Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice
Abstract
1. Introduction
2. Results
2.1. Establishment of a Novel Vps13a Mutant Line on the C57BL/6N Background
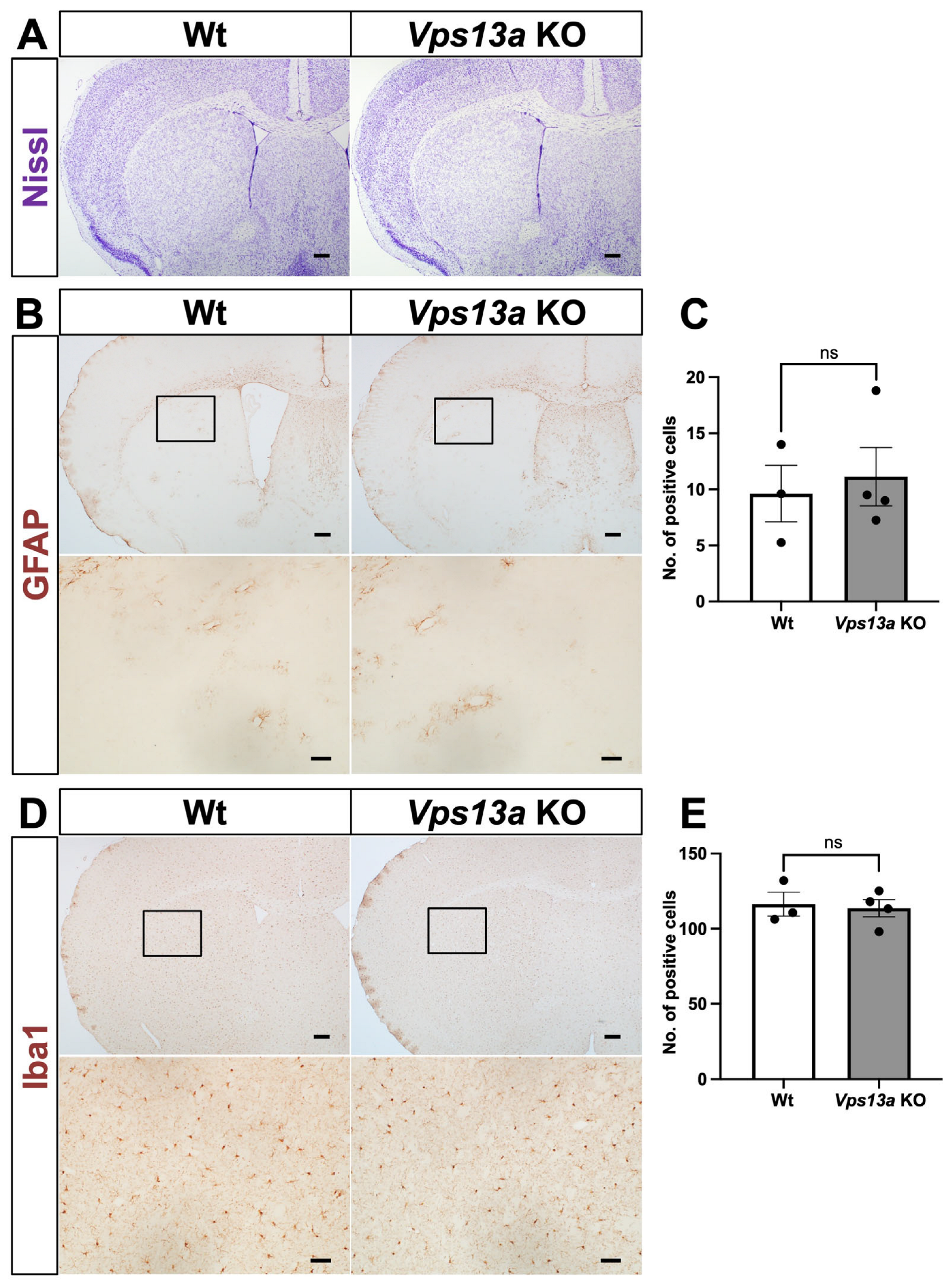
2.2. Neurological Phenotype of Vps13a KO Mice
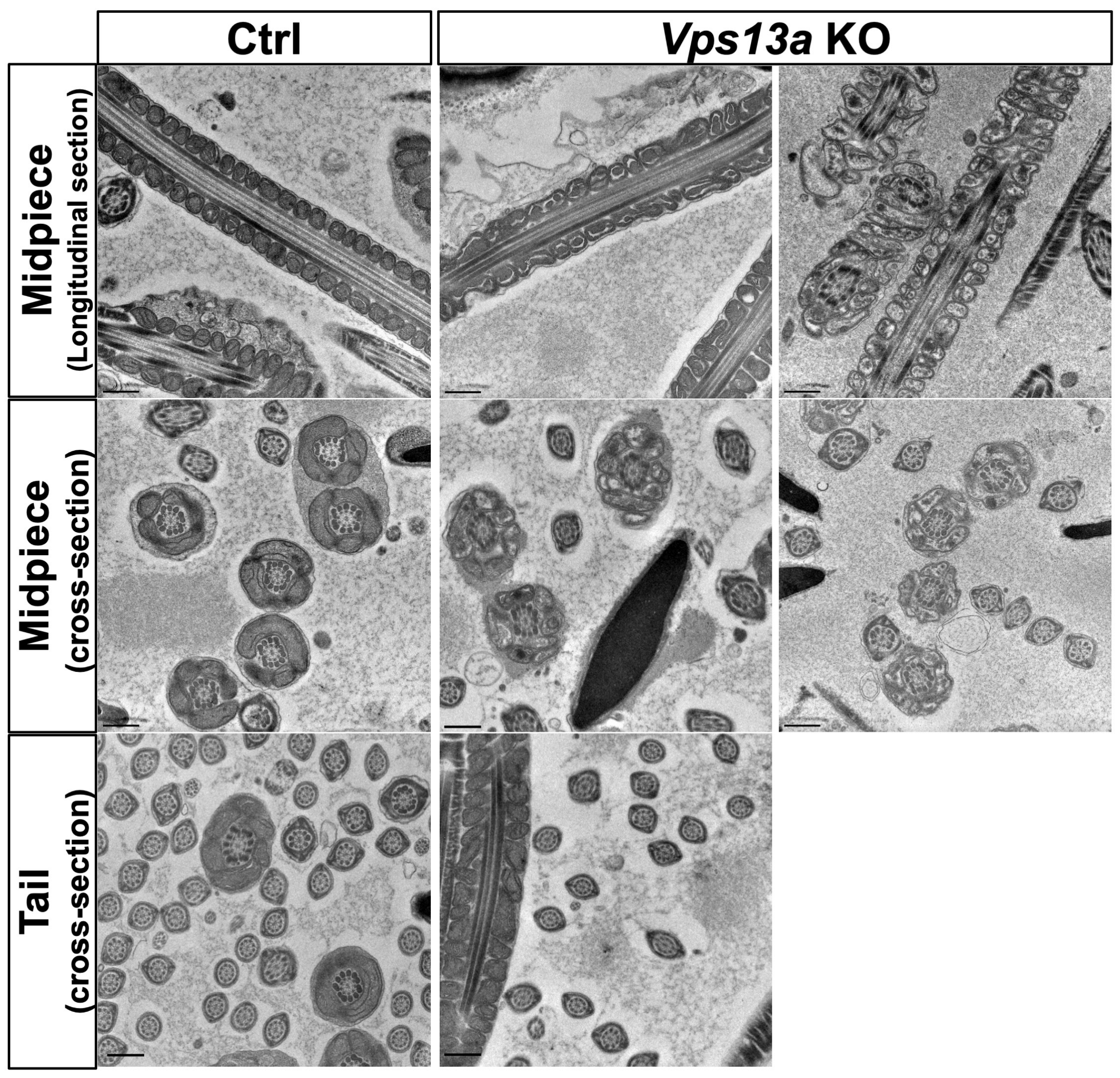
2.3. Transmission Electron Microscopy Analysis of Sperm
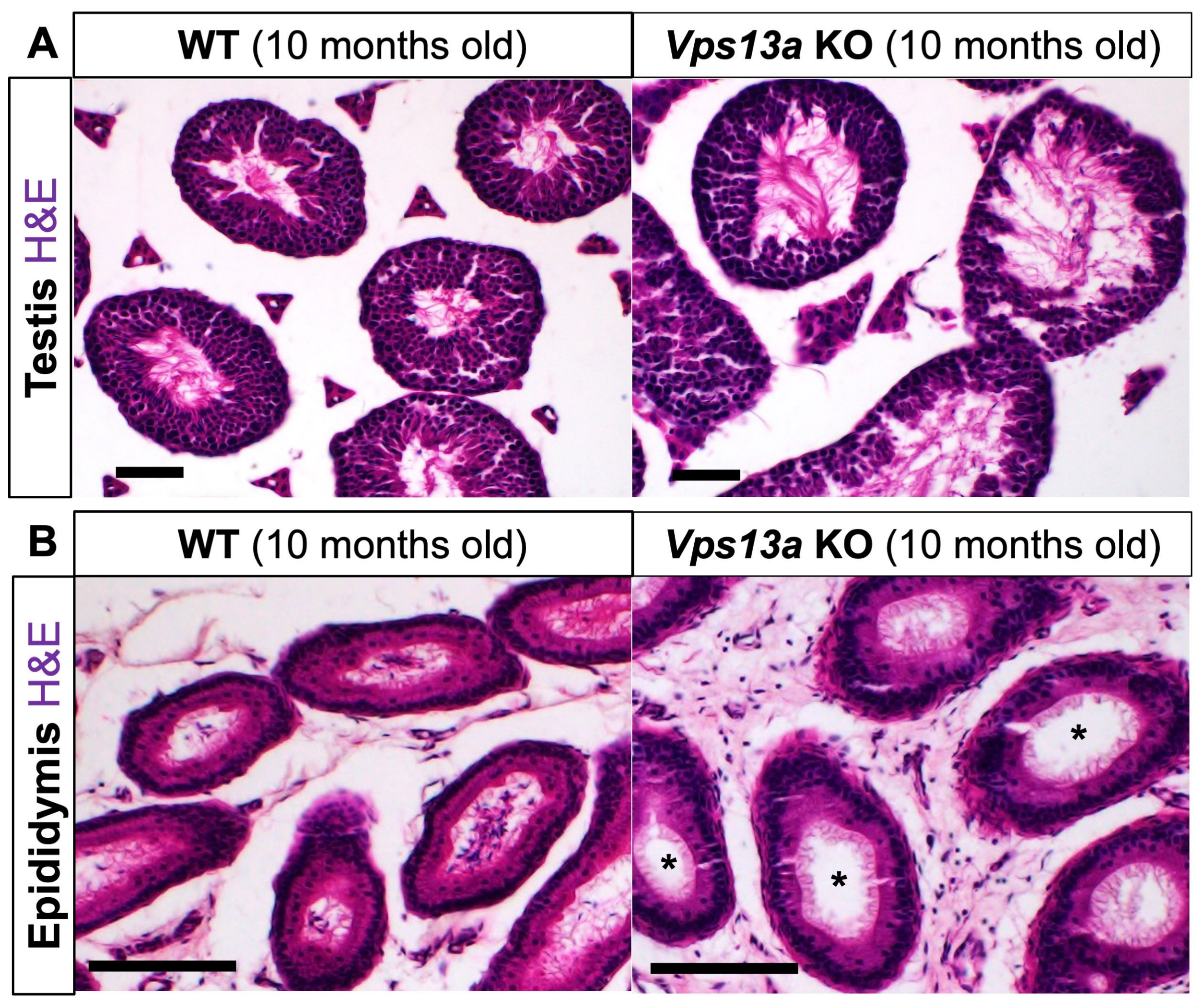
2.4. Histological Analyses of Male Reproductive Organs
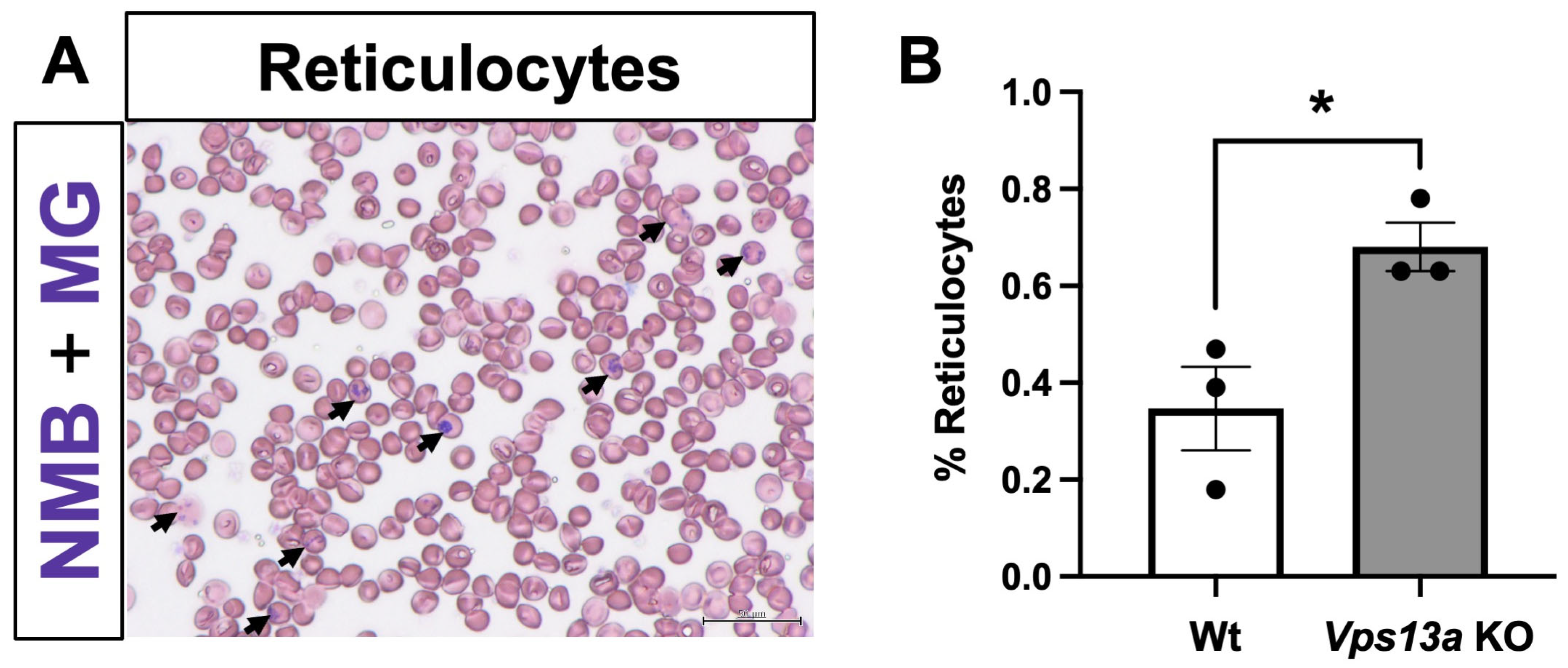
2.5. Analyses of Blood Phenotype
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Genotyping PCR
4.3. Western Blot Analysis
4.4. Histology
4.5. TEM Analysis
4.6. Measurement of the Lumen Area in Seminiferous Tubules
4.7. Blood Smear Test
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danek, A.; Walker, R.H. Neuroacanthocytosis. Curr. Opin. Neurol. 2005, 18, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Sato, N.; Sugiyama, A.; Iijima, K.; Shigemoto, Y.; Morimoto, E.; Kimura, Y.; Fujii, H.; Takahashi, Y.; Nakata, Y.; et al. Chorea-acanthocytosis: Time-dependent changes of symptoms and imaging findings. J. Neuroradiol. 2021, 48, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, L.; Dobson-Stone, C.; Rubio, J.P.; Danek, A.; Chalmers, R.M.; Wood, N.W.; Verellen, C.; Ferrer, X.; Malandrini, A.; Fabrizi, G.M.; et al. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat. Genet. 2001, 28, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, A.; Nakamura, M.; Ichiba, M.; Ueno, S.; Saiki, S.; Morimoto, M.; Kobal, J.; Kageyama, Y.; Inui, T.; Wakabayashi, K.; et al. Novel pathogenic mutations and copy number variations in the VPS13A gene in patients with