Melatonin as a Circadian Marker for Plasmodium Rhythms
Abstract
1. Introduction
2. Plasmodium Rhythms
3. Do Plasmodium Parasites Possess an Intrinsic Clock?
4. Host Cues Involved in Plasmodium Synchronization
5. Melatonin Circadian Production and Release by the Pineal Gland
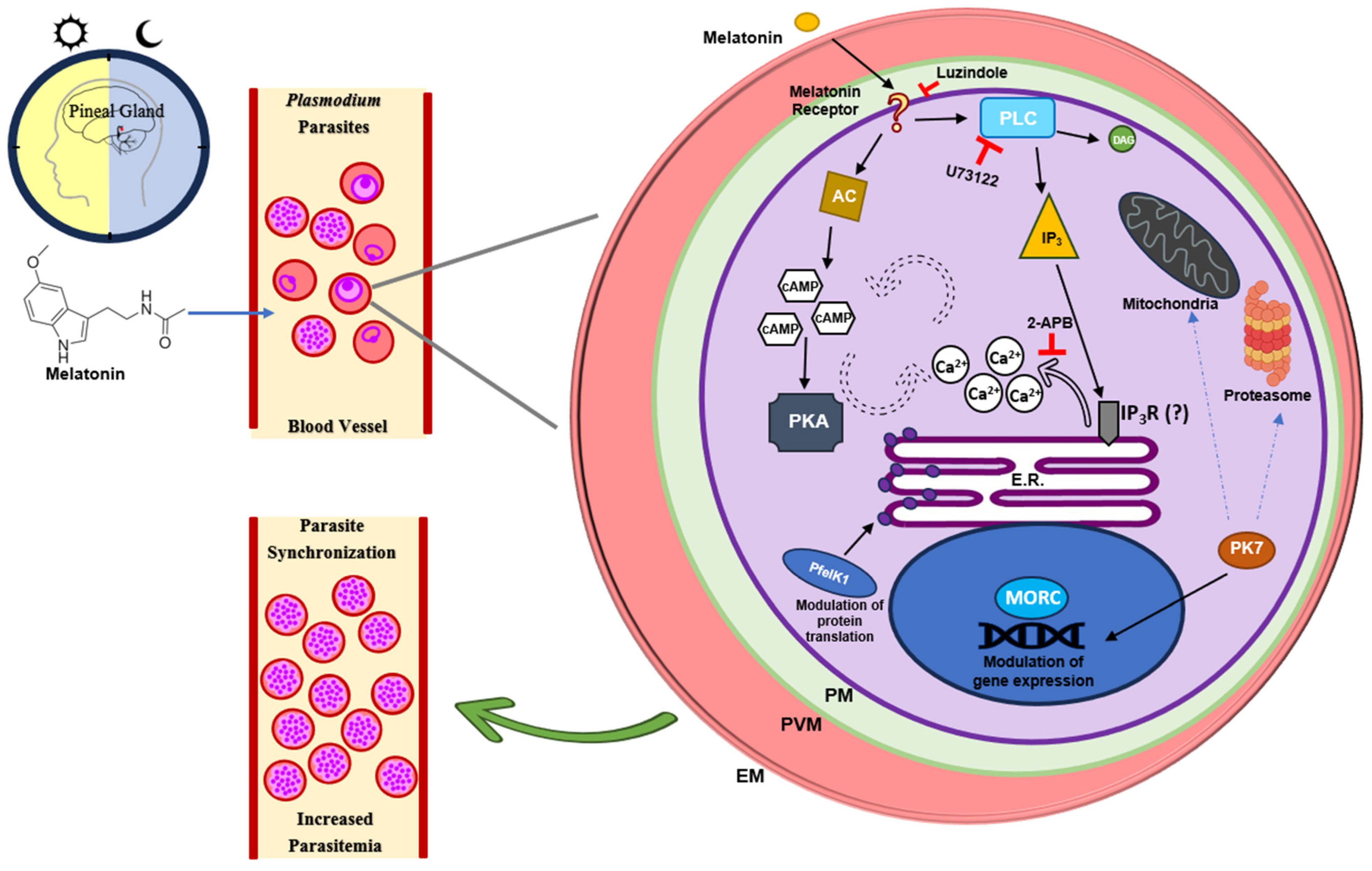
6. Melatonin as a Circadian Host Cue to Synchronize Plasmodium Rhythms

7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023.
