Cyclic Nitroxide 4-Methoxy-Tempo May Decrease Serum Amyloid A-Mediated Renal Fibrosis and Reorganise Collagen Networks in Aortic Plaque
Abstract
1. Introduction
2. Results
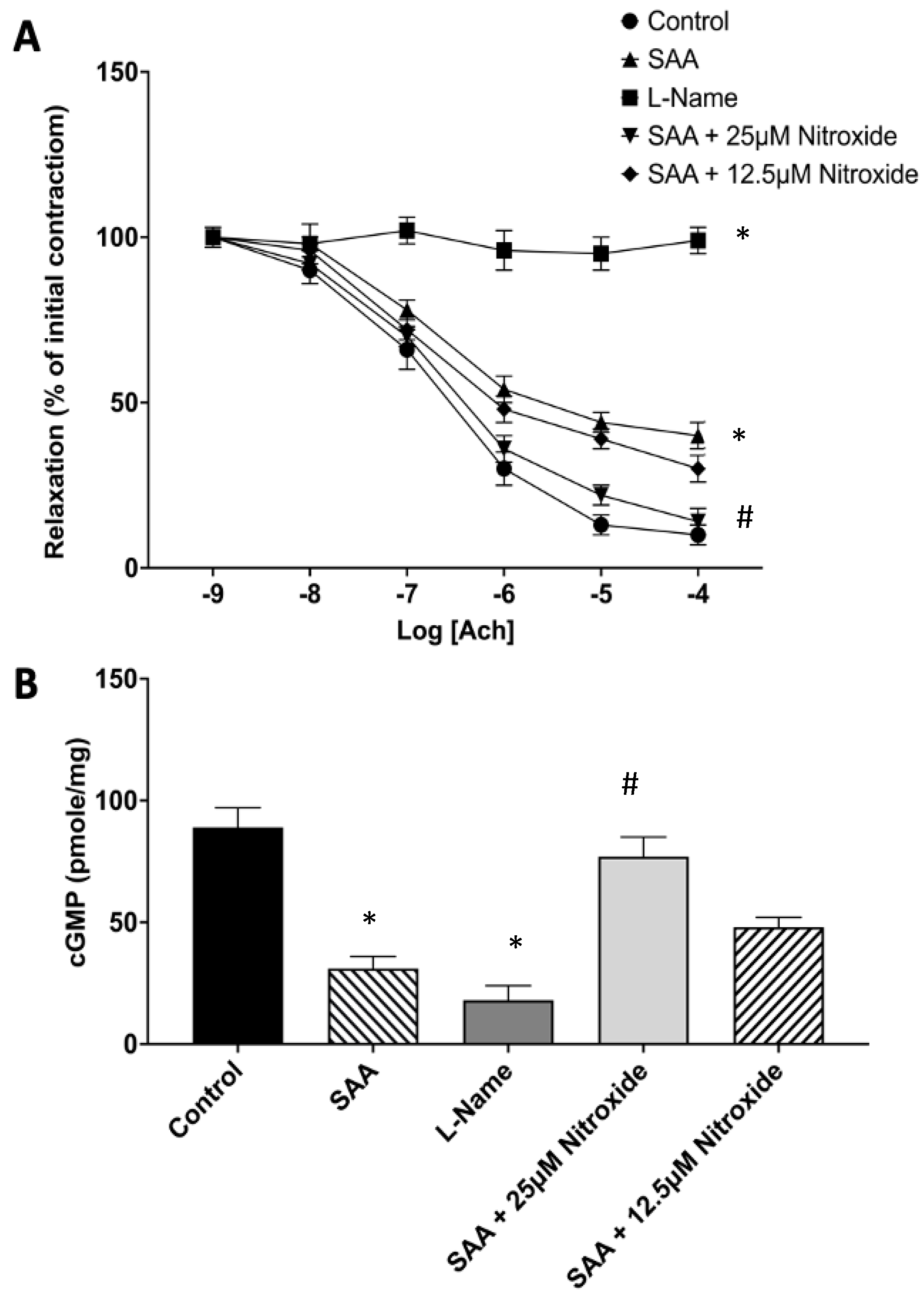
