STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart
Abstract
1. Introduction
2. Results
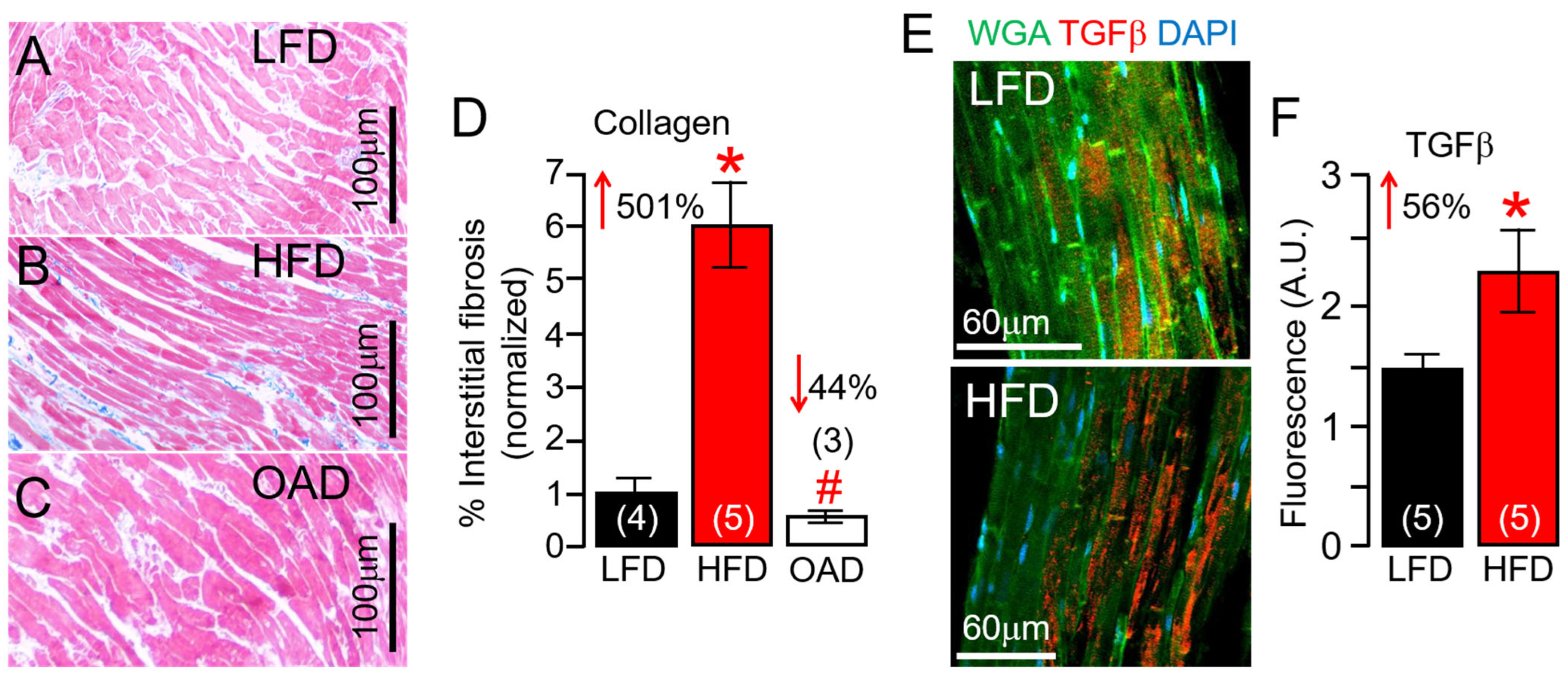
2.1. Effect of HFD on Cardiac Fibrosis in Guinea Pig
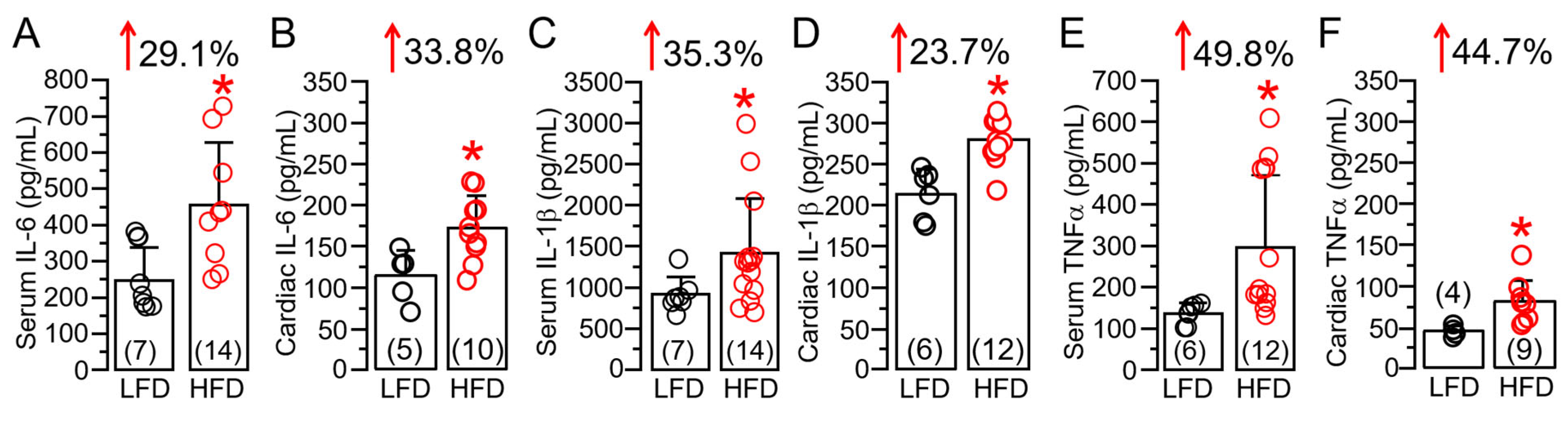
2.2. HFD Feeding Is Associated with Upregulated Cardiac IL-6 Linked Inflammation in Guinea Pig Ventricular Tissue
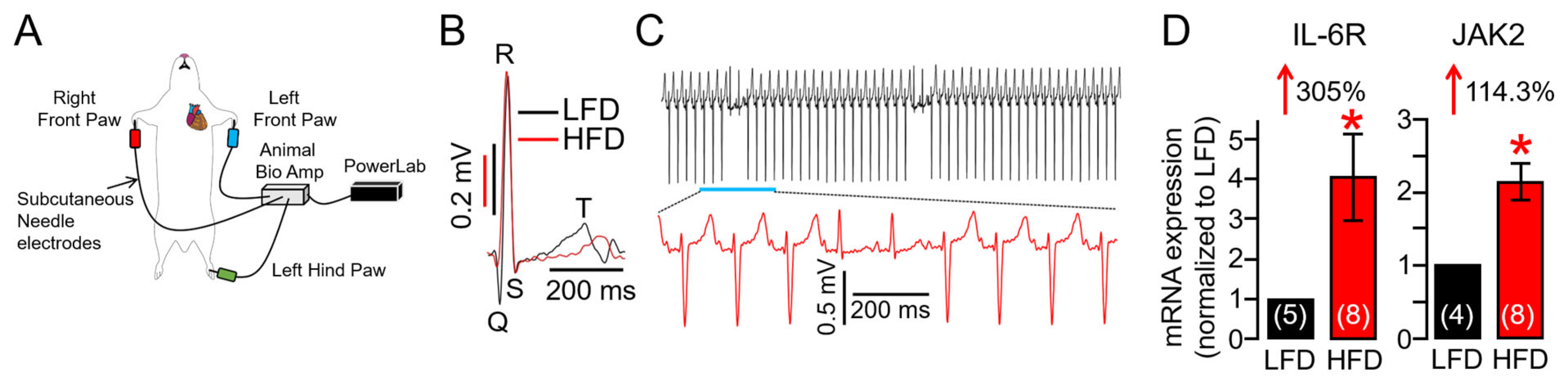
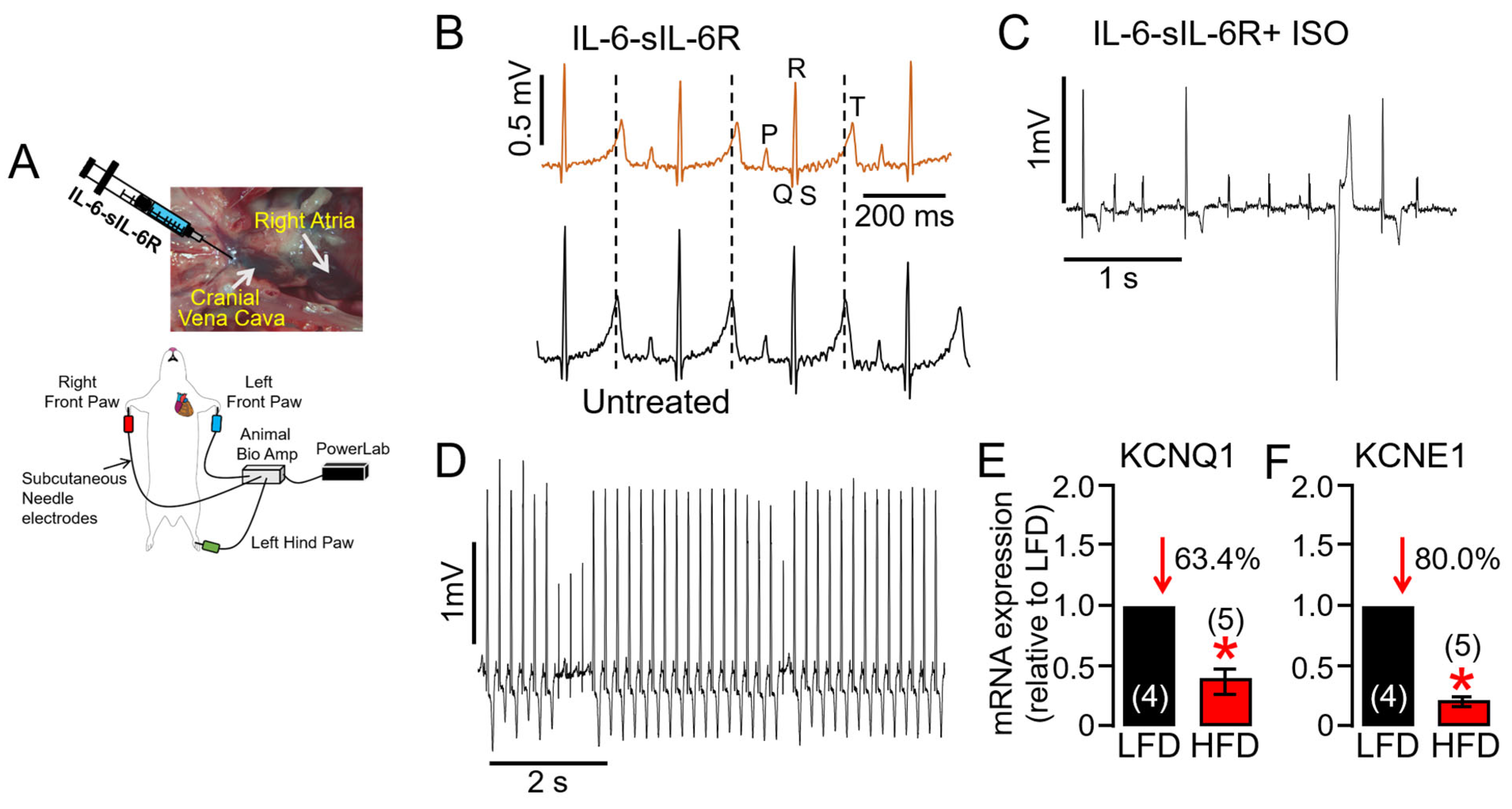
2.3. Overactivated IL-6 Trans-Signaling Causes Pathological Guinea Pig Heart Electrophysiology and Increased Arrhythmic Risk
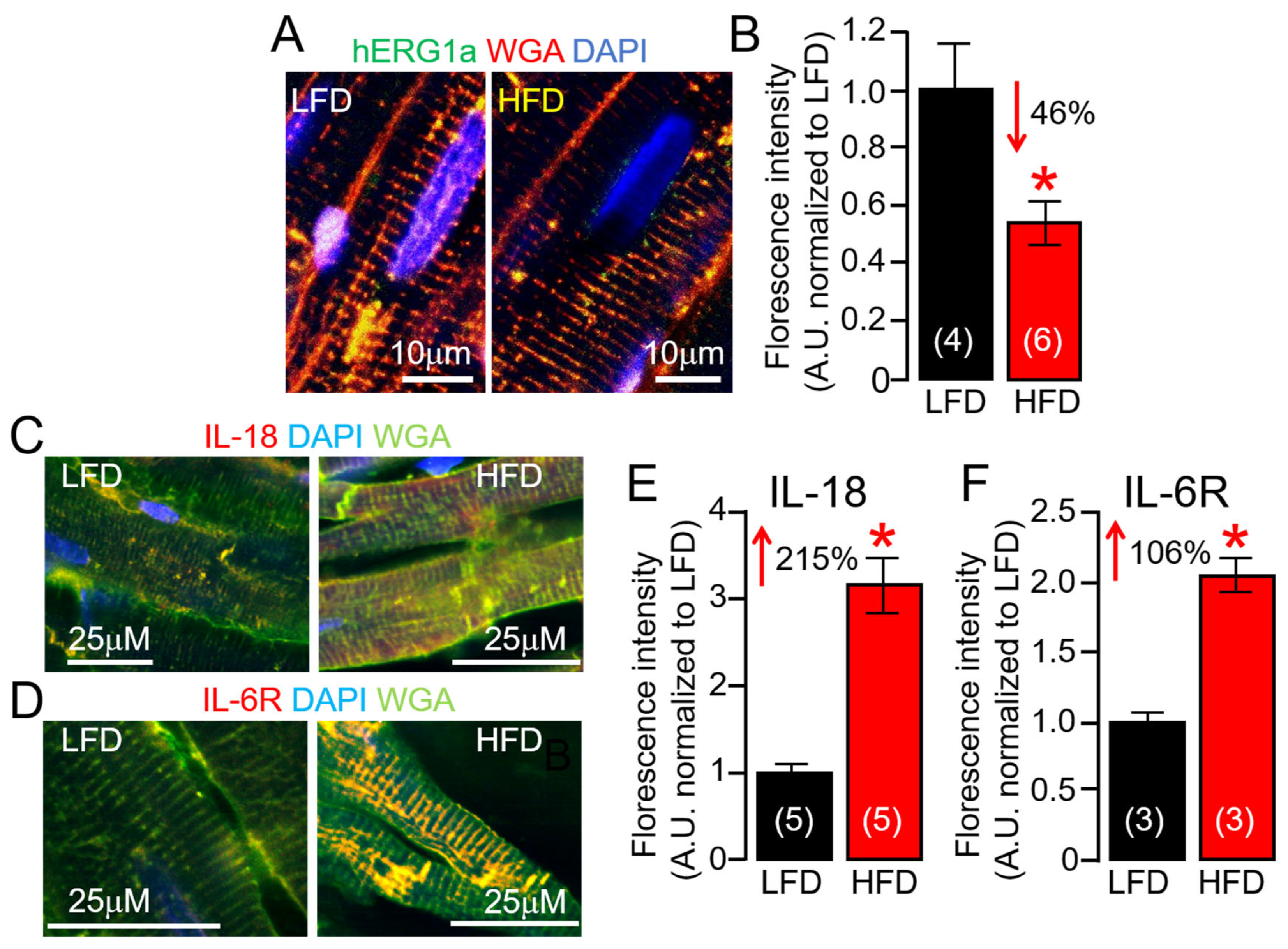
2.4. IL-18 Enhances IL-6 Trans-Signaling Effects on Guinea Pig Ventricular Cardiac Electrophysiology
2.5. Proinflammatory Cytokines Induce Dramatic Ventricular Electrophysiology Remodeling during Adverse Sympathetic Regulation and Are Associated with VT in Guinea Pig
2.6. ERG1a Protein Expression Is Reduced in HFD Hearts
2.7. HFD Feeding Is Associated with Increased STAT4 Expression in Guinea Pig Heart
2.8. Lipotoxicity Promotes Overactivation of IL-6 Trans-Signaling in Guinea Pig Ventricular Myocytes via Lipid Droplet Accumulation
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Animals, Low-Fat Diet, High-Fat Diet (Palmitic-Acid (PA) Diet), and Oleic-Acid Diet (OAD) Feeding
4.2. Electrocardiogram (ECG)
4.3. Estimation of Interstitial Fibrosis in Ventricular Tissue Slices from Guinea Pig
4.4. Enzyme-Linked Immunosorbent (ELISA) Assay
4.5. Preparation of Bovine Serum Albumin (BSA)-Conjugated FFA Solutions
4.6. Guinea Pig Ventricular Myocyte Isolation
4.7. LipidSpot Lipid Droplet Staining
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Immunofluorescence, Confocal Imaging, and Image Analysis
4.10. Statistical Analyses
5. Conclusions
Clinical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERG | Ether-à-go-go related gene |
| CVC | Cranial vena cava |
| FFA | Free fatty acid |
| QTc | Heart rate corrected QT interval |
| HFD | High-fat diet |
| hERG | Human ether-à-go-go related gene |
| hIL-6 | Hyper-IL-6 |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| JAK2 | Janus kinase 2 |
| LFD | Low-fat diet |
| OAD | Oleic acid diet |
| PA | Palmitic acid |
| IKr | Rapidly activating delayed rectifier K current |
| IKs | Slowly activating delayed rectifier K current |
| STAT4 | Signal transducer and activator of transcription 4 |
| sIL-6 | Soluble interleukin-6 receptor |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
References
- Marsman, R.F.; Tan, H.L.; Bezzina, C.R. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat. Rev. Cardiol. 2014, 11, 96–111. [Google Scholar] [CrossRef]
- Kallergis, E.M.; Goudis, C.A.; Simantirakis, E.N.; Kochiadakis, G.E.; Vardas, P.E. Mechanisms, Risk Factors, and Management of Acquired Long QT Syndrome: A Comprehensive Review. Sci. World J. 2012, 2012, 212178. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, N.; Turitto, G.; Boutjdir, M. Acquired Long QT Syndrome and Electrophysiology of Torsade de Pointes. Arrhythm. Electrophysiol. Rev. 2019, 8, 122–130. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Ali, A.; Boutjdir, M.; Aromolaran, A.S. Cardiolipotoxicity, Inflammation, and Arrhythmias: Role for Interleukin-6 Molecular Mechanisms. Front. Physiol. 2018, 9, 1866. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Chowdhury, M.K.H.; Martinez-Mateu, L.; Do, J.; Aromolaran, K.A.; Saiz, J.; Aromolaran, A.S. Macrophage-Dependent Interleukin-6-Production and Inhibition of I(K) Contributes to Acquired QT Prolongation in Lipotoxic Guinea Pig Heart. Int. J. Mol. Sci. 2021, 22, 11249. [Google Scholar] [CrossRef]
