Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum
Abstract
1. Introduction
2. Agronomic Traits of Sorghum under Drought Stress
QTL Mapping of Drought Tolerance in Sorghum Pre- and Post-Flowering under Drought Stress Conditions
3. Mechanism of Physiological Response in Drought-Resistant Sorghum
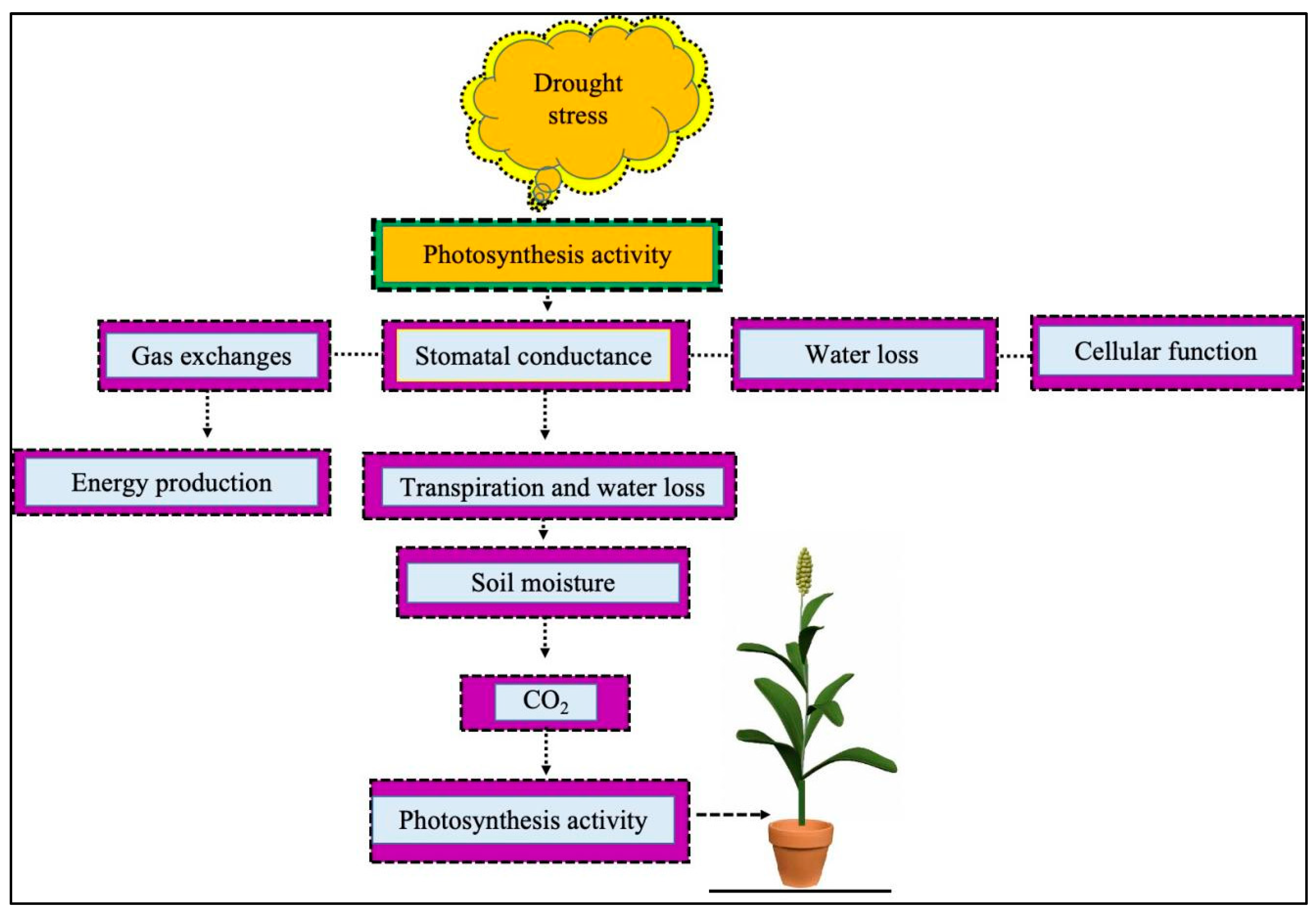
3.1. Response of Photosynthetic System to Drought Stress
3.2. Response of Reactive Oxygen Metabolism System to Drought Stress
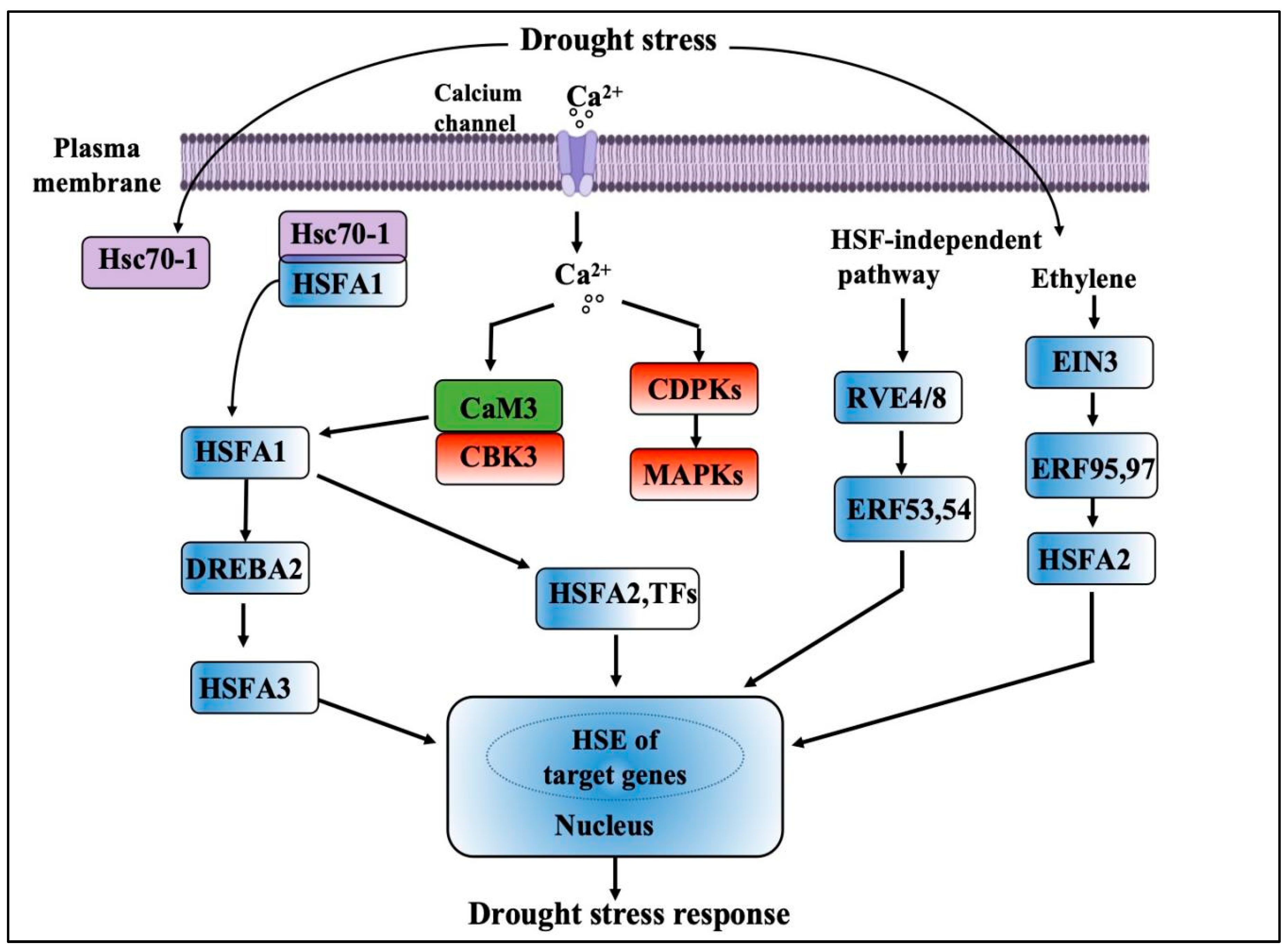
3.2.1. Participation in Signal Transduction
3.2.2. Involvement in ROS Metabolism
4. Transcription Factors Involved in the Drought Stress Response
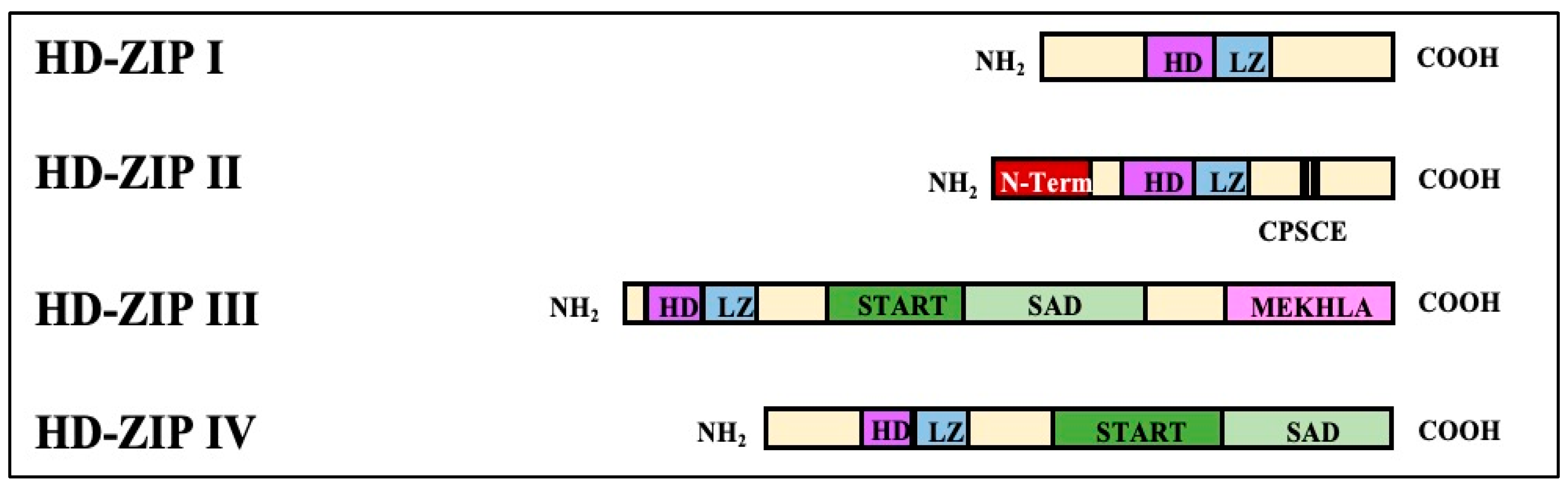
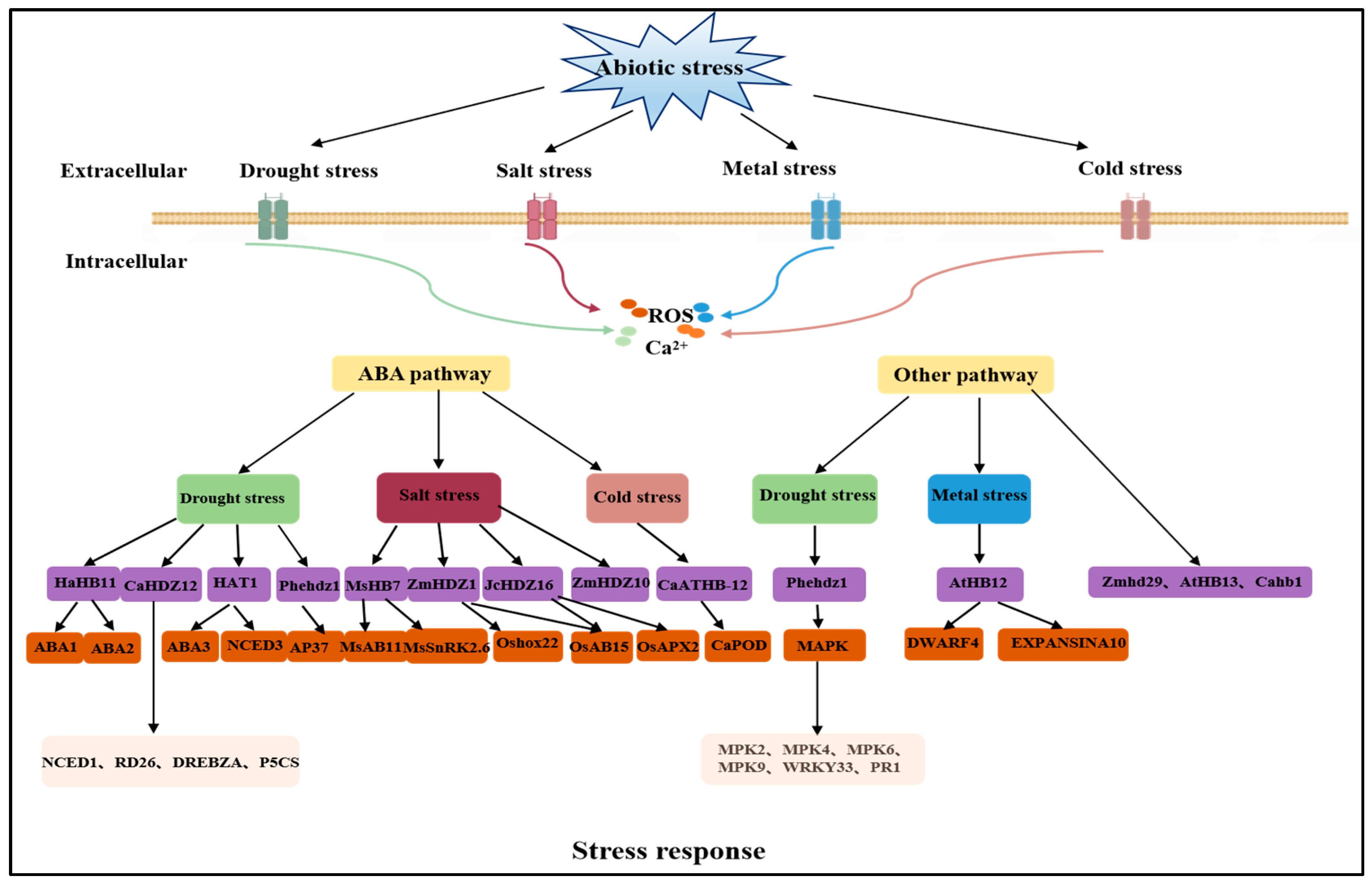
4.1. Response of HD-ZIP Transcription Factors to Drought Stress
4.2. Roles of the MYB Transcription Factors in Sorghum under Drought Stress
4.3. NAC Improves Reactive Oxygen Species’ Scavenging Capacities
5. Genes and Mechanisms Related to Drought Resistance in Sorghum
6. Conclusions
7. Future Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhu, J.H.; Gong, Z.Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Abreha, K.B.; Enyew, M.; Carlsson, A.S.; Vetukuri, R.R.; Feyissa, T.; Motlhaodi, T.; Ng’uni, D.; Geleta, M. Sorghum in dryland: Morphological, physiological, and molecular responses of sorghum under drought stress. Planta 2022, 255, 20. [Google Scholar] [CrossRef]
- Sarshad, A.; Talei, D.; Torabi, M.; Rafei, F.; Nejatkhah, P. Morphological and biochemical responses of Sorghum bicolor (L.) Moench under drought stress. SN Appl. Sci. 2021, 3, 81. [Google Scholar] [CrossRef]
- Hossain, M.S.; Islam, M.N.; Rahman, M.M.; Mostofa, M.G.; Khan, M.A.R. Sorghum: A prospective crop for climatic vulnerability, food and nutritional security. J. Agric. Food Res. 2022, 8, 100300. [Google Scholar] [CrossRef]
- Carr, T.W.; Mkuhlani, S.; Segnon, A.C.; Ali, Z.; Zougmoré, R.; Dangour, A.D.; Green, R.; Scheelbeek, P. Climate change impacts and adaptation strategies for crops in West Africa: A systematic review. Environ. Res. Lett. 2022, 17, 053001. [Google Scholar] [CrossRef]
- Ahmad, R.; Alsahli, A.A.; Alansi, S.; Altaf, M.A. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic efficiency and antioxidant defense system of pea (Pisum sativum L.). Sci. Hortic. 2023, 322, 112431. [Google Scholar] [CrossRef]
- Badigannavar, A.; Teme, N.; de Oliveira, A.C.; Li, G.Y.; Vaksmann, M.; Viana, V.E.; Ganapathi, T.R.; Sarsu, F. Physiological, genetic and molecular basis of drought resilience in sorghum [Sorghum bicolor (L.) Moench]. Indian J. Plant Physiol. 2018, 23, 670–688. [Google Scholar] [CrossRef]
- Ndlovu, E.; van Staden, J.; Maphosa, M. Morpho-physiological effects of moisture, heat and combined stresses on Sorghum bicolor [Moench (L.)] and its acclimation mechanisms. Plant Stress 2021, 2, 100018. [Google Scholar] [CrossRef]
- Rad, R.D.; Sharifabad, H.H.; Torabi, M.; Azizinejad, R.; Salemi, H.; Soltanabadi, M.H. Drought stress tolerance based on selection indices of resistant crops variety. Glob. J. Environ. Sci. Manag. 2023, 9, 287–298. [Google Scholar] [CrossRef]