- Cuenca, P.R.; Key, S.; Jumail, A.; Surendra, H.; Ferguson, H.M.; Drakeley, C.J.; Fornace, K. Epidemiology of the zoonotic malaria Plasmodium knowlesi in changing landscapes. Adv. Parasitol. 2021, 113, 225–286. [Google Scholar] [PubMed]
- Naik, D.G. Plasmodium knowlesi-mediated zoonotic malaria: A challenge for elimination. Trop. Parasitol. 2020, 10, 3–6. [Google Scholar] [PubMed]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Buyon, L.E.; Elsworth, B.; Duraisingh, M.T. The molecular basis of antimalarial drug resistance in Plasmodium vivax. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Minetti, C.; Ingham, V.A.; Ranson, H. Effects of insecticide resistance and exposure on Plasmodium development in Anopheles mosquitoes. Curr. Opin. Insect Sci. 2020, 39, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yewhalaw, D.; Wassie, F.; Steurbaut, W.; Spanoghe, P.; Van Bortel, W.; Denis, L.; Tessema, D.A.; Getachew, Y.; Coosemans, M.; Duchateau, L.; et al. Multiple insecticide resistance: An impediment to insecticide-based malaria vector control program. PLoS ONE 2011, 6, e16066. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; McCall, M.B.B. Correlates of malaria vaccine efficacy. Expert. Rev. Vaccines 2021, 20, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, T.R.; Yang, J.; Freudzon, M.; Rembisz, A.; Craft, S.; Hamilton, M.; Graham, M.; Mlambo, G.; Tripathi, A.K.; Li, Y.; et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat. Commun. 2018, 9, 2908. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Cockburn, I.A. The challenges of a circumsporozoite protein-based malaria vaccine. Expert. Rev. Vaccines 2021, 20, 113–125. [Google Scholar] [CrossRef]
- Okoyo, C.; Githinji, E.; Muia, R.W.; Masaku, J.; Mwai, J.; Nyandieka, L.; Munga, S.; Njenga, S.M.; Kanyi, H.M. Assessment of malaria infection among pregnant women and children below five years of age attending rural health facilities of Kenya: A cross-sectional survey in two counties of Kenya. PLoS ONE 2021, 16, e0257276. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC—Malaria Parasites—Biology. Available online: https://www.cdc.gov/dpdx/malaria/index.html (accessed on 12 July 2024).
