Autotaxin–Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications
Abstract
1. Introduction
2. Autotaxin
2.1. Structure and Isoforms
2.2. Regulation of Autotaxin Expression
2.3. Functions
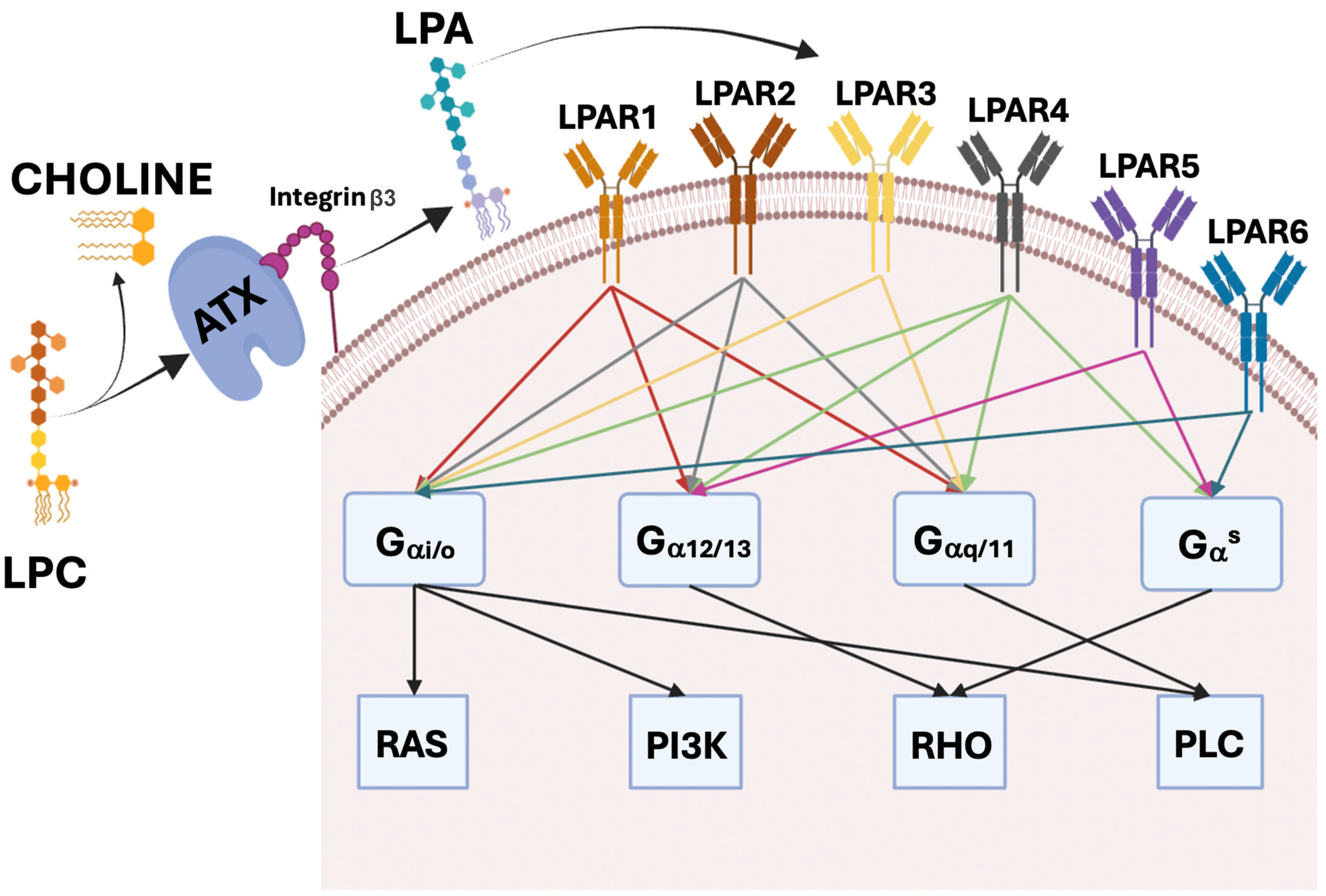
3. LPA and LPAR1–6
4. ATX–LPA Axis in Physiological Conditions
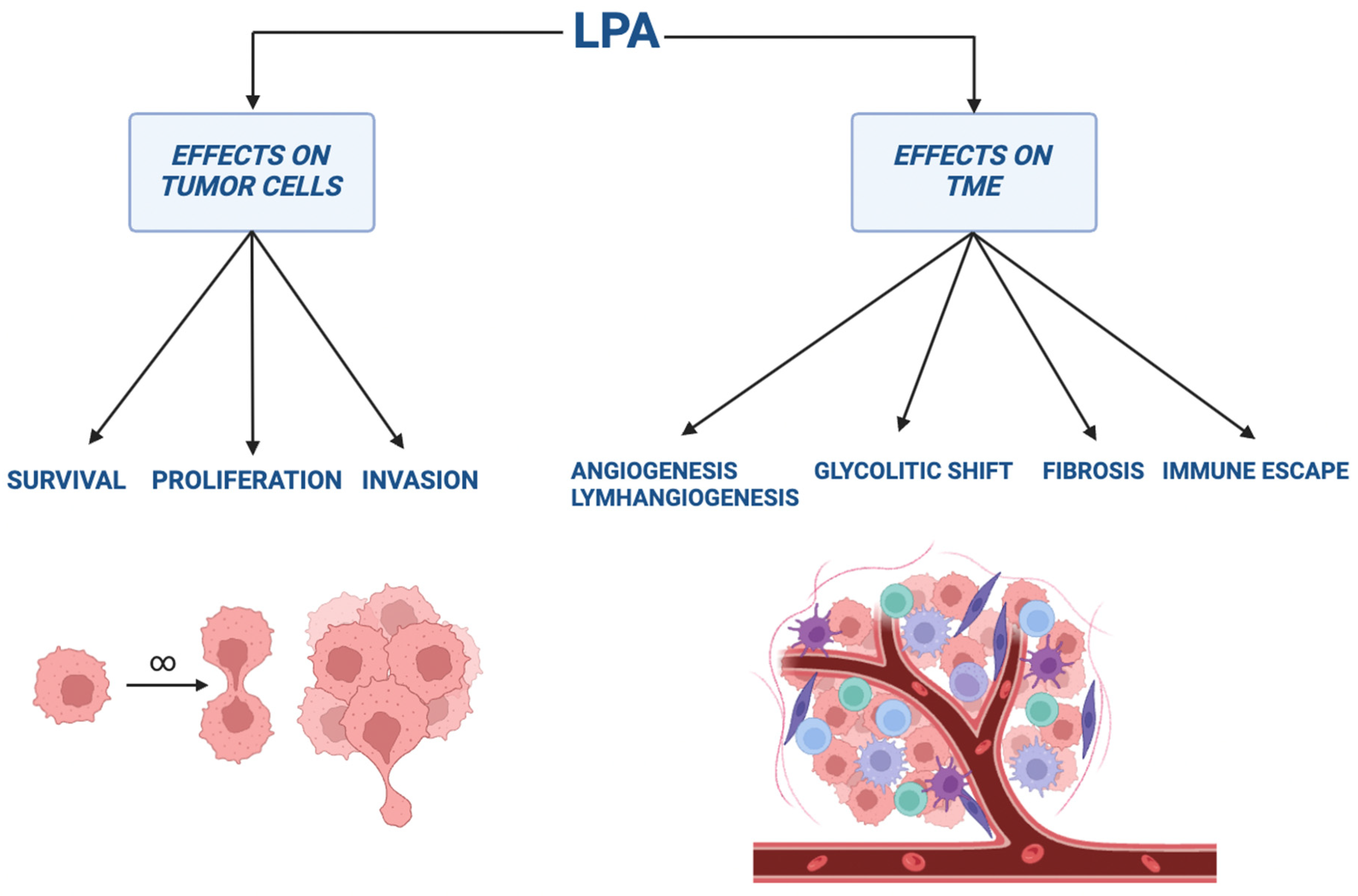
5. ATX–LPA Axis in Cancer
5.1. The Role in Cancer Genesis, Invasion, and Metastasis
5.2. Stimulation of Angiogenesis and Lymphangiogenesis
5.3. The Effect on Tumor Glycolytic Shift and Fibrosis Development
5.4. Immune Escape
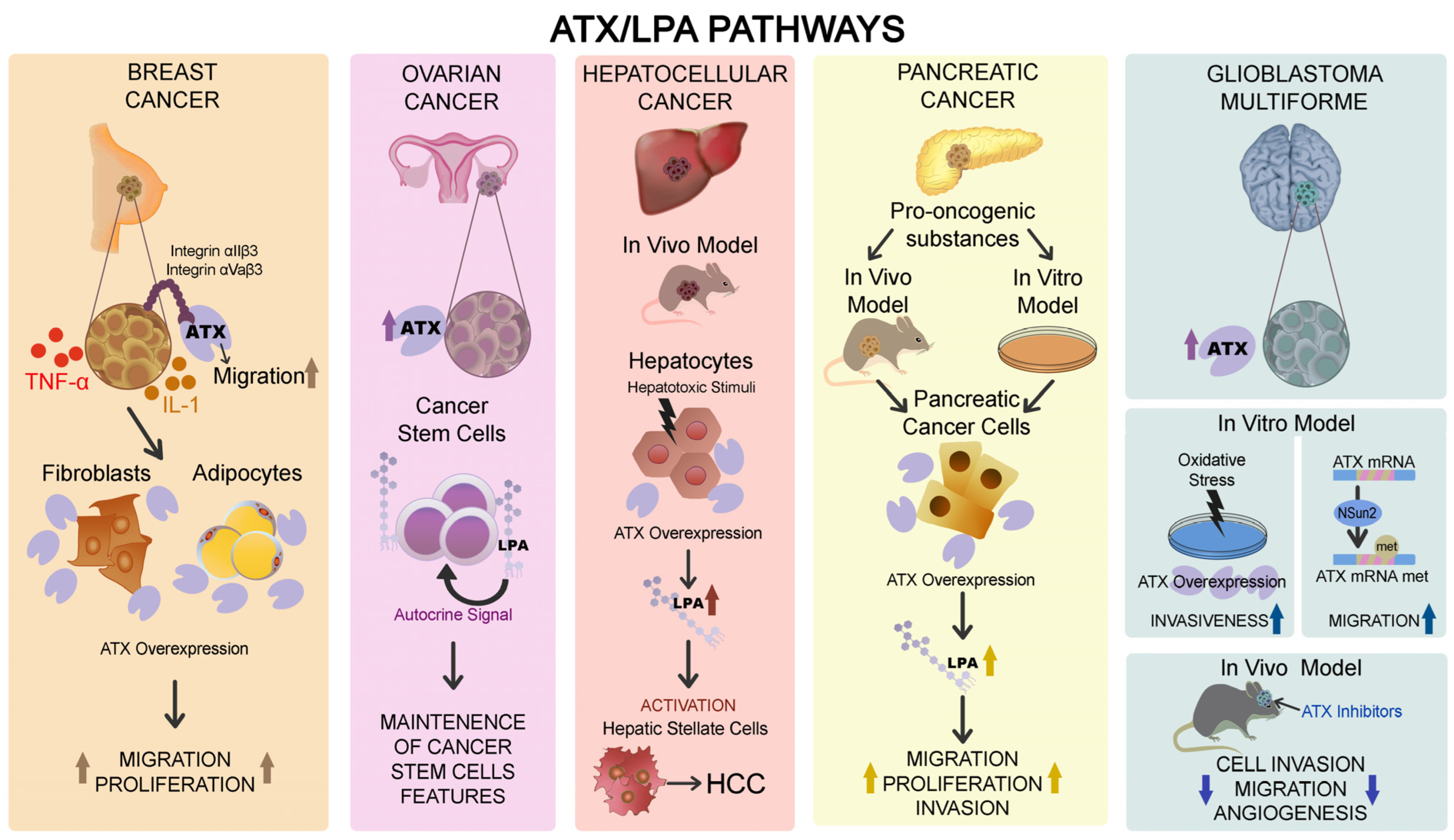
6. The Role of ATX–LPA Pathway in the Different Cancers
6.1. Breast Cancer
6.2. Ovarian Cancer
6.3. Hepatocellular Carcinoma
6.4. Pancreatic Cancer
6.5. Glioblastoma Multiforme
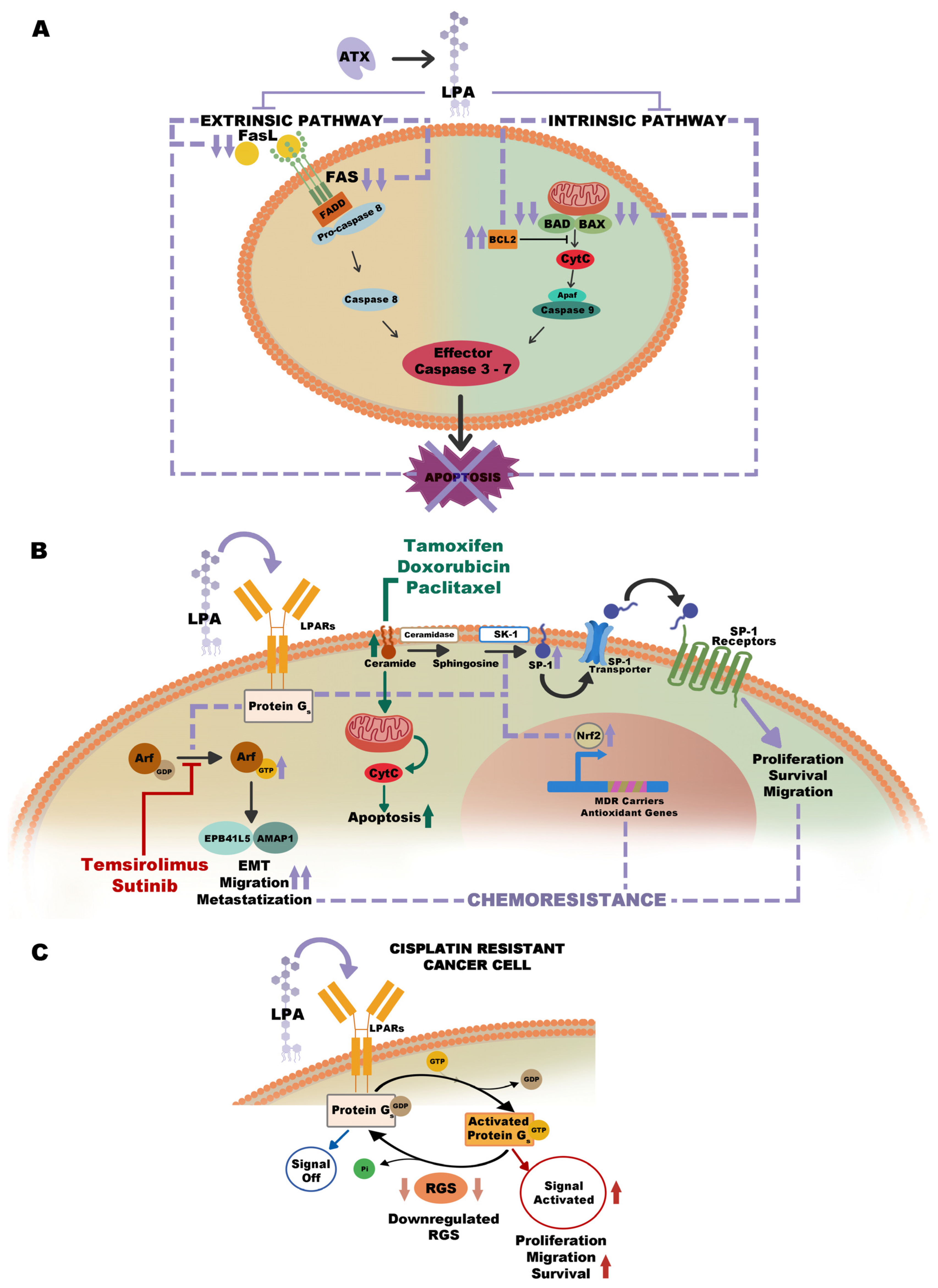
7. The Role of ATX–LPA Pathway in Drug Resistance
8. ATX Inhibitors and Future Clinical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Meeteren, L.A.; Moolenaar, W.H. Regulation and Biological Activities of the Autotaxin–LPA Axis. Prog. Lipid Res. 2007, 46, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Yin, N.; Zhang, J. The Expression Regulation and Biological Function of Autotaxin. Cells 2021, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA Receptors: Subtypes and Biological Actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Tomsig, J.L.; Snyder, A.H.; Berdyshev, E.V.; Skobeleva, A.; Mataya, C.; Natarajan, V.; Brindley, D.N.; Lynch, K.R. Lipid Phosphate Phosphohydrolase Type 1 (LPP1) Degrades Extracellular Lysophosphatidic Acid in Vivo. Biochem. J. 2009, 419, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Benesch, M.G.K.; Brindley, D.N. Lipid Phosphate Phosphatases and Their Roles in Mammalian Physiology and Pathology. J. Lipid Res. 2015, 56, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Dusaulcy, R.; Rancoule, C.; Grès, S.; Wanecq, E.; Colom, A.; Guigné, C.; Van Meeteren, L.A.; Moolenaar, W.H.; Valet, P.; Saulnier-Blache, J.S. Adipose-Specific Disruption of Autotaxin Enhances Nutritional Fattening and Reduces Plasma Lysophosphatidic Acid. J. Lipid Res. 2011, 52, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.A.; Chun, J.; Ueda, H. Initiation of Neuropathic Pain Requires Lysophosphatidic Acid Receptor Signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.S.; Moolenaar, W.H. Autotaxin and LPA Receptor Signaling in Cancer. Cancer Metastasis Rev. 2011, 30, 557–565. [Google Scholar] [CrossRef]
- Okudaira, S.; Yukiura, H.; Aoki, J. Biological Roles of Lysophosphatidic Acid Signaling through Its Production by Autotaxin. Biochimie 2010, 92, 698–706. [Google Scholar] [CrossRef]
- Hemming, D.G.; Brindle, D.N. Signalling by Lysophosphatidate and Its Health Implications. Essays Biochem. 2020, 64, 547–563. [Google Scholar] [CrossRef]
- Brindley, D.N.; Lin, F.T.; Tigyi, G.J. Role of the Autotaxin-Lysophosphatidate Axis in Cancer Resistance to Chemotherapy and Radiotherapy. Biochim. Acta 2013, 1831, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.S.; Van Wijk, X.M.R.; Van Meeteren, L.A.; Van Zeijl, L.; Van De Westerlo, E.M.A.; Hausmann, J.; Fish, A.; Perrakis, A.; Van Kuppevelt, T.H.; Moolenaar, W.H. The Polybasic Insertion in Autotaxin α Confers Specific Binding to Heparin and Cell Surface Heparan Sulfate Proteoglycans. J. Biol. Chem. 2013, 288, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Okudaira, S.; Igarashi, K.; Hama, K.; Yatomi, Y.; Aoki, J. Identification and Biochemical Characterization of a Novel Autotaxin Isoform, ATXδ, with a Four-Amino Acid Deletion. J. Biochem. 2012, 151, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Giganti, A.; Rodriguez, M.; Fould, B.; Moulharat, N.; Coge, F.; Chomarat, P.; Galizzi, J.P.; Valet, P.; Saulnier-Blache, J.S.; Boutin, J.A.; et al. Murine and Human Autotaxin α, β, and γ Isoforms: Gene Organization, Tissue Distribution, and Biochemical Characterization. J. Biol. Chem. 2008, 283, 7776–7789. [Google Scholar] [CrossRef] [PubMed]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-Function and Signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef]
- Nishimasu, H.; Okudaira, S.; Hama, K.; Mihara, E.; Dohmae, N.; Inoue, A.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal Structure of Autotaxin and Insight into GPCR Activation by Lipid Mediators. Nat. Struct. Mol. Biol. 2011, 18, 205–213. [Google Scholar] [CrossRef]
- Tabchy, A.; Tigyi, G.; Mills, G.B. Location, Location, Location: A Crystal-Clear View of Autotaxin Saturating LPA Receptors. Nat. Struct. Mol. Biol. 2011, 18, 117–118. [Google Scholar] [CrossRef][Green Version]
- Fulkerson, Z.; Wu, T.; Sunkara, M.; Vander Kooi, C.; Morris, A.J.; Smyth, S.S. Binding of Autotaxin to Integrins Localizes Lysophosphatidic Acid Production to Platelets and Mammalian Cells. J. Biol. Chem. 2011, 286, 34654–34663. [Google Scholar] [CrossRef]
