Mitochondrial ATP Synthase beta-Subunit Affects Plastid Retrograde Signaling in Arabidopsis
Abstract
:1. Introduction
2. Results
2.1. Isolation, Sequence Analysis, and Expression Pattern of Mitochondrial ATP Synthase beta-Subunit
2.2. Subcellular Localization of the AT5G08670 Protein in Arabidopsis thaliana
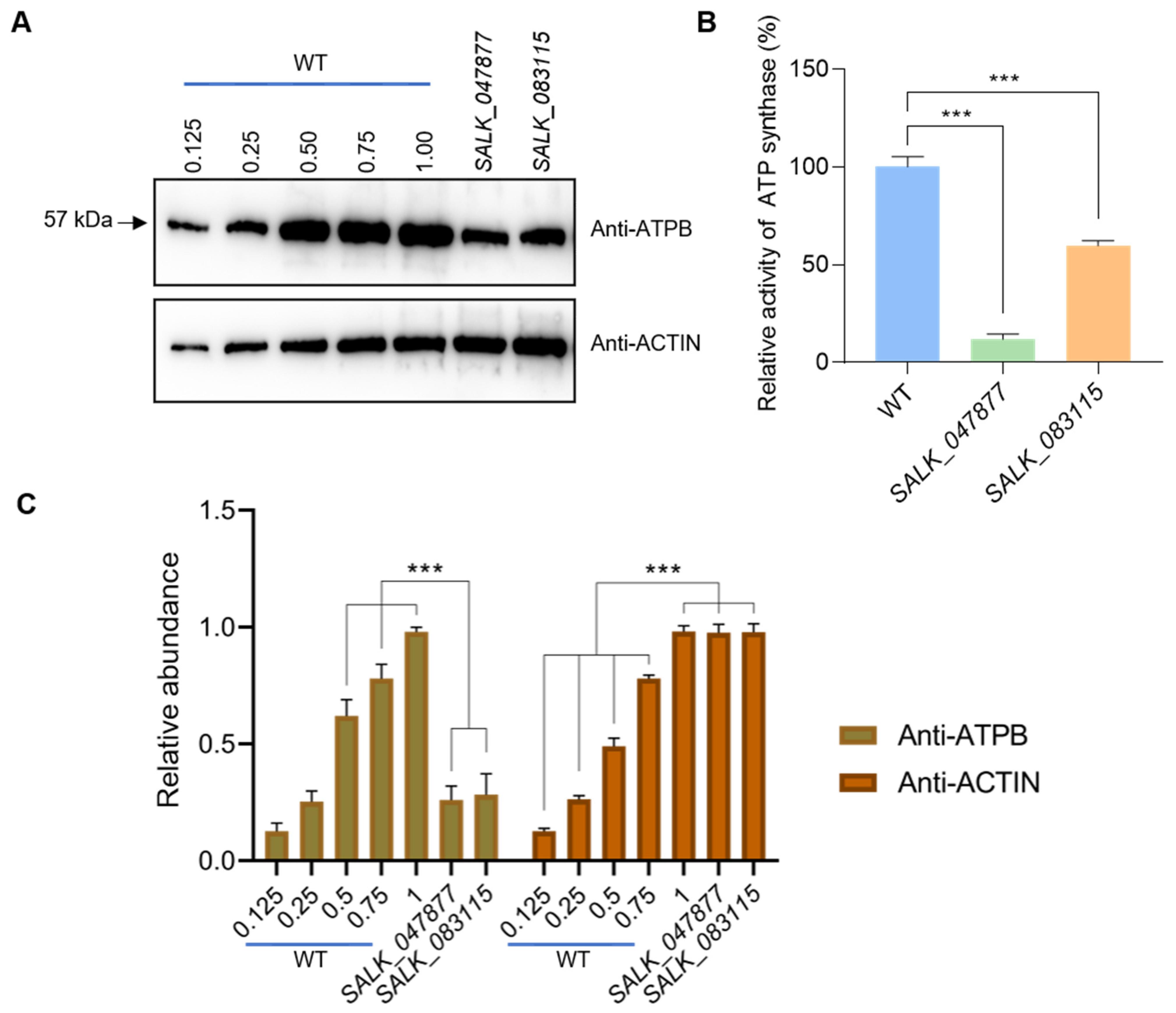
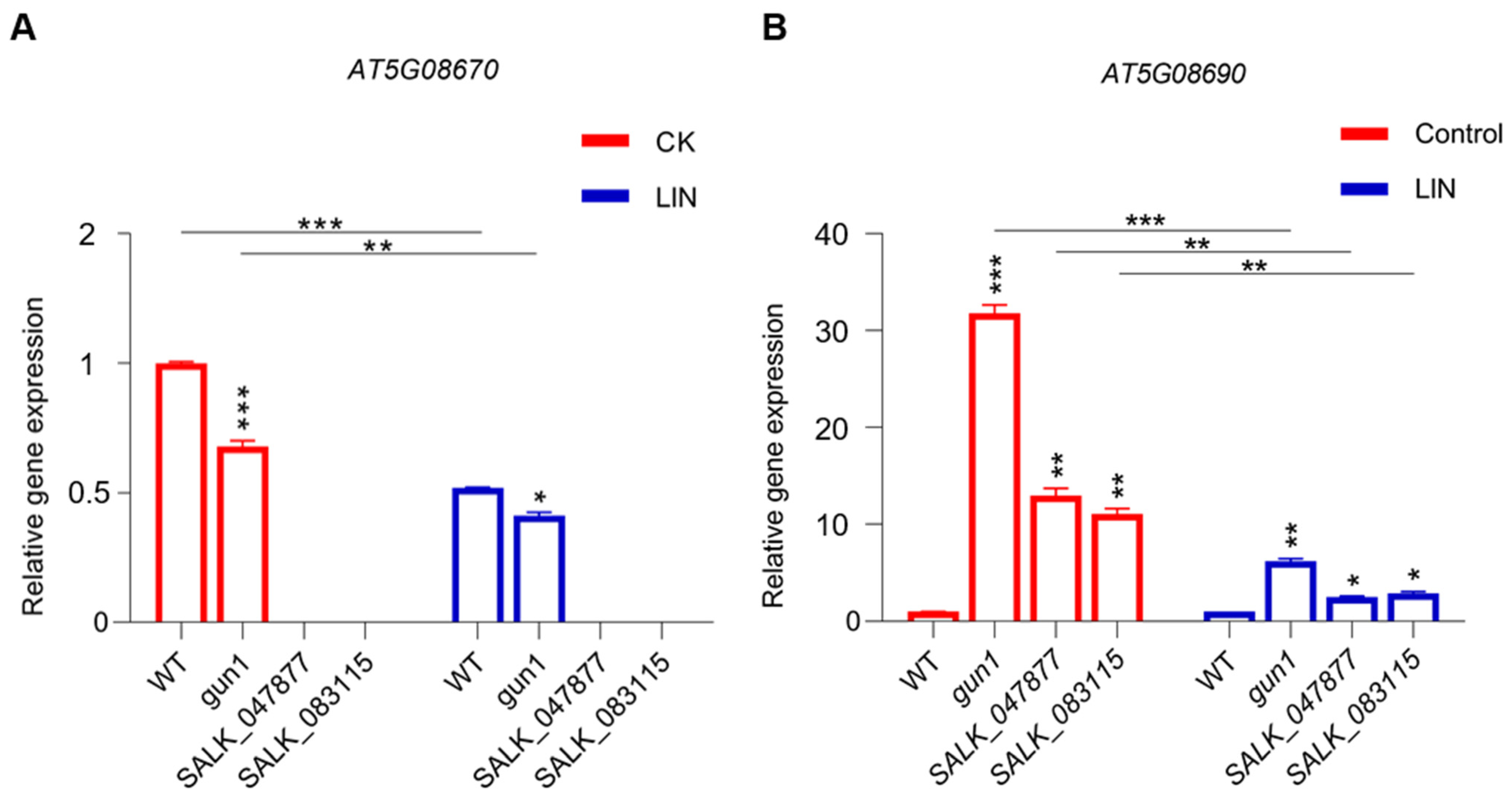
2.3. Loss of Function of AT5G08670 Leads to a Decrease in Mitochondrial ATP Synthase Level and Activity and Affects Plastid Retrograde Signaling
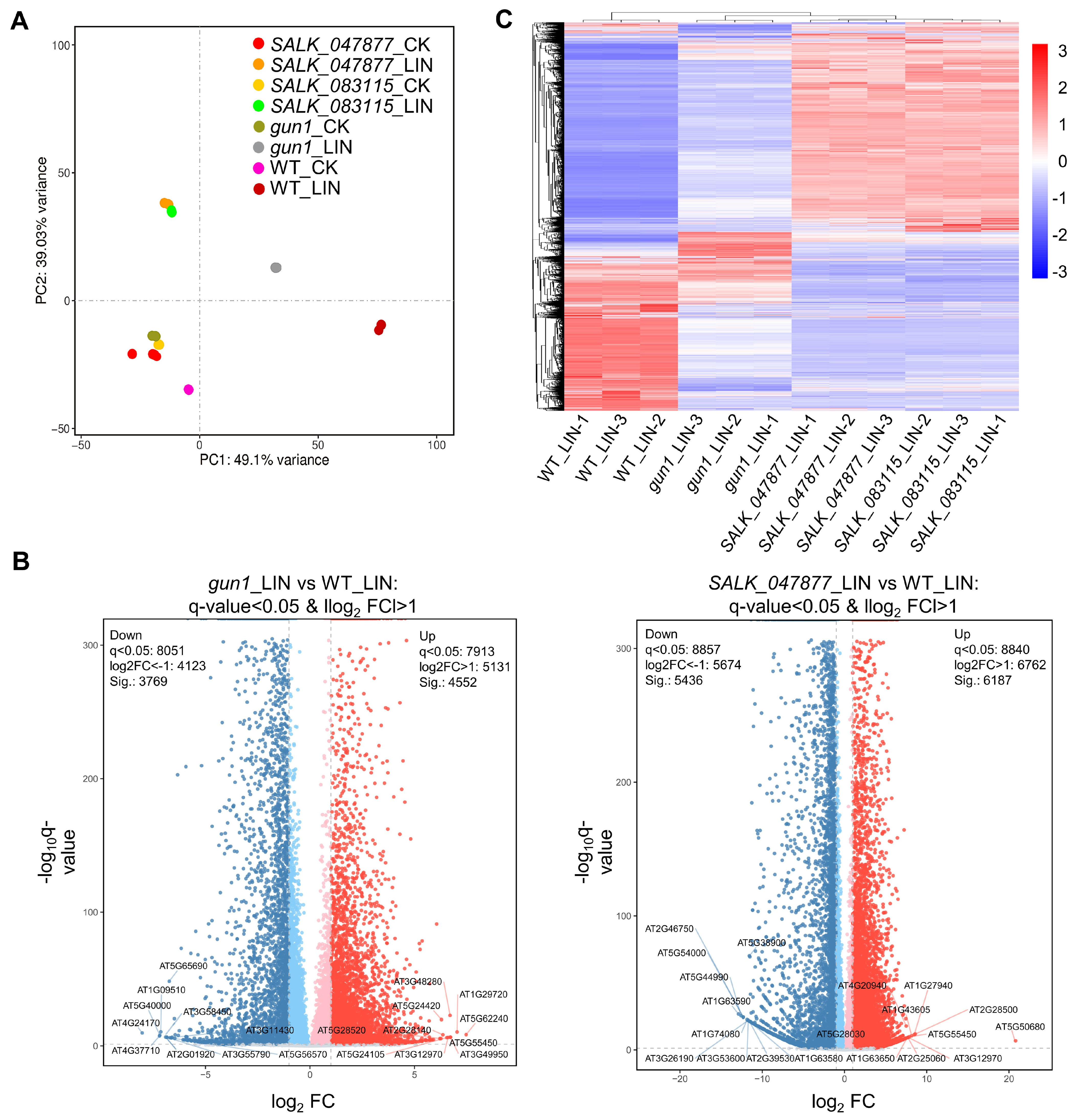
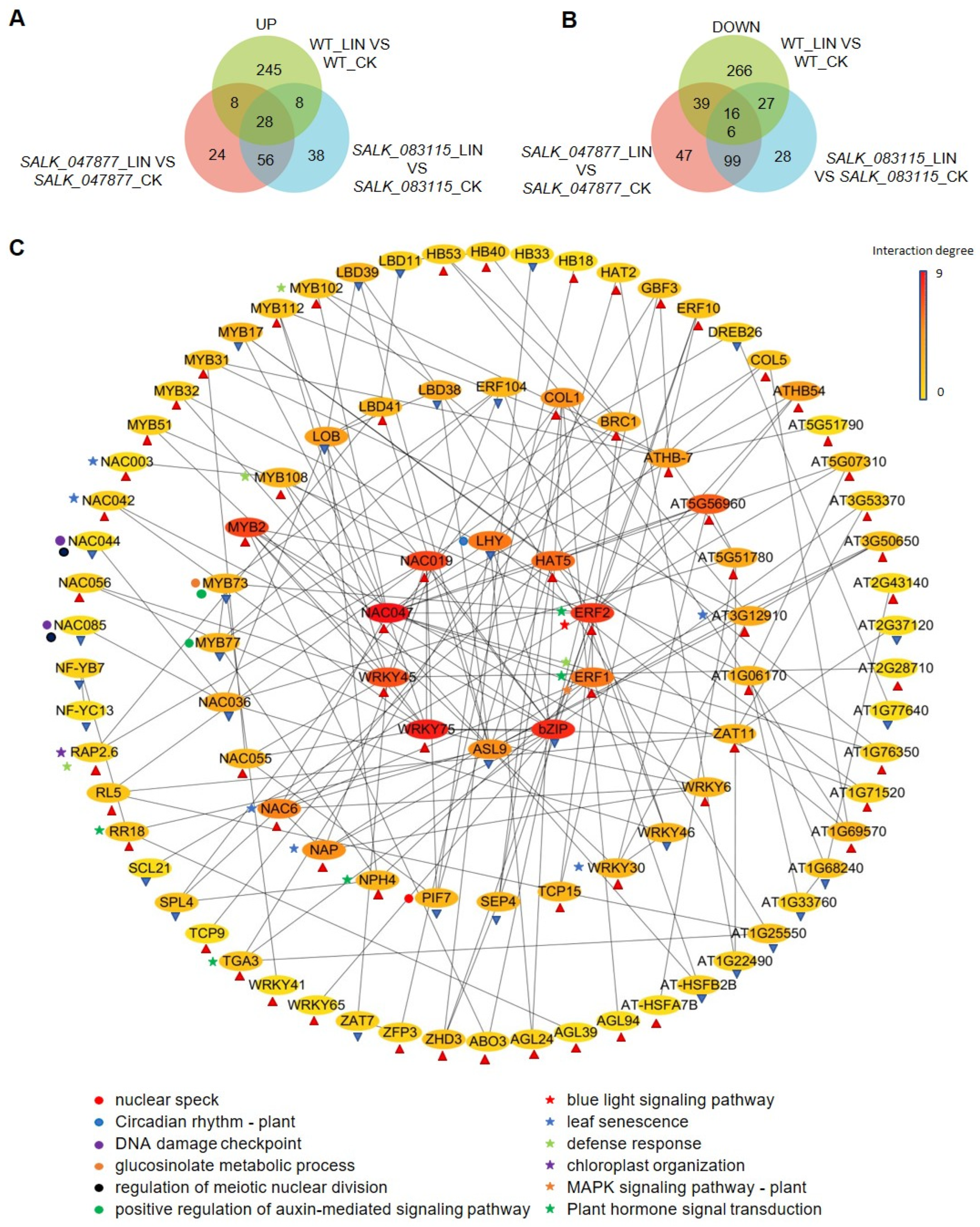
2.4. Detection of Differentially Expressed Genes (DEGs) in AT5G08670 Mutants by RNA-Sequencing (RNA-Seq)
2.5. Gene Ontology (GO) Enrichment Analysis of DEGs between WT and SALK Lines after LIN Treatment
2.6. GO Enrichment Analysis of Plastid-Related DEGs
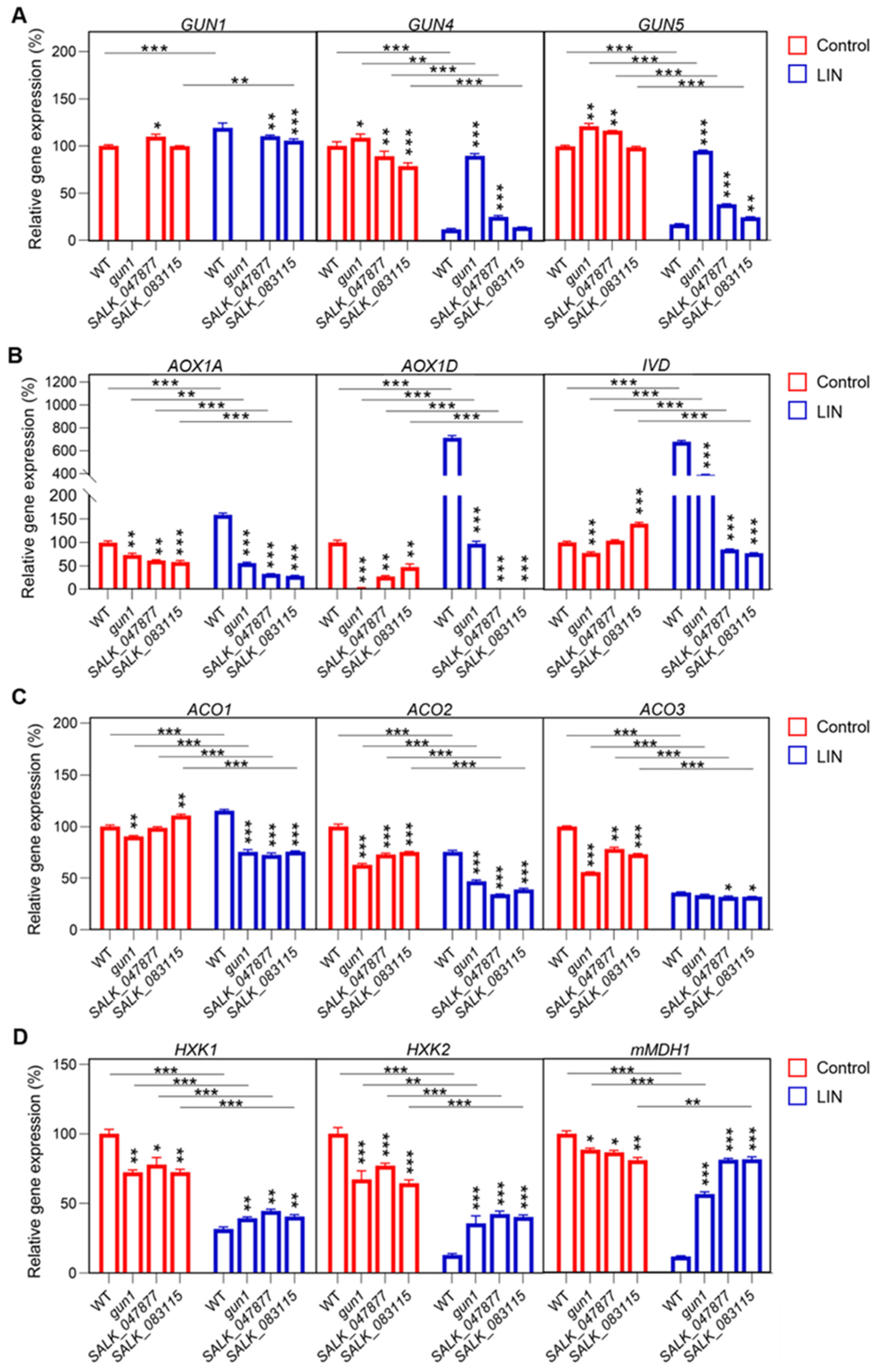
2.7. Expression of Nuclear Genes of Mitochondrial Proteins Is Affected by the Loss of AT5G08670
2.8. Identification of Important TFs Downstream of AT5G08670-Involved Signaling
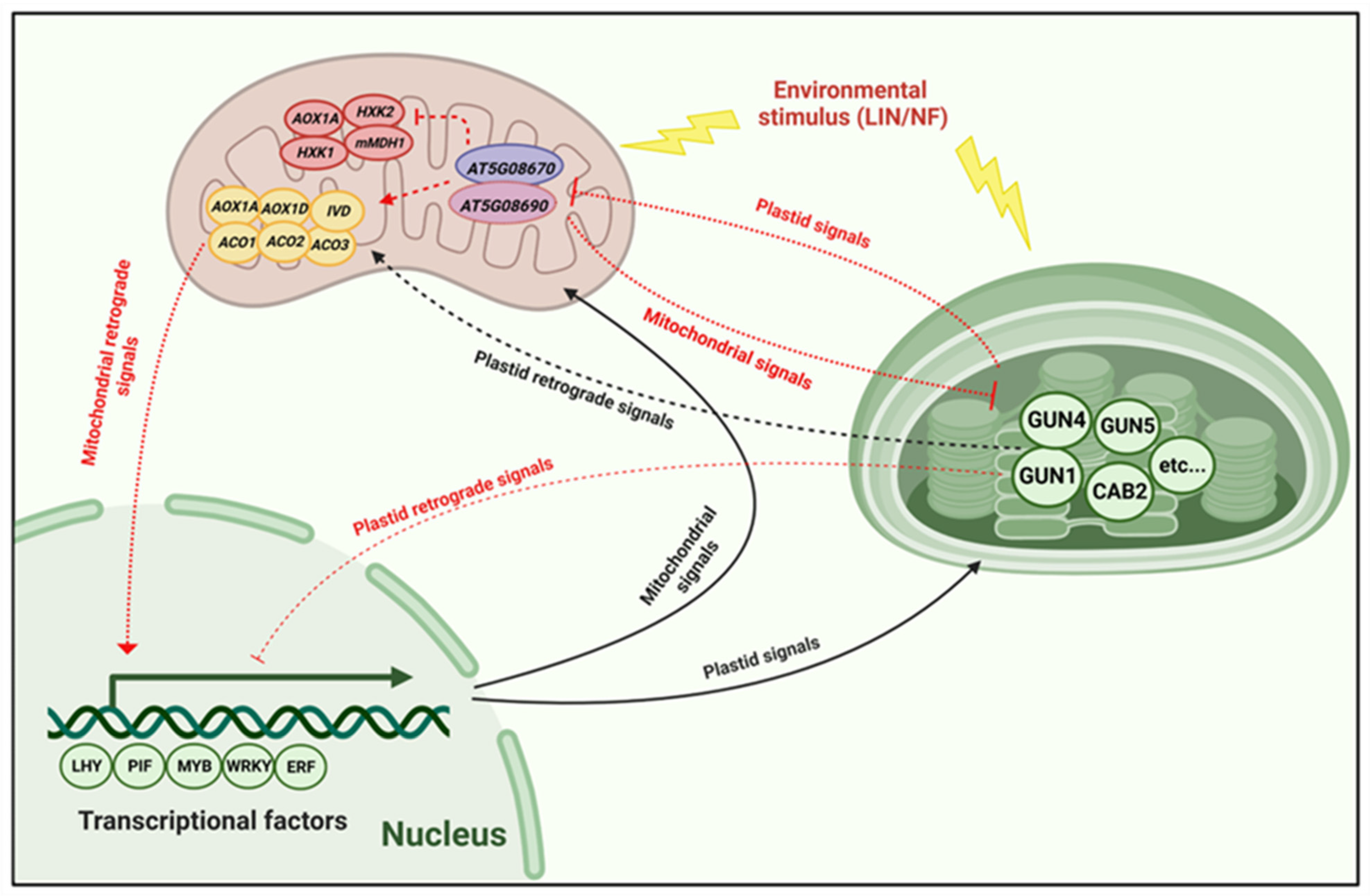
3. Discussion
3.1. AT5G08670: An Impact on PhANG Expression and Its Operational Consequences
3.2. Mitochondrial Proteins Contribute to Plastid Retrograde Signaling
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. DNA Extraction
4.3. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.4. RNA Sequencing Analyses
4.5. Protoplast Transient Expression Assay
4.6. GUS Staining and Histological Analysis
4.7. Total Protein Extraction and Immunoblot Analysis
4.8. Mitochondrial ATP Synthase Activity Assay
4.9. Phylogenetic Analysis of AT5G08670
4.10. Drawing of TF Network Diagram
4.11. Generation of Complementary Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, J.F.; Paula, W.; Puthiyaveetil, S.; Nield, J. A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci. 2011, 16, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2014, 15, 147. [Google Scholar] [CrossRef]
