Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Results
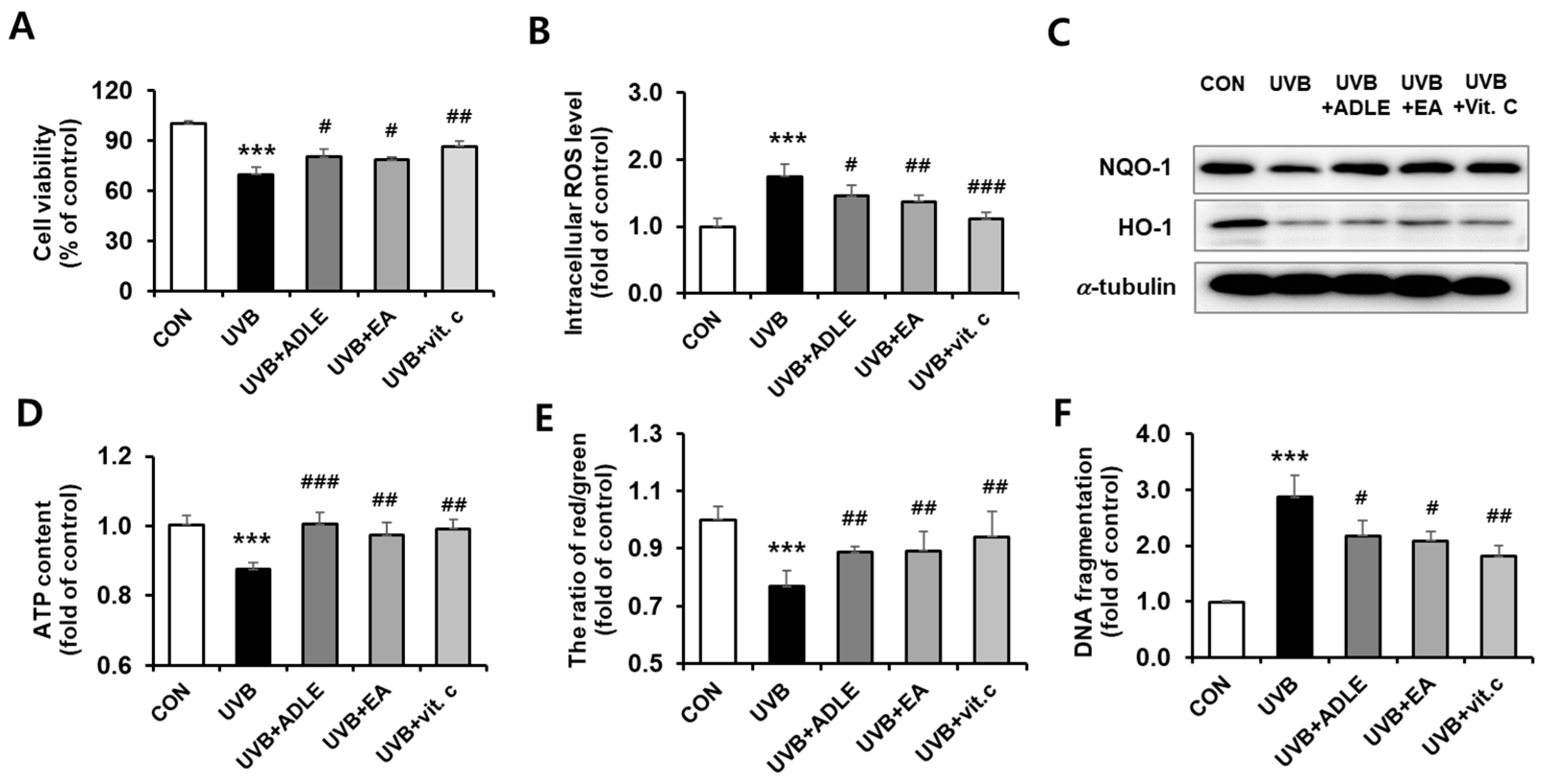
2.1. Cytoprotective Effect of the EA Fraction against UVB-Irradiated Human Dermal Fibroblast (HDF) Cells
2.2. Anti-Aging Effects of the EA Fraction on Senescence-Associated Secretory Phenotype-Based Biomarkers in UVB-Irradiated HDF Cells
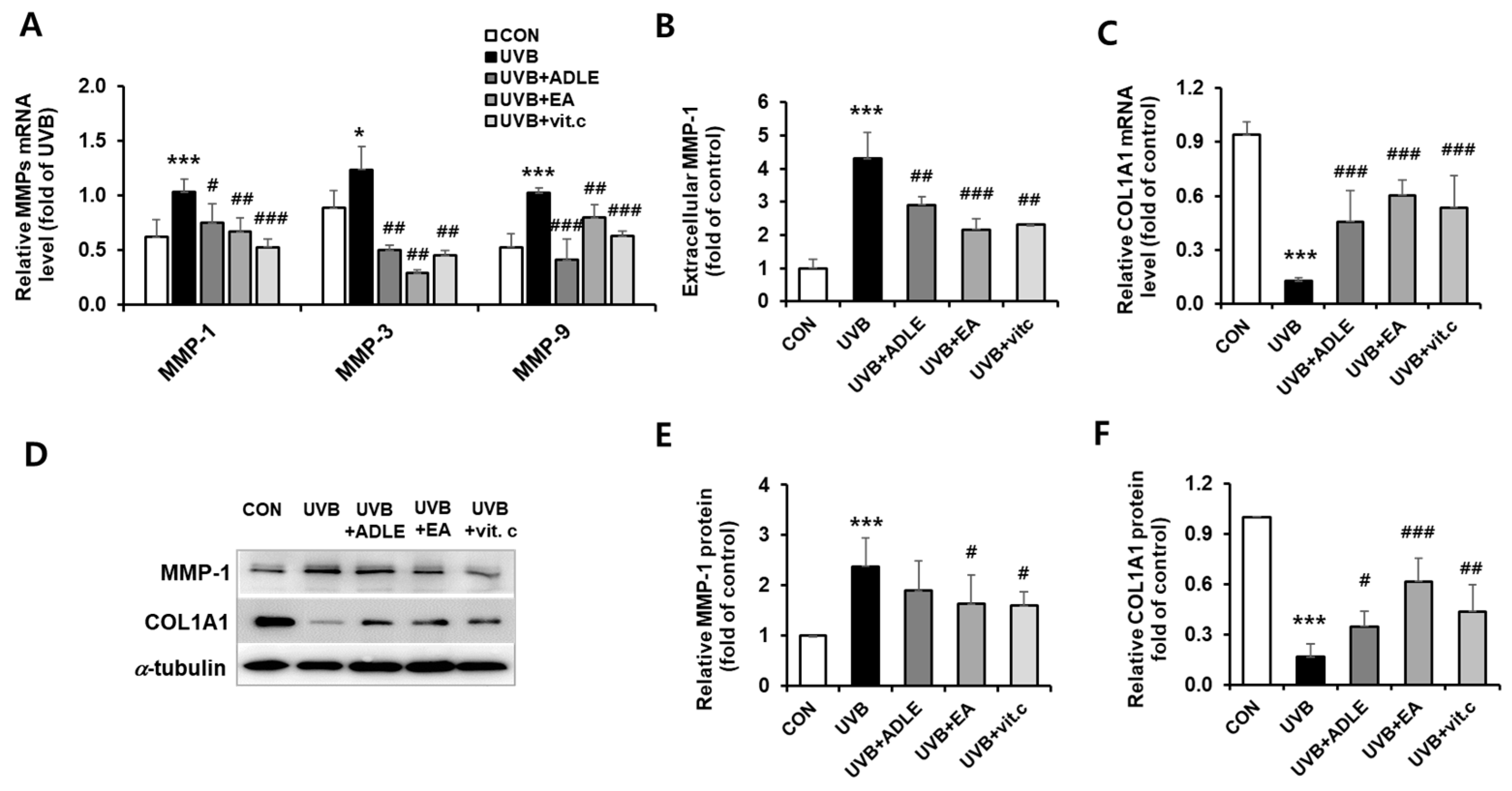
2.3. Regulation of COL1A1 and MMP-1 Expression via EA Treatment in UVB-Irradiated HDF Cells
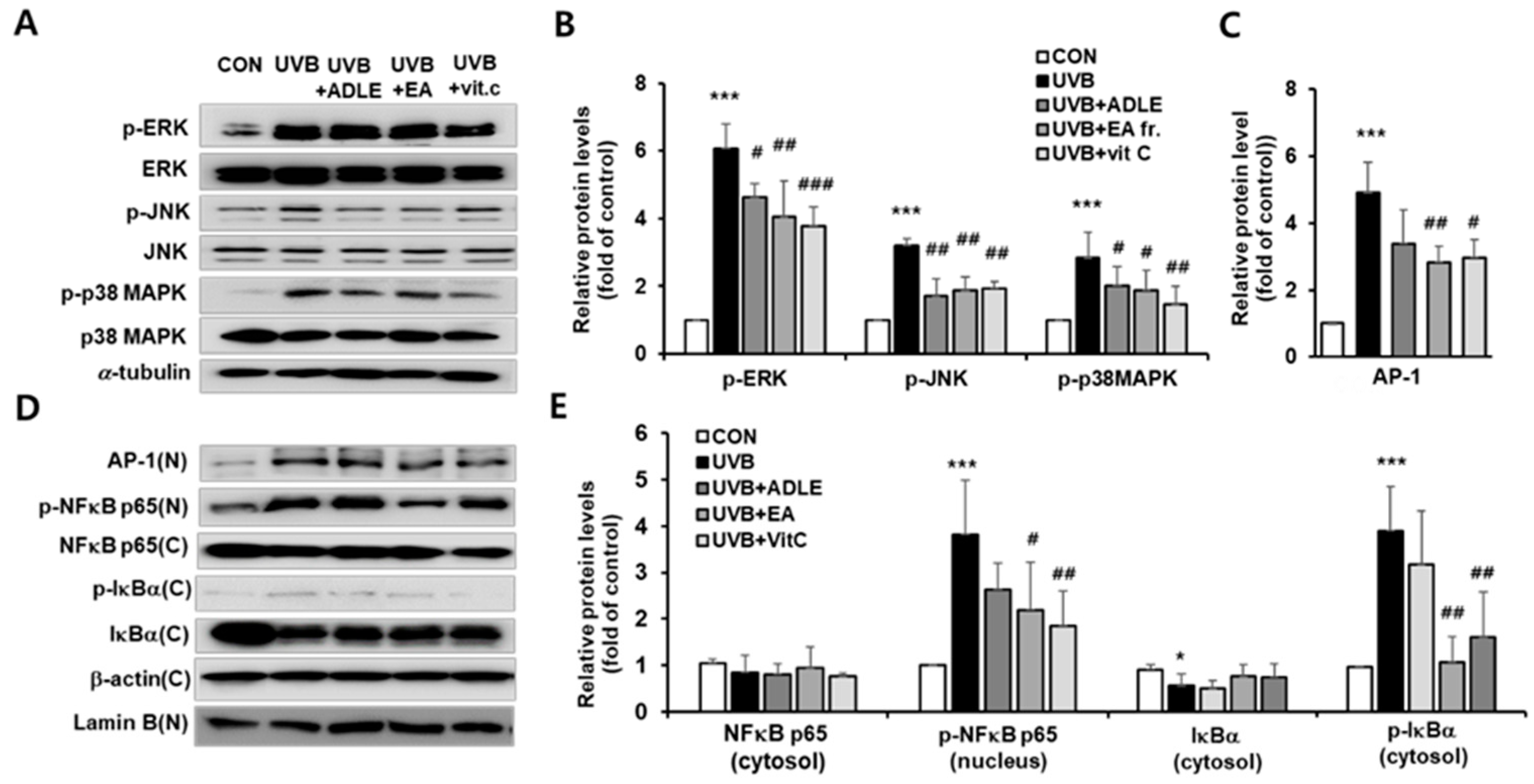
2.4. Effect of EA Fraction on Activation of MAPK and NF-κB/AP-1 Signaling Pathways in UVB-Irradiated HDF Cells
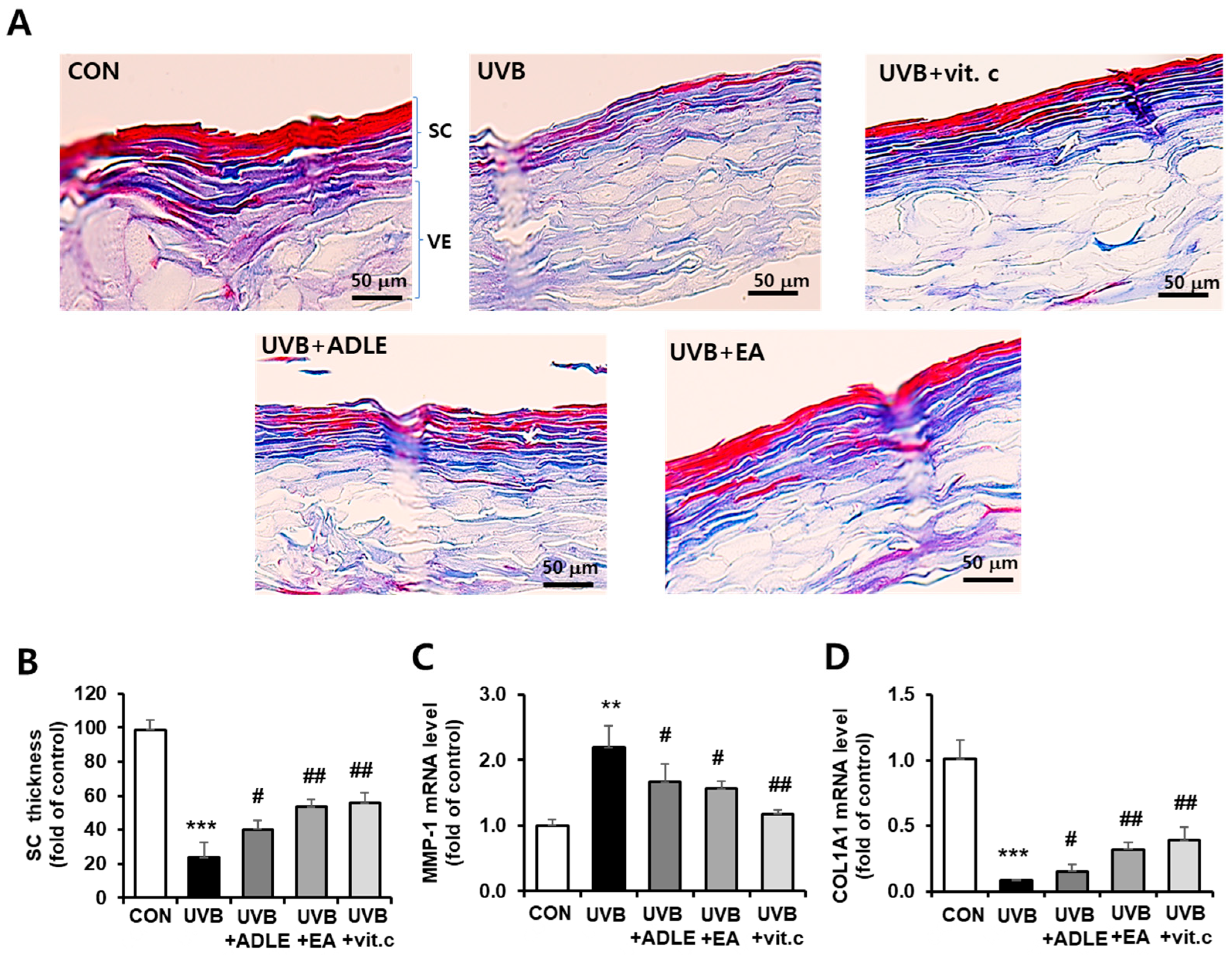
2.5. Inhibitory Effects of the EA Fraction on UVB-Induced Collagen Degradation in a 3D Reconstructed Human Skin Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of the EA Fraction of the ADLE
4.3. Cell Culture, UVB Irradiation, and Cell Viability
4.4. Intracellular ROS Measurement
4.5. Senescence-Associated β-Galactosidase Assay
4.6. ATP Content
4.7. Mitochondrial Membrane Potential Assay
4.8. ELISA
4.9. Nuclear Extract Preparation
4.10. Treatment of a Human Reconstituted Skin Model and Measurement of Collagen Expression Using Histological Analysis and Gene and Protein Expression
4.11. Masson’s Trichrome Stain
4.12. Quantitative Real-Time Polymerase Chain Reaction
4.13. Western Blotting