chorea-acanthocytosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156b, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nakamura, M.; Urata, Y.; Kasamo, K.; Hiwatashi, H.; Yokoyama, I.; Mizobuchi, M.; Sakurai, K.; Osaki, Y.; Morita, Y.; et al. Novel pathogenic VPS13A gene mutations in Japanese patients with chorea-acanthocytosis. Neurol. Genet. 2019, 5, e332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Samander, A.; Wu, Y.; Pineda, S.S.; García, F.J.; Eisen, J.N.; Leonzino, M.; Ugur, B.; Kellis, M.; Heiman, M.; De Camilli, P. A partnership between the lipid scramblase XK and the lipid transfer protein VPS13A at the plasma membrane. Proc. Natl. Acad. Sci. USA 2022, 119, e2205425119. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Braceras, S.; Tornero-Écija, A.R.; Vincent, O.; Escalante, R. VPS13A is closely associated with mitochondria and is required for efficient lysosomal degradation. Dis. Model. Mech. 2019, 12, dmm036681. [Google Scholar] [CrossRef] [PubMed]
- Tomemori, Y.; Ichiba, M.; Kusumoto, A.; Mizuno, E.; Sato, D.; Muroya, S.; Nakamura, M.; Kawaguchi, H.; Yoshida, H.; Ueno, S.; et al. A gene-targeted mouse model for chorea-acanthocytosis. J. Neurochem. 2005, 92, 759–766. [Google Scholar] [CrossRef]
- Sakimoto, H.; Nakamura, M.; Nagata, O.; Yokoyama, I.; Sano, A. Phenotypic abnormalities in a chorea-acanthocytosis mouse model are modulated by strain background. Biochem. Biophys. Res. Commun. 2016, 472, 118–124. [Google Scholar] [CrossRef]