- Hausmann, J.; Kamtekar, S.; Christodoulou, E.; Day, J.E.; Wu, T.; Fulkerson, Z.; Albers, H.M.H.G.; Van Meeteren, L.A.; Houben, A.J.S.; Van Zeijl, L.; et al. Structural Basis of Substrate Discrimination and Integrin Binding by Autotaxin. Nat. Struct. Mol. Biol. 2011, 18, 198–205. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Fanidis, D.; Aidinis, V.; Chatzaki, E. ENPP2 Methylation in Health and Cancer. Int. J. Mol. Sci. 2021, 22, 11958. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Xu, Y.; Zhang, J. Autotaxin Is Induced by TSA through HDAC3 and HDAC7 Inhibition and Antagonizes the TSA-Induced Cell Apoptosis. Mol. Cancer 2011, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Argaud, D.; Boulanger, M.C.; Chignon, A.; Mkannez, G.; Mathieu, P. Enhancer-Mediated Enrichment of Interacting JMJD3–DDX21 to ENPP2 Locus Prevents R-Loop Formation and Promotes Transcription. Nucleic Acids Res. 2019, 47, 8424–8438. [Google Scholar] [CrossRef] [PubMed]
- Braeuer, R.R.; Zigler, M.; Kamiya, T.; Dobroff, A.S.; Huang, L.; Choi, W.; McConkey, D.J.; Shoshan, E.; Mobley, A.K.; Song, R.; et al. Galectin-3 Contributes to Melanoma Growth and Metastasis via Regulation of NFAT1 and Autotaxin. Cancer Res. 2012, 72, 5757–5766. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.R.; Cappabianca, L.; Ruggeri, P.; Di Ianni, N.; Ragone, M.; Merolle, S.; Sano, K.; Stracke, M.L.; Horowitz, J.M.; Gulino, A.; et al. Constitutive Autotaxin Transcription by Nmyc-Amplified and Non-Amplified Neuroblastoma Cells Is Regulated by a Novel AP-1 and SP-Mediated Mechanism and Abrogated by Curcumin. FEBS Lett. 2012, 586, 3681–3691. [Google Scholar] [CrossRef] [PubMed]
- Azare, J.; Doane, A.; Leslie, K.; Chang, Q.; Berishaj, M.; Nnoli, J.; Mark, K.; Al-Ahmadie, H.; Gerald, W.; Hassimi, M.; et al. Stat3 Mediates Expression of Autotaxin in Breast Cancer. PLoS ONE 2011, 6, e278512011. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.J.; Humphreys, I.S.; Rudge, S.A.; Wilson, G.K.; Bhattacharya, B.; Ciaccia, M.; Hu, K.; Zhang, Q.; Mailly, L.; Reynolds, G.M.; et al. Autotaxin-Lysophosphatidic Acid Receptor Signalling Regulates Hepatitis C Virus Replication. J. Hepatol. 2017, 66, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Williams, M.E.; Innis, J.W. Range of HOX/TALE Superclass Associations and Protein Domain Requirements for HOXA13:MEIS Interaction. Dev. Biol. 2005, 277, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Sioletic, S.; Czaplinski, J.; Hu, L.; Fletcher, J.A.; Fletcher, C.D.M.; Wagner, A.J.; Loda, M.; Demetri, G.D.; Sicinska, E.T.; Snyder, E.L. C-Jun Promotes Cell Migration and Drives Expression of the Motility Factor ENPP2 in Soft Tissue Sarcomas. J. Pathol. 2014, 234, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Black, E.J.; Clair, T.; Delrow, J.; Neiman, P.; Gillespie, D.A.F. Microarray Analysis Identifies Autotaxin, a Tumour Cell Motility and Angiogenic Factor with Lysophospholipase D Activity, as a Specific Target of Cell Transformation by v-Jun. Oncogene 2003, 23, 2357–2366. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Lyu, L.; Li, X.; Yao, S.; Zhang, J. Autotaxin Expression Is Regulated at the Post-Transcriptional Level by the RNA-Binding Proteins HuR and AUF1. J. Biol. Chem. 2016, 291, 25823–25836. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, L.; Zhang, X.; Zhang, J. Autotaxin Is a Novel Target of MicroRNA-101-3p. FEBS Open Bio 2019, 9, 707–716. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Zhang, J.; Zhang, X. NSun2 Promotes Cell Migration through Methylating Autotaxin MRNA. J. Biol. Chem. 2020, 295, 18134–18147. [Google Scholar] [CrossRef]
- Wu, J.M.; Xu, Y.; Skill, N.J.; Sheng, H.; Zhao, Z.; Yu, M.; Saxena, R.; Maluccio, M.A. Autotaxin Expression and Its Connection with the TNF-Alpha-NF-KappaB Axis in Human Hepatocellular Carcinoma. Mol. Cancer 2010, 9, 71. [Google Scholar] [CrossRef]
- Song, J.; Guan, M.; Zhao, Z.; Zhang, J. Type I Interferons Function as Autocrine and Paracrine Factors to Induce Autotaxin in Response to TLR Activation. PLoS ONE 2015, 10, e01366292015. [Google Scholar] [CrossRef]
- Pradère, J.P.; Tarnus, E.; Grès, S.; Valet, P.; Saulnier-Blache, J.S. Secretion and Lysophospholipase D Activity of Autotaxin by Adipocytes Are Controlled by N-Glycosylation and Signal Peptidase. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 93–102. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, B.; Xiong, C.; Zhang, X.; Zhang, X.; Zhang, J. Selective Export of Autotaxin from the Endoplasmic Reticulum. J. Biol. Chem. 2017, 292, 7011–7022. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Zhao, Y.Y.; Curtis, J.M.; McMullen, T.P.W.; Brindley, D.N. Regulation of Autotaxin Expression and Secretion by Lysophosphatidate and Sphingosine 1-Phosphate. J. Lipid Res. 2015, 56, 1134–1144. [Google Scholar] [CrossRef]
- Van Meeteren, L.A.; Ruurs, P.; Christodoulou, E.; Goding, J.W.; Takakusa, H.; Kikuchi, K.; Perrakis, A.; Nagano, T.; Moolenaar, W.H. Inhibition of Autotaxin by Lysophosphatidic Acid and Sphingosine 1-Phosphate. J. Biol. Chem. 2005, 280, 21155–21161. [Google Scholar] [CrossRef]
