Selenium Nanoparticle and Melatonin Treatments Improve Melon Seedling Growth by Regulating Carbohydrate and Polyamine
Abstract
:1. Introduction
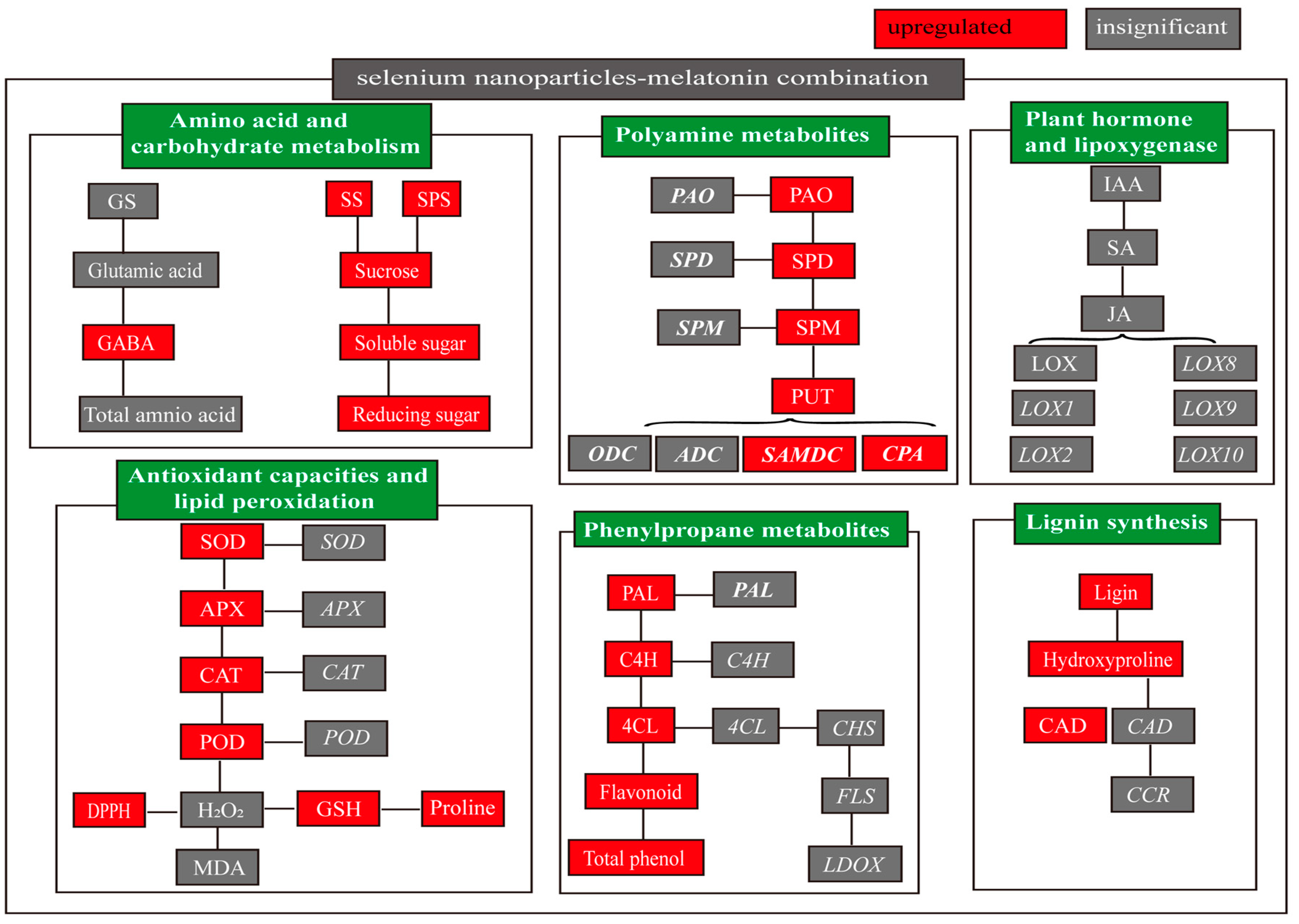
2. Results
2.1. Effects of Selenium Nanoparticles and Melatonin on Plant Biomass
2.2. Lipoxygenase and Plant Hormones after Selenium Nanoparticle and Melatonin Treatments
2.3. Effects of Selenium Nanoparticle and Melatonin Treatments on Amino Acid and Carbohydrate Metabolism
2.4. Effects of Selenium Nanoparticle and Melatonin Treatments on Secondary Metabolism
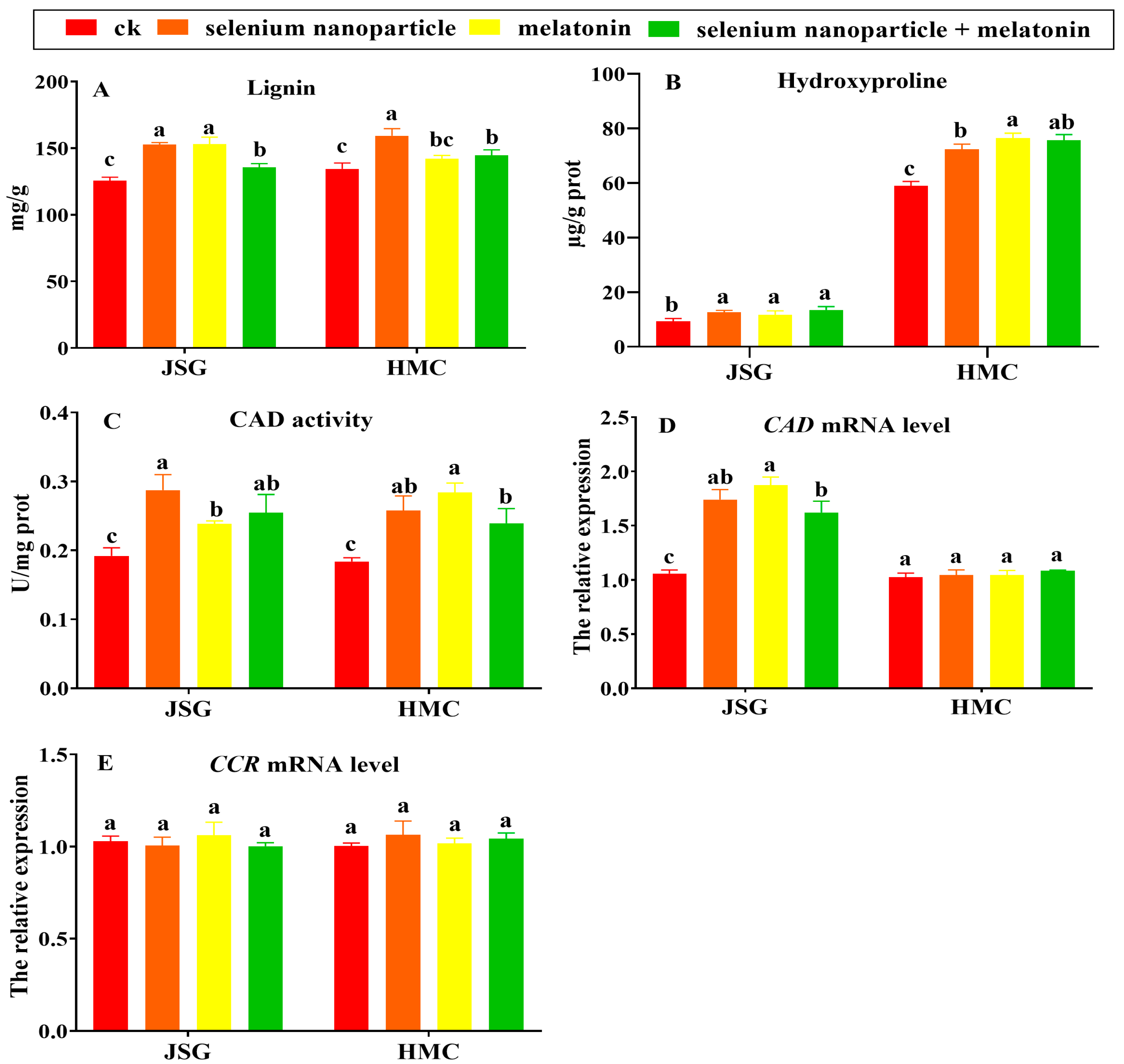
2.4.1. Selenium Nanoparticle and Melatonin Effects on Lignin Synthesis
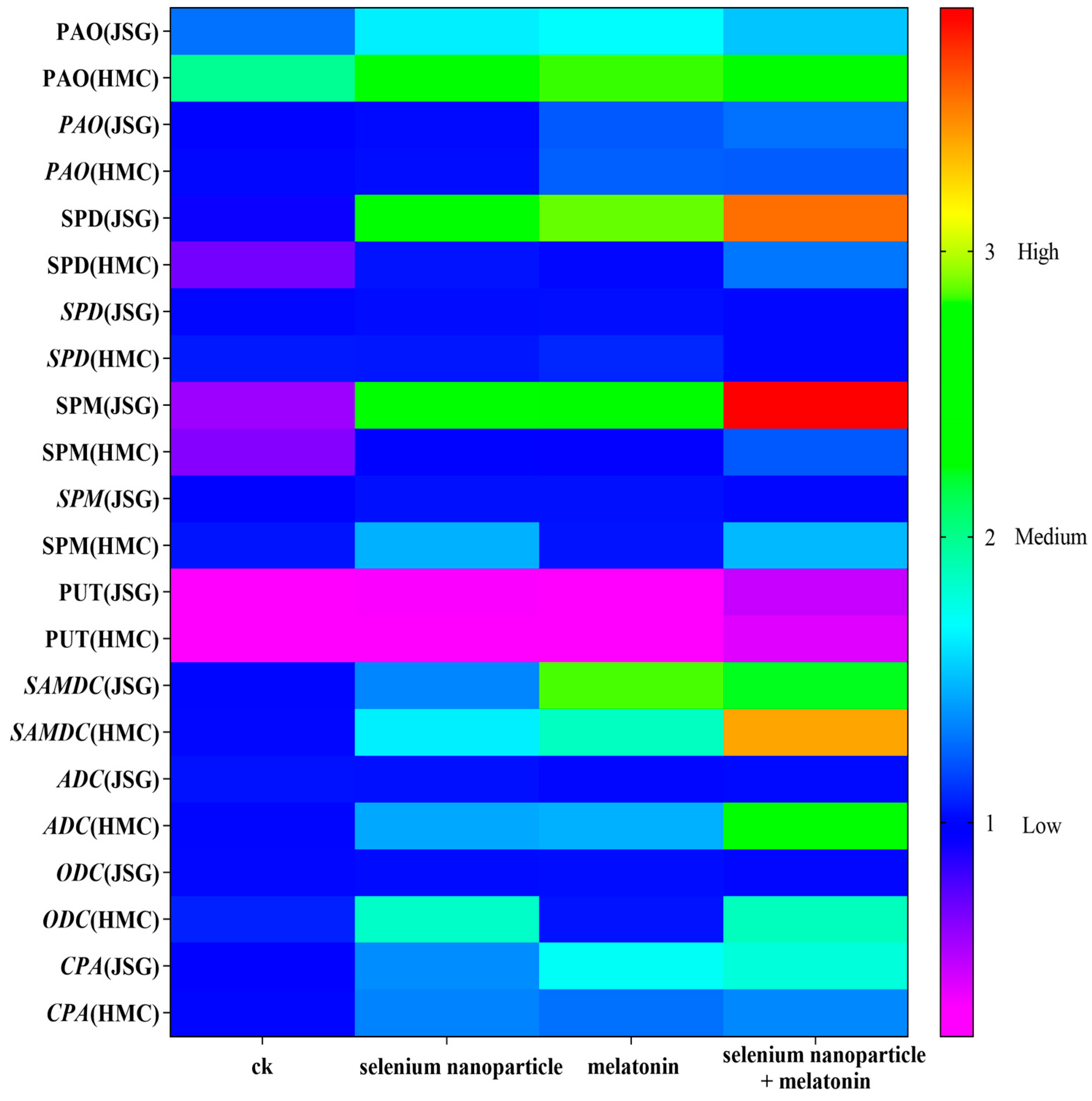
2.4.2. Polyamine Metabolism after Selenium Nanoparticle and Melatonin Treatments
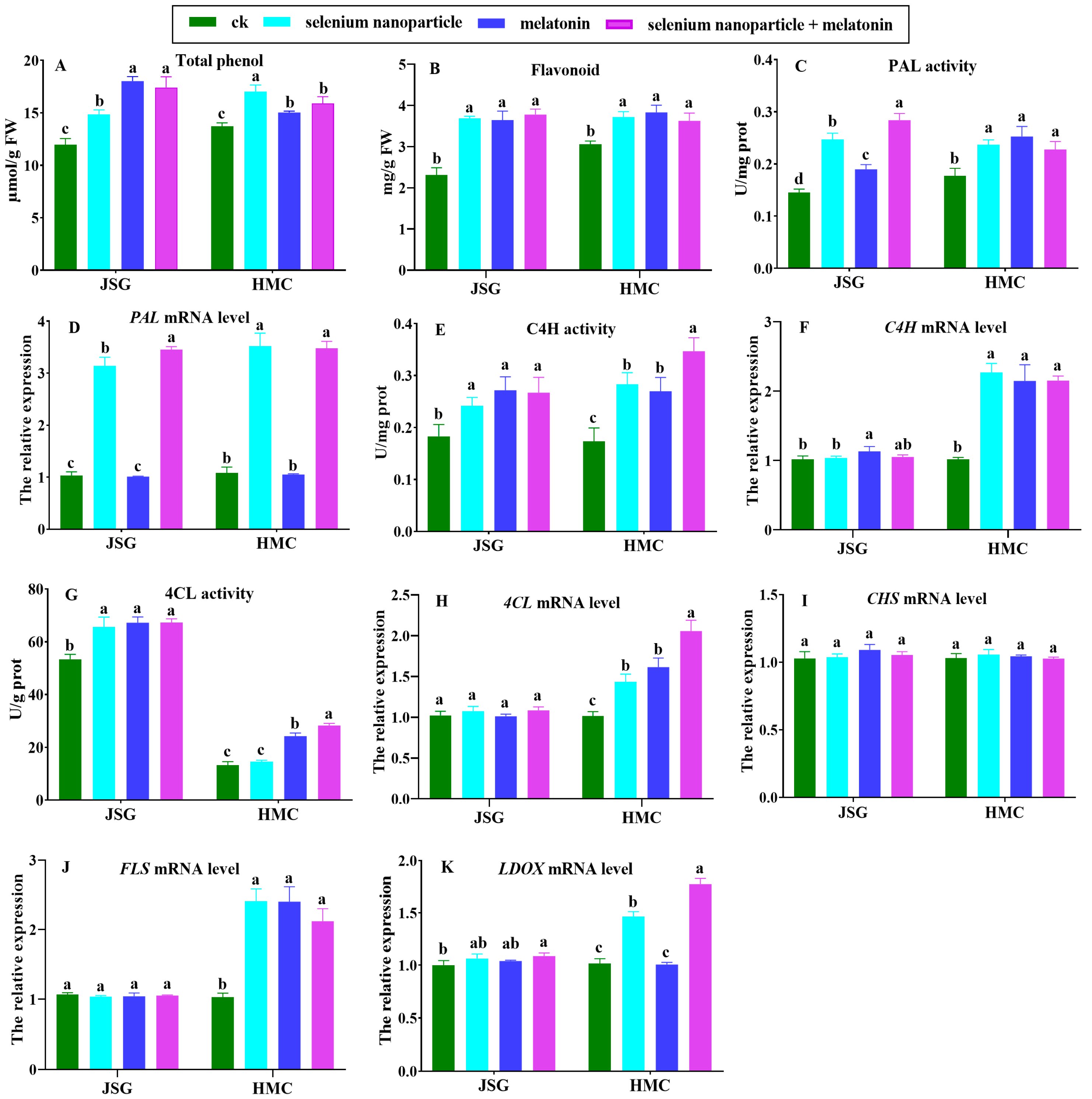
2.4.3. Effects of Selenium Nanoparticles and Melatonin on Phenylpropane Metabolism
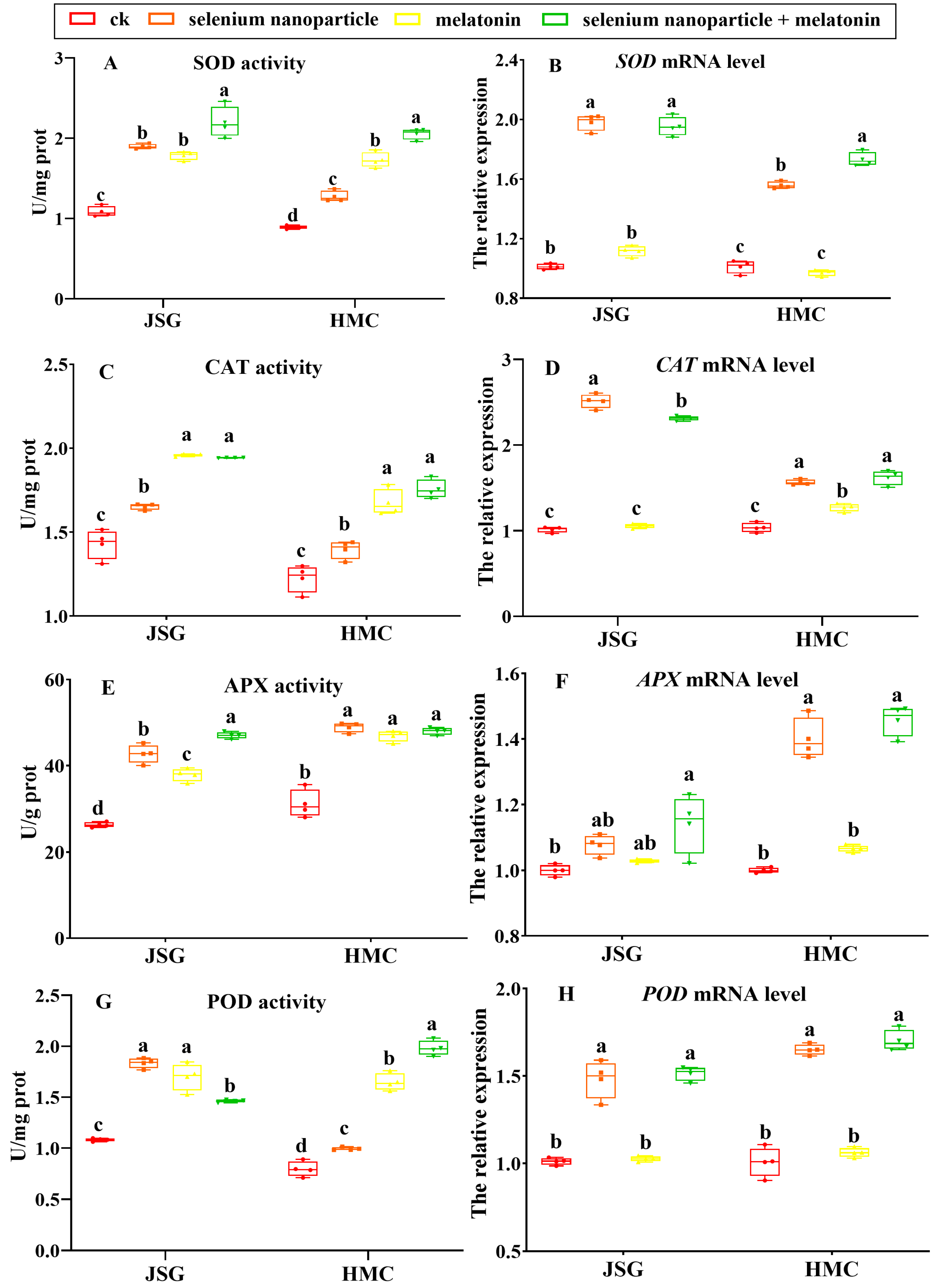
2.5. Effects of Selenium Nanoparticle and Melatonin Treatment on Antioxidant Capacities
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Determination of Lipid Peroxidation Products
4.3. Determinations of Plant Primary and Secondary Metabolites
4.4. Ultra Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC–MS/MS) Analyses of Polyamines, γ-Aminobutyric Acid (GABA), and Plant Hormones
