Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences
Abstract
:1. Introduction
2. Adipose Tissue and Metabolic Health
2.1. WAT and Metabolic Health
2.2. BAT and Metabolic Health
3. Effects of Climate Change on Adipose Tissue
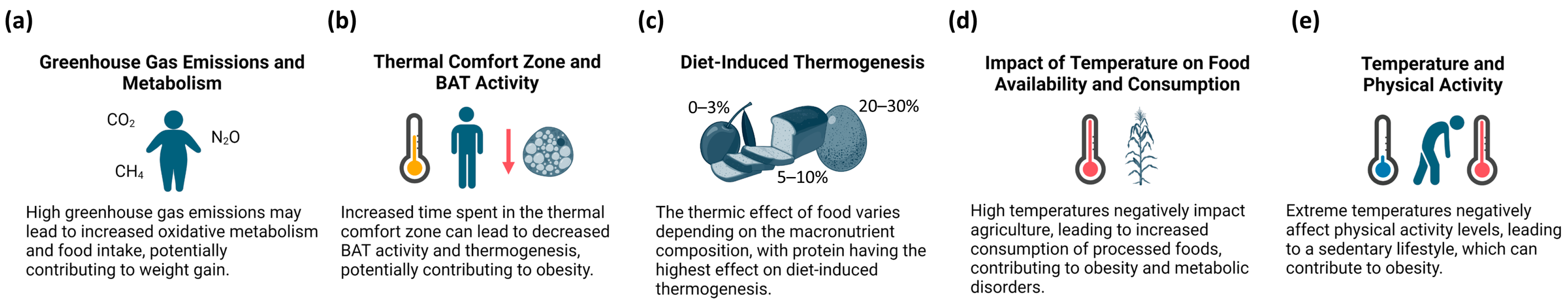
3.1. Climate Change and Obesity
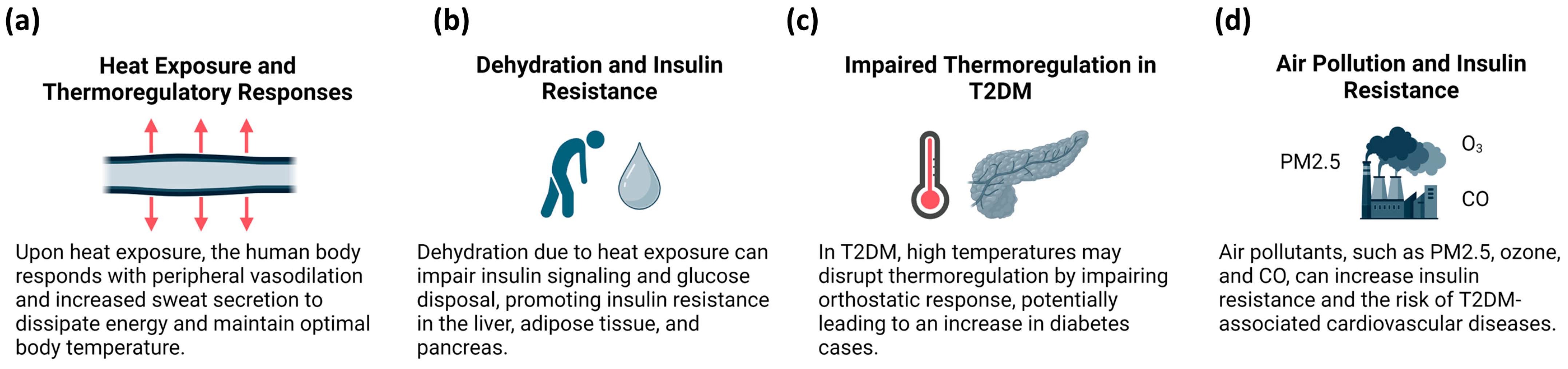
3.2. Climate Change and T2DM
4. Environmental Factors Affecting Adipose Tissue Metabolism
4.1. Air Pollutants and BAT
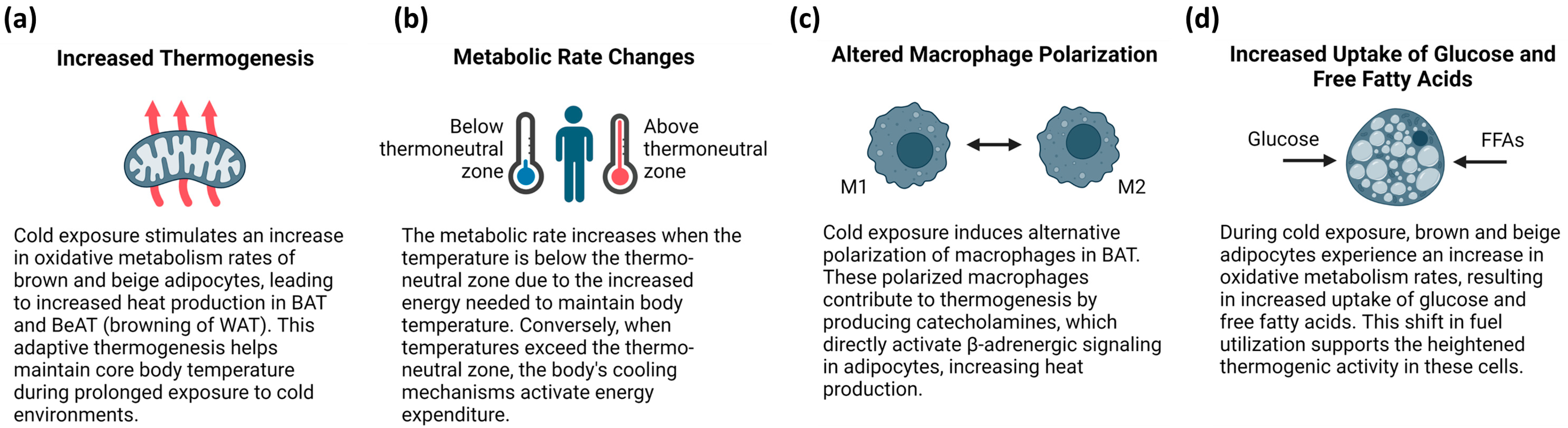
4.2. Temperature-Related Adaptations of BAT Function and Metabolism
4.3. Air Pollutants and WAT Dysfunction
4.4. Association between Climate Change, Air Pollution, and Altered Dietary Patterns
5. Climate Change Adaptation and Mitigation Strategies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AHR | Aryl hydrocarbon receptor |
| ATGL | Adipose triglyceride lipase |
| BAT | Brown adipose tissue |
| BeAT | Beige adipose tissue |
| BMI | Body mass index |
| BPA | Bisphenol A |
| CFC | Chlorofluorocarbon |
| CREB-α | cAMP response element-binding protein alpha |
| CVD | Cardiovascular diseases |
| DDE | Dichlordiphenylethylene |
| DDT | Dichlorodiphenyltrichloroethane |
| DGAT2 | Diacylglycerol O-acyltransferase 2 |
| ER | Endoplasmic reticulum |
| FFA | Free fatty acid |
| FGF21 | Fibroblast growth factor 21 |
| GDF-15 | Growth differentiation factor 15 |
| GHG | Greenhouse gas |
| GLUT1 | Glucose transporter type 1 |
| GLUT4 | Glucose transporter type 4 |
| HFC | Hydrofluorocarbons |
| HDL | High-density lipoprotein |
| HOXC9 | Homeobox protein C9 |
| ICE | Intermittent cold exposure |
| IGFBP3 | Insulin-like growth factor binding protein 3 |
| IL-6 | Interleukin-6 |
| IR | Insulin resistance |
| iWAT | Inguinal WAT |
| LPL | Lipoprotein lipase |
| LYG6 | Lymphocyte antigen 6 complex, locus G |
| MEHP | Mono-2-ethylhexyl phthalate |
| NF-κB | Nuclear factor-kappa B |
| NOx | Nitrogen oxides |
| NST | Non-shivering thermogenesis |
| PDK1 | Phosphoinositide-dependent kinase-1 |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma co-activator-1-alpha |
| PFAS | Polyfluoroalkyl substances |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane sulfonate |
| PI3K | Phosphatidylinositol 3-kinase |
| PKB | Protein kinase B (or Akt) |
| PM | Particulate matter |
| PM2.5 | Particulate matter up to 2.5 μm |
| PPARα | Peroxisome proliferator-activated receptor-alpha |
| PPARγ | Peroxisome proliferator-activated receptor-gamma |
| ROS | Reactive oxygen species |
| sWAT | Subcutaneous WAT |
| T2DM | Type 2 diabetes mellitus |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TG | Triglyceride |
| TN | Thermoneutral |
| TNFα | Tumor necrosis factor alpha |
| UCP1 | Uncoupled protein 1 |
| vWAT | Visceral WAT |
| WAT | White adipose tissue |
References