- Jansen, S.; Andries, M.; Derua, R.; Waelkens, E.; Bollen, M. Domain Interplay Mediated by an Essential Disulfide Linkage Is Critical for the Activity and Secretion of the Metastasis-Promoting Enzyme Autotaxin. J. Biol. Chem. 2009, 284, 14296–14302. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in Cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Andries, M.; Vekemans, K.; Vanbilloen, H.; Verbruggen, A.; Bollen, M. Rapid Clearance of the Circulating Metastatic Factor Autotaxin by the Scavenger Receptors of Liver Sinusoidal Endothelial Cells. Cancer Lett. 2009, 284, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Nakanaga, K.; Hama, K.; Aoki, J. Autotaxin—An LPA Producing Enzyme with Diverse Functions. J. Biochem. 2010, 148, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, K.; Aoki, J.; Taira, A.; Tsujimoto, M.; Arai, H.; Inoue, K. Lysophosphatidic Acid (LPA) Receptors of the EDG Family Are Differentially Activated by LPA Species. FEBS Lett. 2000, 478, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H. Development of Our Current Understanding of Bioactive Lysophospholipids. Ann. NY Acad. Sci. 2000, 905, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Ko, Y.M.; Mcmullen, T.P.W.; Brindley, D.N.; Baksh, S.; Blandino, G.; Just, W. Autotaxin in the Crosshairs: Taking Aim at Cancer and Other Inflammatory Conditions. FEBS Lett. 2014, 588, 2712–2727. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, H.; Soma, O.; Goji, J.; Nishimura, N.; Narita, M.; Inazawa, J.; Nakamura, H.; Sano, K. Molecular Cloning and Chromosomal Assignment of the Human Brain-Type Phosphodiesterase I/Nucleotide Pyrophosphatase Gene (PDNP2). Genomics 1995, 30, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, N.C.; Chun, J. Promising Pharmacological Directions in the World of Lysophosphatidic Acid Signaling. Biomol. Ther. 2015, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; de Spohr, T.C.L.S.; do Amaral, R.F.; da Fonseca, A.C.C.; Garcia, C.; de Mendes, F.A.; Freitas, C.; dos Santos, M.F.; Lima, F.R.S. Role of Lysophosphatidic Acid and Its Receptors in Health and Disease: Novel Therapeutic Strategies. Signal Transduct. Target Ther. 2021, 6, 45. [Google Scholar] [CrossRef]
- Contos, J.J.A.; Ishii, I.; Chun, J. Lysophosphatidic Acid Receptors. Mol. Pharmacol. 2000, 58, 1188–1196. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid Receptor Nomenclature Review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an Intracellular Receptor for Lysophosphatidic Acid (LPA): LPA Is a Transcellular PPARγ Agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hayashibaral, Y.; Matsuda, H.; Onoi, M.; Mitsumori, M.; Tanaka, M.; Aoki, J.; Arai, H.; Noji, S. Diversified Expression Patterns of Autotaxin, a Gene for Phospholipid-Generating Enzyme during Mouse and Chicken Development. Dev. Dyn. 2007, 236, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Bächner, D.; Ahrens, M.; Betat, N.; Schröder, D.; Gross, G. Developmental Expression Analysis of Murine Autotaxin (ATX). Mech. Dev. 1999, 84, 121–125. [Google Scholar] [CrossRef]
- Katsifa, A.; Kaffe, E.; Nikolaidou-Katsaridou, N.; Economides, A.N.; Newbigging, S.; McKerlie, C.; Aidinis, V. The Bulk of Autotaxin Activity Is Dispensable for Adult Mouse Life. PLoS ONE 2015, 10, e01430832015. [Google Scholar] [CrossRef]
- Kanda, H.; Newton, R.; Klein, R.; Morita, Y.; Gunn, M.D.; Rosen, S.D. Autotaxin, an Ectoenzyme That Produces Lysophosphatidic Acid, Promotes the Entry of Lymphocytes into Secondary Lymphoid Organs. Nat. Immunol. 2008, 9, 415–423. [Google Scholar] [CrossRef]
- Sun, S.; Wang, R.; Song, J.; Guan, M.; Li, N.; Zhang, X.; Zhao, Z.; Zhang, J. Blocking Gp130 Signaling Suppresses Autotaxin Expression in Adipocytes and Improves Insulin Sensitivity in Diet-Induced Obesity. J. Lipid Res. 2017, 58, 2102–2113. [Google Scholar] [CrossRef]
- D’Souza, K.; Kane, D.A.; Touaibia, M.; Kershaw, E.E.; Pulinilkunnil, T.; Kienesberger, P.C. Autotaxin Is Regulated by Glucose and Insulin in Adipocytes. Endocrinology 2017, 158, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Ferry, G.; Tellier, E.; Try, A.; Grés, S.; Naime, I.; Simon, M.F.; Rodriguez, M.; Boucher, J.; Tack, I.; Gesta, S.; et al. Autotaxin Is Released from Adipocytes, Catalyzes Lysophosphatidic Acid Synthesis, and Activates Preadipocyte Proliferation. Up-Regulated Expression with Adipocyte Differentiation and Obesity. J. Biol. Chem. 2003, 278, 18162–18169. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Nagasaki, M.; Okudaira, S.; Aoki, J.; Ohmori, T.; Ohkawa, R.; Nakamura, K.; Igarashi, K.; Yamashita, H.; Eto, K.; et al. ENPP2 Contributes to Adipose Tissue Expansion and Insulin Resistance in Diet-Induced Obesity. Diabetes 2014, 63, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Nsaibia, M.J.; Mahmut, A.; Boulanger, M.C.; Arsenault, B.J.; Bouchareb, R.; Simard, S.; Witztum, J.L.; Clavel, M.A.; Pibarot, P.; Bossé, Y.; et al. Autotaxin Interacts with Lipoprotein(a) and Oxidized Phospholipids in Predicting the Risk of Calcific Aortic Valve Stenosis in Patients with Coronary Artery Disease. J. Intern. Med. 2016, 280, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; Mueller, P.; Yang, F.; Brandon, J.A.; Morris, A.J. Arguing the Case for the Autotaxin-Lysophosphatidic Acid-Lipid Phosphate Phosphatase 3-Signaling Nexus in the Development and Complications of Atherosclerosis. Arter. Biol. 2014, 34, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Miyauchi, K.; Ohkawa, R.; Nakamura, K.; Kurano, M.; Kishimoto, T.; Yanagisawa, N.; Ogita, M.; Miyazaki, T.; Nishino, A.; et al. Increased Lysophosphatidic Acid Levels in Culprit Coronary Arteries Ofpatients with Acute Coronary Syndrome. Atherosclerosis 2013, 229, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Rajagopalan, L.E. Rho Kinase-2 Activation in Human Endothelial Cells Drives Lysophosphatidic Acid-Mediated Expression of Cell Adhesion Molecules via NF-ΚB P65. J. Biol. Chem. 2010, 285, 12536–12542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Subramanian, P.; Sevilmis, G.; Globke, B.; Soehnlein, O.; Karshovska, E.; Megens, R.; Heyll, K.; Chun, J.; Saulnier-Blache, J.S.; et al. Lipoprotein-Derived Lysophosphatidic Acid Promotes Atherosclerosis by Releasing CXCL1 from the Endothelium. Cell Metab. 2011, 13, 592–600. [Google Scholar] [CrossRef]