- Aromolaran, A.S.; Colecraft, H.M.; Boutjdir, M. High-fat diet-dependent modulation of the delayed rectifier K(+) current in adult guinea pig atrial myocytes. Biochem. Biophys. Res. Commun. 2016, 474, 554–559. [Google Scholar] [CrossRef]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised interleukin-6 levels in obese patients. Obes. Res. 2000, 8, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- El-Mikkawy, D.M.E.; El-Sadek, M.A.; El-Badawy, M.A.; Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, G.; Goldenberg, I.; McNitt, S.; Moss, A.J.; Zareba, W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J. Cardiovasc. Electrophysiol. 2007, 18, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Goldenberg, I.; Moss, A.J.; McNitt, S.; Glikson, M.; Biton, Y.; Jackson, L.; Polonsky, B.; Zareba, W.; Kutyifa, V. Predictors and Risk of Ventricular Tachyarrhythmias or Death in Black and White Cardiac Patients: A MADIT-CRT Trial Substudy. JACC Clin. Electrophysiol. 2016, 2, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Sidi, Y.; Kivity, S.; Beinart, R.; Glikson, M.; Segev, S.; Goldenberg, I.; Maor, E. Obesity and exercise-induced ectopic ventricular arrhythmias in apparently healthy middle aged adults. Eur. J. Prev. Cardiol. 2016, 23, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Remme, C.A. Sudden Cardiac Death in Diabetes and Obesity: Mechanisms and Therapeutic Strategies. Can. J. Cardiol. 2022, 38, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, R.; Terry, B.E.; Fresen, J.L.; Petruc, M.; Govindarajan, G.; Alpert, M.A. Relation of left ventricular mass to QTc in normotensive severely obese patients. Obesity 2012, 20, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Bertolozzi, I.; Morozzi, G.; Lorenzini, S.; Simpatico, A.; Selvi, E.; Bacarelli, M.R.; Finizola, F.; Vanni, F.; et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 2017, 103, 1821–1829. [Google Scholar] [CrossRef]
- Rakemann, T.; Niehof, M.; Kubicka, S.; Fischer, M.; Manns, M.P.; Rose-John, S.; Trautwein, C. The designer cytokine hyper-interleukin-6 is a potent activator of STAT3-dependent gene transcription in vivo and in vitro. J. Biol. Chem. 1999, 274, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Goldschmitt, J.; Peschel, C.; Brakenhoff, J.P.; Kallen, K.J.; Wollmer, A.; Grotzinger, J.; Rose-John, S.I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 1997, 15, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 signalling in health and disease. F1000Research 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gatsiou, A.; Tual-Chalot, S.; Napoli, M.; Ortega-Gomez, A.; Regen, T.; Badolia, R.; Cesarini, V.; Garcia-Gonzalez, C.; Chevre, R.; Ciliberti, G.; et al. The RNA editor ADAR2 promotes immune cell trafficking by enhancing endothelial responses to interleukin-6 during sterile inflammation. Immunity 2023, 56, 979–997.e11. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Manaenko, A.; Fan, R.; Huang, L.; Enkhjargal, B.; McBride, D.; Ding, Y.; Tang, J.; Xiao, X.; Zhang, J.H. Osteopontin attenuates inflammation via JAK2/STAT1 pathway in hyperglycemic rats after intracerebral hemorrhage. Neuropharmacology 2018, 138, 160–169. [Google Scholar] [CrossRef]
- Goumas, F.A.; Holmer, R.; Egberts, J.H.; Gontarewicz, A.; Heneweer, C.; Geisen, U.; Hauser, C.; Mende, M.M.; Legler, K.; Röcken, C.; et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer 2015, 137, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, A.S.; Boutjdir, M. Cardiac Ion Channel Regulation in Obesity and the Metabolic Syndrome: Relevance to Long QT Syndrome and Atrial Fibrillation. Front. Physiol. 2017, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Heymes, C.; Corbaz, A.; Logeart, D.; Alouani, S.; Cohen-Solal, A.; Seidler, T.; Hasenfuss, G.; Chvatchko, Y.; Shah, A.M.; et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004, 18, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- O–Brien, L.C.; Mezzaroma, E.; Van Tassell, B.W.; Marchetti, C.; Carbone, S.; Abbate, A.; Toldo, S. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol. Med. 2014, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Mezzaroma, E.; O’Brien, L.; Marchetti, C.; Seropian, I.M.; Voelkel, N.F.; Van Tassell, B.W.; Dinarello, C.A.; Abbate, A. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1025–H1031. [Google Scholar] [CrossRef] [PubMed]
- Enoksson, S.L.; Grasset, E.K.; Hagglof, T.; Mattsson, N.; Kaiser, Y.; Gabrielsson, S.; McGaha, T.L.; Scheynius, A.; Karlsson, M.C. The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc. Natl. Acad. Sci. USA 2011, 108, E1399–E1407. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Lopez, F.J.; Celkova, L.; Brennan, K.; Mulfaul, K.; Ozaki, E.; Kenna, P.F.; Kurali, E.; Hudson, N.; Doggett, T.; et al. IL-18 Immunotherapy for Neovascular AMD: Tolerability and Efficacy in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.Y.; Lu, H.H.; Sudhakar, J.N.; Chen, Y.W.; Shih, N.S.; Weng, Y.T.; Shui, J.W. IL-22 initiates an IL-18-dependent epithelial response circuit to enforce intestinal host defence. Nat. Commun. 2022, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.; Eldstrom, J.; Westhoff, M.; McAfee, D.; Balse, E.; Fedida, D. cAMP-dependent regulation of I(Ks) single-channel kinetics. J. Gen. Physiol. 2017, 149, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Jost, N.; Virag, L.; Bitay, M.; Takacs, J.; Lengyel, C.; Biliczki, P.; Nagy, Z.; Bogats, G.; Lathrop, D.A.; Papp, J.G.; et al. Restricting excessive cardiac action potential and QT prolongation: A vital role for IKs in human ventricular muscle. Circulation 2005, 112, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.X.; Sobie, E.A. Quantification of repolarization reserve to understand interpatient variability in the response to proarrhythmic drugs: A computational analysis. Heart Rhythm. 2011, 8, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.F.; Gaspo, R.; Busch, A.E.; Lang, H.J.; Li, G.R.; Nattel, S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998, 38, 441–450. [Google Scholar] [CrossRef]
