Plant Hormone Pathway Is Involved in Regulating the Embryo Development Mechanism of the Hydrangea macrophylla Hybrid
Abstract
:1. Introduction
2. Results
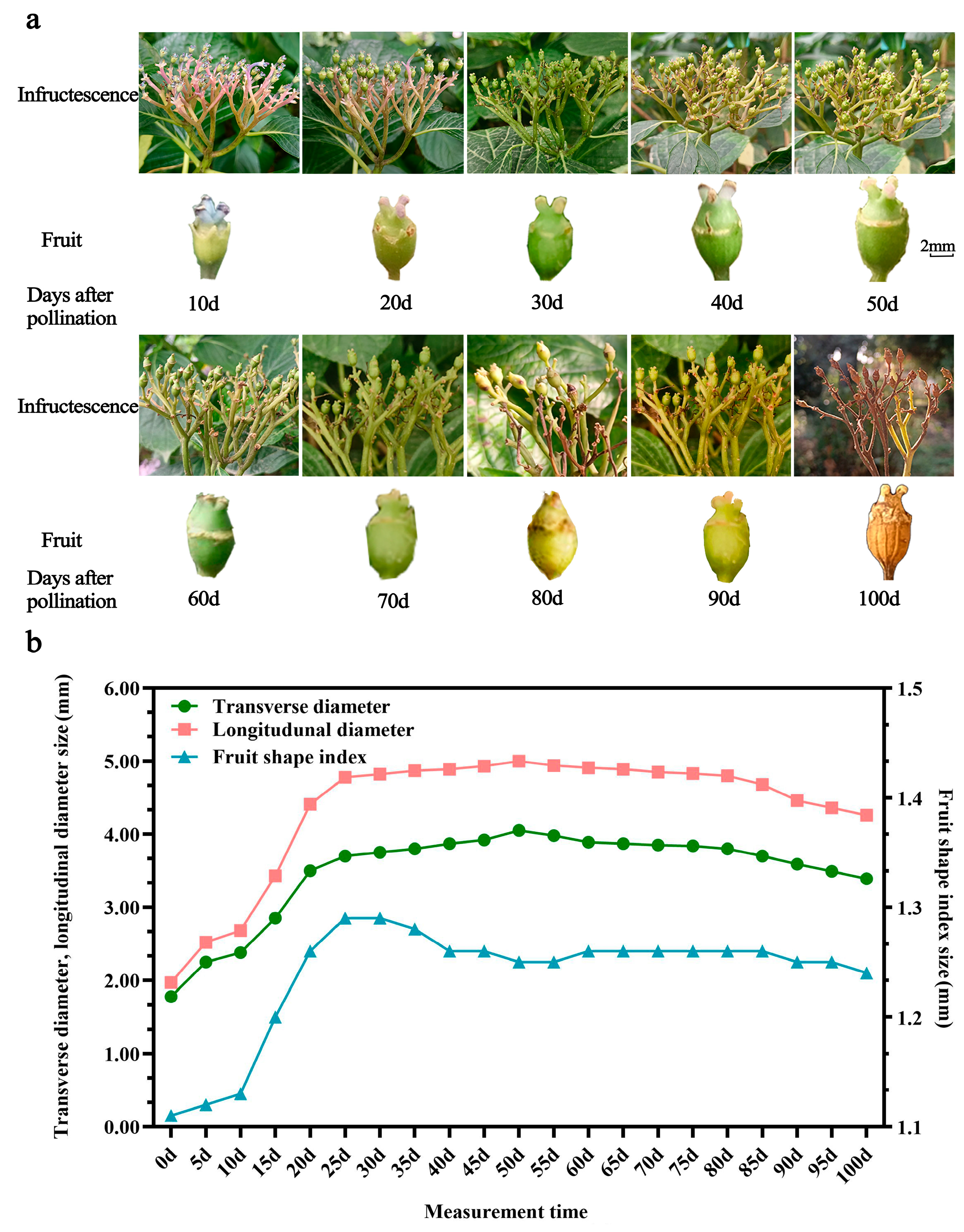
2.1. Fruit Morphological Development
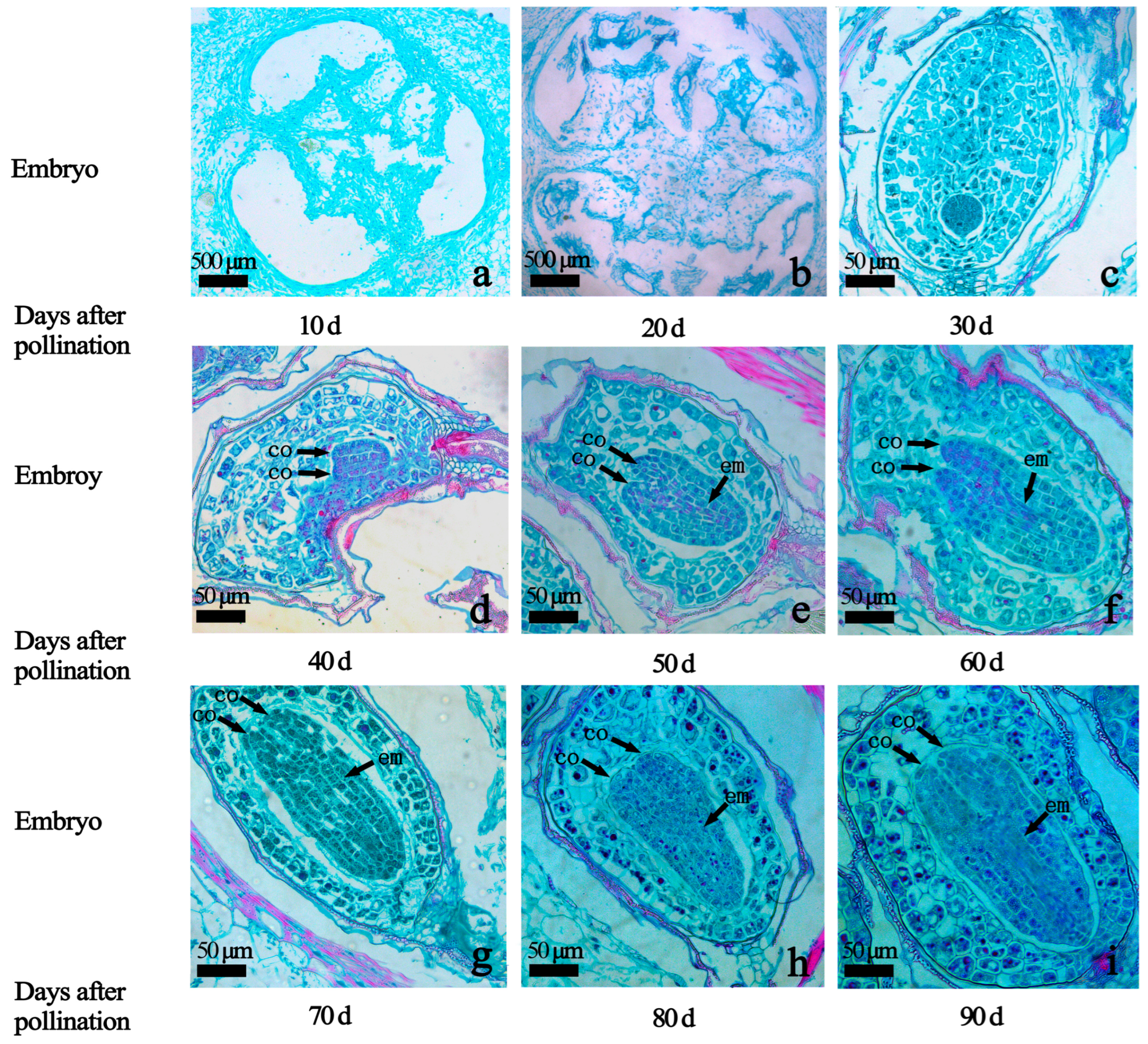
2.2. Embryo Development at Different Embryo Ages
2.3. Analysis of Sequencing Data
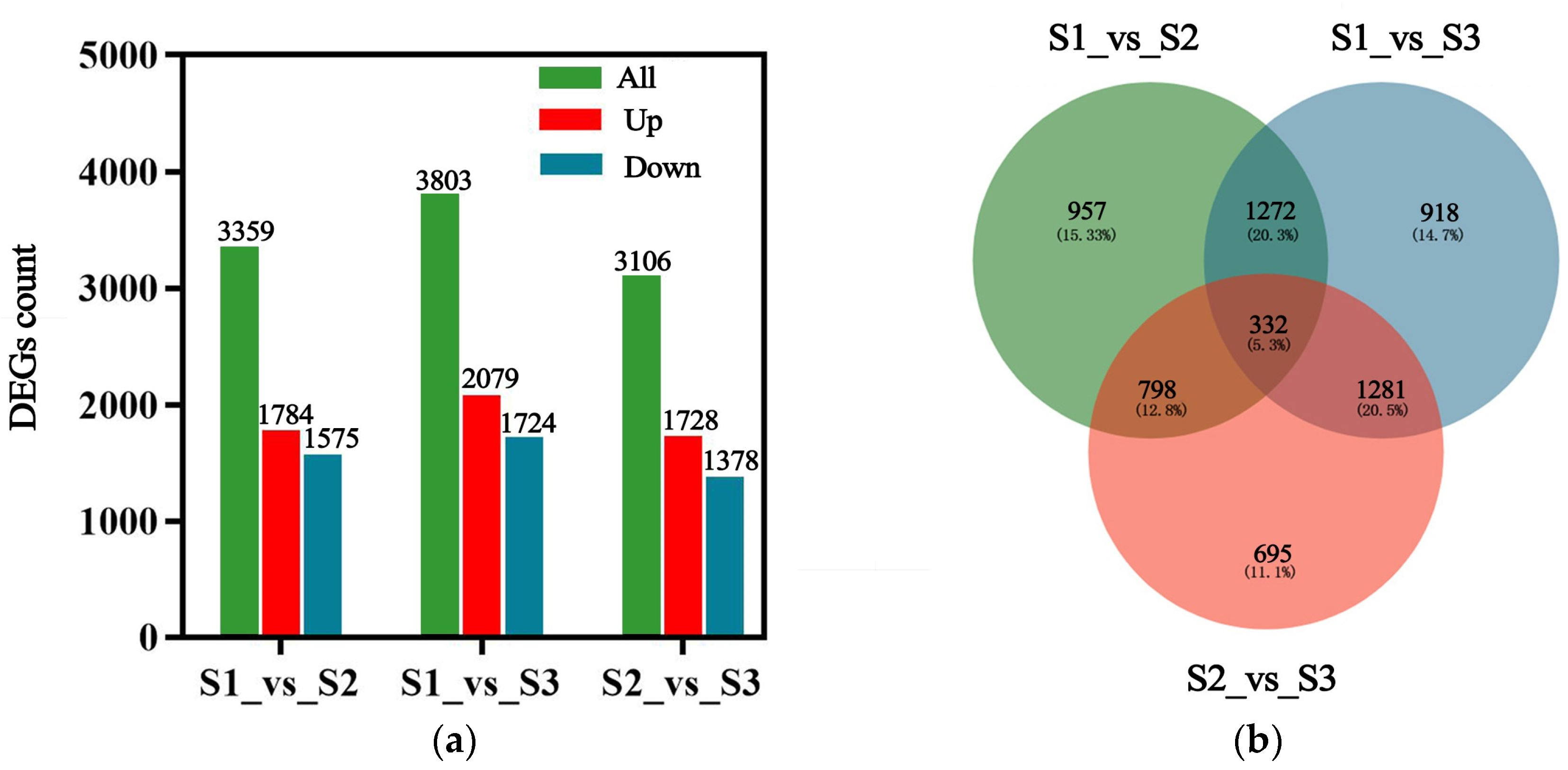
2.3.1. Differential Genes Expression Analysis
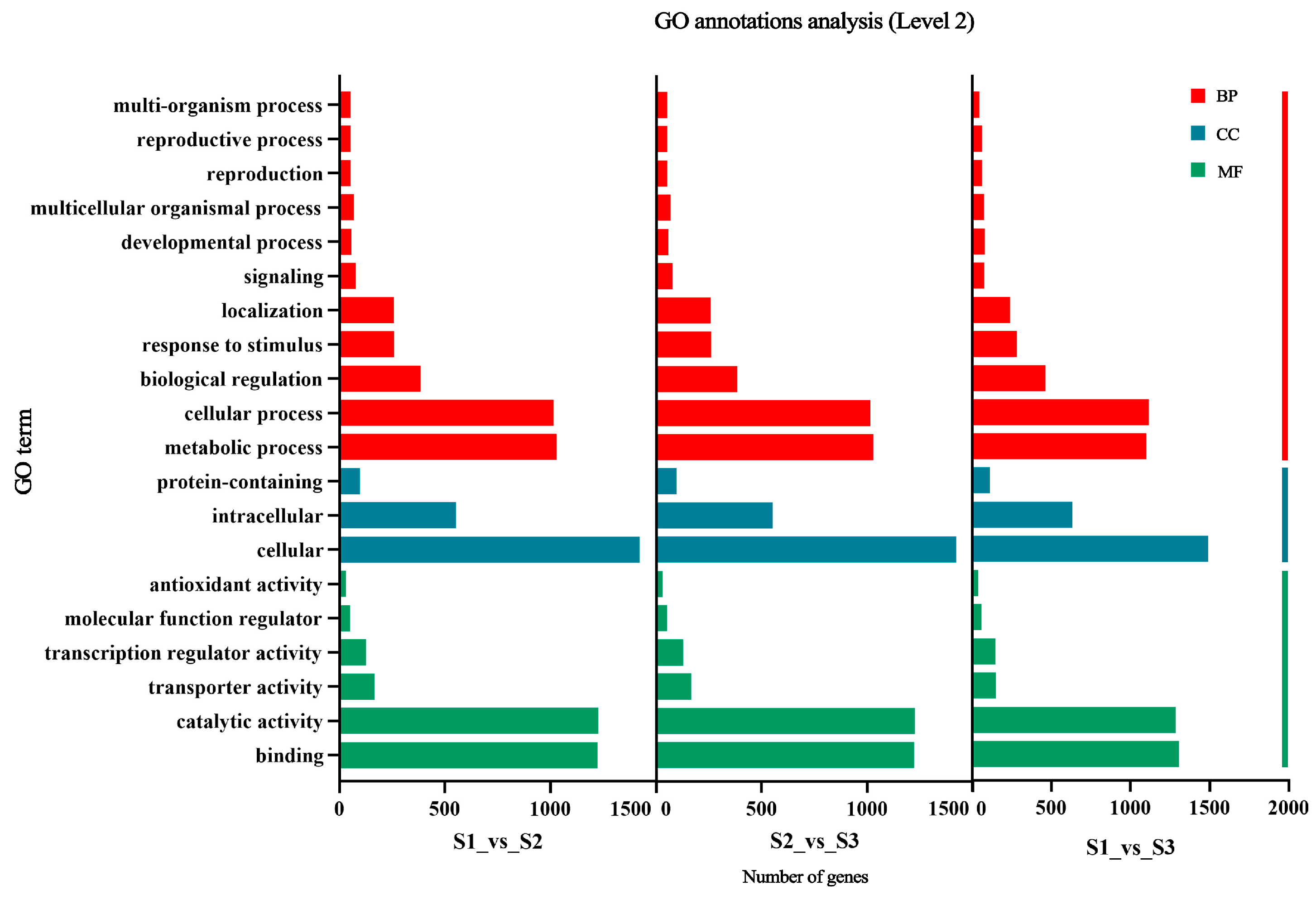
2.3.2. Functional Analysis of Differential Genes with GO Enrichment Analysis
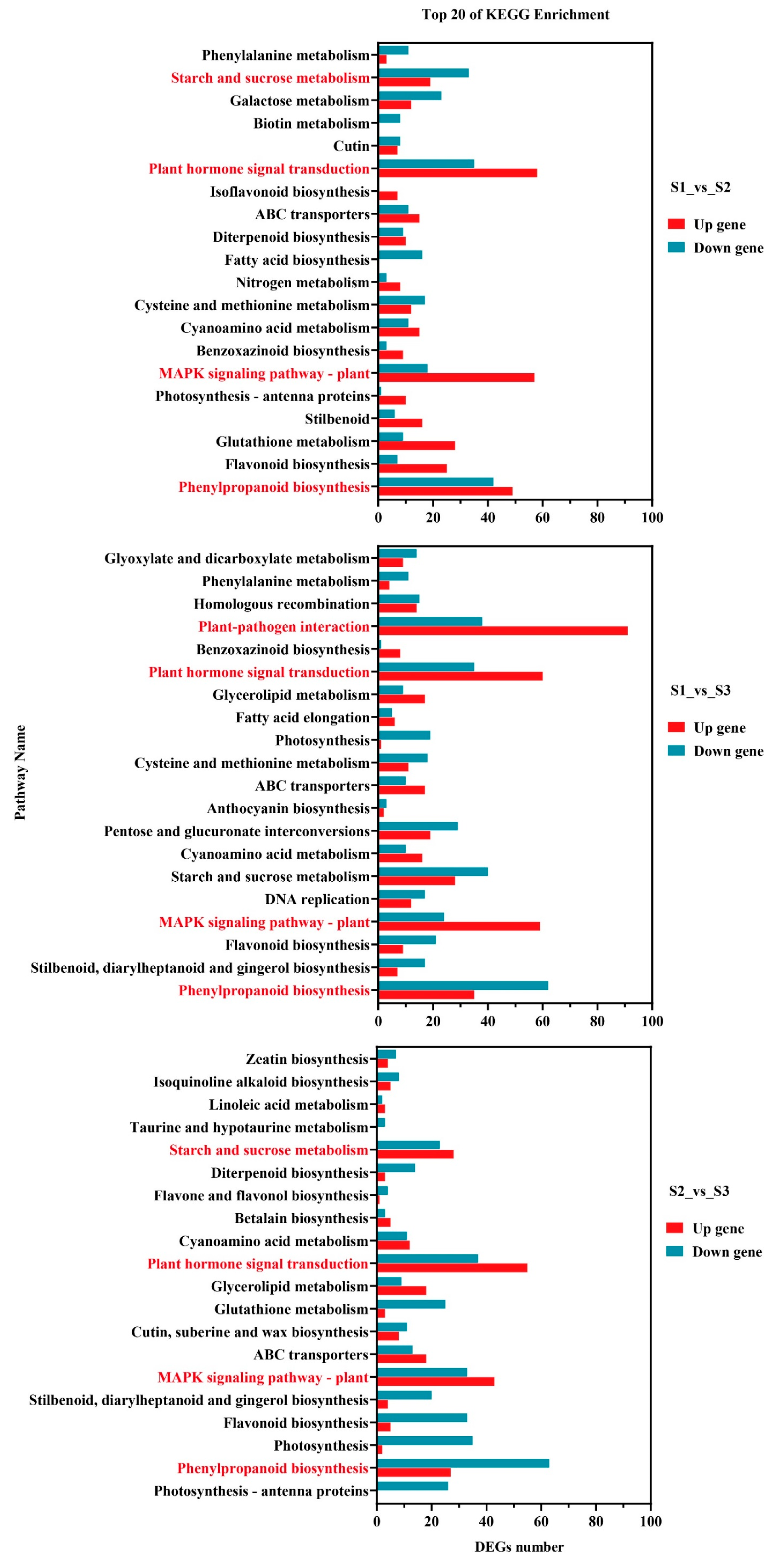
2.3.3. Differentially Expressed Genes Participate in Pathway Analysis
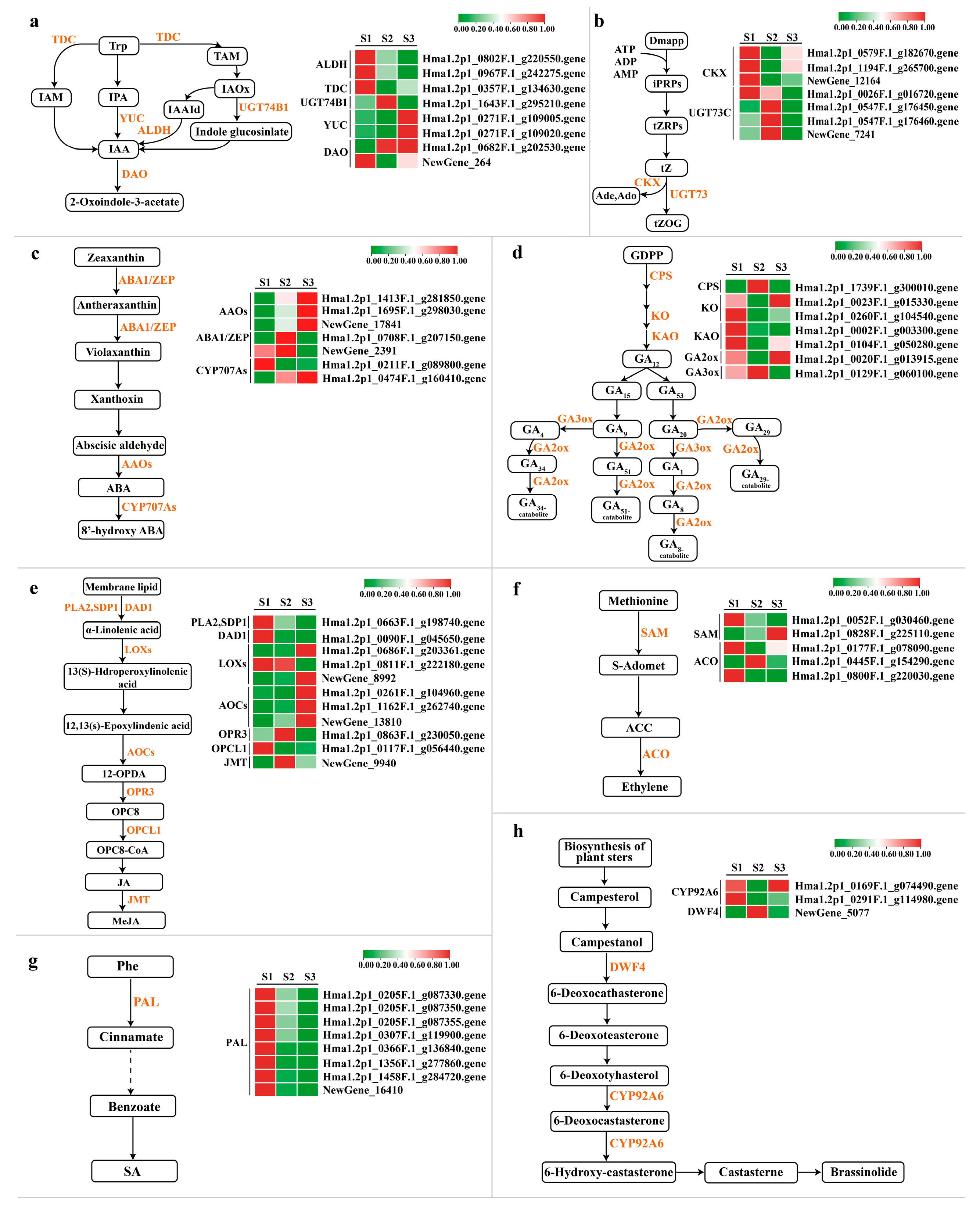
2.4. DEG Analysis of Biosynthetic Pathways and Signal Transduction Pathways of Plant Hormones
2.4.1. Auxin Synthesis Pathway and Signal Transduction Pathway
2.4.2. Cytokinin Synthesis Pathway and Signal Transduction Pathway
2.4.3. Abscisic Acid Synthetic Pathway and Signal Transduction Pathway
2.4.4. Gibberellin Synthesis Pathway and Signal Transduction Pathway
2.4.5. Jasmonic Acid Synthetic Pathway and Signal Transduction Pathway
2.4.6. Ethylene Synthesis Pathway and Signal Transduction Pathway
2.4.7. Salicylic Acid Synthesis Pathway and Signal Transduction Pathway
2.4.8. Brassinolide Synthesis and Signal Transduction Pathway
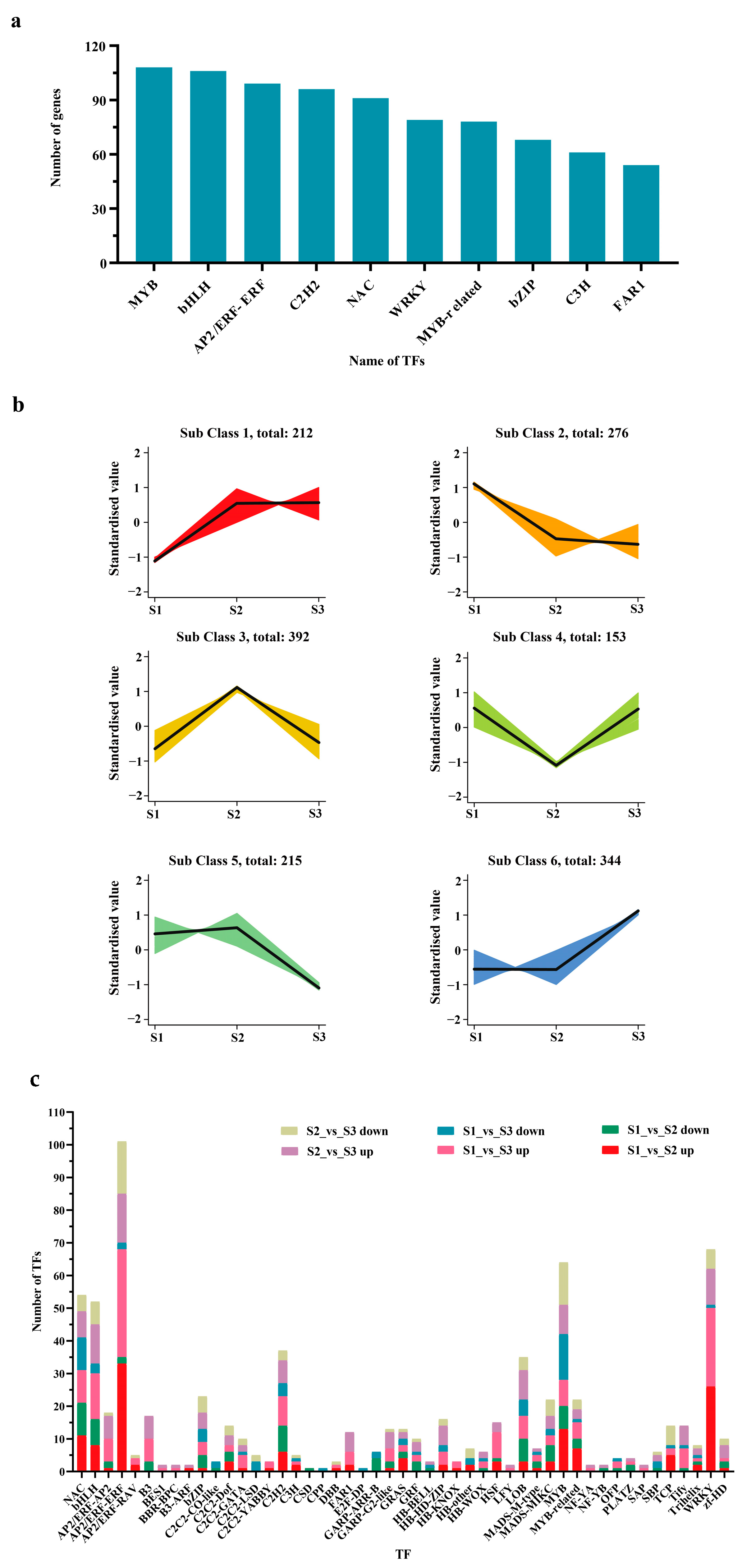
2.5. TFs Involved in the Growth and Development of Hybrid Fruits
2.6. RT-qPCR Verification
3. Discussion
4. Materials and Methods
4.1. Experimental Material
4.2. Growth Index of Hybrid Fruits
4.3. Hybrid Embryo Growth and Development Morphology
4.4. RNA Extraction, Library Construction, and RNA Sequencing
4.5. Transcriptome Analysis
4.6. RT-qPCR Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.-Y.; Chen, C.-M. Early-Flowering Interspecific Hydrangea Progenies from Crossing H. chinensis with H. macrophylla. HortScience 2022, 57, 1487–1489. [Google Scholar] [CrossRef]
- Suyama, T.; Tanigawa, T.; Yamada, A.; Matsuno, T.; Kunitake, T. Cross Compatibility between Hydrangea serrata (Thunb.) Ser. and Hydrangea macrophylla (Thunb.) Ser., and Efficiency Improvement of Hybrid Acquisition with Ovule Culture. Hortic. Res. 2008, 7, 337–343. [Google Scholar] [CrossRef]