- Lin, C.I.; Chen, C.N.; Chen, J.H.; Lee, H. Lysophospholipids Increase IL-8 and MCP-1 Expressions in Human Umbilical Cord Vein Endothelial Cells through an IL-1-Dependent Mechanism. J. Cell Biochem. 2006, 99, 1216–1232. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Niu, J.P.; Zhang, Z.J. Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves P38 Mitogen-Activated Protein Kinase Pathway Activation. Int. J. Mol. Sci. 2009, 10, 3194–3208. [Google Scholar] [CrossRef]
- Bot, M.; De Jager, S.C.A.; MacAleese, L.; Lagraauw, H.M.; Van Berkel, T.J.C.; Quax, P.H.A.; Kuiper, J.; Heeren, R.M.A.; Biessen, E.A.L.; Bot, I. Lysophosphatidic Acid Triggers Mast Cell-Driven Atherosclerotic Plaque Destabilization by Increasing Vascular Inflammation. J. Lipid Res. 2013, 54, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ochoa, L.N.; Kagan, A.; Chai, H.; Liang, Z.; Lin, P.H.; Yao, Q. Lysophosphatidic Acid Causes Endothelial Dysfunction in Porcine Coronary Arteries and Human Coronary Artery Endothelial Cells. Atherosclerosis 2012, 222, 74–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.-C.M.; Krummel, M.F.; Rosen, S.D. Autotaxin through Lysophosphatidic Acid Stimulates Polarization, Motility, and Transendothelial Migration of Naive T Cells. J. Immunol. 2012, 189, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of Human Plasma Lysophospholipase D, a Lysophosphatidic Acid-Producing Enzyme, as Autotaxin, a Multifunctional Phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Nagamatsu, T.; Schust, D.J.; Kawai-Iwasawa, Y.; Kawana, K.; Yamashita, T.; Osuga, Y.; Aoki, J.; Yatomi, Y.; Fujii, T. Placental Autotaxin Expression Is Diminished in Women with Pre-Eclampsia. J. Obstet. Gynaecol. Res. 2015, 41, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Fujii, T.; Iwasawa, Y.; Nakamura, K.; Ohkawa, R.; Igarashi, K.; Okudaira, S.; Ikeda, H.; Kozuma, S.; Aoki, J.; et al. Serum Autotaxin Measurements in Pregnant Women: Application for the Differentiation of Normal Pregnancy and Pregnancy-Induced Hypertension. Clin. Acta 2011, 412, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of Autotaxin and Lysophosphatidic Acid Receptors Increases Mammary Tumorigenesis, Invasion, and Metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yoshikawa, K.; Tanabe, E.; Kitayoshi, M.; Fukui, R.; Fukushima, N.; Tsujiuchi, T. Opposite Roles of LPA1 and LPA3 on Cell Motile and Invasive Activities of Pancreatic Cancer Cells. Tumor. Biol. 2012, 33, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Clair, T.; Campo, C.K.; Lee, H.Y.; Liotta, L.A.; Stracke, M.L. Autotaxin (ATX), a Potent Tumor Motogen, Augments Invasive and Metastatic Potential of Ras-Transformed Cells. Oncogene 2000, 19, 241–247. [Google Scholar] [CrossRef]
- Centonze, M.; Di Conza, G.; Lahn, M.; Fabregat, I.; Dituri, F.; Gigante, I.; Serino, G.; Scialpi, R.; Carrieri, L.; Negro, R.; et al. Autotaxin Inhibitor IOA-289 Reduces Gastrointestinal Cancer Progression in Preclinical Models. J. Exp. Clin. Cancer Res. 2023, 42, 197. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Murph, M.M.; Lu, Y.; Liu, S.; Hall, H.S.; Liu, J.; Stephens, C.; Fang, X.; Mills, G.B. Lysophosphatidic Acid Receptors Determine Tumorigenicity and Aggressiveness of Ovarian Cancer Cells. JNCI J. Natl. Cancer Inst. 2008, 100, 1630–1642. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, K.J.; Panupinthu, N.; Yu, S.; Lee, J.; Han, J.W.; Kim, J.M.; Lee, J.S.; Kang, J.; Park, C.G.; et al. Lysophosphatidic Acid Augments Human Hepatocellular Carcinoma Cell Invasion through LPA1 Receptor and MMP-9 Expression. Oncogene 2010, 30, 1351–1359. [Google Scholar] [CrossRef]
- Peng, W.T.; Sun, W.Y.; Li, X.R.; Sun, J.C.; Du, J.J.; Wei, W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, 1366. [Google Scholar] [CrossRef]
- Mazzocca, A.; Dituri, F.; De Santis, F.; Filannino, A.; Lopane, C.; Betz, R.C.; Li, Y.Y.; Mukaida, N.; Winter, P.; Tortorella, C.; et al. Lysophosphatidic Acid Receptor LPAR6 Supports the Tumorigenicity of Hepatocellular Carcinoma. Cancer Res. 2015, 75, 532–543. [Google Scholar] [CrossRef]
- Popnikolov, N.K.; Dalwadi, B.H.; Thomas, J.D.; Johannes, G.J.; Imagawa, W.T. Association of Autotaxin and Lysophosphatidic Acid Receptor 3 with Aggressiveness of Human Breast Carcinoma. Tumour. Biol. 2012, 33, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Targeting Lysophosphatidic Acid Receptor Type 1 with Debio 0719 Inhibits Spontaneous Metastasis Dissemination of Breast Cancer Cells Independently of Cell Proliferation and Angiogenesis. Available online: https://www.spandidos-publications.com/10.3892/ijo.2011.1309 (accessed on 4 April 2024).