- Vaughan, A.M.; Kappe, S.H.I. Malaria Parasite Liver Infection and Exoerythrocytic Biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025486. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Takeuschi, M.; Yoshioka, K.; Miyamoto, H. Imaging Plasmodium falciparum-infected ghost and parasite by atomic force microscopy. J. Struct. Biol. 1997, 119, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.; Murphy, S.C.; Milner, D.A.; Taylor, T.E. Malaria: Mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu. Rev. Pathol. 2007, 2, 217–249. [Google Scholar] [CrossRef]
- Garcia, C.R.; Markus, R.P.; Madeira, L. Tertian and quartan fevers: Temporal regulation in malarial infection. J. Biol. Rhythms 2001, 16, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bannister, L.H.; Hopkins, J.M.; Fowler, R.E.; Krishna, S.; Mitchell, G.H. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol. Today 2000, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Hentzschel, F.; Valkiunas, G.; Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Microbiol. 2020, 18, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.S.; Jacobs-Lorena, M. Production of Plasmodium falciparum gametocytes in vitro. Methods Mol. Biol. 2013, 923, 17–25. [Google Scholar] [PubMed]
- Carvalho Cabral, P.; Olivier, M.; Cermakian, N. The Complex Interplay of Parasites, Their Hosts, and Circadian Clocks. Front. Cell Infect. Microbiol. 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Mideo, N.; Reece, S.E.; Smith, A.L.; Metcalf, C.J. The Cinderella syndrome: Why do malaria-infected cells burst at midnight? Trends Parasitol. 2013, 29, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.D.; Wijesekera, S.K.; Wanasekera, D.; Mendis, K.N.; Carter, R. The paroxysm of Plasmodium vivax malaria. Trends Parasitol. 2003, 19, 188–193. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Ngernna, S.; Chim-Ong, A.; Roobsoong, W.; Sattabongkot, J.; Cui, L.; Nguitragool, W. Efficient synchronization of Plasmodium knowlesi in vitro cultures using guanidine hydrochloride. Malar. J. 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Hawking, F. The clock of the malaria parasite. Sci. Am. 1970, 222, 123–131. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Chapman, D.; Watt, G. The effects of multiplication and synchronicity on the vascular distribution of parasites in falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.L.; O’Donnell, A.J.; de Bekker, C.; Lively, C.M.; Zuk, M.; Reece, S.E. The evolutionary ecology of circadian rhythms in infection. Nat. Ecol. Evol. 2019, 3, 552–560. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.J.; Schneider, P.; McWatters, H.G.; Reece, S.E. Fitness costs of disrupting circadian rhythms in malaria parasites. Proc. Biol. Sci. 2011, 278, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Bagnaresi, P.; Markus, R.P.; Hotta, C.T.; Pozzan, T.; Garcia, C.R.S. Desynchronizing Cell Cycle Increases Chloroquine Protection at Suboptimal Doses. Open Parasitol. J. 2008, 2, 55–58. [Google Scholar] [CrossRef]
- Schneider, P.; Rund, S.S.C.; Smith, N.L.; Prior, K.F.; O’Donnell, A.J.; Reece, S.E. Adaptive periodicity in the infectivity of malaria gametocytes to mosquitoes. Proc. Biol. Sci. 2018, 285, 20181876. [Google Scholar] [CrossRef]
- Habtewold, T.; Tapanelli, S.; Masters, E.K.G.; Windbichler, N.; Christophides, G.K. The circadian clock modulates Anopheles gambiae infection with Plasmodium falciparum. PLoS ONE 2022, 17, e0278484. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.H. Induced Variations in the Asexual Cycle of Plasmodium cathemerium. Am. J. Hyg. 1929, 9, 181–187. [Google Scholar] [CrossRef]
