Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives
Abstract
1. Introduction
- The CDs have numerous chiral centers (35 in β-CD). These chiral centers differ from each other. The CDs have twisted truncated shapes, resulting in different arrangements (lengths, directions) of the functional groups around every chiral center. This is the reason why the CDs have broader chiral recognition spectra than linear amylose molecules, which have the same arrangements in every glucose unit.
- The added functional groups of derivatized CDs (phosphate, sulfate, amino, naphthyl, acetate) offer further interaction abilities and steric arrangements to selectand enantiomers compared to native CDs.
- The majority of CD derivatives are randomly substituted molecules. They are not uniform products. They differ from each other in their numbers and in the positions of the substituents, resulting in every isomer having a different chiral recognition feature.
- The derivatized CDs have a rather flexible structure. They can change their shapes to interact intimately (more strongly) with functional groups of analytes with the so-called “induced fit” mechanism. The “induced fit” phenomenon further increases the chiral selectivity spectra of CDs.
- The ionizable CDs can broaden their selectivity spectra according to their ionization states.
- The types of mobile phases or background buffers influence the selectivity features of CDs. The CDs can simultaneously include not only the analytes but also a solvent molecule in their cavities.
2. Results
- (a)
- With O(2)CH3 and O(3)CH3 units of the same glucopyranoside unit;
- (b)
- With O(2)CH3 and O(3)CH3 units of adjacent glucopyranoside units;
- (c)
- With different glucopyranoside units of the macrocycle O(2)CH3 and O(2)CH3, or O(3)CH3 and O(3)CH3 separated by two or three units.
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poppe, L.; Nógrádi, M. Stereochemistry and Stereoselective Synthesis: An Introduction, 1st ed.; Wiley-VCH: Weinheim, Germany, 2016; ISBN 3527339019. [Google Scholar]