4.5. RT-qPCR Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Martín, M.; Pérez-de-Castro, A.; Picó, B.; Gómez-Guillamón, M.L. Advanced Genetic Studies on Powdery Mildew Resistance in TGR-1551. Int. J. Mol. Sci. 2022, 23, 2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Li, M.; Yang, Y.; Li, Z.; Xu, Y.; Wang, H.; Wang, D.; Zhang, Y.; Wang, H.; et al. Molecular mapping for fruit-related traits, and joint identification of candidate genes and selective sweeps for seed size in melon. Genomics 2022, 114, 110306. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Leskovar, D.I.; Crosby, K.M.; Volder, A.; Ibrahim, A.M.H. Root growth, yield, and fruit quality responses of reticulatus and inodorus melons (Cucumis melo L.) to deficit subsurface drip irrigation. Agric. Water Manag. 2014, 136, 75–85. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Malik, S.; de Costa, F.; Yousefzadi, M.; Mirjalili, M.H.; Arroo, R.; Bhambra, A.S.; Strnad, M.; Bonfill, M.; Fett-Neto, A.G. Specialized Plant Metabolism Characteristics and Impact on Target Molecule Biotechnological Production. Mol. Biotechnol. 2018, 60, 169–183. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Sadak, M.S.; Thabet, M.S. Induction of Tolerance in Groundnut Plants Against Drought Stress and Cercospora Leaf Spot Disease with Exogenous Application of Arginine and Sodium Nitroprusside Under Field Conditions. J. Soil. Sci. Plant Nutruition 2023, 23, 6612–6631. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, T.; Liu, H.; Zhou, F.; Zhang, J.; Zhang, M.; Liu, X.; Shi, W.; Cao, T. Nano-selenium controlled cadmium accumulation and improved photosynthesis in indica rice cultivated in lead and cadmium combined paddy soils. J. Environ. Sci. 2021, 103, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.-S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; dos Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Kaczyński, P.; Pietkun, M.; Łozowicka, B. Evaluation of titanium and silicon role in mitigation of fungicides toxicity in wheat expressed at the level of biochemical and antioxidant profile. Chemosphere 2022, 308, 136284. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Omar, A.A.; Heikal, Y.M.; Zayed, E.M.; Shamseldin, S.A.M.; Salama, Y.E.; Amer, K.E.; Basuoni, M.M.; Abd Ellatif, S.; Mohamed, A.H. Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials 2023, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Wang, Z.; Chen, Z.; Wu, Y.; Nie, W.; Zhao, S.; Yin, X.; Teng, X. Physiological characteristics, rhizosphere soil properties, and root-related microbial communities of Trifolium repens L. in response to Pb toxicity. Sci. Total Environ. 2024, 907, 167871. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Ge, C.; Wu, H.; Jing, F.; Wu, S.; Li, H.; Zhou, D. Foliar Selenium Nanoparticles Application Promotes the Growth of Maize (Zea mays L.) Seedlings by Regulating Carbon, Nitrogen and Oxidative Stress Metabolism. Sci. Hortic. 2023, 311, 111816. [Google Scholar] [CrossRef]