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, X.-J.; Kim, E.J.; Oh, I.K.; Kim, Y.K.; Park, C.-H.; Chung, J.H. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J. Korean Med. Sci. 2010, 25, 930–937. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, J.-H. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol. Rep. 2008, 19, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Farris, P.K. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–817; discussion 818. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Lee, S.-Y.; Joung, M.-S.; Choi, J.-W. Anti-wrinkle effect of 3-O-cetyl-L-ascorbic acid. J. Soc. Cosmet. Sci. Korea 2008, 34, 303–309. [Google Scholar]
- Wyss, R. Chromatographic and electrophoretic analysis of biomedically important retinoids. J. Chromatogr. B Biomed. Appl. 1995, 671, 381–425. [Google Scholar] [CrossRef]
- Weiss, J.S.; Ellis, C.N.; Headington, J.T.; Voorhees, J.J. Topical tretinoin in the treatment of aging skin. J. Am. Acad. Dermatol. 1988, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-efficient food production to reduce global warming and ecodegradation: The use of edible insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- MacEvilly, M.C. Bugs in the system. Nutr. Bull. 2000, 25, 267–268. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Dossey, A.T. Insects and their chemical weaponry: New potential for drug discovery. Nat. Prod. Rep. 2010, 27, 1737–1757. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, G.D.; Lee, M.; Park, E.-Y.; Baek, D.J.; Kim, C.Y.; Jun, H.-S.; Oh, Y.S. Allomyrina dichotoma larva extract ameliorates the hepatic insulin resistance of high-fat diet-induced diabetic mice. Nutrients 2019, 11, 1522. [Google Scholar] [CrossRef]