- Hancox, J.C.; McPate, M.J.; El Harchi, A.; Zhang, Y.H. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol. Ther. 2008, 119, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Lepailleur, A.; Mignani, S.M.; Dallemagne, P.; Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 2020, 195, 112290. [Google Scholar] [CrossRef] [PubMed]
- Taga, T.; Kishimoto, T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.A.; Rose, N.R.; Cihakova, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef]
- Akira, S.; Isshiki, H.; Sugita, T.; Tanabe, O.; Kinoshita, S.; Nishio, Y.; Nakajima, T.; Hirano, T.; Kishimoto, T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. Embo J. 1990, 9, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Nishio, Y.; Inoue, M.; Wang, X.J.; Wei, S.; Matsusaka, T.; Yoshida, K.; Sudo, T.; Naruto, M.; Kishimoto, T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell 1994, 77, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Naka, T.; Narazaki, M.; Hirata, M.; Matsumoto, T.; Minamoto, S.; Aono, A.; Nishimoto, N.; Kajita, T.; Taga, T.; Yoshizaki, K.; et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 1997, 387, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wen, Z.; Darnell, J.E., Jr. Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994, 264, 95–98. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.M.; Darnell, J.E. The state of the STATs: Recent developments in the study of signal transduction to the nucleus. Curr. Opin. Cell Biol. 1997, 9, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Leonard, W.J. The Jak-STAT pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Koglin, J.; Glysing-Jensen, T.; Gadiraju, S.; Russell, M.E. Attenuated Cardiac Allograft Vasculopathy in Mice with Targeted Deletion of the Transcription Factor STAT4. Circulation 2000, 101, 1034–1039. [Google Scholar] [CrossRef]
- Svenungsson, E.; Gustafsson, J.; Leonard, D.; Sandling, J.; Gunnarsson, I.; Nordmark, G.; Jönsen, A.; Bengtsson, A.A.; Sturfelt, G.; Rantapää-Dahlqvist, S.; et al. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 834–840. [Google Scholar] [CrossRef]
- Meinert, C.; Gembardt, F.; Böhme, I.; Tetzner, A.; Wieland, T.; Greenberg, B.; Walther, T. Identification of intracellular proteins and signaling pathways in human endothelial cells regulated by angiotensin-(1–7). J. Proteom. 2016, 130, 129–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Del Alamo, J.C.; Lemons, D.; Serrano, R.; Savchenko, A.; Cerignoli, F.; Bodmer, R.; Mercola, M. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim. Biophys. Acta 2016, 1863, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, T.; Zembroski, A.S.; Buhman, K.K. Diet Induced Obesity Alters Intestinal Cytoplasmic Lipid Droplet Morphology and Proteome in the Postprandial Response to Dietary Fat. Front. Physiol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mateu, L.; Saiz, J.; Aromolaran, A.S. Differential Modulation of I(K) and I(Ca,L) Channels in High-Fat Diet-Induced Obese Guinea Pig Atria. Front. Physiol. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: Further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020, 41, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Barbarroja, N.; Lopez-Pedrera, R.; Mayas, M.D.; Garcia-Fuentes, E.; Garrido-Sanchez, L.; Macias-Gonzalez, M.; El Bekay, R.; Vidal-Puig, A.; Tinahones, F.J. The obese healthy paradox: Is inflammation the answer? Biochem. J. 2010, 430, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight 2021, 6, e145495. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Tsai, H.Y.; Su, M.Y.; Wu, Y.F.; Hwang, J.J.; Tseng, W.Y.; Lin, J.L.; Lin, L.Y. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis 2015, 241, 607–614. [Google Scholar] [CrossRef]
- Fuller, B.; Garland, J.; Anne, S.; Beh, R.; McNevin, D.; Tse, R. Increased Epicardial Fat Thickness in Sudden Death From Stable Coronary Artery Atherosclerosis. Am. J. Forensic Med. Pathol. 2017, 38, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Vm, M.; Al, S.; Aa, A.; As, Z.; Av, K.; Rs, O.; Im, M.; Ga, K. Circulating interleukin-18: Association with IL-8, IL-10 and VEGF serum levels in patients with and without heart rhythm disorders. Int. J. Cardiol. 2016, 215, 105–109. [Google Scholar] [CrossRef]
- Gupta, A.; Fei, Y.D.; Kim, T.Y.; Xie, A.; Batai, K.; Greener, I.; Tang, H.; Ciftci-Yilmaz, S.; Juneman, E.; Indik, J.H.; et al. IL-18 mediates sickle cell cardiomyopathy and ventricular arrhythmias. Blood 2021, 137, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Despa, S. Na+ transport in cardiac myocytes; Implications for excitation-contraction coupling. IUBMB Life 2009, 61, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Varro, A.; Nanasi, P.P.; Lathrop, D.A. Potassium currents in isolated human atrial and ventricular cardiocytes. Acta Physiol. Scand. 1993, 149, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, A.S.; Subramanyam, P.; Chang, D.D.; Kobertz, W.R.; Colecraft, H.M. LQT1 mutations in KCNQ1 C-terminus assembly domain suppress IKs using different mechanisms. Cardiovasc. Res. 2014, 104, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Puckerin, A.; Aromolaran, K.A.; Chang, D.D.; Zukin, R.S.; Colecraft, H.M.; Boutjdir, M.; Aromolaran, A.S. hERG 1a LQT2 C-terminus truncation mutants display hERG 1b-dependent dominant negative mechanisms. Heart Rhythm. 2016, 13, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.P.; Yuan, C.; Navedo, M.F.; Dixon, R.E.; Nieves-Cintron, M.; Scott, J.D.; Santana, L.F. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ. Res. 2011, 109, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Aromolaran, A.S.; Srivastava, U.; Ali, A.; Chahine, M.; Lazaro, D.; El-Sherif, N.; Capecchi, P.L.; Laghi-Pasini, F.; Lazzerini, P.E.; Boutjdir, M. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS ONE 2018, 13, e0208321. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Miyoshi, S.; Fukuda, K.; Nishiyama, N.; Ikegami, Y.; Tanimoto, K.; Murata, M.; Takahashi, E.; Shimoda, K.; Hirano, T.; et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J. Mol. Cell Cardiol. 