- Salama, D.M.; Abd El-Aziz, M.E.; Rizk, F.A.; Abd Elwahed, M.S.A. Applications of nanotechnology on vegetable crops. Chemosphere 2021, 266, 129026. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.; Bakhoum, G.; Tawfic, M. Chitosan and chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinus termis L plant under drought conditions. Egypt. J. Chem. 2022, 65, 537–549. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakry, B.A.; El-Karamany, M.F. Agrophysiological variation in flax affected by folic acid and Ag nanoparticles foliar applications. Asian J. Plant Sci. 2022, 21, 339–346. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Kalaji, H.M.; Zhang, Z.; Ma, X. Nanoparticles in environment and plant system: A boon or bane. Chemosphere 2022, 308, 136320. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiouny, H.M.S.; Abdallah, M.M.-S.; El-Enany, M.A.M.; Sadak, M.S. Nano-Zinc Oxide and Arbuscular mycorrhiza Effects on Physiological and Biochemical Aspects of Wheat Cultivars under Saline Conditions. Pak. J. Biol. Sci. PJBS 2020, 23, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M. Mitigation of salinity adverse effects of on wheat by grain priming with melatonin. Int. J. ChemTech Res. 2016, 9, 85–97. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S. Nitric oxide and hydrogen peroxide as signaling molecules for better growth and yield of wheat plant exposed to water deficiency. Egypt. J. Chem. 2022, 65, 209–223. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Wu, C.; Hao, W.; Yan, L.; Zhang, H.; Zhang, J.; Liu, C.; Zheng, L. Postharvest melatonin treatment enhanced antioxidant activity and promoted GABA biosynthesis in yellow-flesh peach. Food Chem. 2023, 419, 136088. [Google Scholar] [CrossRef]
- Yang, K.; Sun, H.; Liu, M.; Zhu, L.; Zhang, K.; Zhang, Y.; Li, A.; Zhang, H.; Zhu, J.; Liu, X.; et al. Morphological and Physiological Mechanisms of Melatonin on Delaying Drought-Induced Leaf Senescence in Cotton. Int. J. Mol. Sci. 2023, 24, 7269. [Google Scholar] [CrossRef]
- Promyou, S.; Raruang, Y.; Chen, Z.Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea. Foods 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, J.; Tang, Z.; Yu, J.; Wu, Y.; Liu, Z.; Wang, J.; Wang, G.; Tian, Q. Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods 2022, 11, 4097. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wu, Y.; Jia, Y.; Chen, Z.; Kang, D.; Zhang, L.; Pan, C. Nano-selenium enhances melon resistance to Podosphaera xanthii by enhancing the antioxidant capacity and promoting alterations in the polyamine, phenylpropanoid and hormone signaling pathways. J. Nanobiotechnol. 2023, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Luo, L.; Miao, P.; Dong, Q.; Cheng, H.; Wang, Y.; Li, D.; Pan, C. A novel perspective to investigate how nanoselenium and melatonin lengthen the cut carnation vase shelf. Plant Physiol. Biochem. 