- Kim, M.; Youn, K.; Yun, E.-Y.; Hwang, J.-S.; Ahn, M.-R.; Jeong, W.-S.; Jun, M. Effects of solvent fractions of Allomyrina dichotom larvae through the inhibition of in vitro BACE1 and β-amyloid(25–35)-induced toxicity in rat pheochromocytoma PC12 cells. Entomol. Res. 2014, 44, 23–30. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jo, D.-E.; Lee, A.-J.; Park, H.-K.; Youn, K.; Yun, E.-Y.; Hwang, J.-S.; Jun, M.; Kang, B.H. Hepatoprotective and anticancer activities of Allomyrina dichotoma larvae. J. Life Sci. 2015, 25, 307–316. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, I.W.; Kim, S.Y.; Kim, M.A.; Kim, S.H.; Hwang, J.S. Anti-inflammatory activity of antimicrobial peptide allomyrinasin derived from the dynastid beetle, Allomyrina dichotoma. J. Microbiol. Biotechnol. 2019, 29, 687–695. [Google Scholar] [CrossRef]
- Kim, K.; Kwak, M.-K.; Bae, G.-D.; Park, E.-Y.; Baek, D.-J.; Kim, C.-Y.; Jang, S.-E.; Jun, H.-S.; Oh, Y.S. Allomyrina dichotoma larva extract attenuates free fatty acid-induced lipotoxicity in pancreatic beta cells. Nutr. Res. Pract. 2021, 15, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, E.-Y.; Baek, D.-J.; Lee, C.-S.; Oh, Y.S. Antiphotoaging effects of solvent fractions isolated from Allomyrina dichotoma larvae extract. Biochem. Biophys. Rep. 2024, 38, 101660. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 2003, RE2. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H2O2 signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Reichert, A.S. How to get rid of mitochondria: Crosstalk and regulation of multiple mitophagy pathways. Biol. Chem. 2017, 399, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.R.; Sperker, B.; Fritz, P.; Klotz, U. Does pH 6 beta-galactosidase activity indicate cell senescence? Mech. Ageing Dev. 1999, 109, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, M.; Zhu, W.-H. Advances in fluorescent sensors for β-galactosidase. Mater. Chem. Front. 2021, 5, 763–774. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Rovillain, E.; Mansfield, L.; Lord, C.J.; Ashworth, A.; Jat, P.S. An RNA interference screen for identifying downstream effectors of the p53 and pRB tumour suppressor pathways involved in senescence. BMC Genom. 2011, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, S.; Sakaguchi, M.; Masunaga, S.; Mori, E. Stress-induced cellular senescence contributes to chronic inflammation and cancer progression. Therm. Med. 2019, 35, 41–58. [Google Scholar] [CrossRef]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor kappaB inhibitor, parthenolide. J. Pharmacol. Exp. Ther. 2005, 315, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, Z.; Ozbal, S.; Celik, A.; Ergur, B.U.; Guneli, E.; Ural, C.; Camsari, T.; Guner, G.A. The effects of alpha-lipoic acid on MMP-2 and MMP-9 activities in a rat renal ischemia and re-perfusion model. Biotech. Histochem. 2014, 89, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Ho, M.T.; Song, Y.W.; Cho, M.; Cho, S.K. Camphor induces proliferative and anti-senescence activities in human primary dermal fibroblasts and inhibits UV-induced wrinkle formation in mouse skin. Phytother. Res. 2015, 29, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Jung, T.D.; Cho, B.Y.; Choi, S.H.; Sim, W.S.; Han, X.; Lee, S.J.; Kim, Y.C.; Lee, O.H. Anti-photoaging effect of fermented agricultural by-products on ultraviolet B-irradiated hairless mouse skin. Int. J. Mol. Med. 2019, 44, 559–568. [Google Scholar] [CrossRef]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-photoaging effect of hydrolysates from pacific whiting skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 signaling pathway in UVB-induced human dermal fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef]
- Adler, A.S.; Sinha, S.; Kawahara, T.L.; Zhang, J.Y.; Segal, E.; Chang, H.Y. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007, 21, 3244–3257. [Google Scholar] [CrossRef] [PubMed]
- Brohem, C.A.; Cardeal, L.B.S.; Tiago, M.; Soengas, M.S.; Barros, S.B.M.; Maria-Engler, S.S. Artificial skin in perspective: Concepts and applications. Pigment Cell Melanoma Res. 2011, 24, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.M. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-G.; Chang, T.-L.; Ke, W.-M.; Lo, Y.-F.; Wang, C.-C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Fontaine, K.A.; Camarda, R.; Lagunoff, M. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014, 88, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulgaridou, G.-P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.-I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis extracts inhibit UV-induced photodamage in human experimental in vitro skin models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef]


