Advancements in Personalized CAR-T Therapy: Comprehensive Overview of Biomarkers and Therapeutic Targets in Hematological Malignancies
Abstract
:1. Introduction
2. Acute Lymphoblastic Leukemia (ALL)
3. Non-Hodgkin Lymphoma (NHL)
4. Multiple Myeloma (MM)
5. Minimal/Measurable Residual Disease (MRD) in ALL, NHL, and MM
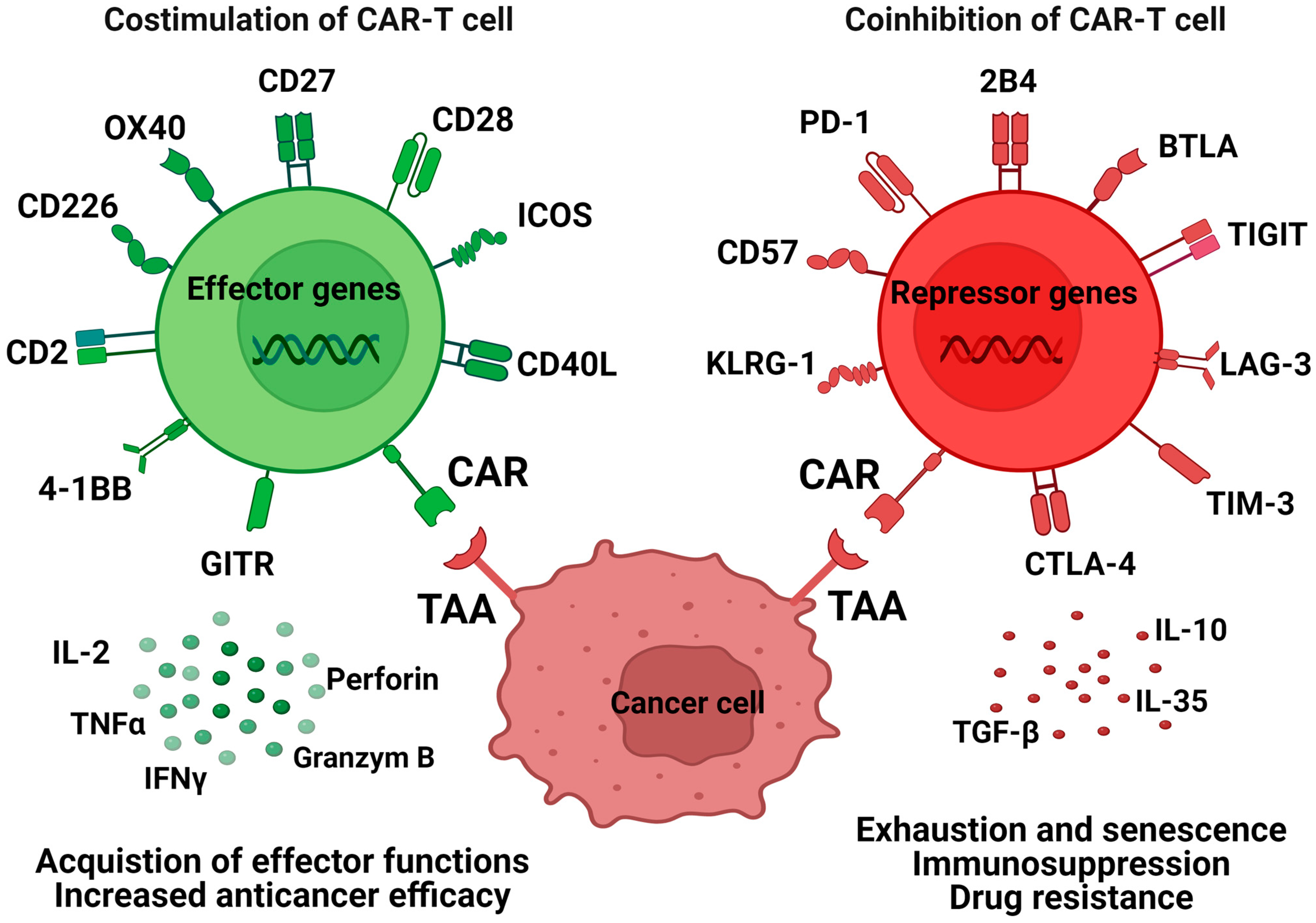
6. Co-Stimulation and Co-Inhibition of CAR-Ts
7. Exhaustion and Senescence Markers in CAR-T Therapy
8. Immunosuppressive Tumor Microenvironment in Hematological Malignancies
9. Cell-Free DNA as a Marker for MRD Monitoring
10. miRNAs as Markers in CAR-T Therapy
11. miRNAs as a Drug Resistance Marker
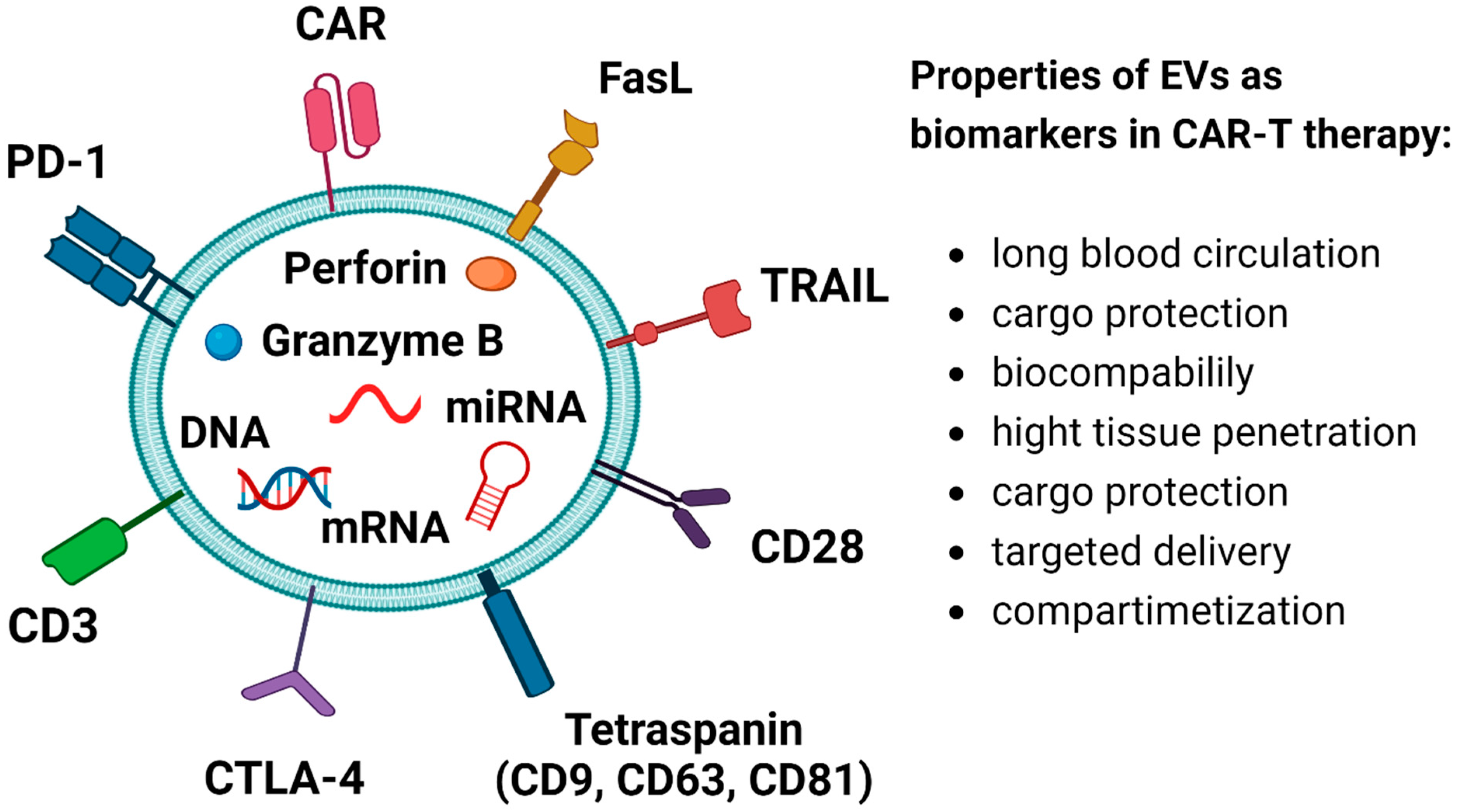
12. EVs as Markers in CAR-T Therapy
13. EVs in Drug Resistance of Hematological Malignancies
14. Combination Immunotherapy with CAR-Ts, Checkpoint Blockade, and Other Drugs
15. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, D.J.; Arany, Z.; Baur, J.A.; Epstein, J.A.; June, C.H. CAR T therapy beyond cancer: The evolution of a living drug. Nature 2023, 619, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Totmaj, M.A.; Abbasi, M.; Hajazimian, S.; Goleij, P.; Behroozi, J.; Shademan, B.; Isazadeh, A.; Baradaran, B. Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies. Cancer Med. 2023, 12, 7844–7858. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T-cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Bourbon, E.; Ghesquieres, H.; Bachy, E. CAR-T-cells, from principle to clinical applications. Bull. Cancer 2021, 108, S4–S17. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Roddie, C.; Bader, P.; Basak, G.W.; Bonig, H.; Bonini, C.; Chabannon, C.; Ciceri, F.; Corbacioglu, S.; Ellard, R.; et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar] [CrossRef]
- Yakoub-Agha, I.; Chabannon, C.; Bader, P.; Basak, G.W.; Bonig, H.; Ciceri, F.; Corbacioglu, S.; Duarte, R.F.; Einsele, H.; Hudecek, M.; et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020, 105, 297–316. [Google Scholar] [CrossRef]
- Tomasik, J.; Jasinski, M.; Basak, G.W. Next generations of CAR-T-cells—New therapeutic opportunities in hematology? Front. Immunol. 2022, 13, 1034707. [Google Scholar] [CrossRef]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem 2022, 3 (Suppl. 1), 6–10. [Google Scholar] [CrossRef]
- Khan, A.N.; Asija, S.; Pendhari, J.; Purwar, R. CAR-T-cell therapy in hematological malignancies: Where are we now and where are we heading for? Eur. J. Haematol. 2024, 112, 6–18. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions Related to CAR-T Cell Therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Gust, J.; Ponce, R.; Liles, W.C.; Garden, G.A.; Turtle, C.J. Cytokines in CAR T-Cell–Associated Neurotoxicity. Front. Immunol. 2020, 11, 577027. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Olejarz, W.; Basak, G. Modern Advances in CARs Therapy and Creating a New Approach to Future Treatment. Int. J. Mol. Sci. 2022, 23, 15006. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T-cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153. [Google Scholar] [CrossRef] [PubMed]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.-C. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef] [PubMed]
- Carniti, C.; Caldarelli, N.M.; Agnelli, L.; Torelli, T.; Ljevar, S.; Jonnalagadda, S.; Zanirato, G.; Fardella, E.; Stella, F.; Lorenzini, D.; et al. Monocytes in leukapheresis products affect the outcome of CD19–targeted CAR T-cell therapy in patients with lymphoma. Blood Adv. 2024, 8, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, S.; Dicataldo, M.; Casadei, B.; Storci, G.; Laprovitera, N.; Arpinati, M.; Maffini, E.; Cortelli, P.; Guarino, M.; Vaglio, F.; et al. Peripheral blood cellular profile at pre-lymphodepletion is associated with CD19-targeted CAR-T-cell-associated neurotoxicity. Front. Immunol. 2023, 13, 1058126. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Young, K.H. Checkpoint inhibitors in hematological malignancies. J. Hematol. Oncol. 2017, 10, 103. [Google Scholar] [CrossRef]
- Liu, D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: A new era of cancer therapy. J. Hematol. Oncol. 2019, 12, 113. [Google Scholar] [CrossRef]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Basak, G. Emerging Therapeutic Targets and Drug Resistance Mechanisms in Immunotherapy of Hematological Malignancies. Cancers 2023, 15, 5765. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gu, C.; Huang, L.; Wu, H.; Shi, J.; Zhang, Z.; Zhou, Y.; Zhou, J.; Gao, Y.; Liu, J.; et al. The third-generation anti-CD30 CAR T-cells specifically homing to the tumor and mediating powerful antitumor activity. Sci. Rep. 2022, 12, 10488. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Cancer biomarkers for targeted therapy. Biomark. Res. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo Clin. Proc. 2016, 91, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.J.; Faderl, S.; Kantarjian, H.M. Adult acute lymphoblastic leukemia. Mayo Clin. Proc. 2005, 80, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Alvarnas, J.C.; Brown, P.A.; Aoun, P.; Ballen, K.K.; Barta, S.K.; Borate, U.; Boyer, M.W.; Burke, P.W.; Cassaday, R.; Castro, J.E.; et al. Acute Lymphoblastic Leukemia, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1240–1279. [Google Scholar] [CrossRef] [PubMed]