- Yahaya, M.A.; Shimelis, H. Drought stress in sorghum: Mitigation strategies, breeding methods and technologies—A review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Emendack, Y.; Herzog, H.; Götz, K.P.; Malinowski, D.P. Mid-Season water stress on yield and water use of millet (Panicum miliaceum) and sorghum (Sorghum bicolour L. Moench). Aust. J. Crop Sci. 2012, 5, 1486–1492. [Google Scholar]
- Bai, X.Q.; Yu, P.P.; Li, Y.L.; Gao, J.M.; Pei, Z.Y.; Luo, F.; Sun, S.J. Genetic Analysis of Agronomic Characters in F2 Population of Sorghum bicolor. Acta Agric. 2019, 34, 107–114. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.B.; Huang, J.; Wu, Y.; Zhang, Y.Q.; Zhang, Z.L.; Zhang, X.C. Genetic Diversity of Sorghum Germplasms Based on Phenotypic Traits. J. Plant Genet. Resour. 2021, 22, 654–664. (In Chinese) [Google Scholar] [CrossRef]
- Meyer, W.S. Resistance to water flow in the Sorghum plant. Plant Physiol. 1980, 65, 33–39. [Google Scholar] [CrossRef]
- Habyarimana, E.; Laureti, D.; de Ninno, A.; Lorenzoni, C. Performance of biomass sorghum (Sorghum bicolor L. Moench) under different water regimes in Mediterranean region. Ind. Crops Prod. 2003, 20, 23–28. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Stone, L.R.; Goodrum, D.E.; Schlegel, A.J.; Jaafar, M.N.; Khan, A.H. Water depletion depth of grain sorghum and sunflower in the central High Plains. Agron. J. 2002, 94, 936–943. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S.N. Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef]
- Demissie, H.S.; Mindaye, T.T.; Teklu, D.N.; Kebede, F.G. Root system architecture analysis of sorghum genotypes and its effect on drought adaptation. Rhizosphere 2023, 27, 100772. [Google Scholar] [CrossRef]
- Borrell, A.K.; Mullet, J.E.; George-Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar] [CrossRef]
- Mwamahonje, A.; Eleblu, J.S.Y.; Ofori, K.; Feyissa, T.; Deshpande, S.; Garcia-Oliveira, A.L.; Bohar, R.; Kigoni, M.; Tongoona, P. Introgression of QTLs for Drought Tolerance into Farmers’ Preferred Sorghum Varieties. Agriculture 2021, 11, 883. [Google Scholar] [CrossRef]
- Somegowda, V.K.; Prasad, K.V.S.V.; Naravula, J.; Vemula, A.; Selvanayagam, S.; Rathore, A.; Jones, C.S.; Gupta, R.; Deshpande, S.P. Genetic Dissection and Quantitative Trait Loci Mapping of Agronomic and Fodder Quality Traits in Sorghum Under Different Water Regimes. Front. Plant Sci. 2022, 13, 810632. [Google Scholar] [CrossRef]
- Pereira, M.G.; Lee, M. Identification of genomic regions affecting plant height in sorghum and maize. Theor. Appl. Genet. 1995, 90, 380–388. [Google Scholar] [CrossRef]
- Tuinstra, M.R.; Ejeta, G.; Goldsbrough, P. Evaluation of near isogenic sorghum lines contrasting for QTL markers associated with drought tolerance. Crop Sci. 1998, 38, 835–842. [Google Scholar] [CrossRef]
- Haussmann, B.I.G.; Mahalakshmi, V.; Reddy, B.V.S.; Seetharama, N.; Hash, C.T.; Geiger, H.H. QTL mapping of stay-green in two sorghum recombinant inbred populations. Theor. Appl. Genet. 2002, 106, 133–142. [Google Scholar] [CrossRef]