- Nagata, O.; Nakamura, M.; Sakimoto, H.; Urata, Y.; Sasaki, N.; Shiokawa, N.; Sano, A. Mouse model of chorea-acanthocytosis exhibits male infertility caused by impaired sperm motility as a result of ultrastructural morphological abnormalities in the mitochondrial sheath in the sperm midpiece. Biochem. Biophys. Res. Commun. 2018, 503, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hossain, M.I.; Yamazaki, M.; Abe, M.; Natsume, R.; Konno, K.; Kageyama, S.; Komatsu, M.; Watanabe, M.; Sakimura, K.; et al. Deletion of exons encoding carboxypeptidase domain of Nna1 results in Purkinje cell degeneration (pcd) phenotype. J. Neurochem. 2018, 147, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Pompolo, S.; Harley, V.R. Localisation of the SRY-related HMG box protein, SOX9, in rodent brain. Brain Res. 2001, 906, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, R.M., Jr.; Walker, R.H.; Nirenberg, M.J.; Tetlow, A.M.; Farrell, K.; Lind-Watson, K.J.; Thorn, E.L.; Dangoor, D.K.; Gordon, R.; De Sanctis, C.; et al. An Autopsy Series of Seven Cases of VPS13A Disease (Chorea-Acanthocytosis). Mov. Disord. 2023, 38, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Nishizawa, Y.; Nagata, O.; Sakimoto, H.; Sasaki, N.; Sano, A.; Nakamura, M. The role of chorein deficiency in late spermatogenesis. Biomedicines 2024, 12, 240. [Google Scholar] [CrossRef]
- Kaminska, J.; Soczewka, P.; Rzepnikowska, W.; Zoladek, T. Yeast as a Model to Find New Drugs and Drug Targets for VPS13-Dependent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5106. [Google Scholar] [CrossRef] [PubMed]
- John Peter, A.T.; Herrmann, B.; Antunes, D.; Rapaport, D.; Dimmer, K.S.; Kornmann, B. Vps13-Mcp1 interact at vacuole-mitochondria interfaces and bypass ER-mitochondria contact sites. J. Cell Biol. 2017, 216, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Yeshaw, W.M.; van der Zwaag, M.; Pinto, F.; Lahaye, L.L.; Faber, A.I.; Gómez-Sánchez, R.; Dolga, A.M.; Poland, C.; Monaco, A.P.; van IJzendoorn, S.C.; et al. Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. Elife 2019, 8, e43561. [Google Scholar] [CrossRef] [PubMed]
- Cloos, A.S.; Daenen, L.G.M.; Maja, M.; Stommen, A.; Vanderroost, J.; Van Der Smissen, P.; Rab, M.; Westerink, J.; Mignolet, E.; Larondelle, Y.; et al. Impaired cytoskeletal and membrane biophysical properties of acanthocytes in hypobetalipoproteinemia - A case study. Front. Physiol. 2021, 12, 638027. [Google Scholar] [CrossRef]
- Sbardella, D.; Tundo, G.R.; Campagnolo, L.; Valacchi, G.; Orlandi, A.; Curatolo, P.; Borsellino, G.; D’Esposito, M.; Ciaccio, C.; Cesare, S.D.; et al. Retention of mitochondria in mature human red blood cells as the result of autophagy impairment in Rett syndrome. Sci. Rep. 2017, 7, 12297. [Google Scholar] [CrossRef]
- Shiokawa, N.; Nakamura, M.; Sameshima, M.; Deguchi, A.; Hayashi, T.; Sasaki, N.; Sano, A. Chorein, the protein responsible for chorea-acanthocytosis, interacts with β-adducin and β-actin. Biochem. Biophys. Res. Commun. 2013, 441, 96–101. [Google Scholar] [CrossRef]
- Mishina, M.; Sakimura, K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci. Res. 2007, 58, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Kurose, M.; Yano, M.; Tran, D.M.; Okuda, S.; Mori-Ochiai, Y.; Horie, M.; Nagai, T.; Nishino, I.; Shibata, S.; et al. Isoform-specific mutation in Dystonin-b gene causes late-onset protein aggregate myopathy and cardiomyopathy. Elife 2022, 11, e78419. [Google Scholar] [CrossRef]
