Role of Oxidative Stress in Sensorineural Hearing Loss
Abstract
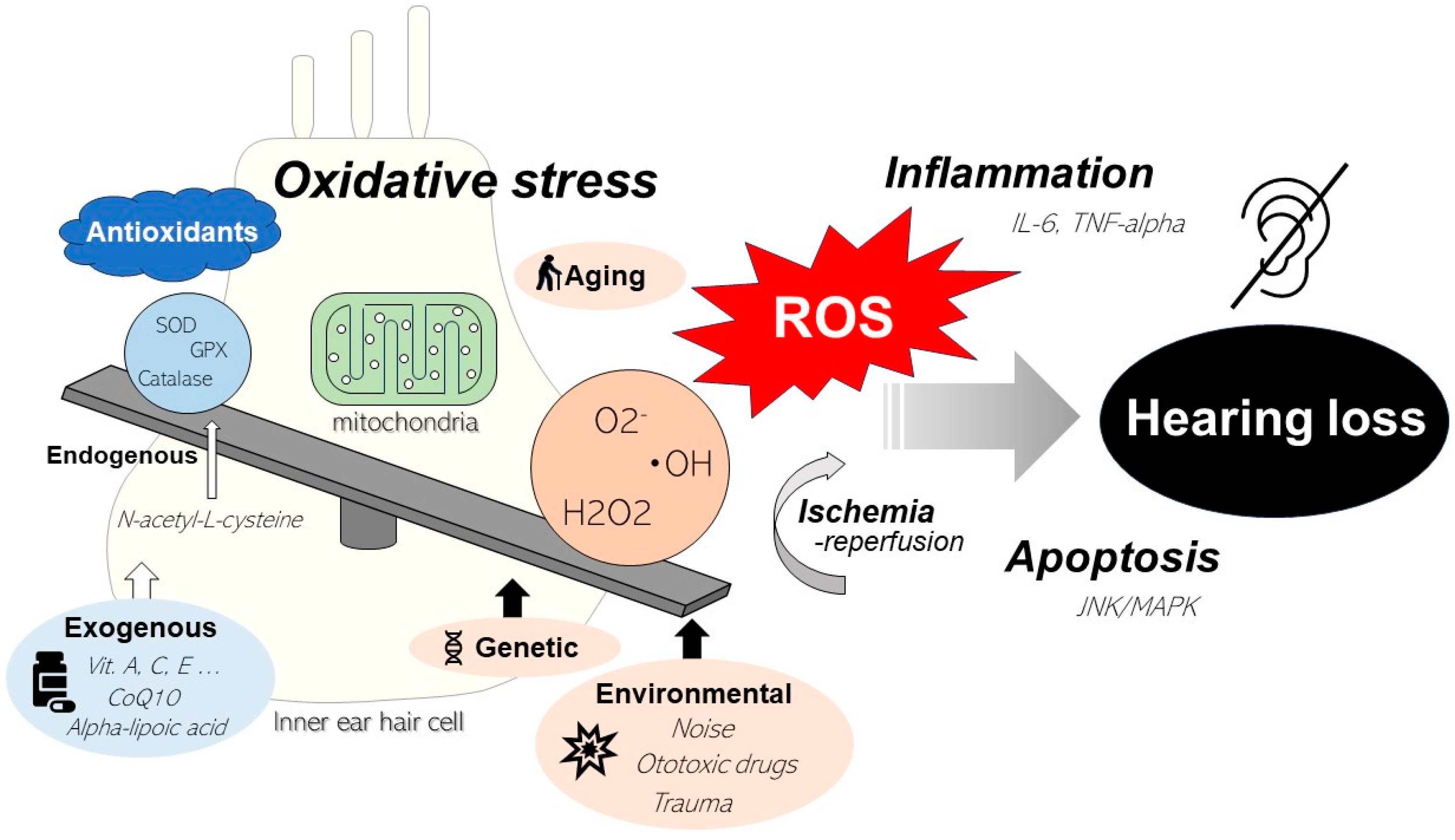
1. Introduction
2. Auditory System and Sensorineural Hearing Loss
3. Reactive Oxygen Species
4. Role of Mitochondrial Oxidative Stress in Hearing Loss
5. Role of Oxidative Stress in ARHL
6. Role of Oxidative Stress on NIHL
7. Role of Oxidative Stress on DIHL
8. Mechanisms of Ischemia–Reperfusion Injury
9. Potential of Antioxidants for the Treatment of Sensorineural Hearing Loss
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamasoba, T.; Someya, S.; Yamada, C.; Weindruch, R.; Prolla, T.A.; Tanokura, M. Role of Mitochondrial Dysfunction and Mitochondrial DNA Mutations in Age-Related Hearing Loss. Hear. Res. 2007, 226, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, W.; Niu, Y.; He, S.; Chai, R.; Yang, J. Autophagy Regulates the Survival of Hair Cells and Spiral Ganglion Neurons in Cases of Noise, Ototoxic Drug, and Age-Induced Sensorineural Hearing Loss. Front. Cell. Neurosci. 2021, 15, 760422. [Google Scholar] [CrossRef] [PubMed]
- Chern, A.; Golub, J.S. Age-Related Hearing Loss and Dementia. Alzheimer Dis. Assoc. Disord. 2019, 33, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Co-hen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, T.D.; Lad, M.; Kumar, S.; Holmes, E.; McMurray, B.; Maguire, E.A.; Billig, A.J.; Sedley, W. How Can Hearing Loss Cause Dementia? Neuron 2020, 108, 401–412. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Hearing—Executive Summary; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Škerková, M.; Kovalová, M.; Rychlý, T.; Tomášková, H.; Šlachtová, H.; Čada, Z.; Maďar, R.; Mrázková, E. Extended High-Frequency Audiometry: Hearing Thresholds in Adults. Eur. Arch. Otorhinolaryngol. 2023, 280, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kociszewska, D.; Vlajkovic, S. Age-Related Hearing Loss: The Link Between Inflammaging, Immunosenescence, and Gut Dysbiosis. Int. J. Mol. Sci. 2022, 23, 7348. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’Andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Perez-Campo, R.; López-Torres, M.; Cadenas, S.; Rojas, C.; Barja, G. The Rate of Free Radical Production as a Determinant of the Rate of Aging: Evidence from the Comparative Approach. J. Comp. Physiol. B 1998, 168, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.T.; Goncalves, S.; Bas, E.; Van De Water, T.R.; Zine, A. Molecular Regulation of Auditory Hair Cell Death and Approaches to Protect Sensory Receptor Cells and/or Stimulate Repair Following Acoustic Trauma. Front. Cell. Neurosci. 2015, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Böttger, E.C.; Schacht, J. The Mitochondrion: A Perpetrator of Acquired Hearing Loss. Hear. Res. 2013, 303, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Ryan, A.F. Mechanisms of Sensorineural Cell Damage, Death and Survival in the Cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Yamasoba, T. Mitochondria-Targeted Antioxidants for Treatment of Hearing Loss: A Systematic Review. Antioxidants 2019, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. How Hearing Happens. Neuron 1997, 19, 947–950. [Google Scholar] [CrossRef]
- Wangemann, P. Supporting Sensory Transduction: Cochlear Fluid Homeostasis and the Endocochlear Potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Schwander, M.; Kachar, B.; Müller, U. Review Series: The Cell Biology of Hearing Review Series. J. Cell Biol. 2010, 190, 9–20. [Google Scholar] [CrossRef]
- Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Cochlear Hair Cells: The Sound-Sensing Machines. FEBS Lett. 2015, 589, 3354–3361. [Google Scholar] [CrossRef]
- Marcotti, W. Functional Assembly of Mammalian Cochlear Hair Cells. Exp. Physiol. 2012, 97, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Von Békésy, G. Experiments in Hearing; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Fuchs, P.A.; Glowatzki, E.; Moser, T. The Afferent Synapse of Cochlear Hair Cells. Curr. Opin. Neurobiol. 2003, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Raphael, Y.; Altschuler, R.A. Structure and Innervation of the Cochlea. Brain Res. Bull. 2003, 60, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Batts, S.; Stankovic, K.M. Noise-Induced Hearing Loss. J. Clin. Med. 2023, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G. Prevention of Noise-Induced Hearing Loss Using Investigational Medicines for the Inner Ear: Previous Trial Outcomes Should Inform Future Trial Design. Antioxid. Redox Signal. 2022, 36, 1171–1202. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E. Noise and Hearing Loss: A Review. J. Sch. Health 2007, 77, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Huth, M.E.; Ricci, A.J.; Cheng, A.G. Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int. J. Otolaryngol. 2011, 2011, 937861. [Google Scholar] [CrossRef] [PubMed]
- Chirtes, F.; Albu, S. Prevention and Restoration of Hearing Loss Associated with the Use of Cisplatin. BioMed Res. Int. 2014, 2014, 925485. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Camilon, M.; Nguyen, S.; Meyer, T.A. Steroids for Treatment of Sudden Sensorineural Hearing Loss: A Meta-Analysis of Randomized Controlled Trials. Laryngoscope 2015, 125, 209–217. [Google Scholar] [CrossRef]