- Klebe, G. Optical Activity and Biological Effect. In Drug Design; Klebe, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Department of Health and Human Services of the USA. Food and Drug Administration’s Policy Statement for the Development of New Stereoisomeric Drugs, Fed. Regist. 57/2 May 1992, 102. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-new-stereoisomeric-drugs (accessed on 5 April 2024).
- Kozma, D. (Ed.) CRC Handbook of Optical Resolutions via Diastereomeric Salt Formation; CRC: Boca Raton, FL, USA, 2001; ISBN 0849300193. [Google Scholar]
- Scriba, G.K.E. (Ed.) Chiral Separations: Methods and Protocols, 2nd ed.; Humana Press: Totowa, NJ, USA, 2013; ISBN 978-1-62703-262-9. [Google Scholar]
- Tarafder, T.; Miller, L. Chiral chromatography method screening strategies: Past, present and future. J. Chromatogr. A 2021, 1638, 461878. [Google Scholar] [CrossRef] [PubMed]
- Dalgliesh, C.E. The optical resolution of aromatic amino acids on paper. J. Chem. Soc. 1952, 137, 3940–3942. [Google Scholar] [CrossRef]
- Schurig, V. Enantiomer Separation by Gas Chromatography on Chiral Phases. J. Chromatogr. A 1994, 666, 111. [Google Scholar] [CrossRef]
- Szejtli, J.; Osa, T. Comprehensive Supramolecular Chemistry: Cyclodextrins; Pergamon: London, UK, 1999; Volume 3, ISBN 9780080427157. [Google Scholar]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Szente, L.; Maklári, D. Cyclodextrins as Dominant Chiral Selective Agents in the Capillary Separation Techniques. Period. Polytech. Chem. Eng. 2021, 65, 580–594. [Google Scholar] [CrossRef]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Iványi, R.; Szente, L. The role of cyclodextrins in chiral capillary electrophoresis. Electrophoresis 2008, 29, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Laczkó, Z.; Iványi, R.; Varga, E. Chiral Separations of Pyrethroic Acids Using Cyclodextrin Selectors. Molecules 2022, 27, 8718. [Google Scholar] [CrossRef] [PubMed]