- Chessells, J.M.; Harrison, G.; Richards, S.M.; Bailey, C.C.; Hill, F.G.; Gibson, B.E.; Hann, I.M. Down’s syndrome and acute lymphoblastic leukaemia: Clinical features and response to treatment. Arch. Dis. Child. 2001, 85, 321–325. [Google Scholar] [CrossRef]
- Bielorai, B.; Fisher, T.; Waldman, D.; Lerenthal, Y.; Nissenkorn, A.; Tohami, T.; Marek, D.; Amariglio, N.; Toren, A. Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia-telangiectasia variant. Pediatr. Hematol. Oncol. 2013, 30, 574–582. [Google Scholar] [CrossRef]
- Geriniere, L.; Bastion, Y.; Dumontet, C.; Salles, G.; Espinouse, D.; Coiffier, B. Heterogeneity of acute lymphoblastic leukemia in HIV-seropositive patients. Ann. Oncol. 1994, 5, 437–440. [Google Scholar] [CrossRef]
- Sehgal, S.; Mujtaba, S.; Gupta, D.; Aggarwal, R.; Marwaha, R.K. High incidence of Epstein Barr virus infection in childhood acute lymphocytic leukemia: A preliminary study. Indian. J. Pathol. Microbiol. 2010, 53, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, D.; Bassan, R.; Dombret, H.; Fielding, A.; Ribera, J.M.; Buske, C.; ESMO Guidelines Committee. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. 5), v69–v82. [Google Scholar] [CrossRef]
- Scavino, H.F.; George, J.N.; Sears, D.A. Remission induction in adult acute lymphocytic leukemia. Use of vincristine and prednisone alone. Cancer 1976, 38, 672–677. [Google Scholar] [CrossRef]
- Myers, R.M.; Li, Y.; Barz Leahy, A.; Barrett, D.M.; Teachey, D.T.; Callahan, C.; Fasano, C.C.; Rheingold, S.R.; DiNofia, A.; Wray, L.; et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2021, 39, 3044–3055. [Google Scholar] [CrossRef]
- Barrett, D.M.; Liu, X.; Jiang, S.; June, C.H.; Grupp, S.A.; Zhao, Y. Regimen-specific effects of RNA-modified chimeric antigen receptor T-cells in mice with advanced leukemia. Hum. Gene Ther. 2013, 24, 717–727. [Google Scholar] [CrossRef]
- Dombret, H.; Gabert, J.; Boiron, J.-M.; Rigal-Huguet, F.; Blaise, D.; Thomas, X.; Delannoy, A.; Buzyn, A.; Bilhou-Nabera, C.; Cayuela, J.-M.; et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood 2002, 100, 2357–2366. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Laetsch, T.W.; Maude, S.L.; Rives, S.; Hiramatsu, H.; Bittencourt, H.; Bader, P.; Baruchel, A.; Boyer, M.; De Moerloose, B.; Qayed, M.; et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J. Clin. Oncol. 2023, 41, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Bishop, M.R.; Oluwole, O.O.; Logan, A.C.; Baer, M.R.; Donnellan, W.B.; O’Dwyer, K.M.; Holmes, H.; Arellano, M.L.; Ghobadi, A.; et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 2021, 138, 11–22. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Nakamura, S.; Tsuzuki, T.; Satou, A. The Immunology of DLBCL. Cancers 2023, 15, 835. [Google Scholar] [CrossRef] [PubMed]
- Vockerodt, M.; Yap, L.-F.; Shannon-Lowe, C.; Curley, H.; Wei, W.; Vrzalikova, K.; Murray, P.G. The Epstein–Barr virus and the pathogenesis of lymphoma. J. Pathol. 2015, 235, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Harnessing the power of the immune system in non-Hodgkin lymphoma: Immunomodulators, checkpoint inhibitors, and beyond. Hematol. Am. Soc. Hematol. Educ. Program. 2017, 2017, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, K.; Brucher, H. Diagnostic and therapeutic problems in non-Hodgkin lymphomas. Blut 1981, 43, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Horning, S.J.; Hoppe, R.T.; Levy, R.; Rosenberg, S.A.; Sigal, B.M.; Warnke, R.A.; Natkunam, Y.; Han, S.S.; Yuen, A.; et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood 2013, 122, 981–987. [Google Scholar] [CrossRef]
- Yuen, A.R.; Kamel, O.W.; Halpern, J.; Horning, S.J. Long-term survival after histologic transformation of low-grade follicular lymphoma. J. Clin. Oncol. 1995, 13, 1726–1733. [Google Scholar] [CrossRef]
- Bastion, Y.; Sebban, C.; Berger, F.; Felman, P.; Salles, G.; Dumontet, C.; Bryon, P.A.; Coiffier, B. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J. Clin. Oncol. 1997, 15, 1587–1594. [Google Scholar] [CrossRef]
- Giraudo, M.F.; Jackson, Z.; Das, I.; Abiona, O.M.; Wald, D.N. Chimeric Antigen Receptor (CAR)-T Cell Therapy for Non-Hodgkin’s Lymphoma. Pathog. Immun. 2024, 9, 1–17. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef]
- Parikh, R.H.; Lonial, S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA Cancer J. Clin. 2023, 73, 275–285. [Google Scholar] [CrossRef]
- Cohen, A.D.; Mateos, M.-V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.W.C.J.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023, 141, 219–230. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.J.; Hildebrandt, M.A.T.; Hashmi, H.; Shune, L.O.; McGuirk, J.P.; Sborov, D.W.; Wagner, C.B.; Kocoglu, M.H.; Rapoport, A.; Atrash, S.; et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. 2023, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Ingegnere, T.; Escure, G.; Prodhomme, C.; Nudel, M.; Mitra, S.; Facon, T. Current state and next-generation CAR-T-cells in multiple myeloma. Blood Rev. 2022, 54, 100929. [Google Scholar] [CrossRef]