- Conlin, A.E.; Parnes, L.S. Treatment of Sudden Sensorineural Hearing Loss: I. A Systematic Review. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 573–581. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.; Cross, C.E. Free Radicals, Antioxidants, and Human Disease: Where Are We Now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar] [PubMed]
- Seidman, M.D. Effects of Dietary Restriction and Antioxidants on Presbyacusis. Laryngoscope 2000, 110, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, J.; Yin, L.; Pu, Y. Metabolomics Analysis Reveals Alterations in Cochlear Metabolic Profiling in Mice with Noise-Induced Hearing Loss. BioMed Res. Int. 2022, 2022, 9548316. [Google Scholar] [CrossRef]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging 2018, 10, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Pekar, T.; Bruckner, K.; Pauschenwein-Frantsich, S.; Gschaider, A.; Oppliger, M.; Willesberger, J.; Ungersbäck, P.; Wendzel, A.; Kremer, A.; Flak, W.; et al. The positive effect of spermidine in older adults suffering from dementia: First results of a 3-month trial. Wien Klin. Wochenschr. 2021, 133, 484–491. [Google Scholar] [CrossRef]
- Barja, G. Updating the Mitochondrial Free Radical Theory of Aging: An Integrated View, Key Aspects, and Confounding Concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef] [PubMed]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive Oxygen Species, Apoptosis, and Mitochondrial Dysfunction in Hearing Loss. BioMed Res. Int. 2015, 2015, 617207. [Google Scholar] [CrossRef]
- Jiang, H.; Talaska, A.E.; Schacht, J.; Sha, S.H. Oxidative Imbalance in the Aging Inner Ear. Neurobiol. Aging 2007, 28, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- McFadden, S.L.; Ding, D.; Salvi, R. Anatomical, Metabolic and Genetic Aspects of Age-Related Hearing Loss in Mice: Aspectos Anatómicos, Metabólicos y Genéticos de la Hipoacusia Relacionada Con la Edad en Ratones. Int. J. Audiol. 2001, 40, 313–321. [Google Scholar] [CrossRef]
- Staecker, H.; Zheng, Q.Y.; Van De Water, T.R. Oxidative Stress in Aging in the C57B16/J Mouse Cochlea. Acta Otolaryngol. 2001, 121, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Pierson, M.G.; Gray, B.H. Superoxide Dismutase Activity in the Cochlea. Hear. Res. 1982, 6, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M.; Canto, C.; Zheng, Q.Y.; Wang, X.; Fischel-Ghodsian, N.; Johnson, K.R. Cu/Zn Superoxide Dismutase and Age-Related Hearing Loss. Hear. Res. 2005, 209, 76–85. [Google Scholar] [CrossRef]
- Menardo, J.; Tang, Y.; Ladrech, S.; Lenoir, M.; Casas, F.; Michel, C.; Bourien, J.; Ruel, J.; Rebillard, G.; Maurice, T.; et al. Oxidative Stress, Inflammation, and Autophagic Stress as the Key Mechanisms of Premature Age-Related Hearing Loss in SAMP8 Mouse Cochlea. Antioxid. Redox Signal. 2012, 16, 263–274. [Google Scholar] [CrossRef]
- Kawamoto, K.; Sha, S.H.; Minoda, R.; Izumikawa, M.; Kuriyama, H.; Schacht, J.; Raphael, Y. Antioxidant Gene Therapy Can Protect Hearing and Hair Cells from Ototoxicity. Mol. Ther. 2004, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- González-González, S. The Role of Mitochondrial Oxidative Stress in Hearing Loss. Neurol. Disord. Therap. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Fujimoto, C.; Yamasoba, T. Oxidative Stresses and Mitochondrial Dysfunction in Age-Related Hearing Loss. Oxid. Med. Cell. Longev. 2014, 2014, 582849. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Hu, Y.J.; Yang, Y.; Kong, W.J. Increased Mitochondrial DNA Damage and Decreased Base Excision Repair in the Auditory Cortex of d-Galactose-Induced Aging Rats. Mol. Biol. Rep. 2011, 38, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Druzhyna, N.M.; Wilson, G.L.; LeDoux, S.P. Mitochondrial DNA Repair in Aging and Disease. Mech. Ageing Dev. 2008, 129, 383–390. [Google Scholar] [CrossRef]
- Bandy, B.; Davison, A.J. Mitochondrial Mutations May Increase Oxidative Stress: Implications for Carcinogenesis and Aging? Free Radic. Biol. Med. 1990, 8, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, Antioxidants, and the Degenerative Diseases of Aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, Y. Mitochondrial Fission and Fusion. Biochem. Soc. Trans. 2016, 44, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; Cunningham, L.L.; Rubel, E.W. Mechanisms of Hair Cell Death and Protection. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 343–348. [Google Scholar] [CrossRef]