- Ruckle, M.E.; DeMarco, S.M.; Larkin, R.M. Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 2007, 19, 3944–3960. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Langdale, J.A. The making of a chloroplast. EMBO J. 2009, 28, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Nott, A.; Jung, H.S.; Koussevitzky, S.; Chory, J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006, 57, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Z.; Wang, J.; Guo, C.; Zhou, Y.; Bawa, G.; Rochaix, J.-D.; Sun, X. COE2 Is Required for the Root Foraging Response to Nitrogen Limitation. Int. J. Mol. Sci. 2022, 23, 861. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, D.; Liu, Z.; Kleine, T.; Leister, D. Functional relationship between mTERF4 and GUN1 in retrograde signaling. J. Exp. Bot. 2016, 67, 3909–3924. [Google Scholar] [CrossRef] [PubMed]
- Susek, R.E.; Ausubel, F.M.; Chory, J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 1993, 74, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Strand, Å.; Asami, T.; Alonso, J.; Ecker, J.R.; Chory, J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 2003, 421, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.M.; Alonso, J.M.; Ecker, J.R.; Chory, J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 2003, 299, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Woodson, J.D.; Perez-Ruiz, J.M.; Chory, J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 2011, 21, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Padmasree, K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003, 8, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Selinski, J.; Mao, C.; Zhu, Y.; Berkowitz, O.; Whelan, J. Linking mitochondrial and chloroplast retrograde signalling in plants. Philos. Trans. R. Soc. B 2020, 375, 20190410. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. The light reactions of photosynthesis. Proc. Natl. Acad. Sci. USA 1971, 68, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.M.; Tikhonov, M.A.; Asiamolova, N.M.; Volkov, M.; Kotov, A.N.; Savchenko, G.E.; Khaĭdakov, K.S. External respiration and gas exchange in space flights. Kosm. Biol. Aviakosm. Med. 1991, 25, 4–8. [Google Scholar] [PubMed]
- Marsh, H.V., Jr.; Galmiche, J.M.; Gibbs, M. Respiration during photosynthesis. Rec. Chem. Prog. 1964, 25, 259–271. [Google Scholar] [PubMed]
- Padmasree, K.; Raghavendra, A. Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.). Physiol. Plant. 1999, 105, 546–553. [Google Scholar] [CrossRef]