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Shoemaker, R.; Larian, N.; Cassis, L. Adipose Tissue as a Site of Toxin Accumulation. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2017; pp. 1085–1135. [Google Scholar]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Chevrier, J.; Rosas, L.G.; Anderson, H.A.; Bornman, M.S.; Bouwman, H.; Chen, A.; Cohn, B.A.; de Jager, C.; Henshel, D.S.; et al. The Pine River Statement: Human Health Consequences of DDT Use. Environ. Health Perspect. 2009, 117, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, Q.; Liu, C. Influencing Factors of Thermogenic Adipose Tissue Activity. Front. Physiol. 2016, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose tissue in health and disease. Open Biol. 2020, 10, 200291. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Tang, X.; Luo, Q.; Xu, D.; Yu, B. Interaction between adipocytes and high-density lipoprotein:new insights into the mechanism of obesity-induced dyslipidemia and atherosclerosis. Lipids Health Dis. 2019, 18, 223. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflug. Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Zhao, S.; Li, N.; Zhu, Y.; Straub, L.; Zhang, Z.; Wang, M.-Y.; Zhu, Q.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Partial leptin deficiency confers resistance to diet-induced obesity in mice. Mol. Metab. 2020, 37, 100995. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2018; pp. 1031–1063. [Google Scholar]
- de Lange, P.; Lombardi, A.; Silvestri, E.; Cioffi, F.; Giacco, A.; Iervolino, S.; Petito, G.; Senese, R.; Lanni, A.; Moreno, M. Physiological Approaches Targeting Cellular and Mitochondrial Pathways Underlying Adipose Organ Senescence. Int. J. Mol. Sci. 2023, 24, 11676. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Blaak, E.E. Adipose tissue dysfunction and impaired metabolic health in human obesity: A matter of oxygen? Front. Endocrinol. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef] [PubMed]
- Giroud, M.; Jodeleit, H.; Prentice, K.J.; Bartelt, A. Adipocyte function and the development of cardiometabolic disease. J. Physiol. 2022, 600, 1189–1208. [Google Scholar] [CrossRef] [PubMed]
- White, U.A.; Fitch, M.D.; Beyl, R.A.; Hellerstein, M.K.; Ravussin, E. Association of In Vivo Adipose Tissue Cellular Kinetics With Markers of Metabolic Health in Humans. J. Clin. Endocrinol. Metab. 2017, 102, 2171–2178. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Ezquerro, S.; Méndez-Giménez, L.; Frühbeck, G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Kobayashi, N.; Matsumura, K.; Nishio, M.; Nakano, K.; Okamura, T.; Okochi, H.; Minamisawa, T.; Shiba, K.; Saeki, K. New Role for Growth/Differentiation Factor 15 in the Survival of Transplanted Brown Adipose Tissues in Cooperation with Interleukin-6. Cells 2020, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Haman, F.; Mantha, O.L.; Cheung, S.S.; DuCharme, M.B.; Taber, M.; Blondin, D.P.; McGarr, G.W.; Hartley, G.L.; Hynes, Z.; Basset, F.A. Oxidative fuel selection and shivering thermogenesis during a 12- and 24-h cold-survival simulation. J. Appl. Physiol. 2016, 120, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Maurya, S.K.; Pani, S.; Sethy, C.; Banerjee, A.; Das, S.; Patnaik, S.; Kundu, C.N. Mild cold induced thermogenesis: Are BAT and skeletal muscle synergistic partners? Biosci. Rep. 2017, 37, BSR20171087. [Google Scholar] [CrossRef] [PubMed]