2007, 43, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zhang, Y.; Gao, H.; Nattel, S.; Wang, Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: Role of reactive oxygen species as a mediator. J. Biol. Chem. 2004, 279, 13289–13292. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, G.; Alarcon, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepulveda, M.; et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Rozanski, G.J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc. Res. 1993, 27, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Sato, K.; Kim, K.J.; Takahashi, N.; Udagawa, N.; Nakamura, I.; Yamaguchi, A.; Kishimoto, T.; Suda, T.; Kashiwazaki, S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J. Bone Min. Res. 1996, 11, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Chen, C.; Bhagat, S.S.; Parker, R.A.; Ostor, A.J. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatology 2011, 50, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Beaulieu, A.; Rubbert-Roth, A.; Ramos-Remus, C.; Rovensky, J.; Alecock, E.; Woodworth, T.; Alten, R.; Investigators, O. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial. Lancet 2008, 371, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Jones, G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev. Recent. Clin. Trials 2006, 1, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, S.Y.; Kawakami, A.; Yamasaki, S.; Imazato, T.; Iwamoto, N.; Fujikawa, K.; Aramaki, T.; Tamai, M.; Nakamura, H.; Ida, H.; et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 2011, 31, 451–456. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, S.; Wang, B.; Chen, H.; Li, Y.; Cao, Q.; Zhong, J.; Xie, M.; Ran, Z.; Tang, T.; et al. 775b Olamkicept, an IL-6 Trans-Signaling Inhibitor, is Effective for Induction of Response and Remission in A Randomized, Placebo-Controlled Trial in Moderate to Severe Ulcerative Colitis. Gastroenterology 2021, 161, e28–e29. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, S.; Wang, B.; Chen, H.; Li, Y.; Cao, Q.; Zhong, J.; Xie, M.; Ran, Z.; Tang, T.; et al. DOP01 Efficacy and safety of the IL-6 trans-signalling inhibitor olamkicept: A phase 2 randomized, placebo-controlled trial in moderately to severely active Ulcerative Colitis. J. Crohn’s Colitis 2021, 15 (Suppl. S1), S041–S042. [Google Scholar] [CrossRef]
- Schreiber, S.; Aden, K.; Bernardes, J.P.; Conrad, C.; Tran, F.; Hoper, H.; Volk, V.; Mishra, N.; Blase, J.I.; Nikolaus, S.; et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients with Active Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2354–2366.e11. [Google Scholar] [CrossRef] [PubMed]
- Schulte, D.M.; Waetzig, G.H.; Schuett, H.; Marx, M.; Schulte, B.; Garbers, C.; Lokau, J.; Vlacil, A.-K.; Schulz, J.; Seoudy, A.K.; et al. Case Report: Arterial Wall Inflammation in Atherosclerotic Cardiovascular Disease is Reduced by Olamkicept (sgp130Fc). Front. Pharmacol. 2022, 13, 758233. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Amin, M.M.; Hassan, A. Effects of oleic acid and/or exercise on diet-induced thermogenesis and obesity in rats: Involvement of beige adipocyte differentiation and macrophage M1 inhibition. Res. Pharm. Sci. 2023, 18, 219–230. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Poggio, P.; Valerio, V.; Cabaro, S.; Campana, P.; Comentale, G.; Attena, E.; Russo, V.; Pilato, E.; et al. Inflammation and Cardiovascular Diseases in the Elderly: The Role of Epicardial Adipose Tissue. Front. Med. 2022, 9, 844266. [Google Scholar] [CrossRef] [PubMed]