- Wang, J.; Ruel, J.; Ladrech, S.; Bonny, C.; van de Water, T.R.; Puel, J.L. Inhibition of the c-Jun N-Terminal Kinase-Mediated Mitochondrial Cell Death Pathway Restores Auditory Function in Sound-Exposed Animals. Mol. Pharmacol. 2007, 71, 654–666. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of Interleukin-6 Signaling Suppressed Cochlear Inflammatory Response and Improved Hearing Impairment in Noise-Damaged Mice Cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M.; Wang, X.; Barkdull, G.C. Tumor Necrosis Factor Alpha Can Induce Recruitment of Inflammatory Cells to the Cochlea. Otol. Neurotol. 2008, 29, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Nuttall, A.L.; Scheibe, F.; Miller, J.M. Sound-Induced Artifact in Cochlear Blood Flow Measurements Using the Laser Doppler Flowmeter. Hear. Res. 1987, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Quirk, W.S.; Shirwany, N.A. Mechanisms of Alterations in the Microcirculation of the Cochlea. Ann. N. Y. Acad. Sci. 1999, 884, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Yan, D. Ageing and Hearing Loss. J. Pathol. 2007, 211, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Mulrow, C.D.; Lichtenstein, M.J. Screening for Hearing Impairment in the Elderly: Rationale and Strategy. J. Gen. Intern. Med. 1991, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Gratton, M.A.; Vázquez, A.E. Age-Related Hearing Loss: Current Research. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.N.; Hanebuth, D.; Probst, R. Prevalence of Age-Related Hearing Loss in Europe: A Review. Eur. Arch. Otorhinolaryngol. 2011, 268, 1101–1107. [Google Scholar] [CrossRef]
- Schuknecht, H.F.; Gacek, M.R. Cochlear Pathology in Presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 1–16. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Boja, E.S.; Tan, W.; Tekle, E.; Fales, H.M.; English, S.; Mieyal, J.J.; Chock, P.B. Reversible Glutathionylation Regulates Actin Polymerization in A431 Cells. J. Biol. Chem. 2001, 276, 47763–47766. [Google Scholar] [CrossRef] [PubMed]
- Rokutan, K.; Johnston, R.B., Jr.; Kawai, K. Oxidative Stress Induces S-thiolation of Specific Proteins in Cultured Gastric Mucosal Cells. Am. J. Physiol. 1994, 266, G247–G254. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Ashraf, S.S.; Rokutan, K.; Johnston, R.B., Jr.; Thomas, J.A. S-thiolation of Individual Human Neutrophil Proteins Including Actin by Stimulation of the Respiratory Burst: Evidence Against a Role for Glutathione Disulfide. Arch. Biochem. Biophys. 1994, 310, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M.; Chung, Y.H.; Kim, M.J.; Lee, E.Y.; Kim, E.G.; Cha, C.I. Age-Related Changes in the Distribution of Nitrotyrosine in the Cerebral Cortex and Hippocampus of Rats. Brain Res. 2002, 931, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Bai, U.; Khan, M.J.; Quirk, W.S. Mitochondrial DNA Deletions Associated with Aging and Presbyacusis. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Khan, M.J.; Bai, U.; Shirwany, N.; Quirk, W.S. Biologic Activity of Mitochondrial Metabolites on Aging and Age-Related Hearing Loss. Am. J. Otol. 2000, 21, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Crawley, B.K.; Keithley, E.M. Effects of Mitochondrial Mutations on Hearing and Cochlear Pathology with Age. Hear. Res. 2011, 280, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.R.; Kuo, M.W.; Stanton, S.G.; Canlon, B.; Krieg, E.; Alagramam, K.N. N-acetyl L-cysteine Does Not Protect Against Premature Age-Related Hearing Loss in C57BL/6J Mice: A Pilot Study. Hear. Res. 2007, 226, 203–208. [Google Scholar] [CrossRef]
- Someya, S.; Xu, J.; Kondo, K.; Ding, D.; Salvi, R.J.; Yamasoba, T.; Rabinovitch, P.S.; Weindruch, R.; Leeuwenburgh, C.; Tanokura, M.; et al. Age-Related Hearing Loss in C57BL/6J Mice Is Mediated by Bak-Dependent Mitochondrial Apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19432–19437. [Google Scholar] [CrossRef]
- Wilson, B.S.; Tucci, D.L.; Merson, M.H.; O’Donoghue, G.M. Global Hearing Health Care: New Findings and Perspectives. Lancet 2017, 390, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C. Noise-Induced Hearing Loss: Permanent Versus Temporary Threshold Shifts and the Effects of Hair Cell Versus Neuronal Degeneration. Adv. Exp. Med. Biol. 2016, 875, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The More the Worse: The Grade of Noise-Induced Hearing Loss Associates with the Severity of Tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Primary Neural Degeneration in Noise-Exposed Human Cochleas: Correlations with Outer Hair Cell Loss and Word-Discrimination Scores. J. Neurosci. 2021, 41, 4439–4447. [Google Scholar] [CrossRef]
- Spoendlin, H. Primary Structural Changes in the Organ of Corti After Acoustic Overstimulation. Acta Otolaryngol. 1971, 71, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Nuttall, A.L. Laser Doppler Measurements of Cochlear Blood Flow During Loud Sound Exposure in the Guinea Pig. Hear. Res. 1987, 27, 1–10. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Yamashita, D.; Minami, S.B.; Yamasoba, T.; Miller, J.M. Mechanisms of Noise-Induced Hearing Loss Indicate Multiple Methods of Prevention. Hear. Res. 2007, 226, 22–43. [Google Scholar] [CrossRef]
- Quirk, W.S.; Avinash, G.; Nuttall, A.L.; Miller, J.M. The Influence of Loud Sound on Red Blood Cell Velocity and Blood Vessel Diameter in the Cochlea. Hear. Res. 1992, 63, 102–107. [Google Scholar] [CrossRef]
- Sahley, T.L.; Anderson, D.J.; Hammonds, M.D.; Chandu, K.; Musiek, F.E. Evidence for a Dynorphin-Mediated Inner Ear Immune/Inflammatory Response and Glutamate-Induced Neural Excitotoxicity: An Updated Analysis. J. Neurophysiol. 2019, 122, 1421–1460. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of Free Radicals in the Guinea Pig Inner Ear After Noise-Induced Acoustic Trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Borg, E.; Canlon, B.; Engström, B. Noise-Induced Hearing Loss. Literature Review and Experiments in Rabbits. Morphological and Electrophysiological Features, Exposure Parameters and Temporal Factors, Variability and Interactions. Scand. Audiol. Suppl. 1995, 40, 1–147. [Google Scholar] [PubMed]