- Hebbelmann, I.; Selinski, J.; Wehmeyer, C.; Goss, T.; Voss, I.; Mulo, P.; Kangasjärvi, S.; Aro, E.M.; Oelze, M.L.; Dietz, K.J.; et al. Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP-malate dehydrogenase. J. Exp. Bot. 2012, 63, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, A.; Mazur, R.; Ostaszewska-Bugajska, M.; Kryzheuskaya, K.; Dziewit, K.; Borysiuk, K.; Wdowiak, A.; Burian, M.; Rasmusson, A.G.; Szal, B. Efficient Photosynthetic Functioning of Arabidopsis thaliana Through Electron Dissipation in Chloroplasts and Electron Export to Mitochondria Under Ammonium Nutrition. Front. Plant. Sci. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Lermontova, I.; Kruse, E.; Mock, H.P.; Grimm, B. Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc. Natl. Acad. Sci. USA 1997, 94, 8895–8900. [Google Scholar] [CrossRef] [PubMed]
- Woodson, J.D.; Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Marienfeld, J.R.; Newton, K.J. The maize NCS2 abnormal growth mutant has a chimeric nad4-nad7 mitochondrial gene and is associated with reduced complex I function. Genetics 1994, 138, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Sabar, M.; De Paepe, R.; de Kouchkovsky, Y. Complex I impairment, respiratory compensations, and photosynthetic decrease in nuclear and mitochondrial male sterile mutants of Nicotiana sylvestris. Plant Physiol. 2000, 124, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V.; Lea, P.J.; Gardeström, P. The role of photorespiration in redox and energy balance of photosynthetic plant cells: A study with a barley mutant deficient in glycine decarboxylase. Physiol. Plant. 2001, 111, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Heineke, D.; Bykova, N.; Gardeström, P.; Bauwe, H. Metabolic response of potato plants to an antisense reduction of the P-protein of glycine decarboxylase. Planta 2001, 212, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, G.; Wang, Y.; Qian, Q.; Xu, J.; Sodmergen; Liu, G.; Zhao, X.; Chen, M.; Zhai, W.; et al. WHITE PANICLE3, a Novel Nucleus-Encoded Mitochondrial Protein, Is Essential for Proper Development and Maintenance of Chloroplasts and Mitochondria in Rice. Front. Plant Sci. 2018, 9, 762. [Google Scholar] [CrossRef]