- Pigeault, R.; Caudron, Q.; Nicot, A.; Rivero, A.; Gandon, S. Timing malaria transmission with mosquito fluctuations. Evol. Lett. 2018, 2, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Motta, F.C.; Chopra, G.; Moch, J.K.; Nerem, R.R.; Cummins, B.; Roche, K.E.; Kelliher, C.M.; Leman, A.R.; Harer, J.; et al. An intrinsic oscillator drives the blood stage cycle of the malaria parasite Plasmodium falciparum. Science 2020, 368, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Acosta-Rodriguez, V.A.; Abel, J.H.; Kornblum, I.; Bento, I.; Kilaru, G.; Klerman, E.B.; Mota, M.M.; Takahashi, J.S. The malaria parasite has an intrinsic clock. Science 2020, 368, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.K.; O’Donnell, A.J.; Ramaprasad, A.; Abkallo, H.M.; Kaushik, A.; Ansari, H.R.; Abdel-Haleem, A.M.; Ben Rached, F.; Kaneko, O.; Culleton, R.; et al. Malaria parasites regulate intra-erythrocytic development duration via serpentine receptor 10 to coordinate with host rhythms. Nat. Commun. 2020, 11, 2763. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Tsang, A.H.; Oster, H. Interaction of central and peripheral clocks in physiological regulation. Prog. Brain Res. 2012, 199, 163–181. [Google Scholar] [PubMed]
- Prior, K.F.; van der Veen, D.R.; O’Donnell, A.J.; Cumnock, K.; Schneider, D.; Pain, A.; Subudhi, A.; Ramaprasad, A.; Rund, S.S.C.; Savill, N.J.; et al. Timing of host feeding drives rhythms in parasite replication. PLoS Pathog. 2018, 14, e1006900. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, X.; Xiao, F.; Zhao, R.; Sun, Z. Circadian clock, diurnal glucose metabolic rhythm, and dawn phenomenon. Trends Neurosci. 2022, 45, 471–482. [Google Scholar] [CrossRef]
- O’Donnell, A.J.; Prior, K.F.; Reece, S.E. Host circadian clocks do not set the schedule for the within-host replication of malaria parasites. Proc. Biol. Sci. 2020, 287, 20200347. [Google Scholar] [CrossRef]
- O’Donnell, A.J.; Greischar, M.A.; Reece, S.E. Mistimed malaria parasites re-synchronize with host feeding-fasting rhythms by shortening the duration of intra-erythrocytic development. Parasite Immunol. 2022, 44, e12898. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.G.; Prior, K.F.; O’Donnell, A.J.; Reece, S.E. Consequences of daily rhythm disruption on host-parasite malaria infection dynamics. bioRxiv 2023, 554632. [Google Scholar] [CrossRef]
- Reece, S.E.; Prior, K.F. Malaria Makes the Most of Mealtimes. Cell Host Microbe 2018, 23, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Hirako, I.C.; Assis, P.A.; Hojo-Souza, N.S.; Reed, G.; Nakaya, H.; Golenbock, D.T.; Coimbra, R.S.; Gazzinelli, R.T. Daily Rhythms of TNFalpha Expression and Food Intake Regulate Synchrony of Plasmodium Stages with the Host Circadian Cycle. Cell Host Microbe 2018, 23, 796–808.e6. [Google Scholar] [CrossRef] [PubMed]
- Rankawat, S.; Kundal, K.; Chakraborty, S.; Kumar, R.; Ray, S. A comprehensive rhythmicity analysis of host proteins and immune factors involved in malaria pathogenesis to decipher the importance of host circadian clock in malaria. Front. Immunol. 2023, 14, 1210299. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Cabral, P.; Weinerman, J.; Olivier, M.; Cermakian, N. Time of day and circadian disruption influence host response and parasite growth in a mouse model of cerebral malaria. iScience 2024, 27, 109684. [Google Scholar] [CrossRef]