- Mazzocca, A.; Dituri, F.; Lupo, L.; Quaranta, M.; Antonaci, S.; Giannelli, G. Tumor-Secreted Lysophostatidic Acid Accelerates Hepatocellular Carcinoma Progression by Promoting Differentiation of Peritumoral Fibroblasts in Myofibroblasts. Hepatology 2011, 54, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in Cancer, Vascular, Rheumatoid and Other Disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Hu, Y.L.; Tee, M.K.; Goetzl, E.J.; Auersperg, N.; Mills, G.B.; Ferrara, N.; Jaffe, R.B. Lysophosphatidic Acid Induction of Vascular Endothelial Growth Factor Expression in Human Ovarian Cancer Cells. JNCI J. Natl. Cancer Inst. 2001, 93, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Miyamoto, S.; Onoyama, I.; Sonoda, K.; Mekada, E.; Nakano, H. Expression of Lysophosphatidic Acid Receptors and Vascular Endothelial Growth Factor Mediating Lysophosphatidic Acid in the Development of Human Ovarian Cancer. Cancer Lett. 2003, 192, 161–169. [Google Scholar] [CrossRef]
- Jeon, E.S.; Heo, S.C.; Lee, I.H.; Choi, Y.J.; Park, J.H.; Choi, K.U.; Park, D.Y.; Suh, D.S.; Yoon, M.S.; Kim, J.H. Ovarian Cancer-Derived Lysophosphatidic Acid Stimulates Secretion of VEGF and Stromal Cell-Derived Factor-1α from Human Mesenchymal Stem Cells. Exp. Mol. Med. 2010, 42, 280–293. [Google Scholar] [CrossRef]
- Bachelier, R.; Confavreux, C.B.; Peyruchaud, O.; Croset, M.; Goehrig, D.; Van Der Pluijm, G.; Clézardin, P. Combination of Anti-Angiogenic Therapies Reduces Osteolysis and Tumor Burden in Experimental Breast Cancer Bone Metastasis. Int. J. Cancer 2014, 135, 1319–1329. [Google Scholar] [CrossRef]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The Role of the VEGF-C/VEGFR-3 Axis in Cancer Progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Burton, J.B.; Priceman, S.J.; Sung, J.L.; Brakenhielm, E.; Dong, S.A.; Pytowski, B.; Alitalo, K.; Wu, L. Suppression of Prostate Cancer Nodal and Systemic Metastasis by Blockade of the Lymphangiogenic Axis. Cancer Res. 2008, 68, 7828–7837. [Google Scholar] [CrossRef]
- Lin, C.E.; Chen, S.U.; Lin, C.C.; Chang, C.H.; Lin, Y.C.; Tai, Y.L.; Shen, T.L.; Lee, H. Lysophosphatidic Acid Enhances Vascular Endothelial Growth Factor-C Expression in Human Prostate Cancer PC-3 Cells. PLoS ONE 2012, 7, e41096. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, C.C.; Chen, W.M.; Lu, K.Y.; Shen, T.L.; Jou, Y.C.; Shen, C.H.; Ohbayashi, N.; Kanaho, Y.; Huang, Y.L.; et al. LPA1/3 Signaling Mediates Tumor Lymphangiogenesis through Promoting CRT Expression in Prostate Cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 1305–1315. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Qiu, G.Z.; Jin, M.Z.; Dai, J.X.; Sun, W.; Feng, J.H.; Jin, W.L. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 669–686. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour Acidosis: From the Passenger to the Driver’s Seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Radhakrishnan, R.; Jayaraman, M.; Yan, M.; Ward, J.D.; Fung, K.M.; Moxley, K.; Sood, A.K.; Isidoro, C.; Mukherjee, P.; et al. Lpa Induces Metabolic Reprogramming in Ovarian Cancer via a Pseudohypoxic Response. Cancer Res. 2018, 78, 1923–1934. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Ha, J.H.; Jayaraman, M.; Liu, J.; Moxley, K.M.; Isidoro, C.; Sood, A.K.; Song, Y.S.; Dhanasekaran, D.N. Ovarian Cancer Cell-Derived Lysophosphatidic Acid Induces Glycolytic Shift and Cancer-Associated Fibroblast-Phenotype in Normal and Peritumoral Fibroblasts. Cancer Lett. 2019, 442, 464–474. [Google Scholar] [CrossRef]
- Mertens, J.C.; Fingas, C.D.; Christensen, J.D.; Smoot, R.L.; Bronk, S.F.; Werneburg, N.W.; Gustafson, M.P.; Dietz, A.B.; Roberts, L.R.; Sirica, A.E.; et al. Therapeutic Effects of Deleting Cancer-Associated Fibroblasts in Cholangiocarcinoma. Cancer Res. 2013, 73, 897–907. [Google Scholar] [CrossRef]
- Cadamuro, M.; Nardo, G.; Indraccolo, S.; Dall’Olmo, L.; Sambado, L.; Moserle, L.; Franceschet, I.; Colledan, M.; Massani, M.; Stecca, T.; et al. Platelet-Derived Growth Factor-D and Rho GTPases Regulate Recruitment of Cancer-Associated Fibroblasts in Cholangiocarcinoma. Hepatology 2013, 58, 1042–1053. [Google Scholar] [CrossRef]
- Dituri, F.; Mancarella, S.; Serino, G.; Chaoul, N.; Lupo, L.G.; Villa, E.; Fabregat, I.; Giannelli, G. Direct and Indirect Effect of TGFβ on Treg Transendothelial Recruitment in HCC Tissue Microenvironment. Int. J. Mol. Sci. 2021, 22, 11765. [Google Scholar] [CrossRef]
- Castellana, M.; Donghia, R.; Guerra, V.; Procino, F.; Castellana, F.; Zupo, R.; Lampignano, L.; Sardone, R.; De Pergola, G.; Romanelli, F.; et al. Fibrosis-4 Index vs Nonalcoholic Fatty Liver Disease Fibrosis Score in Identifying Advanced Fibrosis in Subjects With Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Am. J. Gastroenterol. 2021, 116, 1833–1841. [Google Scholar] [CrossRef]
- Dituri, F.; Cossu, C.; Mancarella, S.; Giannelli, G. The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells 2019, 8, 1130. [Google Scholar] [CrossRef]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef]
- Malfettone, A.; Soukupova, J.; Bertran, E.; Crosas-Molist, E.; Lastra, R.; Fernando, J.; Koudelkova, P.; Rani, B.; Fabra, Á.; Serrano, T.; et al. Transforming Growth Factor-β-Induced Plasticity Causes a Migratory Stemness Phenotype in Hepatocellular Carcinoma. Cancer Lett. 2017, 392, 39–50. [Google Scholar] [CrossRef]
- Dituri, F.; Mancarella, S.; Cigliano, A.; Chieti, A.; Giannelli, G. TGF-β as Multifaceted Orchestrator in HCC Progression: Signaling, EMT, Immune Microenvironment, and Novel Therapeutic Perspectives. Semin. Liver Dis. 2019, 39, 53–69. [Google Scholar] [CrossRef]
- Dituri, F.; Serio, G.; Filannino, D.; Mascolo, A.; Sacco, R.; Villa, E.; Giannelli, G. Circulating TGF-Β1-Related Biomarkers in Patients with Hepatocellular Carcinoma and Their Association with HCC Staging Scores. Cancer Lett. 2014, 353, 264–271. [Google Scholar] [CrossRef]
- Fouassier, L.; Marzioni, M.; Afonso, M.B.; Dooley, S.; Gaston, K.; Giannelli, G.; Rodrigues, C.M.P.; Lozano, E.; Mancarella, S.; Segatto, O.; et al. Signalling Networks in Cholangiocarcinoma: Molecular Pathogenesis, Targeted Therapies and Drug Resistance. Liver Int. 2019, 39 (Suppl. S1), 43–62. [Google Scholar] [CrossRef]
- Mancarella, S.; Gigante, I.; Serino, G.; Pizzuto, E.; Dituri, F.; Valentini, M.F.; Wang, J.; Chen, X.; Armentano, R.; Calvisi, D.F.; et al. Crenigacestat Blocking Notch Pathway Reduces Liver Fibrosis in the Surrounding Ecosystem of Intrahepatic CCA ViaTGF-β Inhibition. J. Exp. Clin. Cancer Res. 2022, 41, 331. [Google Scholar] [CrossRef]
- Mancarella, S.; Krol, S.; Crovace, A.; Leporatti, S.; Dituri, F.; Frusciante, M.; Giannelli, G. Validation of Hepatocellular Carcinoma Experimental Models for TGF-β Promoting Tumor Progression. Cancers 2019, 11, 1510. [Google Scholar] [CrossRef]
- Buechler, M.B.; Turley, S.J. A Short Field Guide to Fibroblast Function in Immunity. Semin. Immunol. 2018, 35, 48–58. [Google Scholar] [CrossRef]
- Eiro, N.; González, L.; Martínez-Ordoñez, A.; Fernandez-Garcia, B.; González, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-Associated Fibroblasts Affect Breast Cancer Cell Gene Expression, Invasion and Angiogenesis. Cell. Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef]
- Bu, L.; Baba, H.; Yoshida, N.; Miyake, K.; Yasuda, T.; Uchihara, T.; Tan, P.; Ishimoto, T. Biological Heterogeneity and Versatility of Cancer-Associated Fibroblasts in the Tumor Microenvironment. Oncogene 2019, 38, 4887–4901. [Google Scholar] [CrossRef]
- Benyahia, Z.; Dussault, N.; Cayol, M.; Sigaud, R.; Berenguer-Daizé, C.; Delfino, C.; Tounsi, A.; Garcia, S.; Martin, P.-M.; Mabrouk, K.; et al. Stromal Fibroblasts Present in Breast Carcinomas Promote Tumor Growth and Angiogenesis through Adrenomedullin Secretion. Oncotarget 2017, 8, 15744–15762. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure—An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 525594. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Reinartz, S.; Lieber, S.; Pesek, J.; Brandt, D.T.; Asafova, A.; Finkernagel, F.; Watzer, B.; Nockher, W.A.; Nist, A.; Stiewe, T.; et al. Cell Type-Selective Pathways and Clinical Associations of Lysophosphatidic Acid Biosynthesis and Signaling in the Ovarian Cancer Microenvironment. Mol. Oncol. 2019, 13, 185–201. [Google Scholar] [CrossRef]
- Ray, R.; Rai, V. Lysophosphatidic Acid Converts Monocytes into Macrophages in Both Mice and Humans. Blood 2017, 129, 1177–1183. [Google Scholar] [CrossRef]
- Slaney, C.Y.; Kershaw, M.H.; Darcy, P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014, 74, 7168–7174. [Google Scholar] [CrossRef]
- Mathew, D.; Kremer, K.N.; Strauch, P.; Tigyi, G.; Pelanda, R.; Torres, R.M. LPA5 Is an Inhibitory Receptor That Suppresses CD8 T-Cell Cytotoxic Function via Disruption of Early TCR Signaling. Front. Immunol. 2019, 10, 464289. [Google Scholar] [CrossRef]
- Oda, S.K.; Strauch, P.; Fujiwara, Y.; Al-Shami, A.; Oravecz, T.; Tigyi, G.; Pelanda, R.; Torres, R.M. Lysophosphatidic Acid Inhibits CD8 T Cell Activation and Control of Tumor Progression. Cancer Immunol. Res. 2013, 1, 245–255. [Google Scholar] [CrossRef]
- Knowlden, S.; Georas, S.N. The Autotaxin–LPA Axis Emerges as a Novel Regulator of Lymphocyte Homing and Inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef]