- Anderson, L.D., Jr. Idecabtagene vicleucel (ide-cel) CAR T-cell therapy for relapsed and refractory multiple myeloma. Future Oncol. 2022, 18, 277–289. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.-V.; Fernandez de Larrea, C.; Martinez-Lopez, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef]
- Othus, M.; Wood, B.L.; Stirewalt, D.L.; Estey, E.H.; Petersdorf, S.H.; Appelbaum, F.R.; Erba, H.P.; Walter, R.B. Effect of measurable (‘minimal’) residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia 2016, 30, 2080–2083. [Google Scholar] [CrossRef] [PubMed]
- Faderl, S.; O’Brien, S.; Pui, C.-H.; Stock, W.; Wetzler, M.; Hoelzer, D.; Kantarjian, H.M. Adult acute lymphoblastic leukemia: Concepts and strategies. Cancer 2010, 116, 1165–1176. [Google Scholar] [CrossRef]
- Della Starza, I.; De Novi, L.A.; Elia, L.; Bellomarino, V.; Beldinanzi, M.; Soscia, R.; Cardinali, D.; Chiaretti, S.; Guarini, A.; Foa, R. Optimizing Molecular Minimal Residual Disease Analysis in Adult Acute Lymphoblastic Leukemia. Cancers 2023, 15, 374. [Google Scholar] [CrossRef]
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054. [Google Scholar] [CrossRef]
- Juarez-Avendano, G.; Mendez-Ramirez, N.; Luna-Silva, N.C.; Gomez-Almaguer, D.; Pelayo, R.; Balandran, J.C. Molecular and cellular markers for measurable residual disease in acute lymphoblastic leukemia. Bol. Med. Hosp. Infant. Mex. 2021, 78, 159–170. [Google Scholar] [CrossRef]
- Knauf, W.U.; Ho, A.D.; Heger, G.; Hoelzer, D.; Hunstein, W.; Thiel, E. Detection of Minimal Residual Disease in Adult Acute Lymphoblastic Leukemia by Analysis of Gene Rearrangements and Correlation with Early Relapses. Leuk. Lymphoma 1991, 5, 57–63. [Google Scholar] [CrossRef]
- Tran, T.H.; Hunger, S.P. The genomic landscape of pediatric acute lymphoblastic leukemia and precision medicine opportunities. Semin. Cancer Biol. 2022, 84, 144–152. [Google Scholar] [CrossRef]
- Pui, C.-H.; Pei, D.; Raimondi, S.C.; Coustan-Smith, E.; Jeha, S.; Cheng, C.; Bowman, W.P.; Sandlund, J.T.; Ribeiro, R.C.; Rubnitz, J.E.; et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia 2017, 31, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Bruggemann, M.; Ritgen, M.; van der Velden, V.H.J.; van Dongen, J.J.M.; Kneba, M. MRD Detection in B-Cell Non-Hodgkin Lymphomas Using Ig Gene Rearrangements and Chromosomal Translocations as Targets for Real-Time Quantitative PCR. Methods Mol. Biol. 2019, 1956, 199–228. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.L.; Armand, P. Minimal residual disease in non-Hodgkin lymphoma—Current applications and future directions. Br. J. Haematol. 2018, 180, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Genuardi, E.; Mazziotta, F.; Iovino, L.; Morabito, F.; Grassi, S.; Ciabatti, E.; Guerrini, F.; Petrini, M. The Minimal Residual Disease in Non-Hodgkin’s Lymphomas: From the Laboratory to the Clinical Practice. Front. Oncol. 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.D.; Fletcher, M.; Whitehouse, H.; Whitby, L.; Yuan, C.; Mazzucchelli, S.; Lin, P.; de Tute, R.; Dorwal, P.; Wallace, P.K.; et al. Assessment of plasma cell myeloma minimal residual disease testing by flow cytometry in an international inter-laboratory study: Is it ready for primetime use? Cytom. Part B Clin. Cytom. 2019, 96, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Roshal, M. Measurable disease evaluation in patients with myeloma. Best Pract. Res. Clin. Haematol. 2020, 33, 101154. [Google Scholar] [CrossRef]
- Flores-Montero, J.; de Tute, R.; Paiva, B.; Perez, J.J.; Bottcher, S.; Wind, H.; Sanoja, L.; Puig, N.; Lecrevisse, Q.; Vidriales, M.B.; et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytom. Part B Clin. Cytom. 2016, 90, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Medina-Herrera, A.; Sarasquete, M.E.; Jimenez, C.; Puig, N.; Garcia-Sanz, R. Minimal Residual Disease in Multiple Myeloma: Past, Present, and Future. Cancers 2023, 15, 3687. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Avet-Loiseau, H.; Malard, F.; Harousseau, J.-L. Potential future direction of measurable residual disease evaluation in multiple myeloma. Blood 2023, 142, 1509–1517. [Google Scholar] [CrossRef]
- Ferla, V.; Antonini, E.; Perini, T.; Farina, F.; Masottini, S.; Malato, S.; Marktel, S.; Lupo Stanghellini, M.T.; Tresoldi, C.; Ciceri, F.; et al. Minimal residual disease detection by next-generation sequencing in multiple myeloma: Promise and challenges for response-adapted therapy. Front. Oncol. 2022, 12, 932852. [Google Scholar] [CrossRef]
- Honikel, M.M.; Olejniczak, S.H. Co-Stimulatory Receptor Signaling in CAR-T Cells. Biomolecules 2022, 12, 1303. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 2021, 18, 715–727. [Google Scholar] [CrossRef]
- Leddon, S.A.; Fettis, M.M.; Abramo, K.; Kelly, R.; Oleksyn, D.; Miller, J. The CD28 Transmembrane Domain Contains an Essential Dimerization Motif. Front. Immunol. 2020, 11, 1519. [Google Scholar] [CrossRef]
