nitert, M.D.; Morris, A.P.; Lindgren, C.M.; Berry, A.; Johnson, P.R.; Hanley, N.; Groop, L.C.; McCarthy, M.I.; et al. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 2013, 62, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Al Hariri, B.; Mustafa, A.R.; Ambra, N.; Amjed, I.; Alharafsheh, A.E.N.; Illahi, M.; Hamuda, S.; Gaafar, M.; Sharif, M. Ceftriaxone-induced hepatotoxicity in patients with common medical infections in Qatar: A retrospective study. Qatar Med. J. 2022, 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Velayudham, L.S.; Farrell, G.C. Drug-induced cholestasis. Expert Opin. Drug Saf. 2003, 2, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.C.; Guo, Y.Y.; Zhang, Y.; Bu, Y.S.; Zhang, F.Y. Factors associated with immune-related thyroid dysfunction induced by PD-1/PD-L1 inhibitors in cancer patients: An observational study. J. Chemother. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.J.; Cosentino, F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur. Heart J. 2019, 40, 3215–3217. [Google Scholar] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Awadhpersad, R.; Pippias, M.; Ferrer-Alamar, M.; Finne, P.; Fraser, S.D.; Heaf, J.G.; Hemmelder, M.H.; Martínez-Castelao, A.; de Meester, J.; et al. International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrology 2019, 24, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- O’shaughnessy, M.M.; Hogan, S.L.; Poulton, C.J.; Falk, R.J.; Singh, H.K.; Nickeleit, V.; Jennette, J.C. Temporal and Demographic Trends in Glomerular Disease Epidemiology in the Southeastern United States, 1986-2015. Clin. J. Am. Soc. Nephrol. 2017, 12, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.E.; Chang, A.; Henriksen, K.J. Medical renal diseases are frequent but often unrecognized in adult autopsies. Mod. Pathol. 2018, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus non-diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular pathways that drive diabetic kidney disease. J. Clin. Investig. 2023, 133, e165654. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Infante, B.; Conserva, F.; Pontrelli, P.; Leo, S.; Stasi, A.; Fiorentino, M.; Troise, D.; Strologo, A.D.; Alfieri, C.; Gesualdo, L.; et al. Recent advances in molecular mechanisms of acute kidney injury in patients with diabetes mellitus. Front. Endocrinol. 2022, 13, 903970. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: An update based on rapidly emerging new evidence. Kidney Int. 2022, 102, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.; Bonifati, C.; Craig, M.; Navaneethan, S.D.; Craig, J.C. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 2006, 2006, CD006257. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Limonte, C.P.; Groop, P.-H.; Heerspink, H.J.L.; Pratley, R.E.; Rossing, P.; Skyler, J.S.; Cherney, D.Z.I. Chronic kidney disease in type 1 diabetes: Translation of novel type 2 diabetes therapeutics to individuals with type 1 diabetes. Diabetologia 2024, 67, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J.; et al. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Sharma, K.; Kalantar-Zadeh, K. Glycemic control in diabetic CKD patients: Where do we stand? Am. J. Kidney Dis. 2008, 52, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes mellitus after kidney transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Werzowa, J.; Haidinger, M.; Hörl, W.H.; Pascual, J.; Budde, K.; Luan, F.L.; Ojo, A.; de Vries, A.P.J.; Porrini, E.; et al. Novel views on new-onset diabetes after transplantation: Development, prevention and treatment. Nephrol. Dial. Transplant. 2013, 28, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, S.; González, E.; López-Revuelta, K.; Ibernon, M.; López, D.; Martín-Gómez, A.; Garcia-Osuna, R.; Linares, T.; Díaz, M.; Martín, N.; et al. Risk factors for non-diabetic renal disease in diabetic patients. Clin. Kidney J. 2020, 13, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Bolignano, D.; Tesar, V.; Pisano, A.; Van Biesen, W.; Tripepi, G.; D’Arrigo, G.; Gesualdo, L. Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol. Dial. Transplant. 2017, 32, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Taverna, A.; Cappelli, D.; Beatrice, G.; Csermely, A.; Sani, E.; Byrne, C.D.; Targher, G. Long-Term Adverse Effect of Liver Stiffness on Glycaemic Control in Type 2 Diabetic Patients with Non-alcoholic Fatty Liver Disease: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 12481. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Seo, M.H.; Shin, H.C.; Ryoo, J.H. Clinical availability of non-alcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology 2013, 57, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Juurinen, L.; Hakkarainen, A.; Westerbacka, J.; Corner, A.; Bergholm, R.; Yki-Järvinen, H. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese non-diabetic subjects. Diabetes Care 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, Y.; He, Y.; Zhang, L.; Liu, J.; Zheng, S.; Bai, Y. The bidirectional relationship between NAFLD and type 2 diabetes: A prospective population-based cohort study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1521–1528. [Google Scholar] [CrossRef]