- Motta, F.C.; McGoff, K.; Moseley, R.C.; Cho, C.Y.; Kelliher, C.M.; Smith, L.M.; Ortiz, M.S.; Leman, A.R.; Campione, S.A.; Devos, N.; et al. The parasite intraerythrocytic cycle and human circadian cycle are coupled during malaria infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2216522120. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocrinol. 2004, 25, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin Signaling a Key Regulator of Glucose Homeostasis and Energy Metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tonon, A.C.; Pilz, L.K.; Markus, R.P.; Hidalgo, M.P.; Elisabetsky, E. Melatonin and Depression: A Translational Perspective from Animal Models to Clinical Studies. Front. Psychiatry 2021, 12, 638981. [Google Scholar] [CrossRef] [PubMed]
- Hotta, C.T.; Gazarini, M.L.; Beraldo, F.H.; Varotti, F.P.; Lopes, C.; Markus, R.P.; Pozzan, T.; Garcia, C.R. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat. Cell Biol. 2000, 2, 466–468. [Google Scholar] [CrossRef]
- Bagnaresi, P.; Alves, E.; Borges da Silva, H.; Epiphanio, S.; Mota, M.M.; Garcia, C.R. Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P. yoelii asynchronous infections are not affected by melatonin. Int. J. Gen. Med. 2009, 2, 47–55. [Google Scholar]
- Beraldo, F.H.; Mikoshiba, K.; Garcia, C.R. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J. Pineal Res. 2007, 43, 360–364. [Google Scholar] [CrossRef]
- Alves, E.; Bartlett, P.J.; Garcia, C.R.; Thomas, A.P. Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J. Biol. Chem. 2011, 286, 5905–5912. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, F.H.; Almeida, F.M.; da Silva, A.M.; Garcia, C.R. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J. Cell Biol. 2005, 170, 551–557. [Google Scholar] [CrossRef]
- Furuyama, W.; Enomoto, M.; Mossaad, E.; Kawai, S.; Mikoshiba, K.; Kawazu, S. An interplay between 2 signaling pathways: Melatonin-cAMP and IP3-Ca2+ signaling pathways control intraerythrocytic development of the malaria parasite Plasmodium falciparum. Biochem. Biophys. Res. Commun. 2014, 446, 125–131. [Google Scholar] [CrossRef]
- Koyama, F.C.; Ribeiro, R.Y.; Garcia, J.L.; Azevedo, M.F.; Chakrabarti, D.; Garcia, C.R. Ubiquitin proteasome system and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum. J. Pineal Res. 2012, 53, 147–153. [Google Scholar] [CrossRef]
- Dias, B.K.M.; Nakabashi, M.; Alves, M.R.R.; Portella, D.P.; Dos Santos, B.M.; Costa da Silva Almeida, F.; Ribeiro, R.Y.; Schuck, D.C.; Jordao, A.K.; Garcia, C.R.S. The Plasmodium falciparum eIK1 kinase (PfeIK1) is central for melatonin synchronization in the human malaria parasite. Melatotosil blocks melatonin action on parasite cell cycle. J. Pineal Res. 2020, 69, e12685. [Google Scholar] [CrossRef] [PubMed]
- Chahine, Z.; Gupta, M.; Lenz, T.; Hollin, T.; Abel, S.; Banks, C.; Saraf, A.; Prudhomme, J.; Florens, L.; Le Roch, K.G. fMORC protein regulates chromatin accessibility and transcriptional repression in the human malaria parasite, P. falciparum. bioRxiv 2023. [Google Scholar] [CrossRef]
- Singh, M.K.; Bonnell, V.A.; Da Silva, I.T.; Santiago, V.F.; Moraes, M.S.; Adderley, J.; Doerig, C.; Palmisano, G.; Llinás, M.; Garcia, C.R. A Plasmodium falciparum MORC protein complex modulates epigenetic control of gene expression through interaction with heterochromatin. eLife 2024, 12. [Google Scholar] [CrossRef]
- Lima, W.R.; Tessarin-Almeida, G.; Rozanski, A.; Parreira, K.S.; Moraes, M.S.; Martins, D.C.; Hashimoto, R.F.; Galante, P.A.F.; Garcia, C.R.S. Signaling transcript profile of the asexual intraerythrocytic development cycle of Plasmodium falciparum induced by melatonin and cAMP. Genes. Cancer 2016, 7, 323–339. [Google Scholar] [CrossRef][Green Version]