- Bizen, N.; Bepari, A.K.; Zhou, L.; Abe, M.; Sakimura, K.; Ono, K.; Takebayashi, H. Ddx20, an Olig2 binding factor, governs the survival of neural and oligodendrocyte progenitor cells via proper Mdm2 splicing and p53 suppression. Cell. Death. Differ. 2022, 29, 1028–1041. [Google Scholar] [CrossRef]
- Zhou, L.; Konno, K.; Yamazaki, M.; Abe, M.; Natsume, R.; Watanabe, M.; Takebayashi, H.; Sakimura, K. Nna1, essential for Purkinje cell survival, is also associated with emotion and memory. Int. J. Mol. Sci. 2022, 23, 12961. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Watanabe, K.; Bepari, A.K.; Nashimoto, J.; Araki, K.; Sano, H.; Chiken, S.; Nambu, A.; Ono, K.; Ikenaka, K.; et al. Disruption of actin-binding domain-containing Dystonin protein causes dystonia musculorum in mice. Eur. J. Neurosci. 2014, 40, 3458–3471. [Google Scholar] [CrossRef]
- Takebayashi, H.; Yoshida, S.; Sugimori, M.; Kosako, H.; Kominami, R.; Nakafuku, M.; Nabeshima, Y. Dynamic expression of basic helix-loop-helix Olig family members: Implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech. Dev. 2000, 99, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Iseda, T.; Mitsuhashi, T.; Oka, A.; Shindo, T.; Moritoki, N.; Nagai, T.; Otsubo, S.; Inoue, T.; Sasaki, E.; et al. Large-area fluorescence and electron microscopic correlative imaging with multibeam scanning electron microscopy. Front. Neural. Circuits 2019, 13, 29. [Google Scholar] [CrossRef]
- Simankova, A.; Bizen, N.; Saitoh, S.; Shibata, S.; Ohno, N.; Abe, M.; Sakimura, K.; Takebayashi, H. Ddx20, DEAD box helicase 20, is essential for the differentiation of oligodendrocyte and maintenance of myelin gene expression. Glia 2021, 69, 2559–2574. [Google Scholar] [CrossRef]