- Klein, B.E.; Cruickshanks, K.J.; Nondahl, D.M.; Klein, R.; Dalton, D.S. Cataract and Hearing Loss in a Population-Based Study: The Beaver Dam Studies. Am. J. Ophthalmol. 2001, 132, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Helzner, E.P.; Cauley, J.A.; Pratt, S.R.; Wisniewski, S.R.; Zmuda, J.M.; Talbott, E.O.; de Rekeneire, N.; Harris, T.B.; Rubin, S.M.; Simonsick, E.M.; et al. Race and Sex Differences in Age-Related Hearing Loss: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005, 53, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Widén, S.E.; Erlandsson, S.I. The Influence of Socio-economic Status on Adolescent Attitude to Social Noise and Hearing Protection. Noise Health 2004, 7, 59–70. [Google Scholar] [PubMed]
- Cruickshanks, K.J.; Klein, R.; Klein, B.E.K.; Wiley, T.L.; Nondahl, D.M.; Tweed, T.S. Cigarette Smoking and Hearing Loss: The Epidemiology of Hearing Loss Study. JAMA 1998, 279, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Kolkhorst, F.W.; Smaldino, J.J.; Wolf, S.C.; Battani, L.R.; Plakke, B.L.; Huddleston, S.; Hensley, L.D. Influence of Fitness on Susceptibility to Noise-Induced Temporary Threshold Shift. Med. Sci. Sports Exerc. 1998, 30, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Lusk, S.L.; Hagerty, B.M.; Gillespie, B.; Caruso, C.C. Chronic Effects of Workplace Noise on Blood Pressure and Heart Rate. Arch. Environ. Health 2002, 57, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early Elevation of Cochlear Reactive Oxygen Species Following Noise Exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Cobley, J.N. Mechanisms of Mitochondrial ROS Production in Assisted Reproduction: The Known, the Unknown, and the Intriguing. Antioxidants 2020, 9, 933. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, H.; Pang, J.; Su, Z.; Lai, L.; Lin, H.; Jian, B.; He, W.; Yang, H.; Zheng, Y. Nrf2 Activation Protects Auditory Hair Cells from Cisplatin-Induced Ototoxicity Independent on Mitochondrial ROS Production. Toxicol. Lett. 2020, 331, 1–10. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.Y.; Schacht, J.; Miller, J.M. Delayed Production of Free Radicals Following Noise Exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Brown, J.N.; Schacht, J. 8-iso-Prostaglandin F(2alpha), a Product of Noise Exposure, Reduces Inner Ear Blood Flow. Audiol. Neurootol. 2003, 8, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Miller, J.M.; Altschuler, R.A.; Schacht, J. Intense Noise Induces Formation of Vasoactive Lipid Peroxidation Products in the Cochlea. Brain Res. 2000, 878, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory Cytokines Expression in Noise-Induced Damaged Cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, S.V.; Sato, K.; Pham, L.; Billings, P.; Keithley, E.M. Immune Cell Recruitment Following Acoustic Trauma. Hear. Res. 2006, 222, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of Noise-Induced Cellular Injury and Repair in the Mouse Cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Miller, J.M.; Schacht, J. Protection from Noise-Induced Lipid Peroxidation and Hair Cell Loss in the Cochlea. Brain Res. 2003, 966, 265–273. [Google Scholar] [CrossRef]
- Duan, M.; Qiu, J.; Laurell, G.; Olofsson, A.; Counter, S.A.; Borg, E. Dose and Time-Dependent Protection of the Antioxidant N-L-acetylcysteine Against Impulse Noise Trauma. Hear. Res. 2004, 192, 1–9. [Google Scholar] [CrossRef]
- Kopke, R.D.; Weisskopf, P.A.; Boone, J.L.; Jackson, R.L.; Wester, D.C.; Hoffer, M.E.; Lambert, D.C.; Charon, C.C.; Ding, D.L.; McBride, D. Reduction of Noise-Induced Hearing Loss Using L-NAC and Salicylate in the Chinchilla. Hear. Res. 2000, 149, 138–146. [Google Scholar] [CrossRef]
- Mukherjea, D.; Ghosh, S.; Bhatta, P.; Sheth, S.; Tupal, S.; Borse, V.; Brozoski, T.; Sheehan, K.E.; Rybak, L.P.; Ramkumar, V.V. Early Investigational Drugs for Hearing Loss. Expert Opin. Investig. Drugs 2015, 24, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Hammill, T.L.; Campbell, K.C. Protection for Medication-Induced Hearing Loss: The State of the Science. Int. J. Audiol. 2018, 57, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. Influence of Genotype and Age on Acute Acoustic Trauma and Recovery in CBA/Ca and C57BL/6J Mice. Acta Otolaryngol. 1992, 112, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Erway, L.C.; Shiau, Y.W.; Davis, R.R.; Krieg, E.F. Genetics of Age-Related Hearing Loss in Mice. III. Susceptibility of Inbred and F1 Hybrid Strains to Noise-Induced Hearing Loss. Hear. Res. 1996, 93, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.R.; Newlander, J.K.; Ling, X.; Cortopassi, G.A.; Krieg, E.F.; Erway, L.C. Genetic Basis for Susceptibility to Noise-Induced Hearing Loss in Mice. Hear. Res. 2001, 155, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Flood, D.G.; Reaume, A.G.; Hoffman, E.K.; Scott, R.W.; Wright, J.S.; Putcha, G.V.; Salvi, R.J. Targeted Deletion of the Cytosolic Cu/Zn-Superoxide Dismutase Gene (Sod1) Increases Susceptibility to Noise-Induced Hearing Loss. Audiol. Neurootol. 