- Suchacki, K.J.; Stimson, R.H. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elía, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Warner, A.; Mittag, J. Breaking BAT: Can browning create a better white? J. Endocrinol. 2016, 228, R19–R29. [Google Scholar] [CrossRef]
- Symonds, M.E.; Farhat, G.; Aldiss, P.; Pope, M.; Budge, H. Brown adipose tissue and glucose homeostasis—The link between climate change and the global rise in obesity and diabetes. Adipocyte 2019, 8, 46–50. [Google Scholar] [CrossRef]
- An, R.; Ji, M.; Zhang, S. Global warming and obesity: A systematic review. Obes. Rev. 2018, 19, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public Health 2020, 17, 7795. [Google Scholar] [CrossRef] [PubMed]
- Annesi-Maesano, I. United Nations Climate Change Conferences: COP21 a lost opportunity for asthma and allergies and preparing for COP22. J. Allergy Clin. Immunol. 2016, 138, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Pérez, M.; Zagmutt, S.; Soler-Vázquez, M.C.; Serra, D.; Mera, P.; Herrero, L. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Periasamy, M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190135. [Google Scholar] [CrossRef] [PubMed]
- van der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.M.A.; Sun, W.; Pires, N.D.; Frontini, A.; Balaz, M.; Jespersen, N.Z.; Feizi, A.; Petrovic, K.; Fischer, A.W.; Bokhari, M.H.; et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat. Metab. 2019, 1, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20160051. [Google Scholar] [CrossRef]
- Pirzgalska, R.M.; Seixas, E.; Seidman, J.S.; Link, V.M.; Sánchez, N.M.; Mahú, I.; Mendes, R.; Gres, V.; Kubasova, N.; Morris, I.; et al. Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 2017, 23, 1309–1318. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 July 2024).
- Ampofo, A.G.; Boateng, E.B. Beyond 2020: Modelling obesity and diabetes prevalence. Diabetes Res. Clin. Pract. 2020, 167, 108362. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Dong, H.; Xiao, Y.; Zhao, Z. The health consequences of greenhouse gas emissions: A potential pathway. Environ. Geochem. Health 2022, 44, 2955–2974. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S. Does global warming contribute to the obesity epidemic? Environ. Res. 2020, 182, 108962. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. The Environmental Foodprint of Obesity. Obesity 2020, 28, 73–79. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.M.; Gonçalves, C.F.L.; Miranda-Alves, L.; Fortunato, R.S.; Carvalho, D.P.; Ferreira, A.C.F. Inhibition of Type 1 Iodothyronine Deiodinase by Bisphenol A. Horm. Metab. Res. 2019, 51, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.E.H.; Boon, M.R.; van der Linden, R.A.D.; Arias-Bouda, L.P.; van Klinken, J.B.; Smit, F.; Verberne, H.J.; Jukema, J.W.; Tamsma, J.T.; Havekes, L.M.; et al. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: A prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2014, 2, 210–217. [Google Scholar] [CrossRef]