- Odorizzi, P.M.; Wherry, E.J. Inhibitory receptors on lymphocytes: Insights from infections. J. Immunol. 2012, 188, 2957–2965. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T-cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T-cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Harjunpaa, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Kuchroo, V.K. Tim-3, Lag-3, and TIGIT. Curr. Top. Microbiol. Immunol. 2017, 410, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor Res. 2015, 42, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2020, 64, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bhattacharya, P.; Prabhakar, B.S. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J. Autoimmun. 2018, 95, 77–99. [Google Scholar] [CrossRef]
- Poorebrahim, M.; Melief, J.; Pico de Coana, Y.; Wickström, S.L.; Cid-Arregui, A.; Kiessling, R. Counteracting CAR T-cell dysfunction. Oncogene 2021, 40, 421–435. [Google Scholar] [CrossRef]
- Chong, E.A.; Alanio, C.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Lacey, S.F.; Ruella, M.; Bhattacharyya, S.; Wherry, E.J.; Schuster, S.J. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood 2022, 139, 1026–1038. [Google Scholar] [CrossRef]
- Wang, C.; Shi, F.; Liu, Y.; Zhang, Y.; Dong, L.; Li, X.; Tong, C.; Wang, Y.; Su, L.; Nie, J.; et al. Anti-PD-1 antibodies as a salvage therapy for patients with diffuse large B cell lymphoma who progressed/relapsed after CART19/20 therapy. J. Hematol. Oncol. 2021, 14, 106. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Sankin, A.I.; Chen, F.; Guan, F.; Zang, X. Immune checkpoint blockade and CAR-T-cell therapy in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef]
- Gazeau, N.; Mitra, S.; Nudel, M.; Tilmont, R.; Chauvet, P.; Srour, M.; Moreau, A.-S.; Varlet, P.; Alidjinou, E.K.; Manier, S.; et al. Safety and efficacy of nivolumab in patients who failed to achieve a complete remission after CD19-directed CAR T-cell therapy in diffuse large B cell lymphoma. Br. J. Haematol. 2023, 202, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; Thompson, P.A.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer 2022, 21, 194. [Google Scholar] [CrossRef]
- Hatic, H.; Sampat, D.; Goyal, G. Immune checkpoint inhibitors in lymphoma: Challenges and opportunities. Ann. Transl. Med. 2021, 9, 1037. [Google Scholar] [CrossRef]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Kobold, S.; Pantelyushin, S.; Rataj, F.; Vom Berg, J. Rationale for Combining Bispecific T Cell Activating Antibodies with Checkpoint Blockade for Cancer Therapy. Front. Oncol. 2018, 8, 285. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef]
- Abaza, Y.; Zeidan, A.M. Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Cells 2022, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. The Next-Generation Immune Checkpoint LAG-3 and Its Therapeutic Potential in Oncology: Third Time’s a Charm. Int. J. Mol. Sci. 2020, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Hoogi, S.; Eisenberg, V.; Mayer, S.; Shamul, A.; Barliya, T.; Cohen, C.J. A TIGIT-based chimeric co-stimulatory switch receptor improves T-cell anti-tumor function. J. Immunother. Cancer 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T-cells in the tumor microenvironment. Cell. Mol. Immunol. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Gumber, D.; Wang, L.D. Improving CAR-T immunotherapy: Overcoming the challenges of T-cell exhaustion. EBioMedicine 2022, 77, 103941. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Hu, Y.; Mei, H. T Cell Exhaustion and CAR-T Immunotherapy in Hematological Malignancies. Biomed. Res. Int. 2021, 2021, 6616391. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T-cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8+ T-cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T-cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hu, H.; Xiao, Y.; Li, Q.; Zhong, Z.; Yang, J.; Zou, P.; Cao, Y.; Meng, F.; Li, W.; et al. Tumor-derived extracellular vesicles induce invalid cytokine release and exhaustion of CD19 CAR-T Cells. Cancer Lett. 2022, 536, 215668. [Google Scholar] [CrossRef]
- Ukrainskaya, V.M.; Musatova, O.E.; Volkov, D.V.; Osipova, D.S.; Pershin, D.S.; Moysenovich, A.M.; Evtushenko, E.G.; Kulakovskaya, E.A.; Maksimov, E.G.; Zhang, H.; et al. CAR-tropic extracellular vesicles carry tumor-associated antigens and modulate CAR T-cell functionality. Sci. Rep. 2023, 13, 463. [Google Scholar] [CrossRef]
- Kasakovski, D.; Xu, L.; Li, Y. T-cell senescence and CAR-T-cell exhaustion in hematological malignancies. J. Hematol. Oncol. 2018, 11, 91. [Google Scholar] [CrossRef]
- Nakagami, H. Cellular senescence and senescence-associated T-cells as a potential therapeutic target. Geriatr. Gerontol. Int. 2020, 20, 97–100. [Google Scholar] [CrossRef]