2.1. The Cyclic Nitroxide 4-MetT Inhibits SAA-Mediated Endothelial Dysfunction in Isolated Aortae
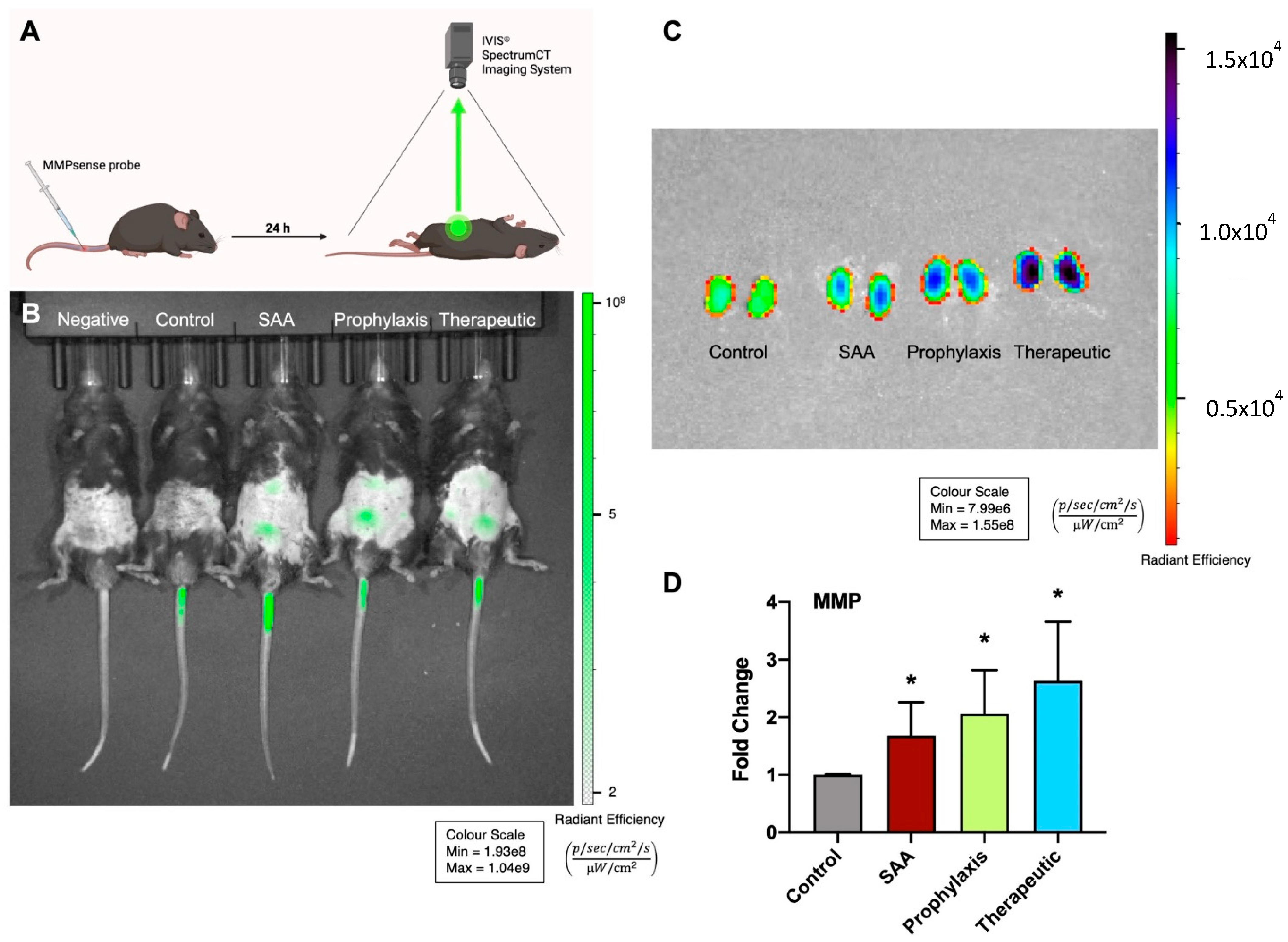
2.2. SAA Stimulates MMP Activity in Mouse Kidneys in the Absence or Presence of 4-MetT