- Repassy, L.; Juvancz, Z.; Bodane-Kendrovics, R.; Kaleta, Z.; Hunyadi, C.; Riszter, G. Structure–Chiral Selectivity Relationships of Various Mandelic Acid Derivatives on Octakis 2,3-di-O-acetyl-6-O-tert-butyldimethylsilyl-gamma-cyclodextrin Containing Gas Chromatographic Stationary. Int. J. Mol. Sci. 2023, 24, 15051. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Ludmerczki, R.; Fiser, B.; Noszál, B.; Tóth, G. Chiral separation of rasagiline using sulfobutylether-β-cyclodextrin: Capillary electrophoresis, NMR and molecular modeling study. Electrophoresis 2019, 40, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Samakashvili, S.; Salgado, A.; Scriba, G.K.E.; Chankvetadze, B. Comparative Enantioseparation of Ketoprofen with Trimethylated α-, β-, and γ-Cyclodextrins in Capillary Electrophoresis and Study of Related Selector–Selectand Interactions Using Nuclear Magnetic Resonance Spectroscopy. Chirality 2013, 25, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bethanis, K.; Christoforides, E.; Andreou, A.; Eliopoulos, E. Molecular Symmetry of Permethylated β-Cyclodextrins upon Complexation. Symmetry 2022, 14, 2214. [Google Scholar] [CrossRef]
- Banik, A.; Saikia, M. Interaction of ibuprofen with β-cyclodextrins: Experimental and molecular modeling studies. J. Indian Chem. Soc. 2013, 90, 1163–1171. [Google Scholar]
- Le, C.A.K.; Ogawa, Y.; Dubreuil, F.; Grimaud, F.; Mazeau, K.; Ziegler, G.R.; Tanwar, S.; Nishiyama, Y.; Potocki-Veronese, G.; Choisnard, L.; et al. Crystal and molecular structure of V-amylose complexed with ibuprofen. Carbohydr. Polym. 2021, 261, 117885. [Google Scholar] [CrossRef] [PubMed]
- Pereva, S.; Sarafska, T.; Bogdanova, S.; Spassov, T. Efficiency of “cyclodextrin-ibuprofen” inclusion complex formation. J. Drug Deliv. Sci. Technol. 2016, 35, 34–39. [Google Scholar] [CrossRef]
- Brown, G.R.; Caira, M.R.; Nassimbeni, L.R.; Oudtshoorn, B. Inclusion of Ibuprofen by Heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin: An X-ray Diffraction and Thermal Analysis Study. J. Incl. Phenom. Mol. Recognit. Chem. 1996, 26, 281–294. [Google Scholar] [CrossRef]