- Montoto, L.G.; Arregui, L.; Sánchez, N.M.; Gomendio, M.; Roldan, E.R.S. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction 2012, 143, 333–346. [Google Scholar] [CrossRef]
- International Committee for Standardization in Haematology. ICSH Guidelines for Reticulocyte Counting by Microscopy on Supra Vitally Stained Preparations; WHO/LBS/92.3; WHO: Geneva, Switzerland, 1992. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinyomahakul, J.; Ise, M.; Kawamura, M.; Yamada, T.; Okuyama, K.; Shibata, S.; Takizawa, J.; Abe, M.; Sakimura, K.; Takebayashi, H. Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice. Int. J. Mol. Sci. 2024, 25, 7776. https://doi.org/10.3390/ijms25147776
Pinyomahakul J, Ise M, Kawamura M, Yamada T, Okuyama K, Shibata S, Takizawa J, Abe M, Sakimura K, Takebayashi H. Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice. International Journal of Molecular Sciences. 2024; 25(14):7776. https://doi.org/10.3390/ijms25147776
Chicago/Turabian StylePinyomahakul, Jitrapa, Masataka Ise, Meiko Kawamura, Takashi Yamada, Kentaro Okuyama, Shinsuke Shibata, Jun Takizawa, Manabu Abe, Kenji Sakimura, and Hirohide Takebayashi. 2024. "Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice" International Journal of Molecular Sciences 25, no. 14: 7776. https://doi.org/10.3390/ijms25147776
APA StylePinyomahakul, J., Ise, M., Kawamura, M., Yamada, T., Okuyama, K., Shibata, S., Takizawa, J., Abe, M., Sakimura, K., & Takebayashi, H. (2024). Analysis of Brain, Blood, and Testis Phenotypes Lacking the Vps13a Gene in C57BL/6N Mice. International Journal of Molecular Sciences, 25(14), 7776. https://doi.org/10.3390/ijms25147776