- Dunne, P.J.; Faint, J.M.; Gudgeon, N.H.; Fletcher, J.M.; Plunkett, F.J.; Soares, M.V.D.; Hislop, A.D.; Annels, N.E.; Rickinson, A.B.; Salmon, M.; et al. Epstein-Barr virus-specific CD8+ T-cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood 2002, 100, 933–940. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.A.R.; Xu, H.; Pan, C.-X.; Zhu, Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T-cell therapy. Theranostics 2022, 12, 6273–6290. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; van Leeuwen, D.G.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; Wang, P.; et al. Targeted delivery of a PD-1-blocking scFv by CAR-T-cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Swatler, J.; Turos-Korgul, L.; Brewinska-Olchowik, M.; De Biasi, S.; Dudka, W.; Le, B.V.; Kominek, A.; Cyranowski, S.; Pilanc, P.; Mohammadi, E.; et al. 4-1BBL-containing leukemic extracellular vesicles promote immunosuppressive effector regulatory T-cells. Blood Adv. 2022, 6, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Dolgalev, I.; Evensen, N.A.; Ma, C.; Chambers, T.; Roberts, K.G.; Sreeram, S.; Dai, Y.; Tikhonova, A.N.; Lasry, A.; et al. Extensive Remodeling of the Immune Microenvironment in B Cell Acute Lymphoblastic Leukemia. Cancer Cell 2020, 37, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Hsu, Y.-M.S.; Schmidt, A.P.; Stock, W.; Fletcher, T.R.; Trinkaus, K.M.; Westervelt, P.; DiPersio, J.F.; Link, D.C. Targeting bone marrow lymphoid niches in acute lymphoblastic leukemia. Leuk. Res. 2015, 39, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, D.B.; Varela, V.A.; Datoguia, T.S.; Caraciolo, V.B.; Lopes, G.H.; Pereira, W.O. The Bone Marrow Microenvironment Mechanisms in Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2021, 9, 764698. [Google Scholar] [CrossRef] [PubMed]
- Autio, M.; Leivonen, S.-K.; Bruck, O.; Karjalainen-Lindsberg, M.-L.; Pellinen, T.; Leppa, S. Clinical Impact of Immune Cells and Their Spatial Interactions in Diffuse Large B-Cell Lymphoma Microenvironment. Clin. Cancer Res. 2022, 28, 781–792. [Google Scholar] [CrossRef]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T-cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T-cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Mao, L.; Xu, H.; Wang, S. Revisiting PD-1/PD-L pathway in T and B cell response: Beyond immunosuppression. Cytokine Growth Factor. Rev. 2022, 67, 58–65. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Marangoni, F.; Zhakyp, A.; Corsini, M.; Geels, S.N.; Carrizosa, E.; Thelen, M.; Mani, V.; Prüßmann, J.N.; Warner, R.D.; Ozga, A.J.; et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell 2021, 184, 3998–4015. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. Adv. Exp. Med. Biol. 2020, 1248, 7–32. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, S.; Fan, L.; Zhang, B.; Xu, S. TIM-3: An update on immunotherapy. Int. Immunopharmacol. 2021, 99, 107933. [Google Scholar] [CrossRef]
- Yue, C.; Gao, S.; Li, S.; Xing, Z.; Qian, H.; Hu, Y.; Wang, W.; Hua, C. TIGIT as a Promising Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2022, 13, 911919. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Snyder, M.W.; Kircher, M.; Hill, A.J.; Daza, R.M.; Shendure, J. Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Cell 2016, 164, 57–68. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Mika, T.; Thomson, J.; Nilius-Eliliwi, V.; Vangala, D.; Baraniskin, A.; Wulf, G.; Klein-Scory, S.; Schroers, R. Quantification of cell-free DNAfor the analysis of CD19-CAR-T-cells during lymphoma treatment. Mol. Ther. Methods Clin. Dev. 2021, 23, 539–550. [Google Scholar] [CrossRef]
- Bastos-Oreiro, M.; Sanz-Villanueva, L.; Muniz, P.; Bailen, R.; Chicano, M.; Oarbeskoa, G.; Gomez, I.; Gutierrez, A.; Iglesia, I.; Carbonell, D.; et al. Cell-Free DNA Dynamic Concentration and Other Variables Are Predictors of Early Progression after Chimeric Antigen Receptor T Cell Therapy in Patients with Diffuse Large B Cell Lymphoma. Transplant. Cell Ther. 2023, 29, 472 e1–472 e4. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.-L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Dawson, S.-J.; Tsui, D.W.Y.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.yF.; Kingsbury, Z.; Wong, A.S.C.; et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef]
- Beaver, J.A.; Jelovac, D.; Balukrishna, S.; Cochran, R.; Croessmann, S.; Zabransky, D.J.; Wong, H.Y.; Toro, P.V.; Cidado, J.; Blair, B.G.; et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin. Cancer Res. 2014, 20, 2643–2650. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W.; Parker, R.L.; Wentworth, J.; Madankumar, R.; Saffer, C.; Das, A.F.; Craig, J.A.; Chudova, D.I.; Devers, P.L.; Jones, K.W.; et al. DNA sequencing versus standard prenatal aneuploidy screening. N. Engl. J. Med. 2014, 370, 799–808. [Google Scholar] [CrossRef]
- Fan, H.C.; Blumenfeld, Y.J.; Chitkara, U.; Hudgins, L.; Quake, S.R. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc. Natl. Acad. Sci. USA 2008, 105, 16266–16271. [Google Scholar] [CrossRef] [PubMed]
- Monick, S.; Rosenthal, A. Circulating Tumor DNA as a Complementary Prognostic Biomarker during CAR-T Therapy in B-Cell Non-Hodgkin Lymphomas. Cancers 2024, 16, 1881. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Goodman, A.M.; Holden, K.A.; Jeong, A.-R.; Kim, L.; Fitzgerald, K.D.; Almasri, E.; McLennan, G.; Eisenberg, M.; Jahromi, A.H.; Hoh, C.; et al. Assessing CAR T-Cell Therapy Response Using Genome-Wide Sequencing of Cell-Free DNA in Patients With B-Cell Lymphomas. Transplant. Cell Ther. 2022, 28, 30 e1–30 e7. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Kassab, J.; Saba, L.; Haroun, E.; Bou Zerdan, M.; Allam, S.; Nasr, L.; Macaron, W.; Mammadli, M.; Abou Moussa, S.; et al. Liquid biopsies and minimal residual disease in lymphoid malignancies. Front. Oncol. 2023, 13, 1173701. [Google Scholar] [CrossRef]