- Deng, Y.; Teng, N.; Chen, S.; Chen, F.; Guan, Z.; Song, A.; Chang, Q. Reproductive Barriers in the Intergeneric Hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 2010, 174, 41–50. [Google Scholar] [CrossRef]
- Yabuya, T.; Yamagata, H. Embryological and Cytological Studies on Seed Development after Reciprocal Crosses between Iris sanguinea Hornem and I. iaevigata Fisch. Jpn. J. Breed. 1978, 28, 211–224. [Google Scholar] [CrossRef]
- Tang, D.; Gallusci, P.; Lang, Z. Fruit Development and Epigenetic Modifications. New Phytol. 2020, 228, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wan, S.; Zhou, J.; Shao, Q.S.; Xing, B. Transcriptional Regulation of Plant Seed Development. Physiol. Plant 2021, 173, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Savadi, S. Molecular Regulation of Seed Development and Strategies for Engineering Seed Size in Crop Plants. Plant Growth Regul. 2018, 84, 401–422. [Google Scholar] [CrossRef]
- Figueiredo, D.D.; Batista, R.A.; Roszak, P.J.; Hennig, L.; Köhler, C. Auxin Production in the Endosperm Drives Seed Coat Development in Arabidopsis. eLife 2016, 5, e20542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Yi, F.; Lai, J.; Chen, J. High Temporal-Resolution Transcriptome Landscapes of Maize Embryo Sac and Ovule during Early Seed Development. Plant Mol. Biol. 2023, 111, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.-C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; et al. A Unique Short-Chain Dehydrogenase/Reductase in Arabidopsis Glucose Signaling and Abscisic Acid Biosynthesis and Functions. Plant Cell 2002, 14, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Godin, B.; Bonnet, M.; Sotta, B.; Marion-Poll, A. Maternal Synthesis of Abscisic Acid Controls Seed Development and Yield in Nicotiana plumbaginifolia. Planta 2004, 218, 958–964. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liang, G.; Lu, S.; Wang, P.; Liu, T.; Ma, Z.; Zuo, C.; Sun, X.; Chen, B.; Mao, J. Genome-Wide Identification and Expression Analysis of GA2ox, GA3ox, and GA20ox Are Related to Gibberellin Oxidase Genes in Grape (Vitis vinifera L.). Genes 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Nguyen, T.