- Lidell, M.E.; Betz, M.J.; Enerbäck, S. Brown adipose tissue and its therapeutic potential. J. Intern. Med. 2014, 276, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Johnson, F.; Mavrogianni, A.; Ucci, M.; Vidal-Puig, A.; Wardle, J. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes. Rev. 2011, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef]
- Donahoo, W.T.; Levine, J.A.; Melanson, E.L. Variability in energy expenditure and its components. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 599–605. [Google Scholar] [CrossRef]
- Morley, N.J.; Lewis, J.W. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 2014, 30, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, T.; Perrier, E.T.; Melander, O. A Journey through the Early Evidence Linking Hydration to Metabolic Health. Ann. Nutr. Metab. 2020, 76, 4–9. [Google Scholar] [CrossRef]
- Nakamura, K.; Velho, G.; Bouby, N. Vasopressin and metabolic disorders: Translation from experimental models to clinical use. J. Intern. Med. 2017, 282, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Blauw, L.L.; Aziz, N.A.; Tannemaat, M.R.; Blauw, C.A.; de Craen, A.J.; Pijl, H.; Rensen, P.C.N. Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res. Care 2017, 5, e000317. [Google Scholar] [CrossRef]
- Ribble, A.; Hellmann, J.; Conklin, D.J.; Bhatnagar, A.; Haberzettl, P. Fine particulate matter (PM2.5)-induced pulmonary oxidative stress contributes to increases in glucose intolerance and insulin resistance in a mouse model of circadian dyssynchrony. Sci. Total Environ. 2023, 877, 162934. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, G.; Edwards, S.; Tran, J.; Rajagopalan, S.; Rao, X. Particulate air pollution exaggerates diet-induced insulin resistance through NLRP3 inflammasome in mice. Environ. Pollut. 2023, 328, 121603. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, H.; Gao, Y.; Liu, S.; Chen, L.; Ni, G.; Guo, X.; Wang, M.; Wang, C.; Chen, Y.; et al. Airborne Nanoplastics Exposure Inducing Irreversible Glucose Increase and Complete Hepatic Insulin Resistance. Environ. Sci. Technol. 2024, 58, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, S.; Wang, X.; Zhong, S.; Yuan, J.; Zhong, Y.; Jiang, Q. Long-term exposure to air pollution and risk of insulin resistance: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 271, 115909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, G.; He, J.; Chen, Z.; Pan, M.; Tong, J.; Liu, F.; Xiang, H. DNA methylation mediates the effects of PM2.5 and O3 on ceramide metabolism: A novel mechanistic link between air pollution and insulin resistance. J. Hazard. Mater. 2024, 469, 133864. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Coleman, N.; Pond, Z.A.; Burnett, R.T. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ. Res. 2020, 183, 108924. [Google Scholar] [CrossRef] [PubMed]