- Maruta, T.; Miyazaki, N.; Nosaka, R.; Tanaka, H.; Padilla-Chacon, D.; Otori, K.; Kimura, A.; Tanabe, N.; Yoshimura, K.; Tamoi, M.; et al. A gain-of-function mutation of plastidic invertase alters nuclear gene expression with sucrose treatment partially via GENOMES UNCOUPLED1-mediated signaling. New Phytol. 2015, 206, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Kropat, J.; Oster, U.; Rüdiger, W.; Beck, C.F. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl. Acad. Sci. USA 1997, 94, 14168–14172. [Google Scholar] [CrossRef] [PubMed]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [PubMed]
- Crisp, P.A.; Smith, A.B.; Ganguly, D.R.; Murray, K.D.; Eichten, S.R.; Millar, A.A.; Pogson, B.J. RNA Polymerase II Read-Through Promotes Expression of Neighboring Genes in SAL1-PAP-XRN Retrograde Signaling. Plant Physiol. 2018, 178, 1614–1630. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Giraud, E.; Duncan, O.; Law, S.R.; Wang, Y.; Xu, L.; Narsai, R.; Carrie, C.; Walker, H.; Day, D.A.; et al. Cyclin-dependent kinase E1 (CDKE1) provides a cellular switch in plants between growth and stress responses. J. Biol. Chem. 2013, 288, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Pimentel, H.; Trapnell, C.; Pachter, L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 2011, 27, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Guo, J.; Li, J.; Tian, Z.; Zhu, Z.; Wang, J.; Wu, R.; Zhang, B.; Hu, Y.; et al. Global Dynamic Molecular Profiling of Stomatal Lineage Cell Development by Single-Cell RNA Sequencing. Mol. Plant 2020, 13, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Sugiyama, M. Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development 2003, 130, 5637–5647. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Stock, D.; Leslie, A.G.; Walker, J.E. Molecular architecture of the rotary motor in ATP synthase. Science 1999, 286, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kim, E.D.; Ha, M.; Lackey, E.; Liu, J.; Zhang, Y.; Sun, Q.; Chen, Z.J. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 2009, 457, 327–331. [Google Scholar] [CrossRef]