- Camus, V.; Jardin, F. Cell-Free DNA for the Management of Classical Hodgkin Lymphoma. Pharmaceuticals 2021, 14, 207. [Google Scholar] [CrossRef]
- Wood, B.; Wu, D.; Crossley, B.; Dai, Y.; Williamson, D.; Gawad, C.; Borowitz, M.J.; Devidas, M.; Maloney, K.W.; Larsen, E.; et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 2018, 131, 1350–1359. [Google Scholar] [CrossRef]
- Ding, L.-W.; Sun, Q.-Y.; Tan, K.-T.; Chien, W.; Thippeswamy, A.M.; Yeoh, A.E.J.; Kawamata, N.; Nagata, Y.; Xiao, J.-F.; Loh, X.-Y.; et al. Mutational Landscape of Pediatric Acute Lymphoblastic Leukemia. Cancer Res. 2017, 77, 390–400. [Google Scholar] [CrossRef]
- Meyer, J.A.; Wang, J.; Hogan, L.E.; Yang, J.J.; Dandekar, S.; Patel, J.P.; Tang, Z.; Zumbo, P.; Li, S.; Zavadil, J.; et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 290–294. [Google Scholar] [CrossRef]
- Hogan, L.E.; Meyer, J.A.; Yang, J.; Wang, J.; Wong, N.; Yang, W.; Condos, G.; Hunger, S.P.; Raetz, E.; Saffery, R.; et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood 2011, 118, 5218–5226. [Google Scholar] [CrossRef]
- Zhang, J.; Mullighan, C.G.; Harvey, R.C.; Wu, G.; Chen, X.; Edmonson, M.; Buetow, K.H.; Carroll, W.L.; Chen, I.-M.; Devidas, M.; et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 2011, 118, 3080–3087. [Google Scholar] [CrossRef]
- Mullighan, C.G. Genomic profiling of B-progenitor acute lymphoblastic leukemia. Best Pract. Res. Clin. Haematol. 2011, 24, 489–503. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B.; et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.-K.; Hartung, K.; Botzen, A.; Brobeil, A.; Rummel, M.; Kurch, L.; Georgi, T.; Jox, T.; Bielack, S.; Burdach, S.; et al. Genotyping circulating tumor DNA of pediatric Hodgkin lymphoma. Leukemia 2020, 34, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Spina, V.; Bruscaggin, A.; Cuccaro, A.; Martini, M.; Di Trani, M.; Forestieri, G.; Manzoni, M.; Condoluci, A.; Arribas, A.; Terzi-Di-Bergamo, L.; et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood 2018, 131, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Cherng, H.-J.J.; Sun, R.; Sugg, B.; Irwin, R.; Yang, H.; Le, C.C.; Deng, Q.; Fayad, L.; Fowler, N.H.; Parmar, S.; et al. Risk assessment with low-pass whole-genome sequencing of cell-free DNA before CD19 CAR T-cell therapy for large B-cell lymphoma. Blood 2022, 140, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Wang, X.; Mann, M.; Adamus, T.P.; Wang, D.; Moreira, D.F.; Zhang, Z.; Ouyang, C.; He, X.; Zhang, B.; et al. Myeloid cell-targeted miR-146a mimic inhibits NF-κB-driven inflammation and leukemia progression in vivo. Blood 2020, 135, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, J.; Zheng, G.; Wang, Q.; Li, X.; Feng, Y.; Shang, F.; He, S.; Jiang, Q.; Shi, B.; et al. Co-Expression of miR155 or LSD1 shRNA Increases the Anti-Tumor Functions of CD19 CAR-T Cells. Front. Immunol. 2021, 12, 811364. [Google Scholar] [CrossRef]
- Gutierrez-Vazquez, C.; Rodriguez-Galan, A.; Fernandez-Alfara, M.; Mittelbrunn, M.; Sanchez-Cabo, F.; Martinez-Herrera, D.J.; Ramirez-Huesca, M.; Pascual-Montano, A.; Sanchez-Madrid, F. miRNA profiling during antigen-dependent T-cell activation: A role for miR-132-3p. Sci. Rep. 2017, 7, 3508. [Google Scholar] [CrossRef]
- Podshivalova, K.; Salomon, D.R. MicroRNA regulation of T-lymphocyte immunity: Modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit. Rev. Immunol. 2013, 33, 435–476. [Google Scholar] [CrossRef] [PubMed]
- Egana-Gorrono, L.; Guardo, A.C.; Bargallo, M.E.; Planet, E.; Vilaplana, E.; Escriba, T.; Perez, I.; Gatell, J.M.; Garcia, F.; Arnedo, M.; et al. MicroRNA Profile in CD8+ T-Lymphocytes from HIV-Infected Individuals: Relationship with Antiviral Immune Response and Disease Progression. PLoS ONE 2016, 11, e0155245. [Google Scholar] [CrossRef]
- Nikhat, S.; Yadavalli, A.D.; Prusty, A.; Narayan, P.K.; Palakodeti, D.; Murre, C.; Pongubala, J.M.R. A regulatory network of microRNAs confers lineage commitment during early developmental trajectories of B and T lymphocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2104297118. [Google Scholar] [CrossRef] [PubMed]
- Longjohn, M.N.; Squires, W.R.B.; Christian, S.L. Meta-analysis of microRNA profiling data does not reveal a consensus signature for B cell acute lymphoblastic leukemia. Gene 2022, 821, 146211. [Google Scholar] [CrossRef]
- Ultimo, S.; Martelli, A.M.; Zauli, G.; Vitale, M.; Calin, G.A.; Neri, L.M. Roles and clinical implications of microRNAs in acute lymphoblastic leukemia. J. Cell Physiol. 2018, 233, 5642–5654. [Google Scholar] [CrossRef]
- Pui, C.-H.; Schrappe, M.; Ribeiro, R.C.; Niemeyer, C.M. Childhood and adolescent lymphoid and myeloid leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2004, 2004, 118–145. [Google Scholar] [CrossRef] [PubMed]
- Musilova, K.; Mraz, M. MicroRNAs in B-cell lymphomas: How a complex biology gets more complex. Leukemia 2015, 29, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Vosa, U.; Vooder, T.; Kolde, R.; Fischer, K.; Valk, K.; Tonisson, N.; Roosipuu, R.; Vilo, J.; Metspalu, A.; Annilo, T. Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes Chromosomes Cancer 2011, 50, 812–822. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Caires, H.R.; Bergantim, R.; Guimaraes, J.E.; Vasconcelos, M.H. miRNAs mediated drug resistance in hematological malignancies. Semin. Cancer Biol. 2022, 83, 283–302. [Google Scholar] [CrossRef]