- Della Guardia, L.; Shin, A.C. White and brown adipose tissue functionality is impaired by fine particulate matter (PM2.5) exposure. J. Mol. Med. 2022, 100, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Burkart, K.; Causey, K.; Cohen, A.J.; Wozniak, S.S.; Salvi, D.D.; Abbafati, C.; Adekanmbi, V.; Adsuar, J.C.; Ahmadi, K.; Alahdab, F.; et al. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM2·5 air pollution, 1990–2019: An analysis of data from the Global Burden of Disease Study 2019. Lancet Planet. Health 2022, 6, e586–e600. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.; Karey, E.; Moshier, E.; Lindtner, C.; La Frano, M.R.; Newman, J.W.; Buettner, C. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring. PLoS ONE 2014, 9, e103337. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-X.; Wang, C.; Zhang, Z.-M.; Jaeger, C.D.; Krager, S.L.; Bottum, K.M.; Liu, J.; Liao, D.-F.; Tischkau, S.A. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int. J. Obes. 2015, 39, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, I.; Busiello, R.A.; Burgos Aceves, M.A.; Lepretti, M.; Paolella, G.; Lionetti, L. Environmental Pollutants Effect on Brown Adipose Tissue. Front. Physiol. 2019, 9, 1891. [Google Scholar] [CrossRef]
- Feige, J.N.; Gerber, A.; Casals-Casas, C.; Yang, Q.; Winkler, C.; Bedu, E.; Bueno, M.; Gelman, L.; Auwerx, J.; Gonzalez, F.J.; et al. The Pollutant Diethylhexyl Phthalate Regulates Hepatic Energy Metabolism via Species-Specific PPARα-Dependent Mechanisms. Environ. Health Perspect. 2010, 118, 234–241. [Google Scholar] [CrossRef]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. Metabolically inert perfluorinated fatty acids directly activate uncoupling protein 1 in brown-fat mitochondria. Arch. Toxicol. 2016, 90, 1117–1128. [Google Scholar] [CrossRef]
- John, L.M.; Petersen, N.; Gerstenberg, M.K.; Torz, L.; Pedersen, K.; Christoffersen, B.Ø.; Kuhre, R.E. Housing-temperature reveals energy intake counter-balances energy expenditure in normal-weight, but not diet-induced obese, male mice. Commun. Biol. 2022, 5, 946. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 2017, 13, 458–465. [Google Scholar] [CrossRef]
- Kaiyala, K.J.; Ogimoto, K.; Nelson, J.T.; Muta, K.; Morton, G.J. Physiological role for leptin in the control of thermal conductance. Mol. Metab. 2016, 5, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, K.; Kotzbeck, P.; Zani, F.; Bauer, M.; Neff, C.; Müller, T.D.; Pfluger, P.T.; Seeley, R.J.; Divanovic, S. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int. J. Obes. 2015, 39, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Kumar, D.; Gupta, S.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, A.P.; Vishwakarma, A.L.; Gayen, J.R.; et al. Role of brown adipose tissue in modulating adipose tissue inflammation and insulin resistance in high-fat diet fed mice. Eur. J. Pharmacol. 2019, 854, 354–364. [Google Scholar] [CrossRef]