- Hennig, L.; Büche, C.; Eichenberg, K.; Schäfer, E. Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings. Plant. Physiol. 1999, 121, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 1999, 400, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Lindemose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci. 2013, 14, 5842–5878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lü, S.; Tran, L.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, J.; Zhang, Y.; Yin, L.; Lu, J. Isolation of a WRKY30 gene from Muscadinia rotundifolia (Michx) and validation of its function under biotic and abiotic stresses. Protoplasma 2015, 252, 1361–1374. [Google Scholar] [CrossRef]
- Hammargren, J.; Rosenquist, S.; Jansson, C.; Knorpp, C. A novel connection between nucleotide and carbohydrate metabolism in mitochondria: Sugar regulation of the Arabidopsis nucleoside diphosphate kinase 3a gene. Plant Cell Rep. 2008, 27, 529–534. [Google Scholar] [CrossRef]
- Roussell, D.L.; Thompson, D.L.; Pallardy, S.G.; Miles, D.; Newton, K.J. Chloroplast Structure and Function Is Altered in the NCS2 Maize Mitochon-drial Mutant. Plant. Physiol. 1991, 96, 232–238. [Google Scholar] [CrossRef]
- Gu, J.; Miles, D.; Newton, K.J. Analysis of Leaf Sectors in the NCS6 Mitochondrial Mutant of Maize. Plant Cell 1993, 5, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Pongthai, P.; Ariyarathne, M.; Teh, O.K.; Fujita, T. AP2/ERF transcription factors regulate salt-induced chloroplast division in the moss Physcomitrella patens. J. Plant Res. 2020, 133, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Hou, L.; Shi, J.; Yang, T.; Liu, Y.; Wei, A. Patterns of Drought Response of 38 WRKY Transcription Factors of Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci. 2018, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Chivasa, S.; Tomé, D.F.; Hamilton, J.M.; Slabas, A.R. Proteomic analysis of extracellular ATP-regulated proteins identifies ATP synthase beta-subunit as a novel plant cell death regulator. Mol. Cell. Proteom. 2011, 10, M110.003905. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Z.; Sun, S.; Qin, A.; Liu, H.; Zhou, Y.; Li, W.; Liu, Y.; Hu, M.; Yang, J.; et al. Identification of bZIP Transcription Factors That Regulate the Development of Leaf Epidermal Cells in Arabidopsis thaliana by Single-Cell RNA Sequencing. Int. J. Mol. Sci. 2024, 25, 2553. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 2013, 502, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Osipenkova, O.V.; Rakhimberdieva, M.G.; Karapetyan, N.V.; Yurina, N.P. Involvement of two plastid signals in the regulation of expression of nuclear gene for the chloroplast protein elip. Dokl. Biochem. Biophys. 