- Rosenow, D.T.; Clark, L.E.; Peterson, G.C.; Odvody, G.N.; Rooney, W.L. Registration of Tx3440 through Tx3482 sorghum germplasm. J. Plant Regist. 2021, 15, 379–387. [Google Scholar] [CrossRef]
- Tuinstra, M.R.; Grote, E.M.; Goldsbrough, P.B.; Ejeta, G. Identification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum. Crop Sci. 1996, 36, 1337–1344. [Google Scholar] [CrossRef]
- Kebede, H.; Subudhi, P.K.; Rosenow, D.T.; Nguyen, H.T. Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 2001, 103, 266–276. [Google Scholar] [CrossRef]
- Hart, G.E.; Schertz, K.F.; Peng, Y.; Syed, N.H. Genetic mapping of Sorghum bicolor (L.) Moench QTLs that control variation in tillering and other morphological characters. Theor. Appl. Genet. 2001, 103, 1232–1242. [Google Scholar] [CrossRef]
- Sabadin, P.K.; Malosetti, M.; Boer, M.P.; Tardin, F.D.; Santos, F.G.; Guimaraes, C.L.T.; Albuquerque, P.E.P.; Caniato, F.F.; Mollinari, M.; Margarido, G.R.A.; et al. Studying the genetic basis of drought tolerance in sorghum by managed stress trials and adjustments for phenological and plant height differences. Theor. Appl. Genet. 2012, 124, 1389–1402. [Google Scholar] [CrossRef]
- Sakhi, S.; Shehzad, T.; Rehman, S.; Okuno, K. Mapping the QTLs underlying drought stress at developmental stage of sorghum [Sorghum bicolor (L.) Moench] by association analysis. Euphytica 2013, 193, 433–450. [Google Scholar] [CrossRef]
- Rami, J.F.; Dufour, P.; Trouche, G.; Fliedel, G.; Mestres, C.; Davrieux, F.; Blanchard, P.; Hamon, P. Quantitative trait loci for grain quality, productivity, morphological and agronomical traits in sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 1998, 97, 605–616. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.Q.; Dong, G.C.; Zhu, G.L.; Zhou, G.S. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef]
- Gruss, S.M.; Souza, A.; Yang, Y.; Dahlberg, J.; Tuinstra, M.R. Expression of stay-green drought tolerance in dhurrin-free sorghum. Crop Sci. 2023, 63, 1270–1283. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Borrell, A.K.; Christopher, J.T.; Kelly, A.; Collins, B.; Chenu, K. Balancing pre- and post-anthesis growth to maximise water-limited yield in cereals. Field Crops Res. 2023, 296, 108919. [Google Scholar] [CrossRef]
- Khanthavong, P.; Yabuta, S.; Malik, A.; Hossain, M.A.; Akagi, I.; Sakagami, J.I. Combinational variation temperature and soil water response of stomata and biomass production in maize, millet, sorghum and rice. Plants 2022, 11, 1039. [Google Scholar] [CrossRef]
- Ortiz, D.; Hu, J.Y.; Fernandez, M.G.S. Genetic architecture of photosynthesis in Sorghum bicolor under non-stress and cold stress conditions. J. Exp. Bot. 2017, 68, 4545–4557. [Google Scholar] [CrossRef]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Lee, S.; Park, C. Regulation of reactive oxygen species generation under drought conditions in arabidopsis. Plant Signal. Behav. 2012, 7, 599–601. [Google Scholar] [CrossRef]