1999, 4, 237–246. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Lear, P.M.; Ho, Y.S. Targeted Mutation of the Gene for Cellular Glutathione Peroxidase (Gpx1) Increases Noise-Induced Hearing Loss in Mice. J. Assoc. Res. Otolaryngol. 2000, 1, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kozel, P.J.; Davis, R.R.; Krieg, E.F.; Shull, G.E.; Erway, L.C. Deficiency in Plasma Membrane Calcium ATPase Isoform 2 Increases Susceptibility to Noise-Induced Hearing Loss in Mice. Hear. Res. 2002, 164, 231–239. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and Aminoglycoside Antibiotics: Hearing Loss and Its Prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Konishi, K.; Chang, K.C.; Ohashi, K.; Morisaki, N.; Minowa, Y.; Morimoto, A. Ototoxicity of the Anticancer Drug Cisplatin. An Experimental Study. Acta Otolaryngol. 1982, 93, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.A.; Ikeda, K.; Oshima, T.; Suzuki, M.; Kawase, T.; Kikuchi, T.; Takasaka, T. Cisplatin-Induced Apoptotic Cell Death in Mongolian Gerbil Cochlea. Hear. Res. 2000, 141, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Nakagawa, T.; Kita, T.; Kim, T.S.; Iguchi, F.; Endo, T.; Shiga, A.; Lee, S.H.; Ito, J. Mechanisms of Apoptosis Induced by Cisplatin in Marginal Cells in Mouse Stria Vascularis. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2004, 66, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Nakagawa, T.; Kim, T.S.; Iguchi, F.; Endo, T.; Dong, Y.; Yuki, K.; Naito, Y.; Lee, S.H.; Ito, J. A Novel Model for Rapid Induction of Apoptosis in Spiral Ganglions of Mice. Laryngoscope 2003, 113, 994–999. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A.; Mukherjea, D.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Hear. Res. 2007, 226, 157–167. [Google Scholar] [CrossRef] [PubMed]
- García-Berrocal, J.R.; Nevado, J.; Ramírez-Camacho, R.; Sanz, R.; González-García, J.A.; Sánchez-Rodríguez, C.; Cantos, B.; España, P.; Verdaguer, J.M.; Trinidad Cabezas, A. The Anticancer Drug Cisplatin Induces an Intrinsic Apoptotic Pathway Inside the Inner Ear. Br. J. Pharmacol. 2007, 152, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of Organotypic Cultures of Corti’s Organ to Study the Protective Effects of Antioxidant Molecules on Cisplatin-Induced Damage of Auditory Hair Cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar] [PubMed]
- Rybak, L.P.; Ravi, R.; Somani, S.M. Mechanism of Protection by Diethyldithiocarbamate Against Cisplatin Ototoxicity: Antioxidant System. Fundam. Appl. Toxicol. 1995, 26, 293–300. [Google Scholar] [CrossRef]
- Ravi, R.; Somani, S.M.; Rybak, L.P. Mechanism of Cisplatin Ototoxicity: Antioxidant System. Pharmacol. Toxicol. 1995, 76, 386–394. [Google Scholar] [CrossRef]
- Forge, A.; Schacht, J. Aminoglycoside Antibiotics. Audiol. Neurootol. 2000, 5, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.A.; Schmitt, B.A.; Seligsohn, R.; Matz, G.J. Comparative Study of Ototoxicity and Nephrotoxicity in Patients Randomly Assigned to Treatment with Amikacin or Gentamicin. Am. J. Med. 1986, 80, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Noack, V.; Pak, K.; Jalota, R.; Kurabi, A.; Ryan, A.F. An Antioxidant Screen Identifies Candidates for Protection of Cochlear Hair Cells from Gentamicin Toxicity. Front. Cell. Neurosci. 2017, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Rauen, U.; de Groot, H.; Lautermann, J. Involvement of the Mitochondrial Permeability Transition in Gentamicin Ototoxicity. Hear. Res. 2002, 169, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Marcotti, W.; Van Netten, S.M.; Kros, C.J. The Aminoglycoside Antibiotic Dihydrostreptomycin Rapidly Enters Mouse Outer Hair Cells Through the Mechano-Electrical Transducer Channels. J. Physiol. 2005, 567, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Tono, T.; Kiyomizu, K.; Matsuda, K.; Komune, S.; Usami, S.; Abe, S.; Shinkawa, H. Different Clinical Characteristics of Aminoglycoside-Induced Profound Deafness with and without the 1555 A→G Mitochondrial Mutation. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2001, 63, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, M.A.; Derakhshandeh-Peykar, P.; Houshmand, M.; Farhadi, M.; Shojaei, A.; Fallah, M.; Mohammadi, E.; Tajdini, A.; Arastoo, S.; Tavakkoly-Bazzaz, J. Novel Nucleotide Changes in Mutational Analysis of Mitochondrial 12SrRNA Gene in Patients with Nonsyndromic and Aminoglycoside-Induced Hearing Loss. Mol. Biol. Rep. 2013, 40, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.J.; Hale, A.B.; Channon, K.M. Dihydrofolate Reductase Protects Endothelial Nitric Oxide Synthase from Uncoupling in Tetrahydrobiopterin Deficiency. Free Radic. Biol. Med. 2011, 50, 1639–1646. [Google Scholar] [CrossRef]
- Rasola, A.; Bernardi, P. The Mitochondrial Permeability Transition Pore and Its Involvement in Cell Death and in Disease Pathogenesis. Apoptosis 2007, 12, 815–833. [Google Scholar] [CrossRef]