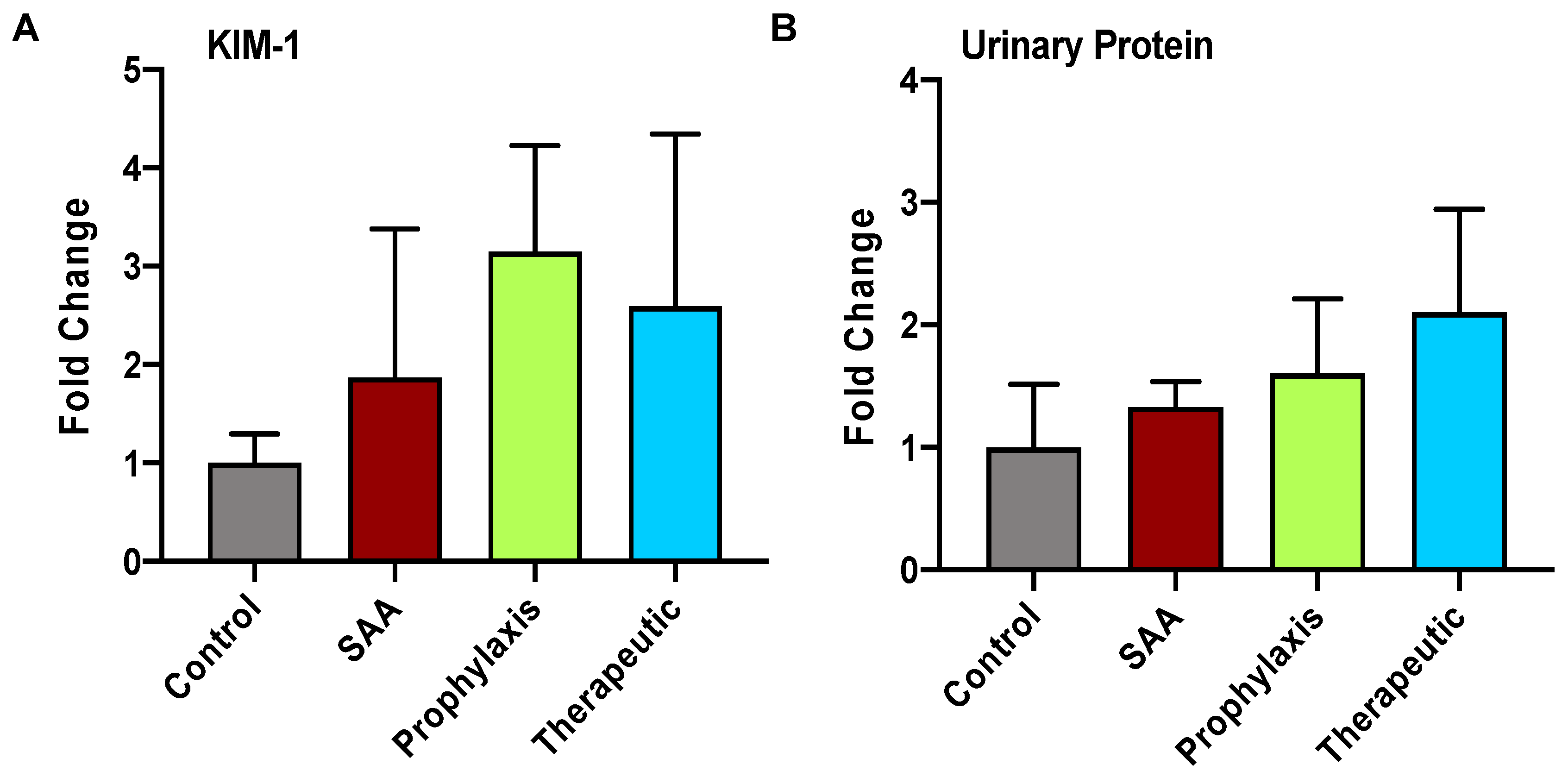
2.3. SAA Elicits Increased Expression of KIM-1 and Total Urinary Protein in the Absence or Presence of 4-MetT
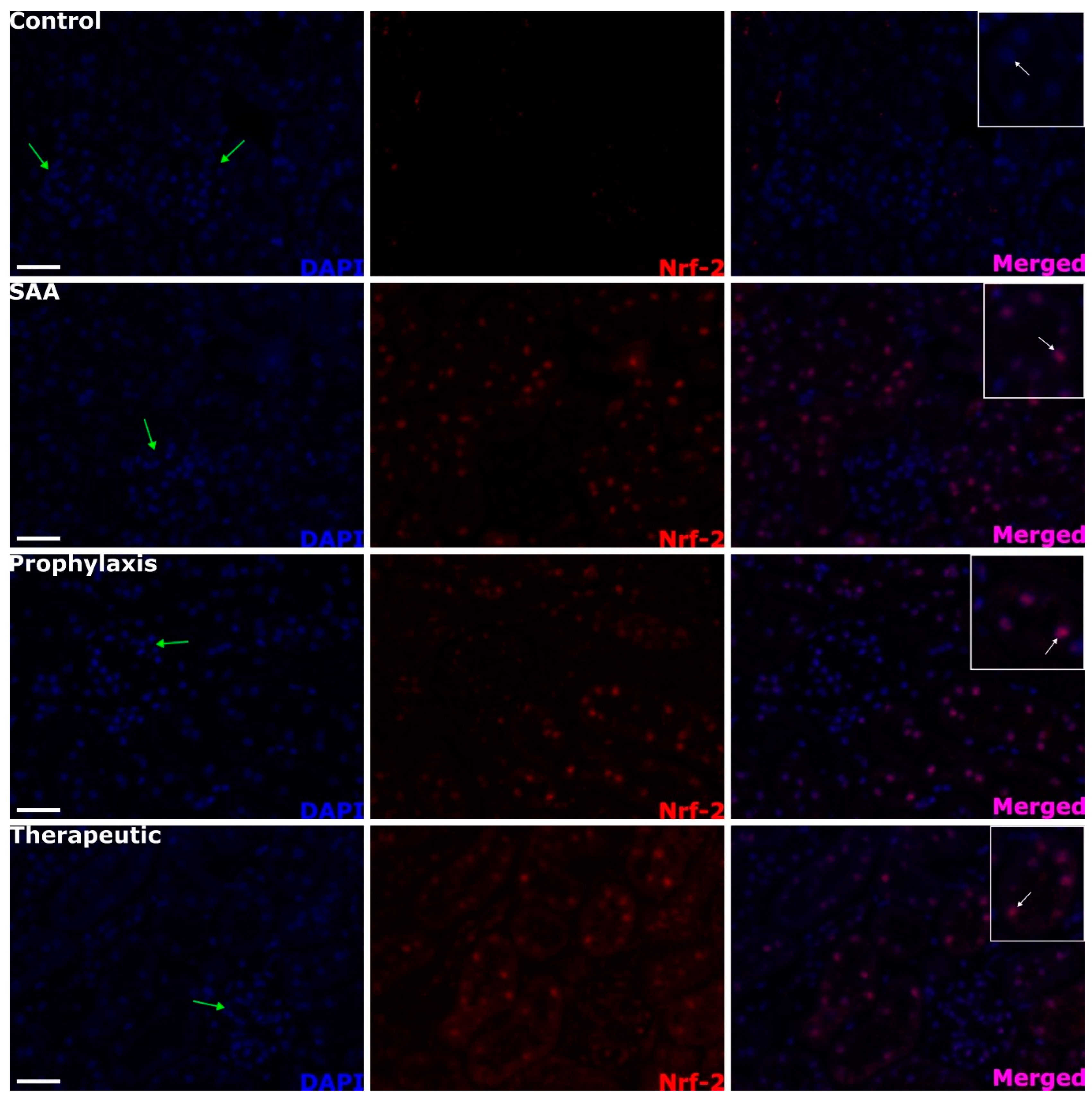