- Saadi, M.I.; Nikandish, M.; Ghahramani, Z.; Valandani, F.M.; Ahmadyan, M.; Hosseini, F.; Rahimian, Z.; Jalali, H.; Tavasolian, F.; Abdolyousefi, E.N.; et al. miR-155 and miR-92 levels in ALL, post-transplant aGVHD, and CMV: Possible new treatment options. J. Egypt. Natl. Cancer Inst. 2023, 35, 18. [Google Scholar] [CrossRef]
- Mensa, E.; Guescini, M.; Giuliani, A.; Bacalini, M.G.; Ramini, D.; Corleone, G.; Ferracin, M.; Fulgenzi, G.; Graciotti, L.; Prattichizzo, F.; et al. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles 2020, 9, 1725285. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Batista, B.S.; Eng, W.S.; Pilobello, K.T.; Hendricks-Munoz, K.D.; Mahal, L.K. Identification of a conserved glycan signature for microvesicles. J. Proteome Res. 2011, 10, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Ofori, K.; Bhagat, G.; Rai, A.J. Exosomes and extracellular vesicles as liquid biopsy biomarkers in diffuse large B-cell lymphoma: Current state of the art and unmet clinical needs. Br. J. Clin. Pharmacol. 2021, 87, 284–294. [Google Scholar] [CrossRef]
- Zare, N.; Haghjooy Javanmard, S.H.; Mehrzad, V.; Eskandari, N.; Andalib, A.R. Effect of Plasma-Derived Exosomes of Refractory/Relapsed or Responsive Patients with Diffuse Large B-Cell Lymphoma on Natural Killer Cells Functions. Cell J. 2020, 22, 40–54. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, K.; Qu, C.; Peng, J.; Yang, L. Non-Coding RNAs Derived from Extracellular Vesicles Promote Pre-Metastatic Niche Formation and Tumor Distant Metastasis. Cancers 2023, 15, 2158. [Google Scholar] [CrossRef]
- Schorey, J.S.; Bhatnagar, S. Exosome function: From tumor immunology to pathogen biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction and Aging: Insights from the Analysis of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Dominiak, A.; Żołnierzak, A.; Kubiak-Tomaszewska, G.; Lorenc, T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020, 2020, 6272498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Cao, X. The exosomes in tumor immunity. OncoImmunology 2015, 4, e1027472. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Mitchell, J.P.; Court, J.; Linnane, S.; Mason, M.D.; Tabi, Z. Human Tumor-Derived Exosomes Down-Modulate NKG2D Expression. J. Immunol. 2008, 180, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Jiang, Y.; Li, M.; Juan, X.; Xu, C. Circulating Exosomal microRNA Signature As a Noninvasive Biomarker for Diagnosis of Diffuse Large B-Cell Lymphoma. Blood 2018, 132 (Suppl. 1), 5406. [Google Scholar] [CrossRef]
- Zhuang, H.; Shen, J.; Zheng, Z.; Luo, X.; Gao, R.; Zhuang, X. MicroRNA-146a rs2910164 polymorphism and the risk of diffuse large B cell lymphoma in the Chinese Han population. Med. Oncol. 2014, 31, 306. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Goldschmidt, N.; Bardugo, A.; Gur-Wahnon, D.; Ben-Dov, I.Z.; Avni, B. Plasma microRNA profiling: Exploring better biomarkers for lymphoma surveillance. PLoS ONE 2017, 12, e0187722. [Google Scholar] [CrossRef]
- Inada, K.; Okoshi, Y.; Cho, Y.; Saito, H.; Iijima, T.; Hori, M.; Kojima, H. Availability of Circulating MicroRNAs as a Biomarker for Early Diagnosis of Diffuse Large B-Cell Lymphoma. Open J. Blood Dis. 2015, 05, 48–58. [Google Scholar] [CrossRef]
- Yan, W.; Song, L.; Wang, H.; Yang, W.; Hu, L.; Yang, Y. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J. Transl. Med. 2021, 19, 511. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef] [PubMed]
- Gluszko, A.; Szczepanski, M.J.; Ludwig, N.; Mirza, S.M.; Olejarz, W. Exosomes in Cancer: Circulating Immune-Related Biomarkers. BioMed Res. Int. 2019, 2019, 1628029. [Google Scholar] [CrossRef] [PubMed]
- Cariello, M.; Squilla, A.; Piacente, M.; Venutolo, G.; Fasano, A. Drug Resistance: The Role of Exosomal miRNA in the Microenvironment of Hematopoietic Tumors. Molecules 2022, 28, 116. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Hong, J.-Y.; Park, H.J.; Lee, S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer 2017, 141, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Izadirad, M.; Huang, Z.; Jafari, F.; Hamidieh, A.A.; Gharehbaghian, A.; Li, Y.-D.; Jafari, L.; Chen, Z.-S. Extracellular Vesicles in Acute Leukemia: A Mesmerizing Journey With a Focus on Transferred microRNAs. Front. Cell Dev. Biol. 2021, 9, 766371. [Google Scholar] [CrossRef]
- Najaflou, M.; Shahgolzari, M.; Khosroushahi, A.Y.; Fiering, S. Tumor-Derived Extracellular Vesicles in Cancer Immunoediting and Their Potential as Oncoimmunotherapeutics. Cancers 2022, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA: A Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Słomka, A.; Kornek, M.; Cho, W.C. Small Extracellular Vesicles and Their Involvement in Cancer Resistance: An Up-to-Date Review. Cells 2022, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, M.; Zeng, S.; Wang, L.; Liu, P.; Xiao, X.; Liu, Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics 2019, 11, 35–51. [Google Scholar] [CrossRef]