-N.; Toora, P.K.; Ayele, B.T. Temporal and Spatial Transcriptional Regulation of Phytohormone Metabolism during Seed Development in Barley (Hordeum vulgare L.). Front. Plant Sci. 2023, 14, 1242913. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Filardo, F.F.; Storey, R.; Jermakow, A.M.; Yamaguchi, S.; Swain, S.M. Overexpression of a Gibberellin Inactivation Gene Alters Seed Development, KNOX Gene Expression, and Plant Development in Arabidopsis. Physiol. Plant 2010, 138, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Park, C.-M. Gibberellic Acid-Mediated Salt Signaling in Seed Germination. Plant Signal Behav. 2008, 3, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Bencivenga, S.; Simonini, S.; Benková, E.; Colombo, L. The Transcription Factors BEL1 and SPL Are Required for Cytokinin and Auxin Signaling During Ovule Development in Arabidopsis. Plant Cell 2012, 24, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Perry, L.; Kisiala, A.; Olechowski, H.; Emery, R.J.N. Cytokinin Activity during Early Kernel Development Corresponds Positively with Yield Potential and Later Stage ABA Accumulation in Field-Grown Wheat (Triticum aestivum L.). Planta 2020, 252, 76. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-B.; Lin, W.-H. Brassinosteroid Functions in Arabidopsis Seed Development. Plant Signal Behav. 2013, 8, e25928. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, H.; Dong, J.; Ma, C.; Li, J.; Li, Z.; Wang, Y.; Ji, J.; Hu, X.; Wu, M.; et al. The Brassinosteroid Biosynthesis Gene TaD11-2A Controls Grain Size and Its Elite Haplotype Improves Wheat Grain Yields. Theor. Appl. Genet. 2022, 135, 2907–2923. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.S.; Park, S.-H.; Koo, Y.J.; Choi, Y.D.; Chung, Y.-Y.; Lee, I.-J.; Kim, J.-K. Methyl Jasmonate Reduces Grain Yield by Mediating Stress Signals to Alter Spikelet Development in Rice. Plant Physiol. 2009, 149, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.E.; Munné-Bosch, S. Salicylic Acid Deficiency in NahG Transgenic Lines and Sid2 Mutants Increases Seed Yield in the Annual Plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Kapoor, S.; Tyagi, A.K. Transcription Factors Regulating the Progression of Monocot and Dicot Seed Development. Bioessays 2011, 33, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, H.; Yao, P.; Li, Q.; Huang, Y.; Li, C.; Chen, H.; Wu, Q. An R2R3-MYB Transcription Factor FtMYB15 Involved in the Synthesis of Anthocyanin and Proanthocyanidins from Tartary Buckwheat. J. Plant Growth Regul. 2018, 37, 76–84. [Google Scholar] [CrossRef]