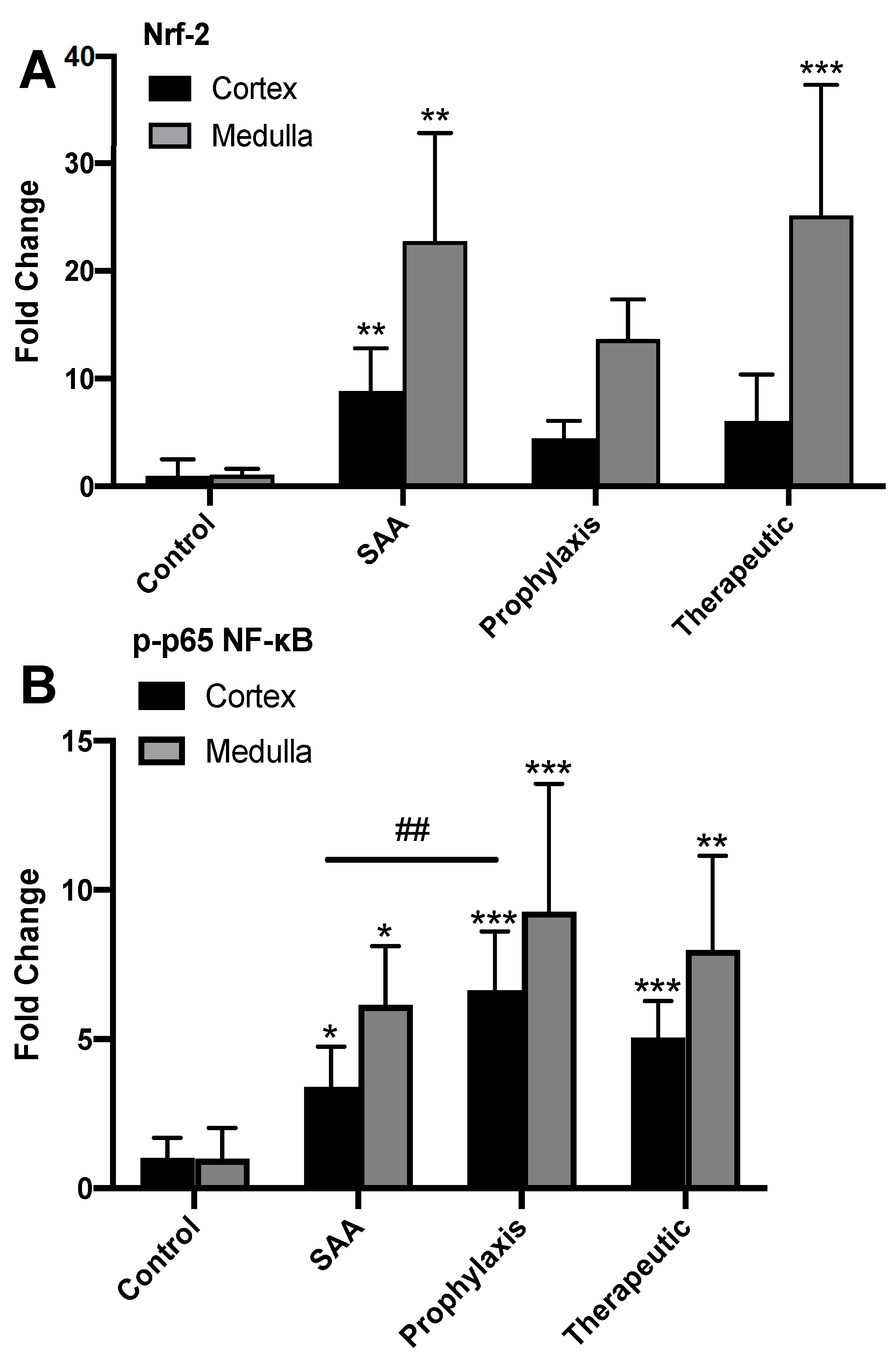
2.4. SAA Stimulates Renal Tubule Epithelium Expression of the Transcription Factor Nuclear Factor Erythroid 2-Related Factor 2 Independent of the