- Ye, C.J.; Zheng, S.Y.; Jiang, D.G.; Lu, J.Q.; Huang, Z.N.; Liu, Z.L.; Zhou, H.; Zhuang, C.X.; Li, J. Initiation and Execution of Programmed Cell Death and Regulation of Reactive Oxygen Species in Plants. Int. J. Mol. Sci. 2021, 22, 12942. [Google Scholar] [CrossRef]
- Cui, S.Y.; Zhang, Z.F.; Fu, X.F.; Liu, J.Q.; Yang, H.S. Key Genes in Response to Drought Stress in Plant Hormone Signal Transduction Pathway of Oat. J. Triticeae Crops 2023, 43, 1384–1393. (In Chinese) [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant. Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Rajarajan, K.; Ganesamurthy, K.; Raveendran, M.; Jeyakumar, P.; Yuvaraja, A.; Sampath, P.; Prathima, P.T.; Senthilraja, C. Differential responses of sorghum genotypes to drought stress revealed by physio-chemical and transcriptional analysis. Mol. Biol. Rep. 2021, 48, 2453–2462. [Google Scholar] [CrossRef]
- Devnarain, N.; Crampton, B.G.; Olivier, N.; van der Westhuyzen, C.; Becher, J.V.M.; O’Kennedy, M.M. Transcriptomic analysis of a Sorghum bicolor landrace identifies a role for beta-alanine betaine biosynthesis in drought tolerance. S. Afr. J. Bot. 2019, 127, 244–255. [Google Scholar] [CrossRef]
- op den Camp, R.G.L.; Przybyla, D.; Ochsenbein, C.; Laloi, C.; Kim, C.H.; Danon, A.; Wagner, D.; Hideg, É.; Göbel, C.; Feussner, I.; et al. Rapid induction of distinct stress responses after the release of singlet oxygen in arabidopsis. Plant Cell 2003, 15, 2320–2332. [Google Scholar] [CrossRef]
- Dietz, K.J. Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Sign. 2011, 15, 1129–1159. [Google Scholar] [CrossRef]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012, 182, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Hu, C.X.; Tan, Q.L.; Li, L.; Shi, K.L.; Zheng, Y.; Sun, X.C. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium Hirsutum). Acta Physiol. Plant. 2015, 37, 167. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wei, Y.Q.; Zhao, Y.R.; Wang, Y.J.; Zou, F.; Huang, S.Q.; Yang, X.L.; Xu, Z.W.; Hu, H. Physiological and transcriptional evaluation of sweet sorghum seedlings in response to single and combined drought and salinity stress. S. Afr. J. Bot. 2022, 146, 459–471. [Google Scholar] [CrossRef]
- Li, H.B.; Li, Y.L.; Ke, Q.B.; Kwak, S.S.; Zhang, S.Q.; Deng, X.P. Physiological and Differential Proteomic Analyses of Imitation Drought Stress Response in Sorghum bicolor Root at the Seedling Stage. Int. J. Mol. Sci. 2021, 21, 9174. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and Physiological Analysis of Drought Stress in Arabidopsis Reveals Early Responses Leading to Acclimation in Plant Growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.C.; Jin, X.Y.; Wang, J.Y.; Chen, W.; Yang, Z.; Chen, Y.X.; Yang, Y.H.; Lu, G.H.; Sun, B. SbNAC9 Improves Drought Tolerance by Enhancing Scavenging Ability of Reactive Oxygen Species and Activating Stress-Responsive Genes of Sorghum. Int. J. Mol. Sci. 2023, 24, 2401. [Google Scholar] [CrossRef]