- Sass, F.; Schlein, C.; Jaeckstein, M.Y.; Pertzborn, P.; Schweizer, M.; Schinke, T.; Ballabio, A.; Scheja, L.; Heeren, J.; Fischer, A.W. TFEB deficiency attenuates mitochondrial degradation upon brown adipose tissue whitening at thermoneutrality. Mol. Metab. 2021, 47, 101173. [Google Scholar] [CrossRef] [PubMed]
- Spiljar, M.; Steinbach, K.; Rigo, D.; Suárez-Zamorano, N.; Wagner, I.; Hadadi, N.; Vincenti, I.; Page, N.; Klimek, B.; Rochat, M.-A.; et al. Cold exposure protects from neuroinflammation through immunologic reprogramming. Cell Metab. 2021, 33, 2231–2246.e8. [Google Scholar] [CrossRef]
- Williams, J.W.; Elvington, A.; Ivanov, S.; Kessler, S.; Luehmann, H.; Baba, O.; Saunders, B.T.; Kim, K.-W.; Johnson, M.W.; Craft, C.S.; et al. Thermoneutrality but Not UCP1 Deficiency Suppresses Monocyte Mobilization Into Blood. Circ. Res. 2017, 121, 662–676. [Google Scholar] [CrossRef]
- Presby, D.M.; Jackman, M.R.; Rudolph, M.C.; Sherk, V.D.; Foright, R.M.; Houck, J.A.; Johnson, G.C.; Orlicky, D.J.; Melanson, E.L.; Higgins, J.A.; et al. Compensation for cold-induced thermogenesis during weight loss maintenance and regain. Am. J. Physiol. Metab. 2019, 316, E977–E986. [Google Scholar] [CrossRef] [PubMed]
- Søberg, S.; Löfgren, J.; Philipsen, F.E.; Jensen, M.; Hansen, A.E.; Ahrens, E.; Nystrup, K.B.; Nielsen, R.D.; Sølling, C.; Wedell-Neergaard, A.-S.; et al. Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men. Cell Rep. Med. 2021, 2, 100408. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Milbank, E.; Diéguez, C.; López, M.; Contreras, C. Glucagon, GLP-1 and Thermogenesis. Int. J. Mol. Sci. 2019, 20, 3445. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Xiao, C.; Gavrilova, O.; Reitman, M.L. Effect of Intermittent Cold Exposure on Brown Fat Activation, Obesity, and Energy Homeostasis in Mice. PLoS ONE 2014, 9, e85876. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Wang, C.; Yuan, Y.; Wang, Z.; Kuang, J.; Yan, X.; Chen, H. Effect of Cold Exposure and Exercise on Insulin Sensitivity and Serum Free Fatty Acids in Obese Rats. Med. Sci. Sport. Exerc. 2023, 55, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.J.; White, A.D.; Bijland, S.; Rutherford, C.; Graham, D.; Richter, E.A.; Viollet, B.; Touyz, R.M.; Palmer, T.M.; Salt, I.P. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol. Cell. Endocrinol. 2017, 440, 44–56. [Google Scholar] [CrossRef]
- Xu, R.; Zhong, Y.; Li, R.; Li, Y.; Zhong, Z.; Liu, T.; Wang, Q.; Lv, Z.; Huang, S.; Duan, Y.-G.; et al. Association between exposure to ambient air pollution and semen quality: A systematic review and meta-analysis. Sci. Total Environ. 2023, 870, 161892. [Google Scholar] [CrossRef]