| Gene | Primer | Sequence (5′→3′) |
|---|---|---|
| MMP-1 | forward reverse | ATTCTACTGATATCGGGGCTTTGA ATGTCCTTGGGGTATCCGTGTAG |
| MMP-3 | forward reverse | TTCGGGATGCCAGGAAAGGTTC AGTTCCTTGGATTGGAGGTGACG |
| MMP-9 | forward reverse | CTGCCAGGACCGCTTCTACT TGGTCCCAGTGGGGATTTAC |
| COL1A1 | forward reverse | CTCGAGGTGGACACCACCCT CAGCTGGATGGCCACATCGG |
| TNFα | forward reverse | TGCTCCTCACCCACACCAT GGAGGTTGACCTTGGTCTGGTA |
| IL-1β | forward reverse | ACGATGCACCTGTACGATCACT CACCAAGCTTTTTTGCTGTGAGT |
| IFNγ | forward reverse | ACTCATCCAAGTGATGGCTGAA TCCTTTTTCGCTTCCCTGTTT |
| p16 | forward reverse | GTGGACCTGGCTGAGGAG CTTTCAATCGGGGATGTCTG |
| p21 | forward reverse | CCGAAGTCAGTTCCTTGTGG CATGGGTTCTGACGGACAT |
| p53 | forward reverse | AGGCCTTGGAACTCAAGGAT CCCTTTTTGGACTTCAGGTG |
| GAPDH | forward reverse | AGGGCTGCTTTTAACTCTGGT CCCCACTTGATTTTGGAGGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, C.-E.; Baek, D.-J.; Park, E.-Y.; Oh, Y.S. Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 7850. https://doi.org/10.3390/ijms25147850
Kim K, Kim C-E, Baek D-J, Park E-Y, Oh YS. Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2024; 25(14):7850. https://doi.org/10.3390/ijms25147850
Chicago/Turabian StyleKim, Kyong, Chae-Eun Kim, Dong-Jae Baek, Eun-Young Park, and Yoon Sin Oh. 2024. "Prevention of UVB-Induced Photoaging by an Ethyl Acetate Fraction from Allomyrina dichotoma Larvae and Its Potential Mechanisms in Human Dermal Fibroblasts" International Journal of Molecular Sciences 25, no. 14: 7850. https://doi.org/10.3390/ijms25147850