Presence or Absence of 4-MetT
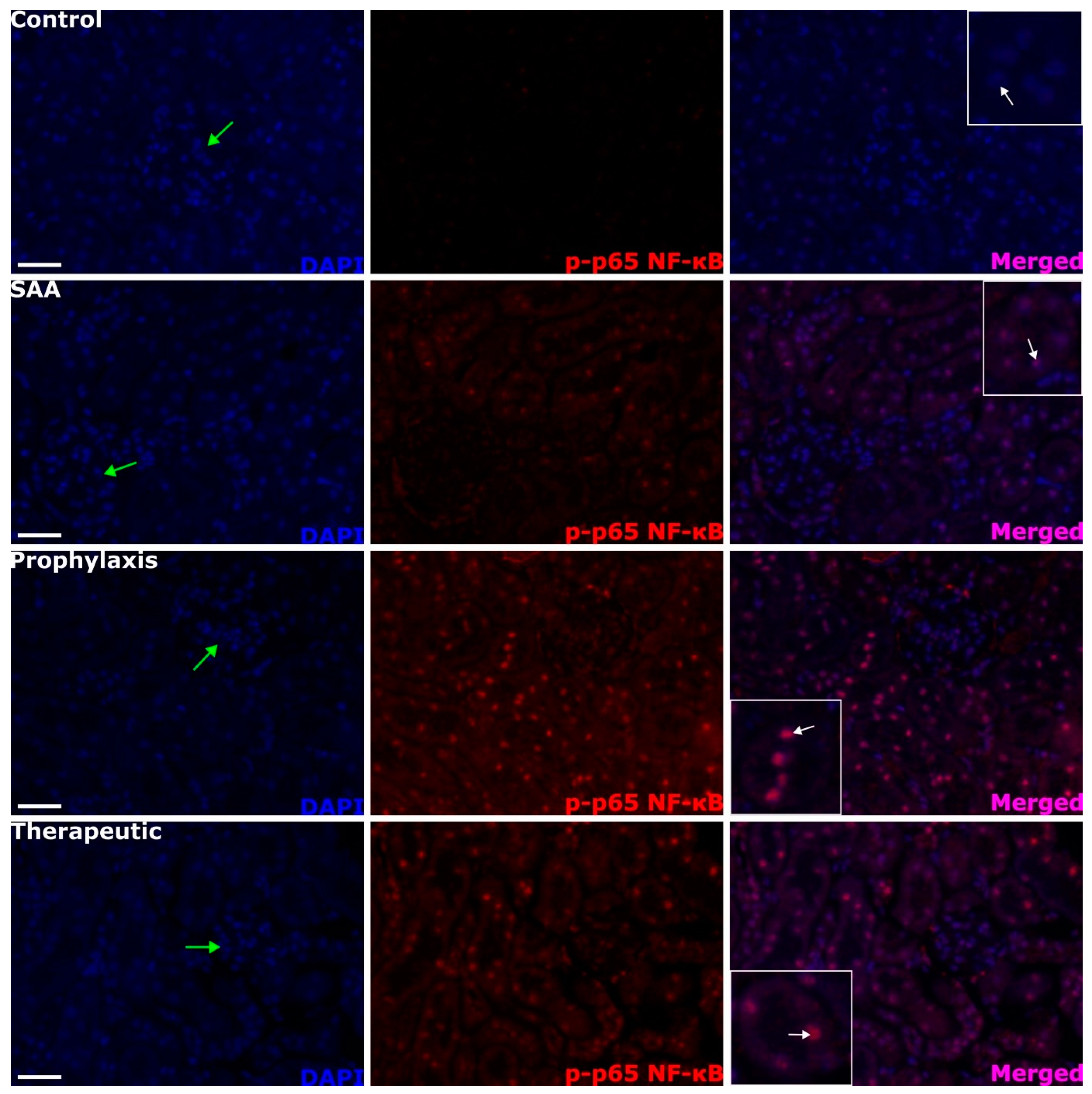
2.5. SAA Stimulates Phospho-NF-kB p-65 Expression in the Renal Tissue Independent of the Presence or Absence of 4-MetT