- Huang, D.Q.; Wilson, L.A.; Behling, C.; Kleiner, D.E.; Kowdley, K.V.; Dasarathy, S.; Amangurbanova, M.; Terrault, N.A.; Diehl, A.M.; Chalasani, N.; et al. Fibrosis Progression Rate in Biopsy-Proven Non-alcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology 2023, 165, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Jarasvaraparn, C.; Vilar-Gomez, E.; Yates, K.P.; Wilson, L.A.; Neuschwander-Tetri, B.; Loomba, R.; Cummings, O.; Vos, M.; Xanthakos, S.; Schwimmer, J.; et al. Age, BMI, and Type 2 Diabetes Modify the Relationship Between PNPLA3 and Advanced Fibrosis in Children and Adults With NAFLD. Clin. Gastroenterol. Hepatol. 2023, 22, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tsuboya, T.; Tsuji, K.; Dohke, M.; Maguchi, H. Independent Association Between Improvement of Non-alcoholic Fatty Liver Disease and Reduced Incidence of Type 2 Diabetes. Diabetes Care 2015, 38, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107684. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan((R)) independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Non-alcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of non-alcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Non-alcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Turino, T.; Lando, M.G.; Gjini, K.; Byrne, C.D.; Zusi, C.; Ravaioli, F.; Colecchia, A.; Maffeis, C.; Salvagno, G.; et al. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab. 2020, 46, 296–303. [Google Scholar] [CrossRef]
- Forlano, R.; Stanic, T.; Jayawardana, S.; Mullish, B.H.; Yee, M.; Mossialos, E.; Goldin, R.; Petta, S.; Tsochatzis, E.; Thursz, M.; et al. A prospective study on the prevalence of MASLD in people with type-2 diabetes in the community. Cost effectiveness of screening strategies. Liver Int. 2024, 44, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.L.; Wan Yusoff, W.N.I.; Vethakkan, S.R.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W.K. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J. Gastroenterol. Hepatol. 2019, 34, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Mantovani, A.; Cespiati, A.; Francione, P.; Maffi, G.; Del Zanna, E.; Maffeis, C.; Colecchia, A.; Passigato, N.; Ferrarese, A.; et al. Evolution of liver fibrosis in diabetic patients with NAFLD in a follow-up study: Hepatoprotective effects of sodium-glucose co-transporter-2 inhibitors. Dig. Liver Dis. 2023, 56, 551–558. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in non-alcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Makker, J.; Tariq, H.; Kumar, K.; Ravi, M.; Shaikh, D.H.; Leung, V.; Hayat, U.; Hassan, M.T.; Patel, H.; Nayudu, S.; et al. Prevalence of advanced liver fibrosis and steatosis in type-2 diabetics with normal transaminases: A prospective cohort study. World J. Gastroenterol. 2021, 27, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Non-alcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on non-alcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Sattar, N.; Welsh, P.; Peters, C.; Zhyzhneuskaya, S.; et al. Clinical and metabolic features of the randomised controlled Diabetes Remission Clinical Trial (DiRECT) cohort. Diabetologia 2018, 61, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and non-alcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Sullivan, S.; Kirk, E.P.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in non-alcoholic fatty liver disease. Hepatology 2012, 55, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Kim, J.Y.; Wild, S.H.; Byrne, C.D. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef]
- Ghani, R.A.; Omar, N.; Koshy, M.; Hanafiah, M.; Hatta, S.F.W.M.; Shah, F.Z.M.; Johari, B.; Zamhuri, I.; Kasim, S.S.; Rahman, T.A. Relationships between severity of steatosis with glycemic control and carotid intima-media thickness among diabetic patients with ischemic heart disease. J. Res. Med. Sci. 2020, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.S.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Non-alcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; A Aldersley, M.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Barritt, A.S.; Marshman, E.; Noureddin, M. Review article: Role of glucagon-like peptide-1 receptor agonists in non-alcoholic steatohepatitis, obesity and diabetes-what hepatologists need to know. Aliment. Pharmacol. Ther. 2022, 55, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Targher, G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Non-alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Dwinata, M.; Putera, D.D.; Hasan, I.; Raharjo, M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: A systematic review. Clin. Exp. Hepatol. 