- Cortinovis, M.; Aiello, S.; Mister, M.; Conde-Knape, K.; Noris, M.; Novelli, R.; Solini, S.; Rodriguez Ordonez, P.Y.; Benigni, A.; Remuzzi, G. Autotaxin Inhibitor Protects from Chronic Allograft Injury in Rat Kidney Allotransplantation. Nephron 2020, 144, 38–48. [Google Scholar] [CrossRef]
- Takeda, A.; Kobayashi, D.; Aoi, K.; Sasaki, N.; Sugiura, Y.; Igarashi, H.; Tohya, K.; Inoue, A.; Hata, E.; Akahoshi, N.; et al. Fibroblastic Reticular Cell-Derived Lysophosphatidic Acid Regulates Confined Intranodal T-Cell Motility. Elife 2016, 5, e10561. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, Y.; He, Y.; Chen, Q.; Liu, H. Serum ATX as a Novel Biomarker for Breast Cancer. Medicine 2019, 98, e14973. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, 1. [Google Scholar] [CrossRef]
- Brindley, D.N.; Tang, X.; Meng, G.; Benesch, M.G.K. Role of Adipose Tissue-Derived Autotaxin, Lysophosphatidate Signaling, and Inflammation in the Progression and Treatment of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5938. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Tang, X.; Dewald, J.; Dong, W.F.; Mackey, J.R.; Hemmings, D.G.; McMullen, T.P.W.; Brindley, D.N. Tumor-Induced Inflammation in Mammary Adipose Tissue Stimulates a Vicious Cycle of Autotaxin Expression and Breast Cancer Progression. FASEB J. 2015, 29, 3990–4000. [Google Scholar] [CrossRef]
- Leblanc, R.; Lee, S.C.; David, M.; Bordet, J.C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clézardin, P.; et al. Interaction of Platelet-Derived Autotaxin with Tumor Integrin AVβ3 Controls Metastasis of Breast Cancer Cells to Bone. Blood 2014, 124, 3141–3150. [Google Scholar] [CrossRef]
- Varqa, A.N. Natural History of Ovarian Cancer. Ecancermedicalscience 2014, 8. [Google Scholar] [CrossRef]
- Schaner, M.E.; Ross, D.T.; Ciaravino, G.; Sørlie, T.; Troyanskaya, O.; Diehn, M.; Wang, Y.C.; Duran, G.E.; Sikic, T.L.; Caldeira, S.; et al. Gene Expression Patterns in Ovarian Carcinomas. Mol. Biol. Cell 2003, 14, 4376–4386. [Google Scholar] [CrossRef]
- Umezu-Goto, M.; Tanyi, J.; Lahad, J.; Liu, S.; Yu, S.; Lapushin, R.; Hasegawa, Y.; Lu, Y.; Trost, R.; Bevers, T.; et al. Lysophosphatidic Acid Production and Action: Validated Targets in Cancer? J. Cell Biochem. 2004, 92, 1115–1140. [Google Scholar] [CrossRef]
- Tokumura, A.; Tominaga, K.; Yasuda, K.; Kanzaki, H.; Kogure, K.; Fukuzawa, K. Lack of Significant Differences in the Corrected Activity of Lysophospholipase D, Producer of Phospholipid Mediator Lysophosphatidic Acid, in Incubated Serum from Women with and without Ovarian Tumors. Cancer 2002, 94, 141–151. [Google Scholar] [CrossRef]
- Ren, J.; Xiao, Y.J.; Singh, L.S.; Zhao, X.; Zhao, Z.; Feng, L.; Rose, T.M.; Prestwich, G.D.; Xu, Y. Lysophosphatidic Acid Is Constitutively Produced by Human Peritoneal Mesothelial Cells and Enhances Adhesion, Migration, and Invasion of Ovarian Cancer Cells. Cancer Res. 2006, 66, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Kwon, Y.W.; Jang, I.H.; Kim, D.K.; Lee, S.I.; Choi, E.J.; Kim, K.H.; Suh, D.S.; Lee, J.H.; Choi, K.U.; et al. Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem. Cells 2016, 34, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Laforgia, M.; Molinari, P.; Ugenti, I.; Gadaleta, C.D.; Porta, C.; Ranieri, G. Hepatic Arterial Infusion of Chemotherapy for Advanced Hepatobiliary Cancers: State of the Art. Cancers 2021, 13, 3091. [Google Scholar] [CrossRef]
- Laface, C.; Fedele, P.; Maselli, F.M.; Ambrogio, F.; Foti, C.; Molinari, P.; Ammendola, M.; Lioce, M.; Ranieri, G. Targeted Therapy for Hepatocellular Carcinoma: Old and New Opportunities. Cancers 2022, 14, 4028. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Ranieri, G.; Maselli, F.M.; Ambrogio, F.; Foti, C.; Ammendola, M.; Laterza, M.; Cazzato, G.; Memeo, R.; Mastrandrea, G.; et al. Immunotherapy and the Combination with Targeted Therapies for Advanced Hepatocellular Carcinoma. Cancers 2023, 15, 654. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.B.; Wu, J.; Lu, D.; Maluccio, M.A. Is Autotaxin (ENPP2) the Link between Hepatitis C and Hepatocellular Cancer? J. Gastrointest. Surg. 2007, 11, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Ikeda, H.; Nakamura, K.; Ohkawa, R.; Kume, Y.; Aoki, J.; Hama, K.; Okudaira, S.; Tanaka, M.; Tomiya, T.; et al. Both Plasma Lysophosphatidic Acid and Serum Autotaxin Levels Are Increased in Chronic Hepatitis C. J. Clin. Gastroenterol. 2007, 41, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte Autotaxin Expression Promotes Liver Fibrosis and Cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Sablone, S.; Fazio, V.; De Ceglia, D.; Porcelli, M.; Molinari, P.; Fucci, L.; Laface, C.; Gadaleta, C.D. A Patient With Stage III Locally Advanced Pancreatic Adenocarcinoma Treated With Intra-Arterial Infusion FOLFIRINOX: Impressive Tumoral Response and Death Due to Legionella Pneumophila Infection: A Unique Case Report. Front. Oncol. 2022, 12, 877334. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Laforgia, M.; Zito, A.F.; Loisi, D.; Zizzo, N.; Tamma, R.; Gadaleta, C.D.; Porcelli, M.; Currò, G.; Ammendola, M.; et al. Chymase-Positive Mast Cells Correlate with Tumor Angiogenesis: First Report in Pancreatic Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6862–6873. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Currò, G.; Laface, C.; Zuccalà, V.; Memeo, R.; Luposella, F.; Laforgia, M.; Zizzo, N.; Zito, A.; Loisi, D.; et al. Mast Cells Positive for C-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells 2021, 10, 444. [Google Scholar] [CrossRef]
- Laface, C.; Laforgia, M.; Molinari, P.; Foti, C.; Ambrogio, F.; Gadaleta, C.D.; Ranieri, G. Intra-Arterial Infusion Chemotherapy in Advanced Pancreatic Cancer: A Comprehensive Review. Cancers 2022, 14, 450. [Google Scholar] [CrossRef]
- Ranieri, G.; Laface, C. Loco-Regional and Systemic Chemotherapies for Hepato-Pancreatic Tumors: Integrated Treatments. Cancers 2020, 12, 2737. [Google Scholar] [CrossRef]
- Laface, C.; Memeo, R.; Maselli, F.M.; Santoro, A.N.; Iaia, M.L.; Ambrogio, F.; Laterza, M.; Cazzato, G.; Guarini, C.; De Santis, P.; et al. Immunotherapy and Pancreatic Cancer: A Lost Challenge? Life 2023, 13, 1482. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.; Cui, J.J.; Feng, X.; Huang, Q. The Critical Role and Potential Target of the Autotaxin/Lysophosphatidate Axis in Pancreatic Cancer. Tumour. Biol. 2017, 39, 1010428317694544. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, L.; Yang, J.; Shi, Z. ATX/LPA Axis Regulates FAK Activation, Cell Proliferation, Apoptosis, and Motility in Human Pancreatic Cancer Cells. Vitr. Cell Dev. Biol. Anim. 2022, 58, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jinno, N.; Yoshida, M.; Hayashi, K.; Naitoh, I.; Hori, Y.; Natsume, M.; Kato, A.; Kachi, K.; Asano, G.; Atsuta, N.; et al. Autotaxin in Ascites Promotes Peritoneal Dissemination in Pancreatic Cancer. Cancer Sci. 2021, 112, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- McLendon, R.E.; Halperin, E.C. Is the Long-Term Survival of Patients with Intracranial Glioblastoma Multiforme Overstated? Cancer 2003, 98, 1745–1748. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Mariani, L.; Wies, J.; Woyke, T.; Berens, T.J.; McDonough, W.S.; Sloan, A.; Coons, S.W.; Berens, M.E. Gene Expression Profile of Glioblastoma Multiforme Invasive Phenotype Points to New Therapeutic Targets. Neoplasia 2005, 7, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Okudaira, S.; Tanaka, M.; Hama, K.; Shida, D.; Kitayama, J.; Yamori, T.; Aoki, J.; Fujimaki, T.; Arai, H. Autotaxin Is Overexpressed in Glioblastoma Multiforme and Contributes to Cell Motility of Glioblastoma by Converting Lysophosphatidylcholine TO Lysophosphatidic Acid. J. Biol. Chem. 2006, 281, 17492–17500. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Nakada, M.; Demuth, T.; Rosensteel, T.; Reavie, L.B.; Berens, M.E. Autotaxin: A Secreted Autocrine/Paracrine Factor That Promotes Glioma Invasion. J. Neurooncol. 2008, 86, 297–309. [Google Scholar] [CrossRef]
- Cholia, R.P.; Dhiman, M.; Kumar, R.; Mantha, A.K. Oxidative Stress Stimulates Invasive Potential in Rat C6 and Human U-87 MG Glioblastoma Cells via Activation and Cross-Talk between PKM2, ENPP2 and APE1 Enzymes. Metab. Brain Dis. 2018, 33, 1307–1326. [Google Scholar] [CrossRef]
- Bhave, S.R.; Dadey, D.Y.A.; Karvas, R.M.; Ferraro, D.J.; Kotipatruni, R.P.; Jaboin, J.J.; Hallahan, A.N.; DeWees, T.A.; Linkous, A.G.; Hallahan, D.E.; et al. Autotaxin Inhibition with PF-8380 Enhances the Radiosensitivity of Human and Murine Glioblastoma Cell Lines. Front. Oncol. 2013, 3, 56616. [Google Scholar] [CrossRef] [PubMed]