2007, 416, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.G.; Taylor, W.C. The chloroplast affects the transcription of a nuclear gene family. Mol. Gen. Genet. 1988, 214, 89–96. [Google Scholar] [CrossRef]
- Chen, N.; Wang, P.; Li, C.; Wang, Q.; Pan, J.; Xiao, F.; Wang, Y.; Zhang, K.; Li, C.; Yang, B.; et al. A Single Nucleotide Mutation of the IspE Gene Participating in the MEP Pathway for Isoprenoid Biosynthesis Causes a Green-Revertible Yellow Leaf Phenotype in Rice. Plant Cell Physiol. 2018, 59, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; He, C.X.; Zhang, Z.W.; Xu, F.; Zhang, D.W.; Wang, X.; Yuan, S.; Lin, H.H. Plastid signals confer Arabidopsis tolerance to water stress. Z. Naturforschung C J. Biosci. 2011, 66, 47–54. [Google Scholar] [CrossRef]
- Surpin, M.; Larkin, R.M.; Chory, J. Signal transduction between the chloroplast and the nucleus. Plant Cell 2002, 14 (Suppl. S1), 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.P.; Wang, Y.; McIntosh, L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. USA 1999, 96, 8271–8276. [Google Scholar] [CrossRef] [PubMed]
- Dojcinovic, D.; Krosting, J.; Harris, A.J.; Wagner, D.J.; Rhoads, D.M. Identification of a region of the Arabidopsis AtAOX1a promoter necessary for mitochondrial retrograde regulation of expression. Plant Mol. Biol. 2005, 58, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Merendino, L.; Courtois, F.; Grübler, B.; Bastien, O.; Straetmanns, V.; Chevalier, F.; Lerbs-Mache, S.; Lurin, C.; Pfannschmidt, T. Retrograde signals from mitochondria reprogramme skoto-morphogenesis in Arabidopsis thaliana via alternative oxidase 1a. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190567. [Google Scholar] [CrossRef] [PubMed]
- Frangedakis, E.; Yelina, N.E.; Billakurthi, K.; Schreier, T.; Dickinson, P.J.; Tomaselli, M.; Haseloff, J.; Hibberd, J.M. Synergistic control of chloroplast biogenesis by MYB-related and Golden2-like transcription factors. bioRxiv 2023. preprint. [Google Scholar]