- Braga, S.S.; Gonçalves, I.S.; Herdtweck, E.; Teixeira-Dias, J.J.C. Solid state inclusion compound of S-ibuprofen in β-cyclodextrin: Structure and characterisation. New J. Chem. 2003, 27, 597–601. [Google Scholar] [CrossRef]
- Crupi, V.; Guella, G.; Majolino, D.; Mancini, I.; Paciaroni, A.; Rossi, B.; Venuti, V.; Verrocchio, P.; Viliani, G. Effect of the chiral discrimination on the vibrational properties of (R)-, (S)- and (R, S)-ibuprofen/methyl-β-cyclodextrin inclusion complexes. Philos. Mag. 2011, 91, 1776–1785. [Google Scholar] [CrossRef][Green Version]
- Rainsford, K.D. (Ed.) Ibuprofen: Development and Therapeutics; Wiley: Hoboken, NJ, USA, 2015; ISBN 9781118743584/111874358X. [Google Scholar]
- Cârje, A.-G.; Juvancz, Z.; Tőkés, B. Quantitative Characterization of Derivatization Effects in Separations Techniques I. Derivatization of Ibuprofen. Acta Polytech. Hung. 2013, 10, 219–230. [Google Scholar]
- Johannsen, M. Separation of enantiomers of ibuprofen on chiral stationary phases by packed column supercritical fluid chromatography. J. Chromatogr. A 2001, 937, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Petersson, P.; Reese, S.L.; Yi, G.; Yun, H.; Malik, A.; Bradshaw, J.S.; Rossiter, B.E.; Lee, M.E.; Markides, K.E. Evaluation of beta-cyclodextrin-based chiral stationary phases for capillary column supercritical fluid chromatography. J. Chromatogr. A. 1994, 684, 297–309. [Google Scholar] [CrossRef]
- Choi, S.; Shim, W.-S.; Yoon, J.; Choi, D.; Lee, J.; Paik, S.-H.; Chung, E.-K.; Lee, K.-T. A Validated Chiral LC-MS/MS Method for the Enantioselective Determination of (S)-(+)- and (R)-(-)-Ibuprofen in Dog Plasma: Its Application to a Pharmacokinetic Study. Pharmaceutics 2023, 15, 824. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral separation using cyclodextrins as mobile phase additives in open-tubular liquid chromatography with a pseudophase coating. J. Sep. Sci. 2022, 45, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Sohajda, T.; Juvancz, Z.; Bodane-Kendrovics, R.; Szekely, E.; Bansaghi, G. Development of Electrophoretic Methods for Simultaneous Determination of Enantiomeric Ratio and Composition of Diastereomeric Salt Mixtures. Chromatographia 2015, 78, 881–888. [Google Scholar] [CrossRef]