- Mendez, R.; Zheng, Z.; Fan, Z.; Rajagopalan, S.; Sun, Q.; Zhang, K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am. J. Transl. Res. 2013, 5, 224–234. [Google Scholar]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol prevents PM2.5-induced adiposity and insulin resistance by restraining oxidative stress related NF-κB pathway and modulation of gut microbiota in a murine model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef]
- Campolim, C.M.; Weissmann, L.; Ferreira, C.K.D.O.; Zordão, O.P.; Dornellas, A.P.S.; de Castro, G.; Zanotto, T.M.; Boico, V.F.; Quaresma, P.G.F.; Lima, R.P.A.; et al. Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice. Sci. Rep. 2020, 10, 10160. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Münzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress—Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef] [PubMed]
- Burgoine, T.; Monsivais, P.; Sharp, S.J.; Forouhi, N.G.; Wareham, N.J. Independent and combined associations between fast-food outlet exposure and genetic risk for obesity: A population-based, cross-sectional study in the UK. BMC Med. 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhen, S.; Yan, Y.; Liu, N.; Ding, D.; Kong, J. Association of dietary patterns with general and central obesity among Chinese adults: A longitudinal population-based study. BMC Public Health 2023, 23, 1588. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.D.S.; Lopes, S.O.; Araújo, S.P.; Priore, S.E.; Alfenas, R.d.C.G.; Hermsdorff, H.H.M. Association between food insecurity and cardiometabolic risk in adults and the elderly: A systematic review. J. Glob. Health 2020, 10, 020402. [Google Scholar] [CrossRef] [PubMed]
- Nkambule, S.J.; Moodley, I.; Kuupiel, D.; Mashamba-Thompson, T.P. Association between food insecurity and key metabolic risk factors for diet-sensitive non-communicable diseases in sub-Saharan Africa: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 5178. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Kalamatianou, S.; Drewnowski, A.; Kulkarni, B.; Kinra, S.; Kadiyala, S. Food Environment Research in Low- and Middle-Income Countries: A Systematic Scoping Review. Adv. Nutr. 2020, 11, 387–397. [Google Scholar] [CrossRef] [PubMed]
- von Braun, J. Economic and Political Innovation for Nutritional Improvement. World Rev. Nutr. Diet. 2018, 118, 1–9. [Google Scholar] [CrossRef]
- Owino, V.; Kumwenda, C.; Ekesa, B.; Parker, M.E.; Ewoldt, L.; Roos, N.; Lee, W.T.; Tome, D. The impact of climate change on food systems, diet quality, nutrition, and health outcomes: A narrative review. Front. Clim. 2022, 4, 941842. [Google Scholar] [CrossRef]
- Hallström, E.; Bajzelj, B.; Håkansson, N.; Sjons, J.; Åkesson, A.; Wolk, A.; Sonesson, U. Dietary climate impact: Contribution of foods and dietary patterns by gender and age in a Swedish population. J. Clean. Prod. 2021, 306, 127189. [Google Scholar] [CrossRef]
- Domingo, N.G.G.; Balasubramanian, S.; Thakrar, S.K.; Clark, M.A.; Adams, P.J.; Marshall, J.D.; Muller, N.Z.; Pandis, S.N.; Polasky, S.; Robinson, A.L.; et al. Air quality–related health damages of food. Proc. Natl. Acad. Sci. USA 2021, 118, e2013637118. [Google Scholar] [CrossRef]