2023, 196, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Melatonin is required for H2 O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, 12379. [Google Scholar] [CrossRef]
- He, H.; He, L.F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant 2020, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef]
- Wang, J.; Cappa, J.J.; Harris, J.P.; Edger, P.P.; Zhou, W.; Pires, J.C.; Adair, M.; Unruh, S.A.; Simmons, M.P.; Schiavon, M.; et al. Transcriptome-wide comparison of selenium hyperaccumulator and nonaccumulator Stanleya species provides new insight into key processes mediating the hyperaccumulation syndrome. Plant Biotechnol. J. 2018. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, W.; Guan, C.; Guan, M.; He, X. Evolution and functional diversity of lipoxygenase (LOX) genes in allotetraploid rapeseed (Brassica napus L.). Int. J. Biol. Macromol. 2021, 188, 844–854. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Wu, T.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of Melatonin Treatment of Postharvest Pear Fruit on Aromatic Volatile Biosynthesis. Molecules 2019, 24, 4233. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Huang, Q.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Hina, F.; Zhou, W. Protective mechanisms of melatonin against selenium toxicity in Brassica napus: Insights into physiological traits, thiol biosynthesis and antioxidant machinery. BMC Plant Biol. 2019, 19, 507. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Lin, L.; Yang, L.; Liao, M.; Wang, X.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H.; et al. Exogenous melatonin promotes growth and sucrose metabolism of grape seedlings. PLoS ONE 2020, 15, e0232033. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Yang, N.; Wan, Q.W.; Teng, R.M.; Duan, A.Q.; Wang, Y.H.; Zhuang, J. Exogenous melatonin positively regulates lignin biosynthesis in Camellia sinensis. Int. J. Biol. Macromol. 2021, 179, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic Identification and Analysis of Extensins in the Plant Kingdom. PLoS ONE 2016, 11, e0150177. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.W.; Khan, A.L.; Lee, I.J. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Wang, G.; Wu, L.; Zhang, H.; Wu, W.; Zhang, M.; Li, X.; Wu, H. Regulation of the Phenylpropanoid Pathway: A Mechanism of Selenium Tolerance in Peanut (Arachis hypogaea L.) Seedlings. J. Agric. Food. Chem. 2016, 64, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Wu, W.; Ba, L.; Ma, C.; Ji, N.; Cao, S. Melatonin Enhances the Postharvest Disease Resistance of Blueberries Fruit by Modulating the Jasmonic Acid Signaling Pathway and Phenylpropanoid Metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Ma, J.; Wu, Z.; Yuan, L.; Lin, Z.Q.; Zhu, R.; Banuelos, G.S.; Reiter, R.J.; Li, M.; Yin, X. Synergistic Effect of Melatonin and Selenium Improves Resistance to Postharvest Gray Mold Disease of Tomato Fruit. Front. Plant Sci. 2022, 13, 903936. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292 Pt B, 118473. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Hu, T.; Wang, Z.; Wu, W.; Liang, Y.; Guo, Y. Sunflower resistance against Sclerotinia sclerotiorum is potentiated by selenium through regulation of redox homeostasis and hormones signaling pathways. Environ. Sci. Pollut. Res. Int. 2022, 29, 38097–38109. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, L.; Jia, Y.; Wu, Y.; Liu, H.; Zhao, D.; Ju, Y.; Pan, C.; Mao, J. Selenium Nanoparticle and Melatonin Treatments Improve Melon Seedling Growth by Regulating Carbohydrate and Polyamine. Int. J. Mol. Sci. 2024, 25, 7830. https://doi.org/10.3390/ijms25147830
Kang L, Jia Y, Wu Y, Liu H, Zhao D, Ju Y, Pan C, Mao J. Selenium Nanoparticle and Melatonin Treatments Improve Melon Seedling Growth by Regulating Carbohydrate and Polyamine. International Journal of Molecular Sciences. 2024; 25(14):7830. https://doi.org/10.3390/ijms25147830
Chicago/Turabian StyleKang, Lu, Yujiao Jia, Yangliu Wu, Hejiang Liu, Duoyong Zhao, Yanjun Ju, Canping Pan, and Jiefei Mao. 2024. "Selenium Nanoparticle and Melatonin Treatments Improve Melon Seedling Growth by Regulating Carbohydrate and Polyamine" International Journal of Molecular Sciences 25, no. 14: 7830. https://doi.org/10.3390/ijms25147830