2.6. Quantifying Renal Expression of Nrf-2 and p-p65 NFκB after Treatment with SAA in the Presence or Absence of 4-MetT
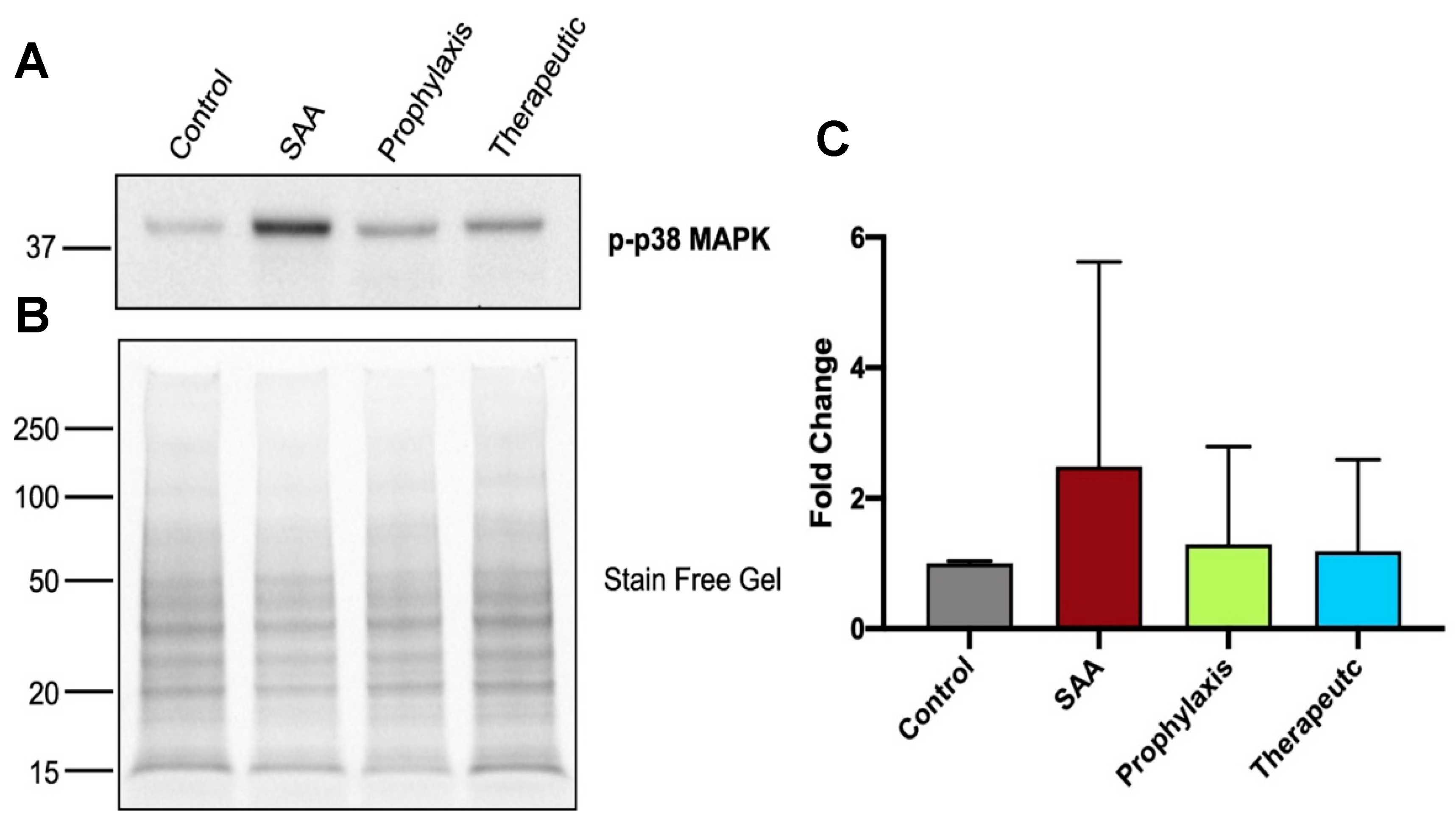
2.7. SAA Marginally Increases p38MAP-Kinase Activation, Whereas