- Li, A.; Zhou, Y.; Jin, C.; Song, W.; Chen, C.; Wang, C. LaAP2L1, a Heterosis-Associated AP2/EREBP Transcription Factor of Larix, Increases Organ Size and Final Biomass by Affecting Cell Proliferation in Arabidopsis. Plant Cell Physiol. 2013, 54, 1822–1836. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, H.; Chen, G.; Li, F.; Wang, Y.; Liao, C.; Hu, Z. The bHLH Transcription Factor SlPRE2 Regulates Tomato Fruit Development and Modulates Plant Response to Gibberellin. Plant Cell Rep. 2019, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, L.; Wan, L.; Huai, D.; Kang, Y.; Shi, L.; Jiang, H.; Lei, Y.; Liao, B. Genome-Wide Systematic Characterization of bZIP Transcription Factors and Their Expression Profiles during Seed Development and in Response to Salt Stress in Peanut. BMC Genom. 2019, 20, 51. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, J.; Xie, Q.; Gong, J.; Shen, H.; Chen, Y.; Chen, G.; Hu, Z. The Basic Helix-Loop-Helix Transcription Factor bHLH95 Affects Fruit Ripening and Multiple Metabolisms in Tomato. J. Exp. Bot. 2020, 71, 6311–6327. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, J.; Ren, Y.; Li, M.; Tian, S.; Yu, Y.; Zuo, Y.; Gong, G.; Zhang, H.; et al. The NAC Transcription Factor ClNAC68 Positively Regulates Sugar Content and Seed Development in Watermelon by Repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 214. [Google Scholar] [CrossRef]
- Liu, X.; He, Z.; Yin, Y.; Xu, X.; Wu, W.; Li, L. Transcriptome Sequencing and Analysis during Seed Growth and Development in Euryale Ferox Salisb. BMC Genom. 2018, 19, 343. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Guo, D.; Guo, L.; Duan, X.; Jia, Q.; Hou, X. Integrated Analysis of Transcriptomic and Proteomic Data from Tree Peony (P. ostii) Seeds Reveals Key Developmental Stages and Candidate Genes Related to Oil Biosynthesis and Fatty Acid Metabolism. Hortic. Res. 2019, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The Main Auxin Biosynthesis Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Wang, X.; Feng, J.; Hong, S.; Liang, Y.; Ren, B.; Zuo, J. Cytokinin Antagonizes Abscisic Acid-Mediated Inhibition of Cotyledon Greening by Promoting the Degradation of Abscisic Acid Insensitive5 Protein in Arabidopsis. Plant Physiol. 