- Iványi, R.; Jicsinszky, L.; Juvancz, Z. Permethyl Monoamino β- Cyclodextrin a New Chiral Selective Agent for Electrophoresis. Chromatographia 2000, 53, 166–172. [Google Scholar] [CrossRef]
- Stein, S.E.; Brown, R.L. Estimation of Normal Boiling Points from Group Contribution. J. Chem. Inf. Comput. Sci. 1994, 34, 581–587. [Google Scholar] [CrossRef]
- Kovats, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retention indices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Berthed, A.; Li, W.; Armstrong, D.W. Multiple Enantioselective Retention Mechanisms on Derivatized Cyclodextrin Gas Chromatographic Chiral Stationary Phases. Anal. Chem. 1992, 64, 873–879. [Google Scholar] [CrossRef]
- Juvancz, Z.; Grolimund, K.; Schurig, V. Unusual overloading phenomenon in GC using Chirasil-dex, an enantioselective stationary phase. J. High-Resolut. Chromatogr. 1993, 16, 202–204. [Google Scholar] [CrossRef]
- Lee, J.-u.; Lee, S.-S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host-Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef] [PubMed]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L.; Lagardère, L.; Schnieders, M.J.; Piquemal, J.-P.; Ren, P.; Ponder, J.W. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef] [PubMed]
- PM7 Implementation of MOPAC2016 (Version: 22.234W). Available online: https://openmopac.net/MOPAC2016.html (accessed on 15 April 2024).
- Mazzarella, D.; Crisenza, G.E.M.; Melchiorre, P. Asymmetric Photocatalytic C–H Functionalization of Toluene and Derivatives. J. Am. Chem. Soc. 2018, 140, 8439–8443. [Google Scholar] [CrossRef]
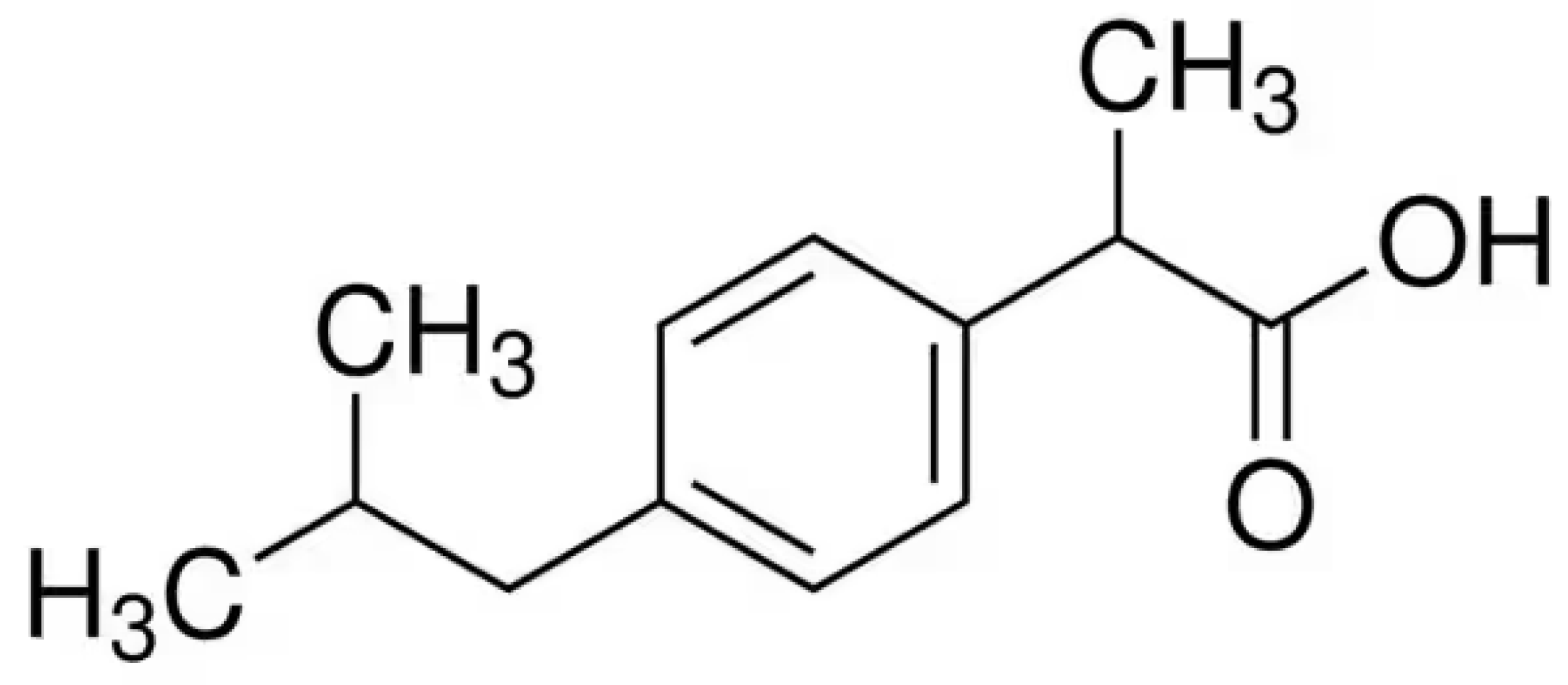
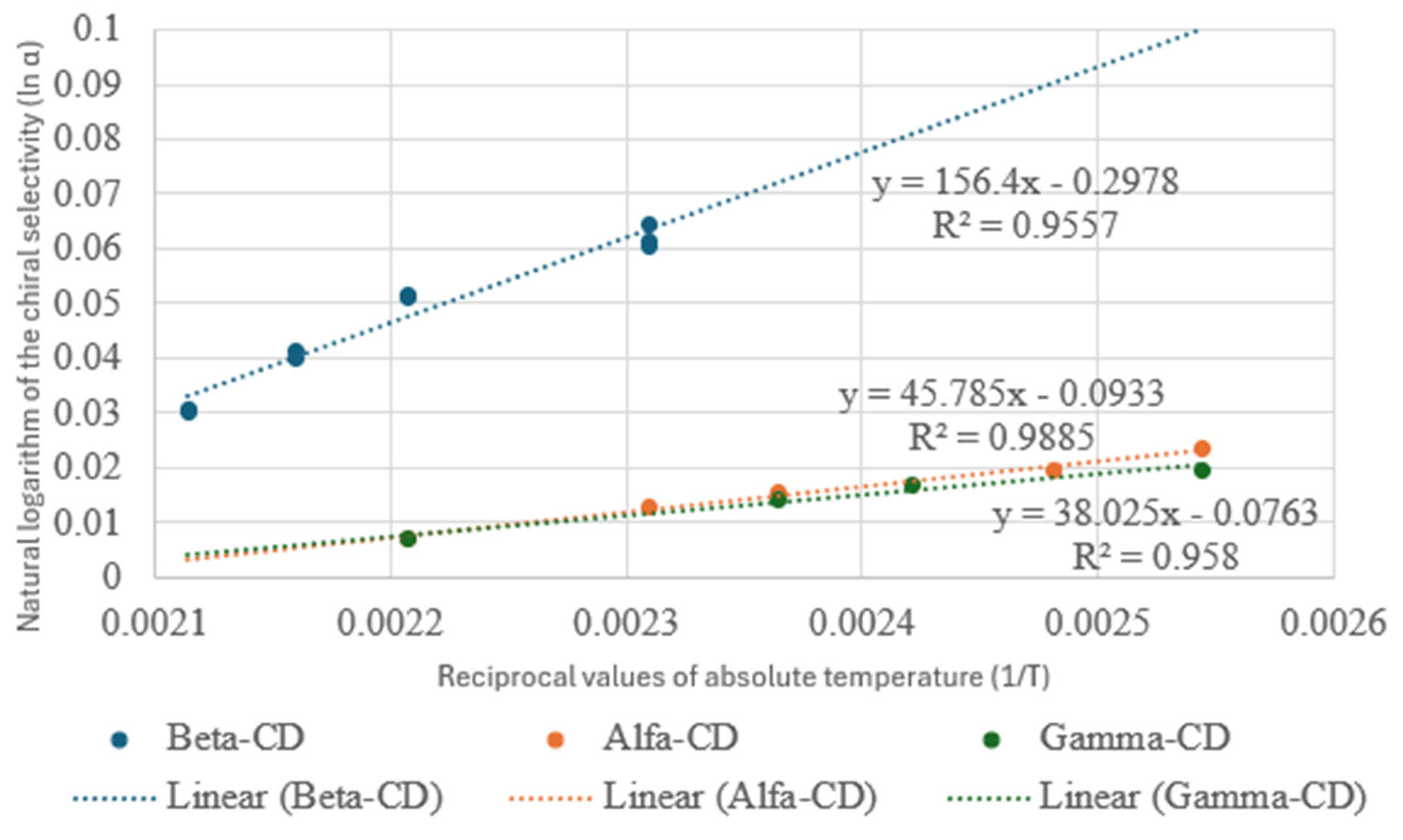
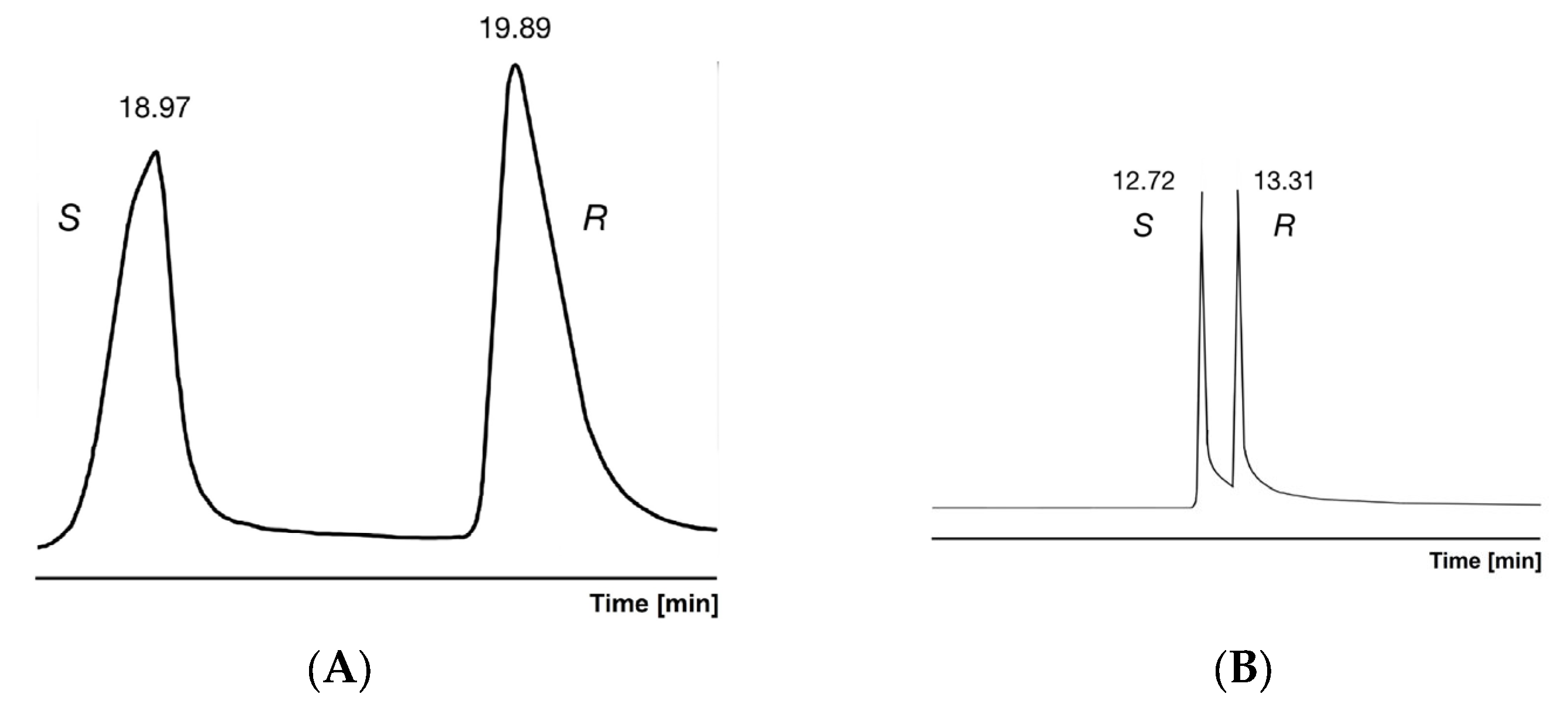

| Compounds | Chiral Selector | Boiling Point | |||||