- Yu, N.; Wang, S.; Wang, P.; Li, Y.; Li, S.; Wang, L.; Chen, H.; Wang, Y. The Calcium Uniporter Regulates the Permeability Transition Pore in Isolated Cortical Mitochondria. Neural Regen. Res. 2012, 7, 109–113. [Google Scholar] [CrossRef]
- Moens, A.L.; Claeys, M.J.; Timmermans, J.P.; Vrints, C.J. Myocardial Ischemia/Reperfusion-Injury, a Clinical View on a Complex Pathophysiological Process. Int. J. Cardiol. 2005, 100, 179–190. [Google Scholar] [CrossRef]
- Shuvy, M.; Atar, D.; Gabriel Steg, P.; Halvorsen, S.; Jolly, S.; Yusuf, S.; Lotan, C. Oxygen Therapy in Acute Coronary Syndrome: Are the Benefits Worth the Risk? Eur. Heart J. 2013, 34, 1630–1635. [Google Scholar] [CrossRef]
- Kontos, H.A.; Wei, E.P. Hydroxyl Radical-Dependent Inactivation of Guanylate Cyclase in Cerebral Arterioles by Methylene Blue and by LY83583. Stroke 1993, 24, 427–434. [Google Scholar] [CrossRef]
- Morizane, I.; Hakuba, N.; Hyodo, J.; Shimizu, Y.; Fujita, K.; Yoshida, T.; Gyo, K. Ischemic Damage Increases Nitric Oxide Production via Inducible Nitric Oxide Synthase in the Cochlea. Neurosci. Lett. 2005, 391, 62–67. [Google Scholar] [CrossRef]
- Ogawa, H.; Okada, M.; Shudou, M.; Gyo, K.; Hato, N. Prevention of Ischemia-Induced Hearing Loss by Intravenous Administration of Hydrogen-Rich Saline in Gerbil. Neurosci. Lett. 2018, 665, 195–199. [Google Scholar] [CrossRef]
- Ackah, S.E.H.; Juhn, S.K.; Huang, T.C.; Wiedmann, T.S. A Combination Antioxidant Therapy Prevents Age-Related Hearing Loss in C57BL/6 Mice. Otolaryngol. Head Neck Surg. 2010, 143, 429–434. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. Role of Oxidative Stress and Antioxidants in Acquired Inner Ear Disorders. Antioxidants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Husain, K.; Whitworth, C.; Somani, S.M.; Rybak, L.P. Partial Protection by Lipoic Acid Against Carboplantin-Induced Ototoxicity in Rats. Biomed. Environ. Sci. 2005, 18, 198–206. [Google Scholar] [PubMed]
- Aabdallah, D.M.; Eid, N.I. Possible Neuroprotective Effects of Lecithin and Alpha-Tocopherol Alone or in Combination against Ischemia/Reperfusion Insult in Rat Brain. J. Biochem. Mol. Toxicol. 2004, 18, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Joachims, H.Z.; Segal, J.; Golz, A.; Netzer, A.; Goldenberg, D. Antioxidants in Treatment of Idiopathic Sudden Hearing Loss. Otol. Neurotol. 2003, 24, 572–575. [Google Scholar] [CrossRef]
- Brown, B.G.; Zhao, X.Q.; Chait, A.; Fisher, L.D.; Cheung, M.C.; Morse, J.S.; Dowdy, A.A.; Marino, E.K.; Bolson, E.L.; Alaupovic, P.; et al. Simvastatin and Niacin, Antioxidant Vitamins, or the Combination for the Prevention of Coronary Disease. N. Engl. J. Med. 2001, 345, 1583–1592. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Kopke, R.D.; Jackson, R.L.; Coleman, J.K.; Liu, J.; Henderson, D. Noise Protection with N-Acetyl-l-Cysteine (NAC) Using a Variety of Noise Exposures, NAC Doses, and Routes of Administration. Acta Otolaryngol. 2007, 127, 914–919. [Google Scholar] [CrossRef]
- Coleman, J.; Huang, X.; Liu, J.; Kopke, R.; Jackson, R. Dosing Study on the Effectiveness of Salicylate/N-Acetylcysteine for Prevention of Noise-Induced Hearing Loss. Noise Health 2010, 12, 159–165. [Google Scholar] [CrossRef]
- Clifford, R.E.; Coleman, J.K.; Balough, B.J.; Liu, J.; Kopke, R.D.; Jackson, R.L. Low-Dose D-methionine and N-acetyl-L-cysteine for Protection from Permanent Noise-Induced Hearing Loss in Chinchillas. Otolaryngol. Head Neck Surg. 2011, 145, 999–1006. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Ralli, M.; Sergi, B.; Parrilla, C.; Troiani, D.; Paludetti, G. Protective Effects of N-Acetylcysteine on Noise-Induced Hearing Loss in Guinea Pigs. Acta Otorhinolaryngol. Ital. 2009, 29, 70–75. [Google Scholar]
- Lorito, G.; Giordano, P.; Petruccelli, J.; Martini, A.; Hatzopoulos, S. Different Strategies in Treating Noiseinduced Hearing Loss with N-Acetylcysteine. Med. Sci. Monit. 2008, 14, BR159–BR164. [Google Scholar]
- Samson, J.; Wiktorek-Smagur, A.; Politanski, P.; Rajkowska, E.; Pawlaczyk-Luszczynska, M.; Dudarewicz, A.; Sha, S.H.; Schacht, J.; Sliwinska-Kowalska, M. Noise-Induced Time-Dependent Changes in Oxidative Stress in the Mouse Cochlea and Attenuation by D-methionine. Neuroscience 2008, 152, 146–150. [Google Scholar] [CrossRef]
- Kundu, S.; Munjal, C.; Tyagi, N.; Sen, U.; Tyagi, A.C.; Tyagi, S.C. Folic Acid Improves Inner Ear Vascularization in Hyperhomocysteinemic Mice. Hear. Res. 2012, 284, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Wang, S.; Zhai, S.; Hu, Y.; Yang, W.; He, L. Effects of Alpha-Tocopherol on Noise-Induced Hearing Loss in Guinea Pigs. Hear. Res. 2003, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scholik, A.R.; Lee, U.S.; Chow, C.K.; Yan, H.Y. Dietary Vitamin E Protects the Fathead Minnow, Pimephales promelas, Against Noise Exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 137, 313–323. [Google Scholar] [CrossRef]
- Kalkanis, J.G.; Whitworth, C.; Rybak, L.P. Vitamin E Reduces Cisplatin Ototoxicity. Laryngoscope 2004, 114, 538–542. [Google Scholar] [CrossRef]
- Teranishi, M.A.; Nakashima, T. Effects of Trolox, Locally Applied on Round Windows, on Cisplatin-Induced Ototoxicity in Guinea Pigs. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 133–139. [Google Scholar] [CrossRef]