- Cao, D.; Cao, X.; Jiang, Y.; Xu, J.; Zheng, Y.; Kang, D.; Xu, C. Circulating exosomal microRNAs as diagnostic and prognostic biomarkers in patients with diffuse large B-cell lymphoma. Hematol. Oncol. 2022, 40, 172–180. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Hu, S.; Cai, Y.; Yang, J.; Ren, S.; Zhao, Y.; Lu, T.; Zhou, X.; Wang, X. Circulating Exosomal MiR-107 Restrains Tumorigenesis in Diffuse Large B-Cell Lymphoma by Targeting 14-3-3η. Front. Cell Dev. Biol. 2021, 9, 667800. [Google Scholar] [CrossRef]
- Choi, Y.; Diefenbach, C.S. Immunotherapy with drugs. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 2020, 598–605. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Lu, Z.; Lei, W.; Zhang, C.; Li, P.; Liang, A.; Young, K.H.; Qian, W. CD19 CAR-T expressing PD-1/CD28 chimeric switch receptor as a salvage therapy for DLBCL patients treated with different CD19-directed CAR T-cell therapies. J. Hematol. Oncol. 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.-E.; Bargou, R.C. T-cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Lasry, A.; Carroll, W.L.; Aifantis, I. Immune-Based Therapies in Acute Leukemia. Trends Cancer 2019, 5, 604–618. [Google Scholar] [CrossRef]
- Viardot, A.; Locatelli, F.; Stieglmaier, J.; Zaman, F.; Jabbour, E. Concepts in immuno-oncology: Tackling B cell malignancies with CD19-directed bispecific T-cell engager therapies. Ann. Hematol. 2020, 99, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, Z.; Jia, H.; Tong, C.; Guo, Y.; Ti, D.; Han, X.; Liu, Y.; Zhang, W.; Wang, C.; et al. Bispecific CAR-T-cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2020, 13, 30. [Google Scholar] [CrossRef]
- Bock, A.M.; Nowakowski, G.S.; Wang, Y. Bispecific Antibodies for Non-Hodgkin Lymphoma Treatment. Curr. Treat. Options Oncol. 2022, 23, 155–170. [Google Scholar] [CrossRef]
- Roselli, E.; Boucher, J.C.; Li, G.; Kotani, H.; Spitler, K.; Reid, K.; Cervantes, E.V.; Bulliard, Y.; Tu, N.; Lee, S.B.; et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T-cells. J. Immunother. Cancer 2021, 9, e003354. [Google Scholar] [CrossRef]
- Kobayashi, R.; Suzuki, D.; Hori, D.; Kishimoto, K.; Sano, H.; Nakazawa, A.; Yasuda, K.; Kobayashi, K. Spontaneous improvement in a pediatric patient with peripheral T-cell lymphoma. Pediatr. Int. 2015, 57, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, G.; Fraietta, J.A.; Gerson, J.N.; Van Deerlin, V.M.; Morrissette, J.J.D.; Caponetti, G.C.; Paruzzo, L.; Harris, J.C.; Chong, E.A.; Susanibar Adaniya, S.P.; et al. T-cell lymphoma and secondary primary malignancy risk after commercial CAR T-cell therapy. Nat. Med. 2024, 30, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhao, M.; Qian, W.; Liang, A.; Li, P.; Liu, H. Secondary myeloid neoplasms after CD19 CAR T therapy in patients with refractory/relapsed B-cell lymphoma: Case series and review of literature. Front. Immunol. 2022, 13, 1063986. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Jazdzewska, A.; Kozlowski, J.; Zacny, A.; Lorenc, T.; Olejarz, W. Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5774. [Google Scholar] [CrossRef] [PubMed]

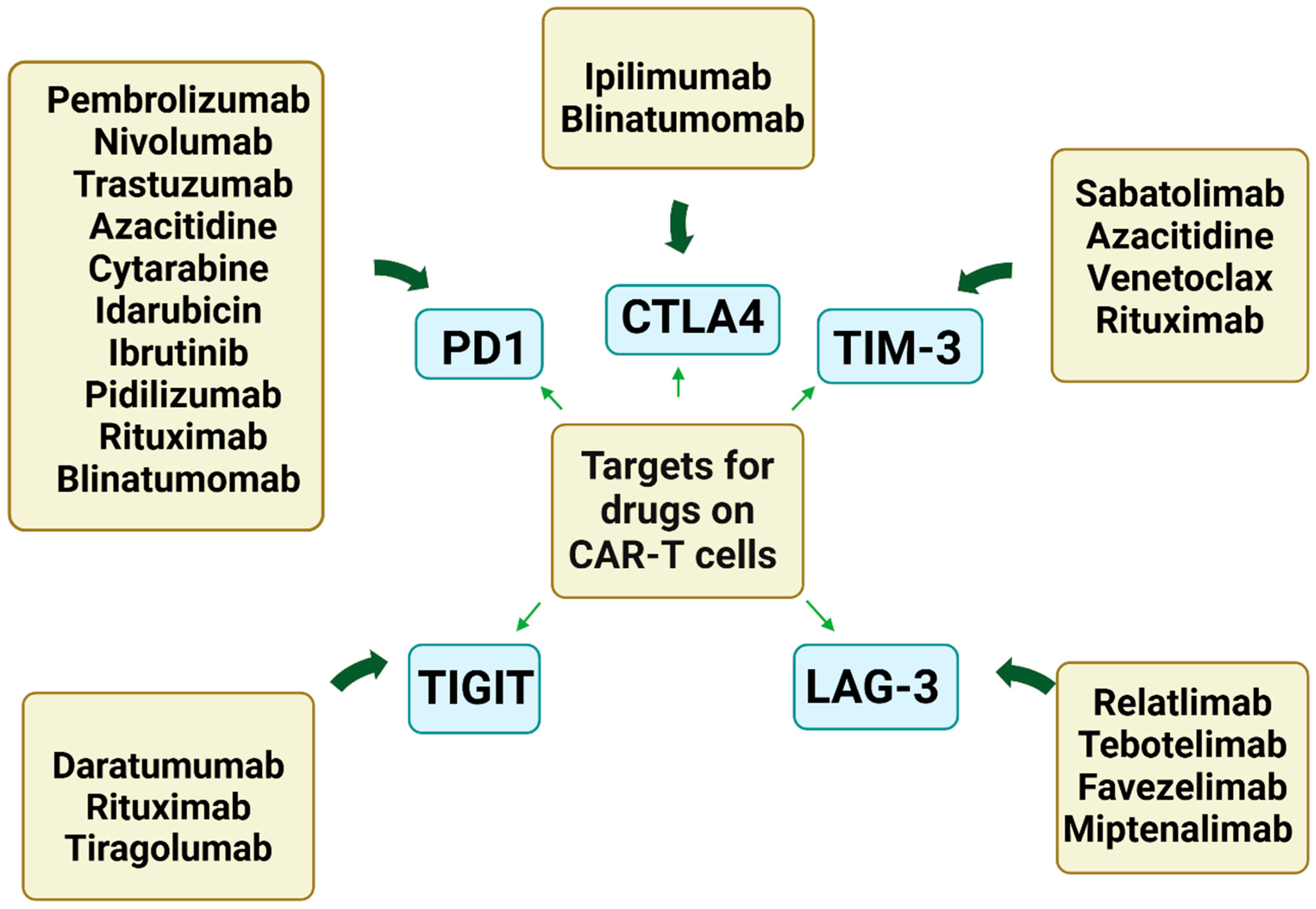
| Receptor | Exhaustion/Senescence | Combination with Immune Checkpoint Inhibitors and Other Drugs | CAR-T Study in Hematological Malignancies [References] |
|---|---|---|---|
| PD-1 | Exhaustion | Pembrolizumab, Nivolumab, Trastuzumab, Azacitidine, Cytarabine, Idarubicin, Ibrutinib, Pidilizumab, Rituximab, Blinatumomab | [97,98,99,100,101,102,103,104,105,106,107,108,109] |
| CTLA-4 | Exhaustion | Ipilimumab, Blinatumomab | [99,107,109,110] |
| TIM-3 | Exhaustion/Senescence | Sabatolimab (MBG453), Azacitidine, Venetoclax, Rituximab | [99,107,111] |
| LAG-3 | Exhaustion/Senescence | Relatlimab (BMS-986016), Favezelimab (MK-4280), Miptenalimab (BI754111), Tebotelimab (MGD013) | [99,107,112] https://clinicaltrials.gov/ (accessed date 11 July 2024) |
| TIGIT | Exhaustion/Senescence | Tiragolumab (MTIG7192A, RG6058), Daratumumab, Rituximab | [99,108,113] https://clinicaltrials.gov/ (accessed date 11 July 2024) |
| Receptor | Immunosuppressive Function | References |
|---|---|---|
| PD-1 |
| [129,139,140,141] |
| CTLA-4 |
| [139,142,143] |
| LAG-3 |
| [21,92,139,143] |
| TIM-3 |
| [21,92,144] |
| TIGIT |
| [21,92,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejarz, W.; Sadowski, K.; Szulczyk, D.; Basak, G. Advancements in Personalized CAR-T Therapy: Comprehensive Overview of Biomarkers and Therapeutic Targets in Hematological Malignancies. Int. J. Mol. Sci. 2024, 25, 7743. https://doi.org/10.3390/ijms25147743
Olejarz W, Sadowski K, Szulczyk D, Basak G. Advancements in Personalized CAR-T Therapy: Comprehensive Overview of Biomarkers and Therapeutic Targets in Hematological Malignancies. International Journal of Molecular Sciences. 2024; 25(14):7743. https://doi.org/10.3390/ijms25147743
Chicago/Turabian StyleOlejarz, Wioletta, Karol Sadowski, Daniel Szulczyk, and Grzegorz Basak. 2024. "Advancements in Personalized CAR-T Therapy: Comprehensive Overview of Biomarkers and Therapeutic Targets in Hematological Malignancies" International Journal of Molecular Sciences 25, no. 14: 7743. https://doi.org/10.3390/ijms25147743