2014, 164, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Guo, H.; Sun, M. Signal Transduction during Wheat Grain Development. Planta 2015, 241, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Yamasaki, Y.; Kanno, Y.; Seo, M.; Ayele, B.T. Transcriptomics of Cytokinin and Auxin Metabolism and Signaling Genes during Seed Maturation in Dormant and Non-Dormant Wheat Genotypes. Sci. Rep. 2019, 9, 3983. [Google Scholar] [CrossRef]
- Dejonghe, W.; Okamoto, M.; Cutler, S.R. Small Molecule Probes of ABA Biosynthesis and Signaling. Plant Cell Physiol. 2018, 59, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.; Xing, L.; Liu, X.; Hou, Y.-J.; Chinnusamy, V.; Wang, P.; Duan, C.; Zhu, J.-K. The Unique Mode of Action of a Divergent Member of the ABA-Receptor Protein Family in ABA and Stress Signaling. Cell Res. 2013, 23, 1380–1395. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular Aspects of Seed Dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, D.M.; Wickramarathna, A.D.; Ozga, J.A.; Kurepin, L.V.; Jin, A.L.; Good, A.G.; Pharis, R.P. Gibberellin 3-Oxidase Gene Expression Patterns Influence Gibberellin Biosynthesis, Growth, and Development in Pea. Plant Physiol. 2013, 163, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; Wang, C.; Jiang, L.; Li, S.; Sun, S.S.M.; He, J.-X. An Interaction between BZR1 and DELLAs Mediates Direct Signaling Crosstalk between Brassinosteroids and Gibberellins in Arabidopsis. Sci. Signal 2012, 5, ra72. [Google Scholar] [CrossRef] [PubMed]
- Zentella, R.; Zhang, Z.-L.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global Analysis of Della Direct Targets in Early Gibberellin Signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Itoh, H.; Gomi, K.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Jeong, D.-H.; An, G.; Kitano, H.; Ashikari, M.; et al. Accumulation of Phosphorylated Repressor for Gibberellin Signaling in an F-Box Mutant. Science 2003, 299, 1896–1898. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Laparra, H.; Romero, S.P.; Schmelz, E.; Hamberg, M.; Mottinger, J.P.; Moreno, M.A.; Dellaporta, S.L. Tasselseed1 Is a Lipoxygenase Affecting Jasmonic Acid Signaling in Sex Determination of Maize. Science 2009, 323, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Sano, R.; Wada, T.; Takabayashi, J.; Okada, K. Jasmonic Acid Control of GLABRA3 Links Inducible Defense and Trichome Patterning in Arabidopsis. Development 2009, 136, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ Family of Repressors Is the Missing Link in Jasmonate Signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. The Ethylene: From Senescence Hormone to Key Player in Plant Metabolism. J. Plant Biochem. Physiol. 2014, 2. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent Advances in Ethylene Research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E. Ethylene and the Regulation of Plant Development. BMC Biol. 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Bulens, I.; Markoula, A.; Hertog, M.L.A.T.M.; Dreesen, R.; Wirtz, M.; Vandoninck, S.; Oppermann, Y.; Keulemans, J.; Hell, R.; et al. Targeted Systems Biology Profiling of Tomato Fruit Reveals Coordination of the Yang Cycle and a Distinct Regulation of Ethylene Biosynthesis during Postclimacteric Ripening. Plant Physiol. 2012, 160, 1498–1514. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; MacKerness, S.A.-H.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic Acid Has a Role in Regulating Gene Expression during Leaf Senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Zeier, J. N-Hydroxypipecolic Acid and Salicylic Acid: A Metabolic Duo for Systemic Acquired Resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Godza, B.; Watanabe, B.; Fujioka, S.; Hategan, L.; Ide, K.; Shibata, K.; Yokota, T.; Szekeres, M.; Mizutani, M.c. CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. J. Biol. Chem. 2012, 287, 31551–31560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential Transphosphorylation of the BRI1/BAK1 Receptor Kinase Complex Impacts Early Events in Brassinosteroid Signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Van Wuytswinkel, O.; Castelain, M.; Bellini, C. Combined Networks Regulating Seed Maturation. Trends Plant Sci. 2007, 12, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Gao, P.; Song, J.; Yang, H.; Qin, L.; Yu, X.; Song, H.; Coulson, J.; Bekkaoui, Y.; Akhov, L.; et al. Spatiotemporal Transcriptomics and Metabolic Profiling Provide Insights into Gene Regulatory Networks during Lentil Seed Development. Plant J. 2023, 115, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D.L. Plant Hormones and Seed Germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Zalabák, D.; Galuszka, P.; Mrízová, K.; Podlešáková, K.; Gu, R.; Frébortová, J. Biochemical Characterization of the Maize Cytokinin Dehydrogenase Family and Cytokinin Profiling in Developing Maize Plantlets in Relation to the Expression of Cytokinin Dehydrogenase Genes. Plant Physiol. Biochem. 2014, 74, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin Synthesized by the YUCCA Flavin Monooxygenases Is Essential for Embryogenesis and Leaf Formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.K.; Ten Hove, C.A.; Xiang, D.; Williams, N.; López, L.G.; Yoshida, S.; Smit, M.; Datla, R.; Weijers, D. Auxin Response Cell-Autonomously Controls Ground Tissue Initiation in the Early Arabidopsis Embryo. Proc. Natl. Acad. Sci. USA 2017, 114, E2533–E2539. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Okada, K.; Fukazawa, J.; Takahashi, Y. DELLA-Dependent and -Independent Gibberellin Signaling. Plant Signal Behav. 2018, 13, e1445933. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Giraldo, C.; Hu, J.; Urbez, C.; Gomez, M.D.; Sun, T.-P.; Perez-Amador, M.A. Role of the Gibberellin Receptors GID1 during Fruit-Set in Arabidopsis. Plant J. 2014, 79, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Hai, J.; Dong, G.; Hui, L.; Qing, Y.; Min, X.; Li, X. Identification and Expression Analysis of Gibberellin Oxidase Gene Fam ily in Flax. Mol. Plant Breed. 2023, 21, 7706–7714. [Google Scholar] [CrossRef]
- Huang, J.; Deng, J.; Shi, T.; Chen, Q.; Liang, C.; Meng, Z.; Zhu, L.; Wang, Y.; Zhao, F.; Yu, S.; et al. Global Transcriptome Analysis and Identification of Genes Involved in Nutrients Accumulation during Seed Development of Rice Tartary Buckwheat (Fagopyrum tararicum). Sci. Rep. 2017, 7, 11792. [Google Scholar] [CrossRef] [PubMed]