- Wang, L.; Tian, Y.; Shi, W.; Yu, P.; Hu, Y.; Lv, J.; Fu, C.; Fan, M.; Bai, M.Y. The miR396-GRFs Module Mediates the Prevention of Photo-oxidative Damage by Brassinosteroids during Seedling De-Etiolation in Arabidopsis. Plant Cell 2020, 32, 2525–2542. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, C.; Dong, X.; Li, H.; Zhang, D.; Zhou, Y.; Jiang, B.; Peng, J.; Qin, X.; Cheng, J.; et al. MYB30 Is a Key Negative Regulator of Arabidopsis Photomorphogenic Development That Promotes PIF4 and PIF5 Protein Accumulation in the Light. Plant Cell 2020, 32, 2196–2215. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, O.; Whelan, J. Comparison of transcriptional changes to chloroplast and mitochondrial perturbations reveals common and specific responses in Arabidopsis. Front. Plant Sci. 2012, 3, 281. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, O.; Zhang, B.; Law, S.; Narsai, R.; Whelan, J. AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiol. 2013, 162, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, L.; De Clercq, I.; Narsai, R.; Xu, Y.; Hartmann, A.; Claros, D.L.; Custovic, E.; Lewsey, M.G.; Whelan, J.; et al. ANAC017 Coordinates Organellar Functions and Stress Responses by Reprogramming Retrograde Signaling. Plant Physiol. 2019, 180, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B.; et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, I.; Vermeirssen, V.; Van Aken, O.; Vandepoele, K.; Murcha, M.W.; Law, S.R.; Inzé, A.; Ng, S.; Ivanova, A.; Rombaut, D.; et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 2013, 25, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, S.; Zheng, Y.; Duan, H.; Xiao, F.; Zhuang, Y.; He, J.; Wu, G.; Zhao, S.; Zhou, H.; et al. SUMOylation of MYB30 enhances salt tolerance by elevating alternative respiration via transcriptionally upregulating AOX1a in Arabidopsis. Plant J. 2020, 102, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.P.; Cheng, Y.Y.; Lee, H.L.; Hsu, T.Y.; Chang, Y.T.; Shen, Y.A. Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12623. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.W.; Anderson, B.D.; Volchenboum, S.L.; Battaile, K.P.; Tiffany, K.; Roberts, D.; Kim, J.J.; Vockley, J. Characterization of molecular defects in isovaleryl-CoA dehydrogenase in patients with isovaleric acidemia. Biochemistry 1998, 37, 10325–10335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Frerman, F.E.; Kim, J.J. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc. Natl. Acad. Sci. USA 2006, 103, 16212–16217. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.W.; Vockley, J. Kinetic and spectral properties of isovaleryl-CoA dehydrogenase and interaction with ligands. Biochimie 2015, 108, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, D.; Absi, G.; Gagliardi, C.; Bandaru, S.S.; Madara, J.C.; Ferrari, L.L.; Arrigoni, E.; Münzberg, H.; Scammell, T.E.; Saper, C.B.; et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018, 9, 4129. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, M.; Li, M.; Guo, X.; Wu, Y.; Wang, J. Identification and Characterization of PLATZ Transcription Factors in Wheat. Int. J. Mol. Sci. 2020, 21, 8934. [Google Scholar] [CrossRef] [PubMed]
- Shapiguzov, A.; Vainonen, J.P.; Hunter, K.; Tossavainen, H.; Tiwari, A.; Järvi, S.; Hellman, M.; Aarabi, F.; Alseekh, S.; Wybouw, B.; et al. Arabidopsis RCD1 coordinates chloroplast and mitochondrial functions through interaction with ANAC transcription factors. eLife 2019, 8, e43284. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, C.; Wollman, F.A.; Bennoun, P. Restoration of phototrophic growth in a mutant of Chlamydomonas reinhardtii in which the chloroplast atpB gene of the ATP synthase has a deletion: An example of mitochondria-dependent photosynthesis. Proc. Natl. Acad. Sci. USA 1988, 85, 1344–1348. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, Z.; Qin, A.; Zhou, Y.; Sun, S.; Liu, Y.; Hu, M.; Yang, J.; Sun, X. Mitochondrial ATP Synthase beta-Subunit Affects Plastid Retrograde Signaling in Arabidopsis. Int. J. Mol. Sci. 2024, 25, 7829. https://doi.org/10.3390/ijms25147829
Liu H, Liu Z, Qin A, Zhou Y, Sun S, Liu Y, Hu M, Yang J, Sun X. Mitochondrial ATP Synthase beta-Subunit Affects Plastid Retrograde Signaling in Arabidopsis. International Journal of Molecular Sciences. 2024; 25(14):7829. https://doi.org/10.3390/ijms25147829
Chicago/Turabian StyleLiu, Hao, Zhixin Liu, Aizhi Qin, Yaping Zhou, Susu Sun, Yumeng Liu, Mengke Hu, Jincheng Yang, and Xuwu Sun. 2024. "Mitochondrial ATP Synthase beta-Subunit Affects Plastid Retrograde Signaling in Arabidopsis" International Journal of Molecular Sciences 25, no. 14: 7829. https://doi.org/10.3390/ijms25147829