- St-Cœur, P.D.; Ferguson, D.; Morin, P.J.; Touaibia, M. PF-8380 and Closely Related Analogs: Synthesis and Structure–Activity Relationship towards Autotaxin Inhibition and Glioma Cell Viability. Arch. Pharm. 2013, 346, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xu, Y.; Fujiwara, Y.; Tsukahara, T.; Tsukahara, R.; Gajewiak, J.; Tigyi, G.; Prestwich, G.D. α-Substituted Phosphonate Analogues of Lysophosphatidic Acid (LPA) Selectively Inhibit Production and Action of LPA. ChemMedChem 2007, 2, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D.; Gajewiak, J.; Zhang, H.; Xu, X.; Yang, G.; Serban, M. Phosphatase-Resistant Analogues of Lysophosphatidic Acid: Agonists Promote Healing, Antagonists and Autotaxin Inhibitors Treat Cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2008, 1781, 588–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vidot, S.; Witham, J.; Agarwal, R.; Greenhough, S.; Bamrah, H.S.; Tigyi, G.J.; Kaye, S.B.; Richardson, A. Autotaxin Delays Apoptosis Induced by Carboplatin in Ovarian Cancer Cells. Cell. Signal. 2010, 22, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Tang, X.; Yang, Z.; Benesch, M.G.K.; Marshall, A.; Murray, D.; Hemmings, D.G.; Wuest, F.; McMullen, T.P.W.; Brindley, D.N. Implications for Breast Cancer Treatment from Increased Autotaxin Production in Adipose Tissue after Radiotherapy. FASEB J. 2017, 31, 4064–4077. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, D.A.; Gosmanova, E.; Johnson, L.R.; Tigyi, G. LPA Protects Intestinal Epithelial Cells from Apoptosis by Inhibiting the Mitochondrial Pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G821–G829. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Yang, Y.; Wang, J.; Li, Y.; Ma, H.; Cai, H.; Liu, X.; Zhang, Y.; Wang, S.; Li, Z.; et al. Lysophosphatidic Acid Inhibits Apoptosis Induced by Cisplatin in Cervical Cancer Cells. Biomed. Res. Int. 2015, 2015, 598386. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Kang, S.; Fishman, D.A. Lysophosphatidic Acid Inhibits Anti-Fas-Mediated Apoptosis Enhanced by Actin Depolymerization in Epithelial Ovarian Cancer. FEBS Lett. 2005, 579, 1311–1319. [Google Scholar] [CrossRef]
- Kang, Y.C.; Kim, K.M.; Lee, K.S.; Namkoong, S.; Lee, S.J.; Han, J.A.; Jeoung, D.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Serum Bioactive Lysophospholipids Prevent TRAIL-Induced Apoptosis via PI3K/Akt-Dependent CFLIP Expression and Bad Phosphorylation. Cell Death Differ. 2004, 11, 1287–1298. [Google Scholar] [CrossRef]
- Rusovici, R.; Ghaleb, A.; Shim, H.; Yang, V.W.; Yun, C.C. Lysophosphatidic Acid Prevents Apoptosis of Caco-2 Colon Cancer Cells via Activation of Mitogen-Activated Protein Kinase and Phosphorylation of Bad. Biochim. Biophys. Acta (BBA) Gen. Subj. 2007, 1770, 1194–1203. [Google Scholar] [CrossRef][Green Version]
- Huang, W.-C.; Chen, C.-L.; Lin, Y.-S.; Lin, C.-F. Apoptotic Sphingolipid Ceramide in Cancer Therapy. J. Lipids 2011, 2011, 565316. [Google Scholar] [CrossRef]
- Martínez, R.; Navarro, R.; Lacort, M.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B. Doxorubicin Induces Ceramide and Diacylglycerol Accumulation in Rat Hepatocytes through Independent Routes. Toxicol. Lett. 2009, 190, 86–90. [Google Scholar] [CrossRef]
- Charles, A.G.; Han, T.Y.; Liu, Y.Y.; Hansen, N.; Giuliano, A.E.; Cabot, M.C. Taxol-Induced Ceramide Generation and Apoptosis in Human Breast Cancer Cells. Cancer Chemother. Pharmacol. 2001, 47, 444–450. [Google Scholar] [CrossRef]
- Parra, V.; Eisner, V.; Chiong, M.; Criollo, A.; Moraga, F.; Garcia, A.; Härtel, S.; Jaimovich, E.; Zorzano, A.; Hidalgo, C.; et al. Changes in Mitochondrial Dynamics during Ceramide-Induced Cardiomyocyte Early Apoptosis. Cardiovasc. Res. 2008, 77, 387–397. [Google Scholar] [CrossRef]
- Gómez-Muñoz, A.; Waggoner, D.W.; O’Brien, L.; Brindley, D.N. Interaction of Ceramides, Sphingosine, and Sphingosine 1-Phosphate in Regulating DNA Synthesis and Phospholipase D Activity. J. Biol. Chem. 1995, 270, 26318–26325. [Google Scholar] [CrossRef]
- Frankel, A.; Mills, G.B. Peptide and Lipid Growth Factors Decrease Cis-Diamminedichloroplatinum- Induced Cell Death in Human Ovarian Cancer Cells. Clin. Cancer Res. 1996, 2, 1307–1313. [Google Scholar]
- Venkatraman, G.; Benesch, M.G.K.; Tang, X.; Dewald, J.; McMullen, T.P.W.; Brindley, D.N. Lysophosphatidate Signaling Stabilizes Nrf2 and Increases the Expression of Genes Involved in Drug Resistance and Oxidative Stress Responses: Implications for Cancer Treatment. FASEB J. 2015, 29, 772–785. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Tang, X.; Maeda, T.; Ohhata, A.; Zhao, Y.Y.; Kok, B.P.C.; Dewald, J.; Hitt, M.; Curtis, J.M.; McMullen, T.P.W.; et al. Inhibition of Autotaxin Delays Breast Tumor Growth and Lung Metastasis in Mice. FASEB J. 2014, 28, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Ko, Y.M.; Tang, X.; Dewald, J.; Lopez-Campistrous, A.; Zhao, Y.Y.; Lai, R.; Curtis, J.M.; Brindley, D.N.; McMullen, T.P.W. Autotaxin Is an Inflammatory Mediator and Therapeutic Target in Thyroid Cancer. Endocr. Relat. Cancer 2015, 22, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Kreuter, M.; Lederer, D.J.; Brown, K.K.; Wuyts, W.; Verbruggen, N.; Stutvoet, S.; Fieuw, A.; Ford, P.; Abi-Saab, W.; et al. Rationale, Design and Objectives of Two Phase III, Randomised, Placebo-Controlled Studies of GLPG1690, a Novel Autotaxin Inhibitor, in Idiopathic Pulmonary Fibrosis (ISABELA 1 and 2). BMJ Open Respir. Res. 2019, 6, e0004222019. [Google Scholar] [CrossRef]
- Tang, X.; Wuest, M.; Benesch, M.G.K.; Dufour, J.; Zhao, Y.Y.; Curtis, J.M.; Monjardet, A.; Heckmann, B.; Murray, D.; Wuest, F.; et al. Inhibition of Autotaxin with GLPG1690 Increases the Efficacy of Radiotherapy and Chemotherapy in a Mouse Model of Breast Cancer. Mol. Cancer Ther. 2020, 19, 63–74. [Google Scholar] [CrossRef]
- Hashimoto, S.; Mikami, S.; Sugino, H.; Yoshikawa, A.; Hashimoto, A.; Onodera, Y.; Furukawa, S.; Handa, H.; Oikawa, T.; Okada, Y.; et al. Lysophosphatidic Acid Activates Arf6 to Promote the Mesenchymal Malignancy of Renal Cancer. Nat. Commun. 2016, 7, 10656. [Google Scholar] [CrossRef]
- Okabe, K.; Hayashi, M.; Kato, K.; Okumura, M.; Fukui, R.; Honoki, K.; Fukushima, N.; Tsujiuchi, T. Lysophosphatidic Acid Receptor-3 Increases Tumorigenicity and Aggressiveness of Rat Hepatoma RH7777 Cells. Mol. Carcinog. 2013, 52, 247–254. [Google Scholar] [CrossRef]
- Gnocchi, D.; Kurzyk, A.; Mintrone, A.; Lentini, G.; Sabba, C.; Mazzocca, A. Inhibition of LPAR6 overcomes sorafenib resistance by switching glycolysis into oxidative phosphorylation in hepatocellular carcinoma. Biochimie 2022, 202, 180–189. [Google Scholar] [CrossRef]
- Hurst, J.H.; Hooks, S.B. Regulator of G-Protein Signaling (RGS) Proteins in Cancer Biology. Biochem. Pharmacol. 2009, 78, 1289–1297. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, N.; Jiang, Y.; Huang, Y.; Lian, Y.Y.; Liu, T.; Li, N.; Guan, G. RGS17 Inhibits Tumorigenesis and Improves 5-Fluorouracil Sensitivity in Nasopharyngeal Carcinoma. Onco Targets Ther. 2018, 11, 7591–7600. [Google Scholar] [CrossRef]
- Desroy, N.; Housseman, C.; Bock, X.; Joncour, A.; Bienvenu, N.; Cherel, L.; Labeguere, V.; Rondet, E.; Peixoto, C.; Grassot, J.M.; et al. Discovery of 2-[[2-Ethyl-6-[4-[2-(3-Hydroxyazetidin-1-Yl)-2-Oxoethyl]Piperazin-1-Yl]-8-Methylimidazo[1,2-a]Pyridin-3-Yl]Methylamino]-4-(4-Fluorophenyl)Thiazole-5-Carbonitrile (GLPG1690), a First-in-Class Autotaxin Inhibitor Undergoing Clinical Evaluation for the Treatment of Idiopathic Pulmonary Fibrosis. J. Med. Chem. 2017, 60, 3580–3590. [Google Scholar]
- Maher, T.M.; van der Aar, E.M.; Van de Steen, O.; Allamassey, L.; Desrivot, J.; Dupont, S.; Fagard, L.; Ford, P.; Fieuw, A.; Wuyts, W. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of GLPG1690, a Novel Autotaxin Inhibitor, to Treat Idiopathic Pulmonary Fibrosis (FLORA): A Phase 2a Randomised Placebo-Controlled Trial. Lancet Respir. Med. 2018, 6, 627–635. [Google Scholar] [CrossRef]
- Kato, K.; Ikeda, H.; Miyakawa, S.; Futakawa, S.; Nonaka, Y.; Fujiwara, M.; Okudaira, S.; Kano, K.; Aoki, J.; Morita, J.; et al. Structural Basis for Specific Inhibition of Autotaxin by a DNA Aptamer. Nat. Struct. Mol. Biol. 2016, 23, 395–401. [Google Scholar] [CrossRef]
- Albers, H.M.H.G.; Hendrickx, L.J.D.; Van Tol, R.J.P.; Hausmann, J.; Perrakis, A.; Ovaa, H. Structure-Based Design of Novel Boronic Acid-Based Inhibitors of Autotaxin. J. Med. Chem. 2011, 54, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lee, S.; Norman, D.D.; Tigyi, G.J. Designing Dual Inhibitors of Autotaxin-LPAR GPCR Axis. Molecules 2022, 27, 5487. [Google Scholar] [CrossRef] [PubMed]
- Swaney, J.S.; Chapman, C.; Correa, L.D.; Stebbins, K.J.; Bundey, R.A.; Prodanovich, P.C.; Fagan, P.; Baccei, C.S.; Santini, A.M.; Hutchinson, J.H.; et al. A Novel, Orally Active LPA(1) Receptor Antagonist Inhibits Lung Fibrosis in the Mouse Bleomycin Model. Br. J. Pharmacol. 2010, 160, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Distler, O.; Jagerschmidt, A.; Illiano, S.; Ledein, L.; Boitier, E.; Agueusop, I.; Denton, C.P.; Khanna, D. Lysophosphatidic Acid Receptor 1 Antagonist SAR100842 for Patients With Diffuse Cutaneous Systemic Sclerosis: A Double-Blind, Randomized, Eight-Week Placebo-Controlled Study Followed by a Sixteen-Week Open-Label Extension Study. Arthritis Rheumatol. 2018, 70, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Ledein, L.; Léger, B.; Dees, C.; Beyer, C.; Distler, A.; Vettori, S.; Boukaiba, R.; Bidouard, J.P.; Schaefer, M.; Pernerstorfer, J.; et al. Translational Engagement of Lysophosphatidic Acid Receptor 1 in Skin Fibrosis: From Dermal Fibroblasts of Patients with Scleroderma to Tight Skin 1 Mouse. Br. J. Pharmacol. 2020, 177, 4296–4309. [Google Scholar] [CrossRef]
- Aiello, S.; Casiraghi, F. Lysophosphatidic Acid: Promoter of Cancer Progression and of Tumor Microenvironment Development. A Promising Target for Anticancer Therapies? Cells 2021, 10, 1390. [Google Scholar] [CrossRef]