- Ngara, R.; Ramulifho, E.; Movahedi, M.; Shargie, N.G.; Brown, A.P.; Chivasa, S. Identifying differentially expressed proteins in sorghum cell cultures exposed to osmotic stress. Sci. Rep. 2018, 8, 8671. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Johannesson, H.; Wang, Y.; Engström, P. DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant Mol. Biol. 2001, 45, 63–73. [Google Scholar] [CrossRef]
- Roodbarkelari, F.; Groot, E.P. Regulatory function of homeodomain-leucine zipper (HD-ZIP) family proteins during embryogenesis. New Phytol. 2017, 213, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Henriksson, E.; Soderman, E.; Henriksson, K.N.; Sundberg, E.; Engstrom, P. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev. Biol. 2003, 264, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.F.; Hong, Y.G.; Yin, M.G.; Li, C.Y.; Zhang, K.; Grierson, D. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008, 55, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Liu, W.; Li, Q.; Li, J.; Wang, L.N.; Ren, Z.H. Comprehensive analysis of the homeodomain-leucine zipper IV transcription factor family in Cucumis sativus. Genome 2013, 56, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Nishiyama, T.; Kato, M.; Hasebe, M. Isolation of homeodomain-leucine zipper genes from the moss Physcomitrella patens and the evolution of homeodomain-leucine zipper genes in land plants. Mol. Biol. Evol. 2001, 18, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, Y.F.; Li, Y.; Hu, C.; Li, J.Y.; Lyu, A. Genome-wide identification and bioinformatics analysis of the WD40 transcription factor family and candidate gene screening for anthocyanin biosynthesis in Rhododendron simsii. BMC Genom. 2023, 24, 488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F. Transcriptomic profiling of sorghum leaves and roots responsive to drought stress at the seedling stage. J. Integr. Agric. 2021, 20, 1980–1995. [Google Scholar] [CrossRef]
- Yan, H.P. Study on Drought Resistence Functions of Two HD-Zip Transcription Factors Zmhdz13 and Zmhdz14 in Maize (Zea mays L.); Gansu Agricultural University: Lanzhou, China, 2016. (In Chinese) [Google Scholar]
- Cabello, J.V.; Chan, R.L. The homologous homeodomain-leucine zipper transcription factors HaHB1 and AtHB13 confer tolerance to drought and salinity stresses via the induction of proteins that stabilize membranes. Plant Biotechnol. J. 2012, 10, 815–825. [Google Scholar] [CrossRef]
- Wu, J.D.; Zhou, W.; Gong, X.F.; Cheng, B.J. Expression of ZmHDZ4, a Maize Homeodomain-Leucine Zipper I Gene, Confers Tolerance to Drought Stress in Transgenic Rice. Plant Mol. Biol. Rep. 2016, 34, 845–853. [Google Scholar] [CrossRef]
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngare, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [Google Scholar] [CrossRef]
- Söderman, E.; Hjellström, M.; Fahleson, J.; Engstrom, P. The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol. Biol. 1999, 40, 1073–1083. [Google Scholar] [CrossRef]
- Lechner, E.; Leonhardt, N.; Eisler, H.; Parmentier, Y.; Ailoua, M.; Jacquet, H.; Leung, J.; Genschik, P. MATH/BTB CRL3 receptors target the homeodomain-leucine zipper ATHB6 to modulate abscisic acid signaling. Dev. Cell 2011, 21, 1116–1128. [Google Scholar] [CrossRef]