2020, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Haruki, U.; Yoshikata, K.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; et al. Antifibrotic effect and long-term outcome of SGLT2 inhibitors in patients with NAFLD complicated by diabetes mellitus. Hepatol. Commun. 2022, 6, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; Okubo, T.; Hayama, K.; et al. Effect of sodium-glucose cotransporter 2 inhibitor in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: A propensity score-matched analysis of real-world data. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211000243. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Noureddin, N.; Ajmera, V.; Amangurbanova, M.; Bettencourt, R.; Truong, E.; Gidener, T.; Siddiqi, H.; Majzoub, A.M.; Nayfeh, T.; et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: An individual participant-level data meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 829–836. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, A.; Tavaglione, F.; Jamialahmadi, O.; Picardi, A.; Incalzi, R.A.; Valenti, L.; Romeo, S.; Vespasiani-Gentilucci, U. A Polygenic Risk Score to Refine Risk Stratification and Prediction for Severe Liver Disease by Clinical Fibrosis Scores. Clin. Gastroenterol. Hepatol. 2022, 20, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Non-alcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Torbenson, M.; Wu, T.T.; Yeh, M.M. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: A clinical and pathological study. J. Gastroenterol. Hepatol. 2013, 28, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; King, L.Y.; Chong, D.Q.; Nguyen, L.H.; Ma, Y.; VoPham, T.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology 2018, 67, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, P.; Wang, B.; Fu, Z.J.; Zhao, W.J.; Yan, S.L. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2014, 9, e95485. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2022, 71, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Lombardi, R.; Cattazzo, F.; Zusi, C.; Cappelli, D.; Dalbeni, A. MAFLD and CKD: An Updated Narrative Review. Int. J. Mol. Sci. 2022, 23, 7007. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Roh, J.H.; Lee, S.; Yoon, J.H. Clinical implications of renin-angiotensin system inhibitors for development and progression of non-alcoholic fatty liver disease. Sci. Rep. 2021, 11, 2884. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Tighiouart, H.; Adingwupu, O.M.; Shlipak, M.G.; Doria, A.; Estrella, M.M.; Froissart, M.; Gudnason, V.; Grubb, A.; Kalil, R.; et al. CKD-EPI and EKFC GFR Estimating Equations: Performance and Other Considerations for Selecting Equations for Implementation in Adults. J. Am. Soc. Nephrol. 2023, 34, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity; Clinical Practice Guideline Panel. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary. Diabetologia 2024, in press. [Google Scholar]
| Assessment | Abnormal Cut-Offs | Endpoint | Complications to Avoid |
|---|---|---|---|
Non-invasive scores
| >60 >36 >2.67 | Hepatic steatosis Hepatic steatosis Hepatic fibrosis |
|
| Liver ultrasound | Bright pattern compared to the kidney | Hepatic steatosis | |
| Fibroscan >8 kPa | >8 kPa | Hepatic fibrosis | |
| GFR by creatinine clearance (Urinary creatinine (mg/dL) urine volume mL)/(Plasmatic creatinine mg/dL) or CKD-EPI formula | <60 mL/min | CKD |
|
| Microalbuminuria | >30 mg/dL in the morning spot urine | CKD | |
| Glycated hemoglobin | >53 mmol/mol | T2DM control |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, C.M.; Molinari, P.; Cinque, F.; Vettoretti, S.; Cespiati, A.; Bignamini, D.; Nardelli, L.; Fracanzani, A.L.; Castellano, G.; Lombardi, R. What Not to Overlook in the Management of Patients with Type 2 Diabetes Mellitus: The Nephrological and Hepatological Perspectives. Int. J. Mol. Sci. 2024, 25, 7728. https://doi.org/10.3390/ijms25147728
Alfieri CM, Molinari P, Cinque F, Vettoretti S, Cespiati A, Bignamini D, Nardelli L, Fracanzani AL, Castellano G, Lombardi R. What Not to Overlook in the Management of Patients with Type 2 Diabetes Mellitus: The Nephrological and Hepatological Perspectives. International Journal of Molecular Sciences. 2024; 25(14):7728. https://doi.org/10.3390/ijms25147728
Chicago/Turabian StyleAlfieri, Carlo Maria, Paolo Molinari, Felice Cinque, Simone Vettoretti, Annalisa Cespiati, Daniela Bignamini, Luca Nardelli, Anna Ludovica Fracanzani, Giuseppe Castellano, and Rosa Lombardi. 2024. "What Not to Overlook in the Management of Patients with Type 2 Diabetes Mellitus: The Nephrological and Hepatological Perspectives" International Journal of Molecular Sciences 25, no. 14: 7728. https://doi.org/10.3390/ijms25147728