- Konwerski, M.; Gąsecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Swifka, J.; Weiss, J.; Addicks, K.; Eckel, J.; Rosen, P. Epicardial fat from guinea pig: A model to study the paracrine network of interactions between epicardial fat and myocardium? Cardiovasc. Drugs Ther. 2008, 22, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Kofahi, Y.; Lassoued, W.; Lee, W.; Roysam, B. Improved automatic detection and segmentation of cell nuclei in histopathology images. IEEE Trans. Biomed Eng. 2010, 57, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Yin, L.; Ingelsson, E. Cyclooxygenase-2 inhibitors and cardiovascular risk in a nation-wide cohort study after the withdrawal of rofecoxib. Eur. Heart J. 2012, 33, 1928–1933. [Google Scholar] [CrossRef]
- Schmidt, M.; Christiansen, C.F.; Mehnert, F.; Rothman, K.J.; Sorensen, H.T. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: Population based case-control study. BMJ 2011, 343, d3450. [Google Scholar] [CrossRef] [PubMed]
- van der Hooft, C.S.; Heeringa, J.; Brusselle, G.G.; Hofman, A.; Witteman, J.C.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H. Corticosteroids and the risk of atrial fibrillation. Arch. Intern. Med. 2006, 166, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Ruigomez, A.; Rodriguez, L.A. Long-term use of anti-inflammatory drugs and risk of atrial fibrillation. Arch. Intern. Med. 2010, 170, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Granier, M.; Massin, F.; Pasquie, J.L. Pro- and anti-arrhythmic effects of anti-inflammatory drugs. Antiinflamm Antiallergy Agents Med. Chem. 2013, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Kossyvakis, C.; Efremidis, M.; Panagopoulou, V.; Kaoukis, A.; Raisakis, K.; Bouras, G.; Angelidis, C.; Theodorakis, A.; et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: A randomized controlled study. J. Am. Coll. Cardiol. 2012, 60, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Beard, C.W.; Khosla, J.; Clifton, S.; Anwaar, M.F.; Ghani, A.; Farhat, K.; Pyrpyris, N.; Momani, J.; Munir, M.B.; et al. Safety and efficacy of colchicine for the prevention of post-operative atrial fibrillation in patients undergoing cardiac surgery: A meta-analysis of randomized controlled trials. Europace 2023, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Ke Wang, M.; Popova, E.; Chan, M.T.V.; Landoni, G.; Cata, J.P.; Reimer, C.; McLean, S.R.; Srinathan, S.K.; Reyes, J.C.T.; et al. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): An international randomised trial. Lancet 2023, 402, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Guo, W.; Tang, X.; Pan, J.; Yu, P.; Fan, H.; Wang, X.; Jiang, R.; Jiang, C.; Liang, P. Colchicine attenuates the electrical remodeling of post-operative atrial fibrillation through inhibited expression of immune-related hub genes and stabilization of microtubules. Int. J. Biol. Sci. 2023, 19, 2934–2956. [Google Scholar] [CrossRef] [PubMed]