- Benech-Arnold, R.L.; Giallorenzi, M.C.; Frank, J.; Rodriguez, V. Termination of Hull-Imposed Dormancy in Developing Barley Grains Is Correlated with Changes in Embryonic ABA Levels and Sensitivity. Seed Sci. Res. 1999, 9, 39–47. [Google Scholar] [CrossRef]
- Xie, F.; Hua, Q.; Chen, C.; Zhang, Z.; Zhang, R.; Zhao, J.; Hu, G.; Chen, J.; Qin, Y. Genome-Wide Characterization of R2R3-MYB Transcription Factors in Pitaya Reveals a R2R3-MYB Repressor HuMYB1 Involved in Fruit Ripening through Regulation of Betalain Biosynthesis by Repressing Betalain Biosynthesis-Related Genes. Cells 2021, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Kondou, Y.; Nakazawa, M.; Kawashima, M.; Ichikawa, T.; Yoshizumi, T.; Suzuki, K.; Ishikawa, A.; Koshi, T.; Matsui, R.; Muto, S.; et al. RETARDED GROWTH OF EMBRYO1, a New Basic Helix-Loop-Helix Protein, Expresses in Endosperm to Control Embryo Growth. Plant Physiol. 2008, 147, 1924–1935. [Google Scholar] [CrossRef]
- Xue, Z.; Yang, R.; Wang, Y.; Ma, Y.; Lin, Y.; Li, Z.; Song, Y.; Feng, X.; Li, L. Candidate Gene Mining of GA-Mediated Regulation of Pear Fruit Shape. Hortic. Environ. Biotechnol. 2024, 65, 403–412. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zeng, X.; Zhu, T.; Jiang, H.; Lei, P.; Zhang, H.; Chen, H. Plant Hormone Pathway Is Involved in Regulating the Embryo Development Mechanism of the Hydrangea macrophylla Hybrid. Int. J. Mol. Sci. 2024, 25, 7812. https://doi.org/10.3390/ijms25147812
Zhu Y, Zeng X, Zhu T, Jiang H, Lei P, Zhang H, Chen H. Plant Hormone Pathway Is Involved in Regulating the Embryo Development Mechanism of the Hydrangea macrophylla Hybrid. International Journal of Molecular Sciences. 2024; 25(14):7812. https://doi.org/10.3390/ijms25147812
Chicago/Turabian StyleZhu, Yali, Xiaoman Zeng, Tingting Zhu, Hui Jiang, Penghu Lei, Huijun Zhang, and Haixia Chen. 2024. "Plant Hormone Pathway Is Involved in Regulating the Embryo Development Mechanism of the Hydrangea macrophylla Hybrid" International Journal of Molecular Sciences 25, no. 14: 7812. https://doi.org/10.3390/ijms25147812
APA StyleZhu, Y., Zeng, X., Zhu, T., Jiang, H., Lei, P., Zhang, H., & Chen, H. (2024). Plant Hormone Pathway Is Involved in Regulating the Embryo Development Mechanism of the Hydrangea macrophylla Hybrid. International Journal of Molecular Sciences, 25(14), 7812. https://doi.org/10.3390/ijms25147812