|---|---|---|---|---|---|---|---|
| Permethylated α-CD | Permethylated β-CD | Permethylated γ-CD | °C, 760 Hgmm [31] | ||||
| alfa * | KI ** | alfa * | KI ** | alfa * | KI ** | ||
| Underivatized ibuprofen | 1.027 | 2033 | 1.129 | 1906 | 1.029 | 2043 | 323 |
| Ibuprofen methyl ester | <1.01 | 1686 | 1.027 | 1652 | <1.01 | 1717 | 282 |
| Ibuprofen ethyl ester | <1.01 | 1738 | ~1.01 | 1696 | <1.01 | 1762 | 297 |
| Ibuprofen isopropyl ester | <1.01 | 1724 | 1.013 | 1703 | <1.01 | 1760 | 303 |
| Ibuprofen propyl ester | <1.01 | 1786 | 1.11 | 1782 | <1.01 | 1845 | 312 |
| Ibuprofen isobutyl ester | <1.01 | 1821 | <1.01 | 1825 | 1.012 | 1881 | 317 |
| Ibuprofen butyl ester | <1.01 | 1913 | <1.01 | 1866 | <1.01 | 1931 | 325 |
| Ibuprofen isoamyl ester | <1.01 | 1953 | <1.01 | 1915 | <1.01 | 1940 | 330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juvancz, Z.; Bodane-Kendrovics, R.; Agoston, C.; Czegledi, B.; Kaleta, Z.; Jicsinszky, L.; Riszter, G. Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives. Int. J. Mol. Sci. 2024, 25, 7802. https://doi.org/10.3390/ijms25147802
Juvancz Z, Bodane-Kendrovics R, Agoston C, Czegledi B, Kaleta Z, Jicsinszky L, Riszter G. Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives. International Journal of Molecular Sciences. 2024; 25(14):7802. https://doi.org/10.3390/ijms25147802
Chicago/Turabian StyleJuvancz, Zoltan, Rita Bodane-Kendrovics, Csaba Agoston, Balazs Czegledi, Zoltan Kaleta, Laszlo Jicsinszky, and Gergo Riszter. 2024. "Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives" International Journal of Molecular Sciences 25, no. 14: 7802. https://doi.org/10.3390/ijms25147802
APA StyleJuvancz, Z., Bodane-Kendrovics, R., Agoston, C., Czegledi, B., Kaleta, Z., Jicsinszky, L., & Riszter, G. (2024). Chiral Selectivities of Permethylated α-, β-, and γ-Cyclodextrins Containing Gas Chromatographic Stationary Phases towards Ibuprofen and Its Derivatives. International Journal of Molecular Sciences, 25(14), 7802. https://doi.org/10.3390/ijms25147802