- Sergi, B.; Fetoni, A.R.; Ferraresi, A.; Troiani, D.; Azzena, G.B.; Paludetti, G.; Maurizi, M. The Role of Antioxidants in Protection from Ototoxic Drugs. Acta Oto-Laryngol. Suppl. 2004, 552, 42–45. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Sergi, B.; Ferraresi, A.; Paludetti, G.; Troiani, D. Alpha-Tocopherol Protective Effects on Gentamicin Ototoxicity: An Experimental Study. Int. J. Audiol. 2004, 43, 166–171. [Google Scholar] [CrossRef]
- Sato, K. Pharmacokinetics of Coenzyme Q10 in Recovery of Acute Sensorineural Hearing Loss Due to Hypoxia. Acta Otolaryngol. Suppl. 1988, 458, 95–102. [Google Scholar] [CrossRef]
- Sergi, B.; Fetoni, A.R.; Paludetti, G.; Ferraresi, A.; Navarra, P.; Mordente, A.; Troiani, D. Protective Properties of Idebenone in Noise-Induced Hearing Loss in the Guinea Pig. NeuroReport 2006, 17, 857–861. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Piacentini, R.; Fiorita, A.; Paludetti, G.; Troiani, D. Water-Soluble Coenzyme Q10 Formulation (Q-ter) Promotes Outer Hair Cell Survival in a Guinea Pig Model of Noise Induced Hearing Loss (NIHL). Brain Res. 2009, 1257, 108–116. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Hughes, L.F.; Miller, J.M. Free Radical Scavengers Vitamins A, C, and E plus Magnesium Reduce Noise Trauma. Free Radic. Biol. Med. 2007, 42, 1454–1463. [Google Scholar] [CrossRef]
- Sebastian, C.; Mostoslavsky, R. SIRT3 in Calorie Restriction: Can You Hear Me Now? Cell 2010, 143, 667–668. [Google Scholar] [CrossRef]
- Guarente, L. Mitochondria--A Nexus for Aging, Calorie Restriction, and Sirtuins? Cell 2008, 132, 171–176. [Google Scholar] [CrossRef]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss Under Caloric Restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef]
- Curhan, S.G.; Stankovic, K.M.; Eavey, R.D.; Wang, M.; Stampfer, M.J.; Curhan, G.C. Carotenoids, Vitamin A, Vitamin C, Vitamin E, and Folate and Risk of Self-Reported Hearing Loss in Women. Am. J. Clin. Nutr. 2015, 102, 1167–1175. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Curhan, S.G.; Eavey, R.; Curhan, G.C. A Prospective Study of Vitamin Intake and the Risk of Hearing Loss in Men. Otolaryngol. Head Neck Surg. 2010, 142, 231–236. [Google Scholar] [CrossRef]
- Gopinath, B.; Flood, V.M.; McMahon, C.M.; Burlutsky, G.; Spankovich, C.; Hood, L.J.; Mitchell, P. Dietary Antioxidant Intake Is Associated with the Prevalence but Not Incidence of Age-Related Hearing Loss. J. Nutr. Health Aging 2011, 15, 896–900. [Google Scholar] [CrossRef]
- Kramer, S.; Dreisbach, L.; Lockwood, J.; Baldwin, K.; Kopke, R.; Scranton, S.; O’Leary, M. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J. Am. Acad. Audiol. 2006, 17, 265–278. [Google Scholar] [CrossRef]
- Feldman, L.; Efrati, S.; Eviatar, E.; Abramsohn, R.; Yarovoy, I.; Gersch, E.; Averbukh, Z.; Weissgarten, J. Gentami-cin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007, 72, 359–363. [Google Scholar] [CrossRef]
- Kharkheli, E.; Kevanishvili, Z.; Maglakelidze, T.; Davitashvili, O.; Schacht, J. Does vitamin E prevent gentami-cin-induced ototoxicity? Georgian Med. News. 2007, 146, 14–17. [Google Scholar]
- Yıldırım, M.; Inançlı, H.M.; Samancı, B.; Oktay, M.F.; Enöz, M.; Topçu, I. Preventing cisplatin induced ototoxicity by N-acetylcysteine and salicylate. Kulak Burun Bogaz Ihtis Derg. 2010, 20, 173–183. [Google Scholar]
- Lin, C.Y.; Wu, J.L.; Shih, T.S.; Tsai, P.J.; Sun, Y.M.; Ma, M.C.; Guo, Y.L. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear. Res. 2010, 269, 42–47. [Google Scholar] [CrossRef]
- Tokgoz, B.; Ucar, C.; Kocyigit, I.; Somdas, M.; Unal, A.; Vural, A.; Sipahioglu, M.; Oymak, O.; Utas, C. Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol. Dial. Transplant. 2011, 26, 4073–4078. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Ko, M.T.; Peng, J.P.; Hwang, C.F. Zinc in the treatment of idiopathic sudden sensorineural hearing loss. Laryngoscope 2011, 121, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, M.E.; Balaban, C.; Slade, M.D.; Tsao, J.W.; Hoffer, B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: A double-blind, placebo controlled study. PLoS ONE 2013, 8, e54163. [Google Scholar] [CrossRef] [PubMed]
- Doosti, A.; Lotfi, Y.; Moossavi, A.; Bakhshi, E.; Talasaz, A.H.; Hoorzad, A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014, 16, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Park, J.J.; Ahn, S.K.; Hur, D.G.; Kim, H.Y. Effect of high dose intravenous vitamin C on idiopathic sudden sensorineural hearing loss: A prospective single-blind randomized controlled trial. Eur. Arch. Otorhinolaryngol. 2013, 270, 2631–2636. [Google Scholar] [CrossRef]
- Kopke, R.; Slade, M.D.; Jackson, R.; Hammill, T.; Fausti, S.; Lonsbury-Martin, B.; Sanderson, A.; Dreisbach, L.; Rabinowitz, P.; Torre, P., 3rd; et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: A randomized clinical trial. Hear. Res. 2015, 323, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Zucchella, C.; Cristalli, G.; Galiè, E.; Bianco, F.; Giannarelli, D.; Carpano, S.; Spriano, G.; Pace, A. Vitamin E neuroprotection against cisplatin ototoxicity: Preliminary results from a randomized, placebo-controlled trial. Head Neck 2016, 38 (Suppl. S1), E2118–E2121. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Chen, L.; Krailo, M.D.; Knight, K.; Villaluna, D.; Bliss, B.; Pollock, B.H.; Ramdas, J.; Lange, B.; Van Hoff, D.; et al. Effects of sodium thiosulfate versus observation on devel-opment of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cispla-tin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef]