- Zhang, S.X.; Haider, I.; Kohlen, W.; Jiang, L.; Bouwmeester, H.; Meijer, A.H.; Schluepmann, H.; Liu, C.M.; Ouwerkerk, P.B.F. Function of the HD-Zip I gene OsHOX22 in ABA-mediated drought and salt tolerances in rice. Plant Mol. Biol. 2013, 80, 571–585. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Q.; Jin, X.L.; Peng, X.L.; Liu, J.Y.; Deng, L.; Yan, H.W.; Sheng, L.; Jiang, H.Y.; Cheng, B.J. A Novel Maize Homeodomain-Leucine Zipper (HD-Zip) I Gene, Zmhdz10, Positively Regulates Drought and Salt Tolerance in Both Rice and Arabidopsis. Plant Cell Physiol. 2014, 55, 1142–1156. [Google Scholar] [CrossRef]
- Sessa, G.; Carabelli, M.; Sassi, M.; Ciolfi, A.; Possenti, M.; Mittempergher, F.; Becker, J.; Morelli, G.; Ruberti, I. A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Gene Dev. 2005, 19, 2811–2815. [Google Scholar] [CrossRef]
- Morelli, G.; Ruberti, I. Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci. 2002, 7, 399–404. [Google Scholar] [CrossRef]
- Steindler, C.; Matteucci, A.; Sessa, G.; Weimar, T.; Ohgishi, M.; Aoyama, T.; Morelli, G.; Ruberti, I. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 1999, 126, 4235–4245. [Google Scholar] [CrossRef]
- Rueda, E.C.; Dezar, C.A.; Gonzalez, D.H.; Chan, R.L. Hahb-10, a sunflower homeobox-leucine zipper gene, is regulated by light quality and quantity, and promotes early flowering when expressed in Arabidopsis. Plant Cell Physiol. 2005, 46, 1954–1963. [Google Scholar] [CrossRef]
- Qin, Y.F.; Li, D.D.; Wu, Y.J.; Liu, Z.H.; Zhang, J.; Zheng, Y.; Li, X.B. Three cotton homeobox genes are preferentially expressed during early seedling development and in response to phytohormone signaling. Plant Cell Rep. 2010, 29, 1147–1156. [Google Scholar] [CrossRef]
- Smith, Z.R.; Long, J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 2010, 464, 423–426. [Google Scholar] [CrossRef]
- Emery, J.F.; Floyd, S.K.; Alvarez, J.; Eshed, Y.; Hawker, N.P.; Izhaki, A.; Baum, S.F.; Bowman, J.L. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 2003, 13, 1768–1774. [Google Scholar] [CrossRef]
- Byrne, M.E. Shoot meristem function and leaf polarity: The role of class III HD-ZIP genes. PLoS Gene 2006, 2, 785–790. [Google Scholar] [CrossRef]
- Singh, A.; Roy, S.; Singh, S.; Das, S.S.; Gautam, V.; Yadav, S.; Kumar, A.; Singh, A.; Sarkar, A.K. Phytohormonal crosstalk modulates the expression of miR166/165s, target Class III HD-ZIPs, and KANADI genes during root growth in Arabidopsis thaliana. Sci. Rep. 2017, 7, 3408. [Google Scholar] [CrossRef]
- Williams, L.; Grigg, S.P.; Xie, M.T.; Christensen, S.; Fletcher, J.C. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 2005, 132, 3657–3668. [Google Scholar] [CrossRef]
- Guan, X.Y.; Li, Q.J.; Shan, C.M.; Wang, S.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2. Physiol. Plant. 2008, 134, 174–182. [Google Scholar] [CrossRef]