| Cancer | ATX/LPA Levels | ATX Inhibitors | ATX–LPA Pathway in Drug Resistance | Clinical Application |
|---|---|---|---|---|
| Breast cancer | - ATX blood levels are higher in BC patients - Threefold higher levels of ATX in tissue microenvironment of BC patients with respect to normal tissue from healthy individuals | - ONO-8430506 - GLPG1690 | - Downregulation of Nrf2 transcription factor | GLPG1690 has been evaluated in phase III clinical studies as a treatment of idiopathic pulmonary fibrosis (IPF) |
| Ovarian cancer | - ATX levels are twofold higher in cancer tissue than in normal tissue - High LPA levels in malignant ovarian ascites | - PF8380 - S32826 - BBT-877 | - Enhanced LPARs activity by downregulation of RGS10 and RGS17 - ATX plays a pivotal role in the maintenance of the CSC-like properties of epithelial ovarian tumors (PTX resistance) | ------ |
| Hepatocellular carcinoma | - Serum ATX and plasma LPA levels higher in HCC patients | - 9xanthenylacetate | - Resistance of HCC cells to sorafenib promoted by LPAR6 (switch from oxidative phosphorylation to lactic acid fermentation | ------ |
| Pancreatic cancer | - LPA levels are higher in serum and ascites in PC patients | - IOA-289 | - Role of LPAR3 in expression of genes correlated with multidrug resistance | A Phase 1b clinical trial is evaluating the safety and clinical efficacy of the ATX inhibitor IOA-289 alone and in combination with Gemcitabine/Nab-paclitaxel in patients with metastatic pancreatic cancer |
| Glioblastoma multiforme | - High levels of ATX in GMB cells | - PF-8380 | ------ |
| Research Topic | Setup of Treatment | Cancer Model | Role of ATX/LPA as Biomarker/Therapeutic Target | Role of ATX/LPA in Carcinogenesis | Role of ATX/LPA in Cancer Progression/Metastasis | Role of ATX/LPA in Drug Resistance | Reference |
|---|---|---|---|---|---|---|---|
| ENPP2 gene expression in breast cancer (BC) | Activation of ATX promoter by hormones | Transgenic mammary glands in mouse models | ATX/LPA overexpression | Induction of precancerous and cancerous lesions | Induction of lymph node metastases | - | [75] |
| Role of LPAR1/2/3 receptors in: - Pancreatic (PC), ovarian (OC), and liver (HCC) cancer cells, - Hamster pancreatic duct adenocarcinomas (PDAs) - breast cancer (BC) mouse model - BC patients | - LPAR 1/3 knockdown cells - Induction of PDAs by a nitroso compound - Forced over expression of LPAR3 in BC model - LPAR1 inhibition by Bebio-0719 - LPA stimulation of OC cells - Inhibition of LPAR1/3 by Ki16425 in PC mouse models | - PC, OC, and HCC cells - Hamster model - BC mouse model - BC patients | - LPA1 down regulation enhances cell motility - LPA3 down regulation inhibits cell motility - LPAR3 as prognostic marker in BC mouse model - Prognostic value of LPAR3 in BC patients | In PDA models, LPA1 is lower expressed, LPA3 is over-expressed | - LPA1/3 are involved in cell motility/invasion - LPAR3 induces metastasis in BC mouse model - LPAR3 higher expression correlates with higher tumor stage and lymph node metastases in BC patients - LPAR1 expression correlates with BC metastasis - LPAR2/3 stimulated VEGF expression in OC cells and OC biopsies - LPAR1/3 stimulated linphoangiogenesis through VEGFC expression in PC models - LPAR stimulate clycolytic shift in OC models | - | [75,76,79,80,81,83,84,88,89,94,95,99,100] |
| Role of LPAR6 in HCC patients and xenograft mouse model | RNAi-mediated attenuation of LPAR6 | - 128 HCC patient cohort - Xenograft mouse model | - Prognostic role of LPAR6 in HCC patients | Role of LPAR6 in HCC carcinogenesis | - | - | [82] |
| Role of ATX expression in different cancer models: - PDAs model - Different cancer models (HCC, PC, glioblastoma multiforme (GMB) - ATX overexpression in different human tumor tissues (BC, OC, HCC, PC, glioblastoma multiforme (GMB)) and in malignant ovarian and pancreatic ascites | - ATX-transfected NIH-3T3 cells - ENPP2 knockdown - ATX transfected/RAS-transformed NIH-3T3 cells - IOA-286 ATX-inhibitor - Oxidative stress - Pro-oncogenic substances - PF-8380 ATX-inhibitor - Alpha-bromomethylene phosphonate LPA (BrP-LPA) (ATX inhibitor) - ATX inhibitors (PF8380 or S32826) - Hepatotoxic stimuli | - PDA mouse models - Different tumor cell lines - Mouse models - Human tissues - Malignant ovarian ascites - Human PC serum an ascites - Ovarian cancer stem cells (CSCs) - Mouse models of chronic liver diseases | ATX expression as biomarkers of cancer cell growth - ATX as therapeutic target | - Treatment of PC cell with pro-oncogenic substances increased ATX levels - LPA stimulated profibrotic signals and activation of hepatic stellate cells playing key role in HCC carcinogenesis | - ATX expression in PDA growth and metastasis - ATX expression induced angiogenesis - ATX–LPA axis involved tumor growth and invasiveness - ATX may influence the maintenance of ovarian CSCs by LPA-mediated autocrine mechanism | - ATX-specific inhibitors suppressed radiation-induced tumor new vessel genesis | [32,77,78,129,136,137,138,139,140,144,145,146,153,154,155,158,159,160,161,162,163,164,165] |
| Role of LPA in fibroblast transformation in OC and HCC models | Tumor-derived LPA | Lung, ovarian, and HCC fibroblast | - Targeted inhibition of LPA-mediated metabolic reprogramming in CAFs | - | - LPA induced cancer associated fibroblast (CAFs) in peri tumor tissues in OC and HCC models | - Reduction of drug delivery to tumor | [85,90,100,117] |
| Role of ATX/LPA in immunosuppressive microenvironment | - Tumor-derived LPA - LPAR5−/− tumor-specific CD8+ T lymphocytes | - OC patients - Mouse models - Rat models | - Targeted inhibition of LPA-mediated tumor associated macrophages (TAMs) conversion | - ATX/LPA signals are involved in immune escape and carcinogenesis | - LPA induced monocytes conversion into TAMs - LPA-mediated LPAR5 stimulation suppressed CD8+ T cells activation - ATX inhibition decreased allograft infiltration of CD4+ and CD8+ T cells - LPA interaction with LPAR2 on naïve T lymphocytes improved T-cell migration across very small pores | [120,121,123,124,125,126,127] | |
| Role of ATX/LPA axis in drug resistance mechanisms | - Carboplatin - TSA - Radiotherapy - Camptothecin - Cisplatin - Anti-FAS - TRAIL - Etoposide - Cis-Diamminedichloroplatinum - Carboplatin - Doxorubicin+ ATX inhibitor(GLPG1690) - Sunitinib - Temsirolimus - ATX inhibitor (PF-8380) - ATX inhibitor (ONO-8430506) | - OC cells - BC, cervix, lung cell lines - BC patients - Rat intestinal epithelial cells (IEC-6) - CRC cells - Idiopathic pulmonary fibrosis (IPF) patients - BC mouse model - Renal cancer (RC) cells - PC cells - Human and murine GBM cell lines | ATX as therapeutic target: - Doxorubicin+GLPG1690 in BC mouse model and in idiopathic pulmonary fibrosis phase III clinical trial. - ATX inhibitor PF-8380 enhanced radiation effects in human and murine GBM cell lines. - ATX inhibitor ONO-8430506 in BC and thyroid cancers model reduced cell growth and inflammation. - LPAR1 antagonists (e.g., AM966, BMS-986020, SAR100842) tested on non-oncologic clinical trial, such as cutaneous systemic sclerosis and IPF | - ATX over-expression is involved in cancer progression and metastasis | - ATX counteracted apoptosis induced by: carboplatin, TSA, radiotherapy, camptothecin, anti-FAS, TRAIL, etoposide, Cis-Diamminedichloroplatinum, carboplatin - LPA induced genes involved in multi drug resistance - GLPG1690 increased doxorubicin effects in idiopathic pulmonary fibrosis - Knockdown of LPAR2 restored sensitivity to Sunitinib and Temsirolimus in RC model - LPAR3 induced multi drug resistance genes in PC cells - Lower level of RGSs proteins leaded to up regulation of LPAR inducing drug resistance in OC cells | [21,162,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,193,194,195,196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laface, C.; Ricci, A.D.; Vallarelli, S.; Ostuni, C.; Rizzo, A.; Ambrogio, F.; Centonze, M.; Schirizzi, A.; De Leonardis, G.; D’Alessandro, R.; et al. Autotaxin–Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7737. https://doi.org/10.3390/ijms25147737
Laface C, Ricci AD, Vallarelli S, Ostuni C, Rizzo A, Ambrogio F, Centonze M, Schirizzi A, De Leonardis G, D’Alessandro R, et al. Autotaxin–Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications. International Journal of Molecular Sciences. 2024; 25(14):7737. https://doi.org/10.3390/ijms25147737
Chicago/Turabian StyleLaface, Carmelo, Angela Dalia Ricci, Simona Vallarelli, Carmela Ostuni, Alessandro Rizzo, Francesca Ambrogio, Matteo Centonze, Annalisa Schirizzi, Giampiero De Leonardis, Rosalba D’Alessandro, and et al. 2024. "Autotaxin–Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications" International Journal of Molecular Sciences 25, no. 14: 7737. https://doi.org/10.3390/ijms25147737
APA StyleLaface, C., Ricci, A. D., Vallarelli, S., Ostuni, C., Rizzo, A., Ambrogio, F., Centonze, M., Schirizzi, A., De Leonardis, G., D’Alessandro, R., Lotesoriere, C., & Giannelli, G. (2024). Autotaxin–Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications. International Journal of Molecular Sciences, 25(14), 7737. https://doi.org/10.3390/ijms25147737