- Sundram, T.K.M.; Tan, E.S.S.; Lim, H.S.; Amini, F.; Bustami, N.A.; Tan, P.Y.; Rehman, N.; Ho, Y.B.; Tan, C.K. Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers. Nutrients 2022, 14, 4858. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; MacCarthy, D.S. Climate change impacts on crop yields. Nat. Rev. Earth Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- Young, B.; Birney, C.; Ingwersen, W.W. Dataset of 2012-2020 U.S. National- and State-Level Greenhouse Gas Emissions by Sector. Data Br. 2024, 53, 110173. [Google Scholar] [CrossRef] [PubMed]
- Flint, E.; Webb, E.; Cummins, S. Change in commute mode and body-mass index: Prospective, longitudinal evidence from UK Biobank. Lancet Public Health 2016, 1, e46–e55. [Google Scholar] [CrossRef]
- Haines, A.; Ebi, K. The Imperative for Climate Action to Protect Health. N. Engl. J. Med. 2019, 380, 263–273. [Google Scholar] [CrossRef]
- Hirvonen, K.; Bai, Y.; Headey, D.; Masters, W.A. Affordability of the EAT–Lancet reference diet: A global analysis. Lancet Glob. Health 2020, 8, e59–e66. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Jay, O.; Capon, A.; Berry, P.; Broderick, C.; de Dear, R.; Havenith, G.; Honda, Y.; Kovats, R.S.; Ma, W.; Malik, A.; et al. Reducing the health effects of hot weather and heat extremes: From personal cooling strategies to green cities. Lancet 2021, 398, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; Salvi, S.; Wong, G.W.K.; Chung, K.F. Personal strategies to minimise effects of air pollution on respiratory health: Advice for providers, patients and the public. Eur. Respir. J. 2020, 55, 1902056. [Google Scholar] [CrossRef] [PubMed]
- Dain, K.; Hadley, L. Diabetes and climate change—Two interconnected global challenges. Diabetes Res. Clin. Pract. 2012, 97, 337–339. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojchevski, R.; Chandrasekaran, P.; Hadzi-Petrushev, N.; Mladenov, M.; Avtanski, D. Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences. Int. J. Mol. Sci. 2024, 25, 7849. https://doi.org/10.3390/ijms25147849
Stojchevski R, Chandrasekaran P, Hadzi-Petrushev N, Mladenov M, Avtanski D. Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences. International Journal of Molecular Sciences. 2024; 25(14):7849. https://doi.org/10.3390/ijms25147849
Chicago/Turabian StyleStojchevski, Radoslav, Preethi Chandrasekaran, Nikola Hadzi-Petrushev, Mitko Mladenov, and Dimiter Avtanski. 2024. "Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences" International Journal of Molecular Sciences 25, no. 14: 7849. https://doi.org/10.3390/ijms25147849
APA StyleStojchevski, R., Chandrasekaran, P., Hadzi-Petrushev, N., Mladenov, M., & Avtanski, D. (2024). Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences. International Journal of Molecular Sciences, 25(14), 7849. https://doi.org/10.3390/ijms25147849