- Ito, M.; Sentoku, N.; Nishimura, A.; Hong, S.K.; Sato, Y.; Matsuoka, M. Position dependent expression of GL2-type homeobox gene, Roc1: Significance for protoderm differentiation and radial pattern formation in early rice embryogenesis. Plant J. 2002, 29, 497–507. [Google Scholar] [CrossRef]
- Yu, L.H.; Chen, X.; Wang, Z.; Wang, S.M.; Wang, Y.P.; Zhu, Q.S.; Li, S.G.; Xiang, C.B. Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 2013, 162, 1378–1391. [Google Scholar] [CrossRef]
- Cao, Y.J.; Wei, Q.; Liao, Y.; Song, H.L.; Li, X.; Xiang, C.B.; Kuai, B.K. Ectopic overexpression of AtHDG11 in tall fescue resulted in enhanced tolerance to drought and salt stress. Plant Cell Rep. 2009, 28, 579–588. [Google Scholar] [CrossRef]
- Ling, L.; Song, L.L.; Wang, Y.J.; Guo, C.H. Genome-wide analysis and expression patterns of the NAC transcription factor family in Medicago truncatula. Physiol. Mol. Biol. Plants 2017, 23, 343–356. [Google Scholar] [CrossRef]
- Vernoud, V.; Laigle, G.; Rozier, F.; Meeley, R.B.; Perez, P.; Rogowsky, P.M. The HD-ZIP IV transcription factor OCL4 is necessary for trichome patterning and anther development in maize. Plant J. 2009, 59, 883–894. [Google Scholar] [CrossRef]
- Henriksson, E.; Olsson, A.S.B.; Johannesson, H.; Hanson, J.; Engstrom, P.; Soderman, E. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005, 139, 509–518. [Google Scholar] [CrossRef]
- Agalou, A.; Purwantomo, S.; Overnas, E.; Johannesson, H.; Zhu, X.; Estiati, A.; de Kam, R.J.; Engstroem, P.; Slamet-Loedin, I.H.; Zhu, Z.; et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 2008, 66, 87–103. [Google Scholar] [CrossRef]
- Cote, C.L.; Boileau, F.; Roy, V.; Ouellet, M.; Levasseur, C.; Morency, M.J.; Cooke, J.E.K.; Seguin, A.; MacKay, J.J. Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010, 10, 273. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Fang, Y.J.; Liao, K.F.; Du, H.; Xu, Y.; Song, H.Z.; Li, X.H.; Xiong, L.Z. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Scully, E.D.; Gries, T.; Sarath, G.; Palmer, N.A.; Baird, L.; Serapiglia, M.J.; Dien, B.S.; Boateng, A.A.; Ge, Z.X.; Funnell-Harris, D.L.; et al. Overexpression of SbMyb60 impacts phenylpropanoid biosynthesis and alters secondary cell wall composition in Sorghum bicolor. Plant J. 2016, 85, 378–395. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Li, J.J.; Liu, P.L.; Duan, J.Z.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.L.; Ali, J.; Li, Z.C. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Casaretto, J.A.; El-Kereamy, A.; Zeng, B.; Stiegelmeyer, S.M.; Chen, X.; Bi, Y.M.; Rothstein, S.J. Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genom. 2016, 17, 312. [Google Scholar] [CrossRef]
- Tang, Y.H.; Bao, X.X.; Zhi, Y.L.; Wu, Q.; Guo, Y.R.; Yin, X.H.; Zeng, L.Q.; Li, J.; Zhang, J.; He, W.L.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef]
- Dai, X.Y.; Xu, Y.Y.; Ma, Q.B.; Xu, W.Y.; Wang, T.; Xue, Y.B. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef]
- Xiang, Y.; Sun, X.J.; Bian, X.L.; Wei, T.H.; Han, T.; Yan, J.W.; Zhang, A.Y. The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J. Exp. Bot. 2021, 72, 1399–1410. [Google Scholar] [CrossRef]