- Benz, A.P.; Amit, G.; Connolly, S.J.; Singh, J.; Acosta-Vélez, J.G.; Conen, D.; Deif, B.; Divakaramenon, S.; McIntyre, W.F.; Mtwesi, V.; et al. Colchicine to Prevent Atrial Fibrillation Recurrence After Catheter Ablation: A Randomized, Placebo-Controlled Trial. Circ. Arrhythm. Electrophysiol. 2024, 17, e01238. [Google Scholar] [CrossRef] [PubMed]
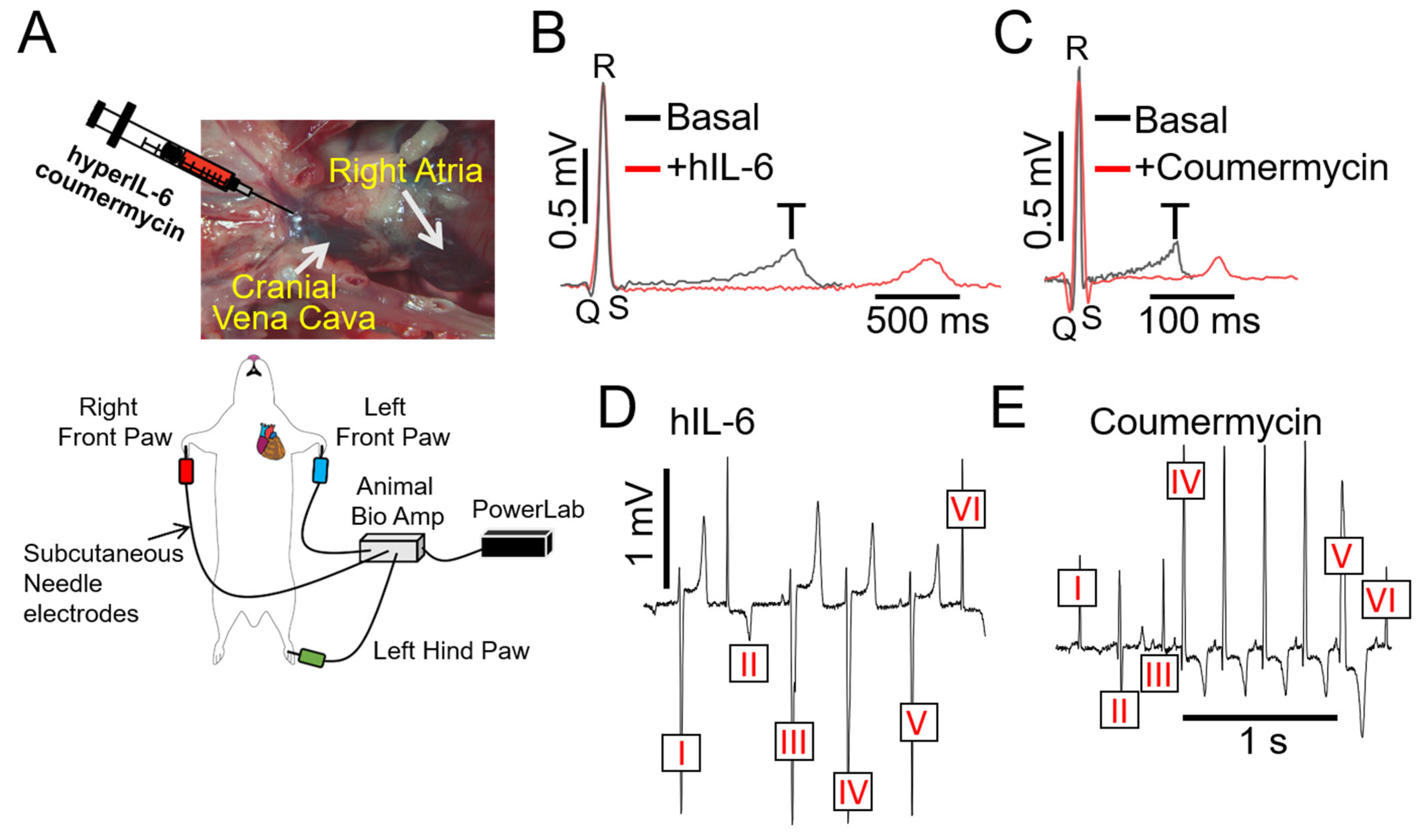

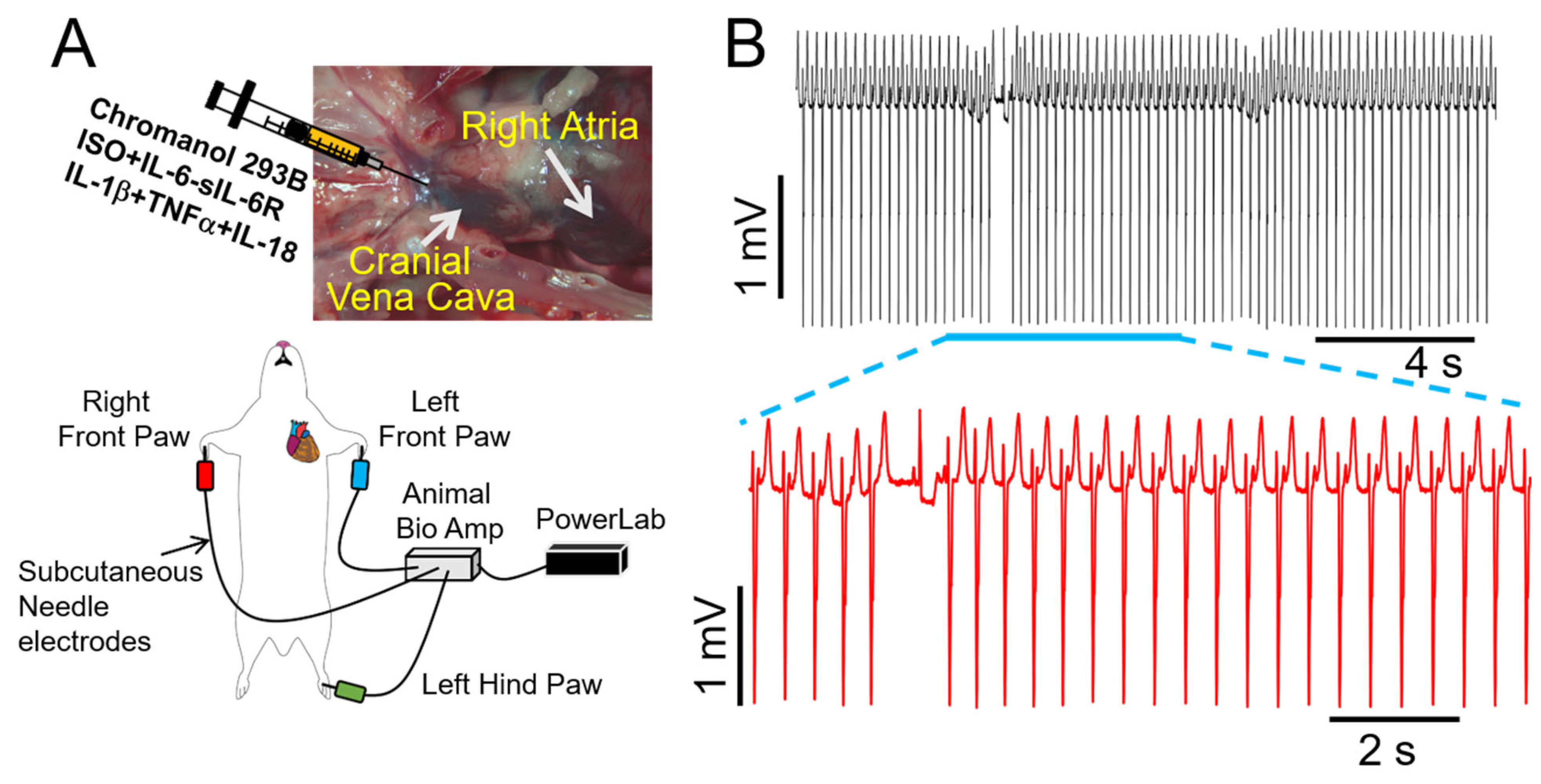


| Conditions | QTc (ms) (Basal) | QTc (ms) (Post-Intervention) | p Value | ΔQTc (ms) | n |
|---|---|---|---|---|---|
| Low-fat diet (LFD) | 279.8 ± 15.2 | 269.4 ± 18.1 | 0.665 | −10.5 ± 4.51 | 8 |
| High-fat diet (HFD) | 260.4 ± 15.1 | 323.9 ± 15.2 * | 0.0018 | 63.32 ± 10.9 | 15 |
| Oleic acid diet (OAD) | 294.7 ± 6.14 | 307.8 ± 5.58 | 0.137 | 13.1 ± 8.09 | 8 |
| Conditions | QTc (ms) (Basal) | QTc (ms) (Post-Intervention) | p Value | ΔQTc (ms) | n |
|---|---|---|---|---|---|
| Vehicle | 297.3 ± 2.54 | 302.7 ± 3.94 | 0.298 | 5.42 ± 1.45 | 4 |
| IL-6-sIL-6R | 289.3 ± 0.72 | 310.9 ± 4.22 * | 0.003 | 21.66 ± 6.12 | 5 |
| hyperIL-6 (hIL-6) | 299.7 ± 2.61 | 333.9 ± 8.63 * | 0.004 | 34.8 ± 8.15 | 8 |
| hIL-6 + Olamkicept | 292.9 ± 5.47 | 293.8 ± 4.24 | 0.899 | 0.93 ± 8.30 | 3 |
| Coumermycin | 299.1 ± 5.40 | 337.5 ± 10.7 * | 0.01 | 38.4 ± 10.9 | 7 |
| IL-6-sIL-6R + IL-18 | 292.3 ± 10.7 | 319.2 ± 10.7 * | 0.0004 | 26.95 ± 3.98 | 6 |
| Chromanol293B | 308.6 ± 4.24 | 327.9 ± 5.68 * | 0.018 | 19.23 ± 3.35 | 8 |
| Chromanol293B + ISO- 10 min | 308.6 ± 4.24 | 342.5 ± 7.69 * | 0.004 | 33.0 ± 10.4 | 6 |
| Chromanol293B + ISO + Cytomix- 10 min | 308.6 ± 4.24 | 363.6 ± 6.48 * | 0.0036 | 54.2 ± 11.02 | 3 |
| Chromanol293B + ISO + Cytomix- 30 min | 308.6 ± 4.24 | 377.8 ± 15.3 * | 0.03 | 68.4 ± 17.8 | 3 |
| Gene | Sequence (5′- > 3′) | Gene ID |
|---|---|---|
| IL-6R | sense GGGTCGGGCTTCAAGATGTTA antisense AACGGTGCCTGTATTCTGGG | 100730490 |
| JAK2 | sense CTTAGATTACGCCGCCCAGC antisense TGTGCCGGTATGACCCTCTA | 100722908 |
| KCNQ1 | sense GCTGTTCTCTGAGGGTCTTCCA antisense CCATCCACCCTGAACTCTTTCT | 100379230 |
| KCNE1 | sense TCCCAGGAAAACTGTCAGCTC antisense CGGTTCTGAGGAAGCGGATT | 100135562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbin, A.; Aromolaran, K.A.; Aromolaran, A.S. STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart. Int. J. Mol. Sci. 2024, 25, 7813. https://doi.org/10.3390/ijms25147813
Corbin A, Aromolaran KA, Aromolaran AS. STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart. International Journal of Molecular Sciences. 2024; 25(14):7813. https://doi.org/10.3390/ijms25147813
Chicago/Turabian StyleCorbin, Andrea, Kelly A. Aromolaran, and Ademuyiwa S. Aromolaran. 2024. "STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart" International Journal of Molecular Sciences 25, no. 14: 7813. https://doi.org/10.3390/ijms25147813
APA StyleCorbin, A., Aromolaran, K. A., & Aromolaran, A. S. (2024). STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart. International Journal of Molecular Sciences, 25(14), 7813. https://doi.org/10.3390/ijms25147813