- Scarpelli, P.H.; Tessarin-Almeida, G.; Vicoso, K.L.; Lima, W.R.; Borges-Pereira, L.; Meissner, K.A.; Wrenger, C.; Raffaello, A.; Rizzuto, R.; Pozzan, T.; et al. Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure. J. Pineal Res. 2019, 66, e12484. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Komatsuya, K.; Imamura, S.; Nozaki, T.; Watanabe, Y.I.; Sato, S.; Dodd, A.N.; Kita, K.; Tanaka, K. Coordination of apicoplast transcription in a malaria parasite by internal and host cues. Proc. Natl. Acad. Sci. USA 2023, 120, e2214765120. [Google Scholar] [CrossRef]
- Cestari, I.; Stuart, K. The phosphoinositide regulatory network in Trypanosoma brucei: Implications for cell-wide regulation in eukaryotes. PLoS Negl. Trop. Dis. 2020, 14, e0008689. [Google Scholar] [CrossRef] [PubMed]
- Hortua Triana, M.A.; Marquez-Nogueras, K.M.; Vella, S.A.; Moreno, S.N.J. Calcium signaling and the lytic cycle of the Apicomplexan parasite Toxoplasma gondii. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865 Pt B, 1846–1856. [Google Scholar] [CrossRef]
- Wengelnik, K.; Daher, W.; Lebrun, M. Phosphoinositides and their functions in apicomplexan parasites. Int. J. Parasitol. 2018, 48, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Lovett, J.L.; Marchesini, N.; Moreno, S.N.; Sibley, L.D. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J. Biol. Chem. 2002, 277, 25870–25876. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.S.; Alves, E.; Pereira, P.H.S.; Bartlett, P.J.; Thomas, A.P.; Mikoshiba, K.; Plattner, H.; Sibley, L.D. InsP3 Signaling in Apicomplexan Parasites. Curr. Top. Med. Chem. 2017, 17, 2158–2165. [Google Scholar] [CrossRef]
- Schuck, D.C.; Jordao, A.K.; Nakabashi, M.; Cunha, A.C.; Ferreira, V.F.; Garcia, C.R. Synthetic indole and melatonin derivatives exhibit antimalarial activity on the cell cycle of the human malaria parasite Plasmodium falciparum. Eur. J. Med. Chem. 2014, 78, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Lunga, M.J.; Chisango, R.L.; Weyers, C.; Isaacs, M.; Taylor, D.; Edkins, A.L.; Khanye, S.D.; Hoppe, H.C.; Veale, C.G.L. Expanding the SAR of Nontoxic Antiplasmodial Indolyl-3-ethanone Ethers and Thioethers. ChemMedChem 2018, 13, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Luthra, T.; Nayak, A.K.; Bose, S.; Chakrabarti, S.; Gupta, A.; Sen, S. Indole based antimalarial compounds targeting the melatonin pathway: Their design, synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 168, 11–27. [Google Scholar] [CrossRef]
- Mallaupoma, L.R.C.; Dias, B.K.M.; Singh, M.K.; Honorio, R.I.; Nakabashi, M.; Kisukuri, C.M.; Paixao, M.W.; Garcia, C.R.S. Decoding the Role of Melatonin Structure on Plasmodium falciparum Human Malaria Parasites Synchronization Using 2-Sulfenylindoles Derivatives. Biomolecules 2022, 12, 638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, B.K.M.; Mohanty, A.; Garcia, C.R.S. Melatonin as a Circadian Marker for Plasmodium Rhythms. Int. J. Mol. Sci. 2024, 25, 7815. https://doi.org/10.3390/ijms25147815
Dias BKM, Mohanty A, Garcia CRS. Melatonin as a Circadian Marker for Plasmodium Rhythms. International Journal of Molecular Sciences. 2024; 25(14):7815. https://doi.org/10.3390/ijms25147815
Chicago/Turabian StyleDias, Bárbara K. M., Abhinab Mohanty, and Célia R. S. Garcia. 2024. "Melatonin as a Circadian Marker for Plasmodium Rhythms" International Journal of Molecular Sciences 25, no. 14: 7815. https://doi.org/10.3390/ijms25147815
APA StyleDias, B. K. M., Mohanty, A., & Garcia, C. R. S. (2024). Melatonin as a Circadian Marker for Plasmodium Rhythms. International Journal of Molecular Sciences, 25(14), 7815. https://doi.org/10.3390/ijms25147815