- Sanjari, S.; Shirzadian-Khorramabad, R.; Shobbar, Z.S.; Shahbazi, M. Systematic analysis of NAC transcription factors’ gene family and identification of post-flowering drought stress responsive members in sorghum. Plant Cell Rep. 2019, 38, 361–376. [Google Scholar] [CrossRef]
- Li, X.; Chang, Y.; Ma, S.Q.; Shen, J.Q.; Hu, H.H.; Xiong, L.Z. Genome-Wide Identification of SNAC1-Targeted Genes Involved in Drought Response in Rice. Front. Plant Sci. 2019, 10, 982. [Google Scholar] [CrossRef]
- Hu, H.H.; You, J.; Fang, Y.J.; Zhu, X.Y.; Qi, Z.Y.; Qi, Z.Y.; Xiong, L.Z. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008, 67, 169–181, Erratum in Plant Mol. Biol. 2010, 72, 567–568. [Google Scholar] [CrossRef]
- Pooam, M.; El-Ballat, E.M.; Jourdan, N.; Ali, H.M.; Hano, C.; Ahmad, M.; El-Esawi, M.A. SNAC3 Transcription Factor Enhances Arsenic Stress Tolerance and Grain Yield in Rice (Oryza sativa L.) through Regulating Physio-Biochemical Mechanisms, Stress-Responsive Genes, and Cryptochrome 1b. Plants 2023, 12, 2713. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Maruyama, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Role of the ZFHD1 and NAC transcription factors in drought-inducible expression of the early responsive to dehydration stress 1 (ERD1) gene of Arabidopsis. Plant Cell Physiol. 2006, 47, S226. (In Chinese) [Google Scholar]
- Liu, Y.; Yao, X.Z.; Lv, L.T.; Lei, Y.T.; Dai, T.T.; Zhao, D.G. Cloning of SbSKIP Gene from Sorghum (Sorghum bicolor) and Analysis of Drought-resistant Function in Tobacco (Nicotiana tabacum). J. Agric. Biotechnol. 2016, 24, 1500–1511. (In Chinese) [Google Scholar] [CrossRef]
- Wu, R.; Kong, L.X.; Wu, X.; Gao, J.; Niu, T.L.; Li, J.Y.; Li, Z.J.; Dai, L.Y. GsNAC2 gene enhances saline-alkali stress tolerance by promoting plant growth and regulating glutathione metabolism in Sorghum bicolor. Funct. Plant Biol. 2023, 50, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Wang, Y.R.; Tao, Q.B. Advances of seed hormonal priming. Pratacultural Sci. 2017, 33, 2494–2502. (In Chinese) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, X.; Wu, H.; Zhu, Y.; Ahmad, I.; Dong, G.; Zhou, G.; Wu, Y. Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum. Int. J. Mol. Sci. 2024, 25, 6464. https://doi.org/10.3390/ijms25126464
Liu J, Wang X, Wu H, Zhu Y, Ahmad I, Dong G, Zhou G, Wu Y. Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum. International Journal of Molecular Sciences. 2024; 25(12):6464. https://doi.org/10.3390/ijms25126464
Chicago/Turabian StyleLiu, Jiao, Xin Wang, Hao Wu, Yiming Zhu, Irshad Ahmad, Guichun Dong, Guisheng Zhou, and Yanqing Wu. 2024. "Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum" International Journal of Molecular Sciences 25, no. 12: 6464. https://doi.org/10.3390/ijms25126464
APA StyleLiu, J., Wang, X., Wu, H., Zhu, Y., Ahmad, I., Dong, G., Zhou, G., & Wu, Y. (2024). Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum. International Journal of Molecular Sciences, 25(12), 6464. https://doi.org/10.3390/ijms25126464