- Rolland, V.; Meyer, F.; Guitton, M.J.; Bussières, R.; Philippon, D.; Bairati, I.; Leclerc, M.; Côté, M. A randomized controlled trial to test the efficacy of trans-tympanic injections of a sodium thiosulfate gel to prevent cisplatin-induced oto-toxicity in patients with head and neck cancer. J. Otolaryngol. Head Neck Surg. 2019, 48, 4. [Google Scholar] [CrossRef] [PubMed]
| Summary of RCTs of Antioxidants on Hearing Loss in Humans | ||||||
|---|---|---|---|---|---|---|
| Author | Year | Antioxidants | Type of Hearing Loss | Objectives | Sample Size (Patients vs. Control) | Main Findings |
| Kramer S et al. [178] | 2006 | N-acetylcysteine | Loud noise | Normal hearing participants | 31 (N/A) | No statistically significant differences |
| L Feldman et al. [179] | 2007 | N-acetylcysteine | Gentamicin-induced ototoxicity | Hemodialysis patients | 40 (20/20) | Significantly more patients exhibiting ototoxicity in the control group |
| E Kharkheli et al. [180] | 2007 | Vitamin E | Gentamicin-induced ototoxicity | Acute pulmonary infections | 52 (23/29) | No statistically significant differences |
| Yıldırım M et al. [181] | 2010 | Salicylate/N-acetylcysteine | Cisplatin-induced ototoxicity | Solid organ tumors | 54 (18/18/18) | No difference detected between N-acetylcysteine or salicylate |
| Lin CY et al. [182] | 2010 | N-acetylcysteine | Noise-induced temporary threshold shift | Male workers | 53 (25/28) | NAC significantly reduced TTS (p = 0.03) Effects were more prominent both GSTM1-null and GSTT1-null genotypes. |
| Tokgoz B et al. [183] | 2011 | N-acetylcysteine | Ototoxicity drug-induced (Aminoglycosides and vancomycin) | Continuous ambulatory peritoneal dialysis treatment | 60 (30/30) | Patients taking NAC had better hearing function test results 4 weeks after the treatment (p < 0.05) |
| Yang CH et al. [184] | 2011 | Zinc | Idiopathic sudden sensorineural hearing loss | SSNHL patients | 66 (33/33) | A significantly larger hearing gain, an increased percentage of recovery, and an increased rate of successful recovery |
| Hoffer ME et al. [185] | 2013 | N-acetylcysteine | Blast exposure | Active duty service members | 81 (41/40) | In a seven day symptom resolution rate of 86% as compared to 11% |
| Doosti A et al. [186] | 2014 | N-Acetylcysteine/Ginseng | Noise-induced | Textile workers | 48 (16/16/16) | Reduced noise-induced TTS for NAC and ginseng groups at 4, 6 and 16 kHz (p < 0.001) |
| Kang HS et al. [187] | 2014 | Vitamin C | Idiopathic sudden sensorineural hearing loss | SSNHL patients | 67 (35/32) | HDVC group showed significantly greater complete and partial recovery improvement (p = 0.035) |
| Kopke R et al. [188] | 2015 | N-acetylcysteine | Military noise during weapons training | Healthy Marine Corps recruit volunteers | 566 (277/289) | No significant differences were found for the primary outcome |
| Villani V et al. [189] | 2016 | Vitamin E | Cisplatin-induced ototoxicity | Solid malignancies | 23 (13/10) | At 1 month, a significant hearing loss at 2k and 8k HZ k was detected in placebo group |
| Freyer DR et al. [190] | 2017 | Sodium thiosulfate | Cisplatin-induced | Aged 1–18 years with newly diagnosed cancer | 125 (61/64) | The likelihood of hearing loss was significantly lower in the sodium thiosulfate group (p = 0.0036) |
| Kil J et al. [191] | 2017 | Ebselen | Calibrated sound challenge | Healthy adults aged 18–31 years | 83 (22/20/21/20) | Mean TTS at 4 kHz was in the 400 mg ebselen group representing a significant reduction of 68% (p = 0.0025) |
| Brock PR et al. [192] | 2018 | Sodium thiosulfate | Cisplatin-induced ototoxicity | Hepatoblastoma patients | 109 (57/52) | 48% lower incidence of hearing loss in the cisplatin-sodium thiosulfate group (p = 0.002) |
| Rolland V et al. [193] | 2019 | Sodium thiosulfate | Cisplatin-induced ototoxicity | Stage III or IV squamous cell carcinoma | 13 (N/A) | Not statistically nor clinically significant differences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teraoka, M.; Hato, N.; Inufusa, H.; You, F. Role of Oxidative Stress in Sensorineural Hearing Loss. Int. J. Mol. Sci. 2024, 25, 4146. https://doi.org/10.3390/ijms25084146
Teraoka M, Hato N, Inufusa H, You F. Role of Oxidative Stress in Sensorineural Hearing Loss. International Journal of Molecular Sciences. 2024; 25(8):4146. https://doi.org/10.3390/ijms25084146
Chicago/Turabian StyleTeraoka, Masato, Naohito Hato, Haruhiko Inufusa, and Fukka You. 2024. "Role of Oxidative Stress in Sensorineural Hearing Loss" International Journal of Molecular Sciences 25, no. 8: 4146. https://doi.org/10.3390/ijms25084146
APA StyleTeraoka, M., Hato, N., Inufusa, H., & You, F. (2024). Role of Oxidative Stress in Sensorineural Hearing Loss. International Journal of Molecular Sciences, 25(8), 4146. https://doi.org/10.3390/ijms25084146