Supplementing with 4-MetT Tended to Diminish This SAA Activity
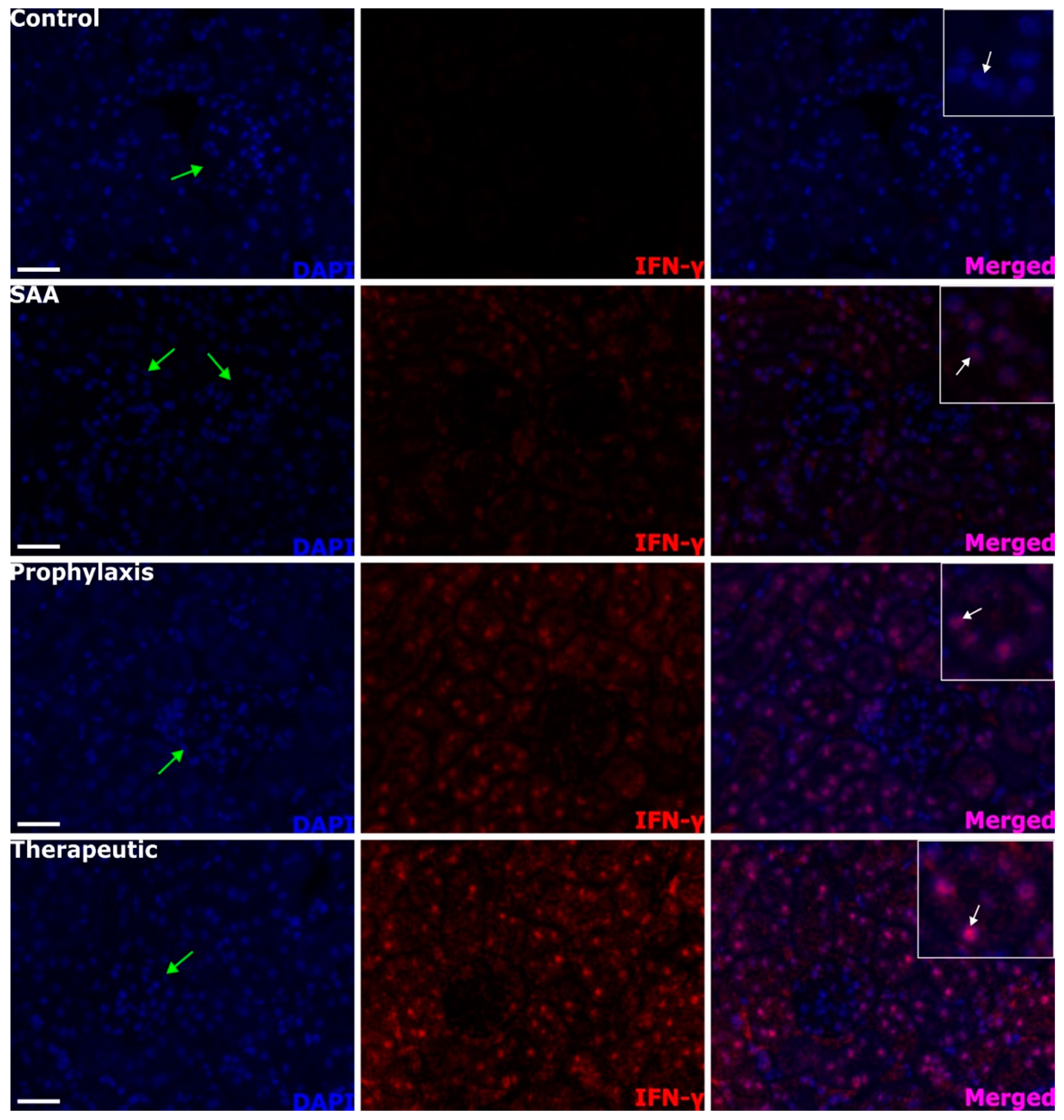
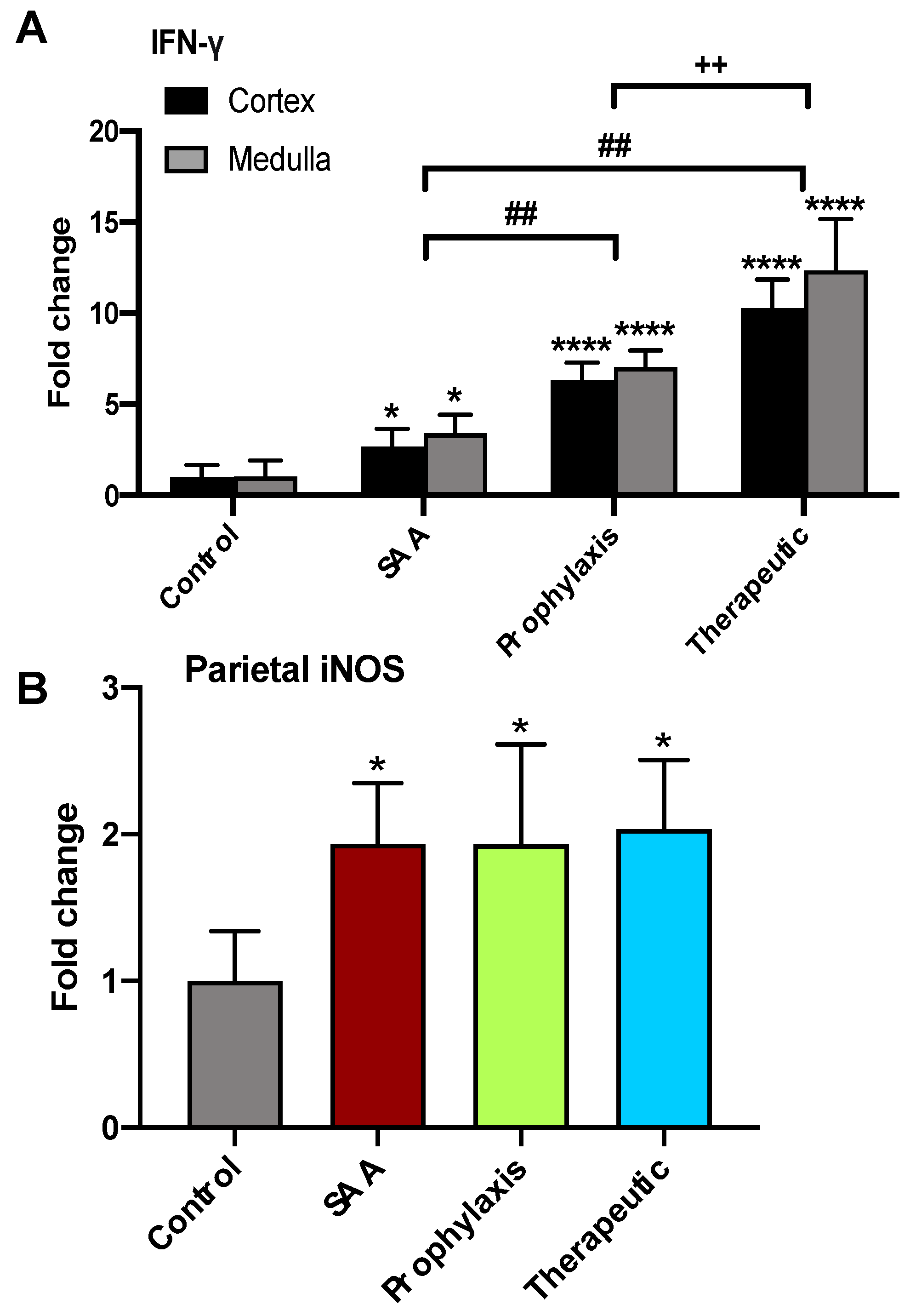
2.8. SAA Stimulates IFN-γ Expression in the Renal Tissue in the Presence or Absence of 4-MetT
2.9. SAA Stimulates Inducible iNOS Expression in the Renal Tissue in the Presence or Absence of 4-MetT
2.10. Quantification of iNOS and IFN-g in the Renal Cortex
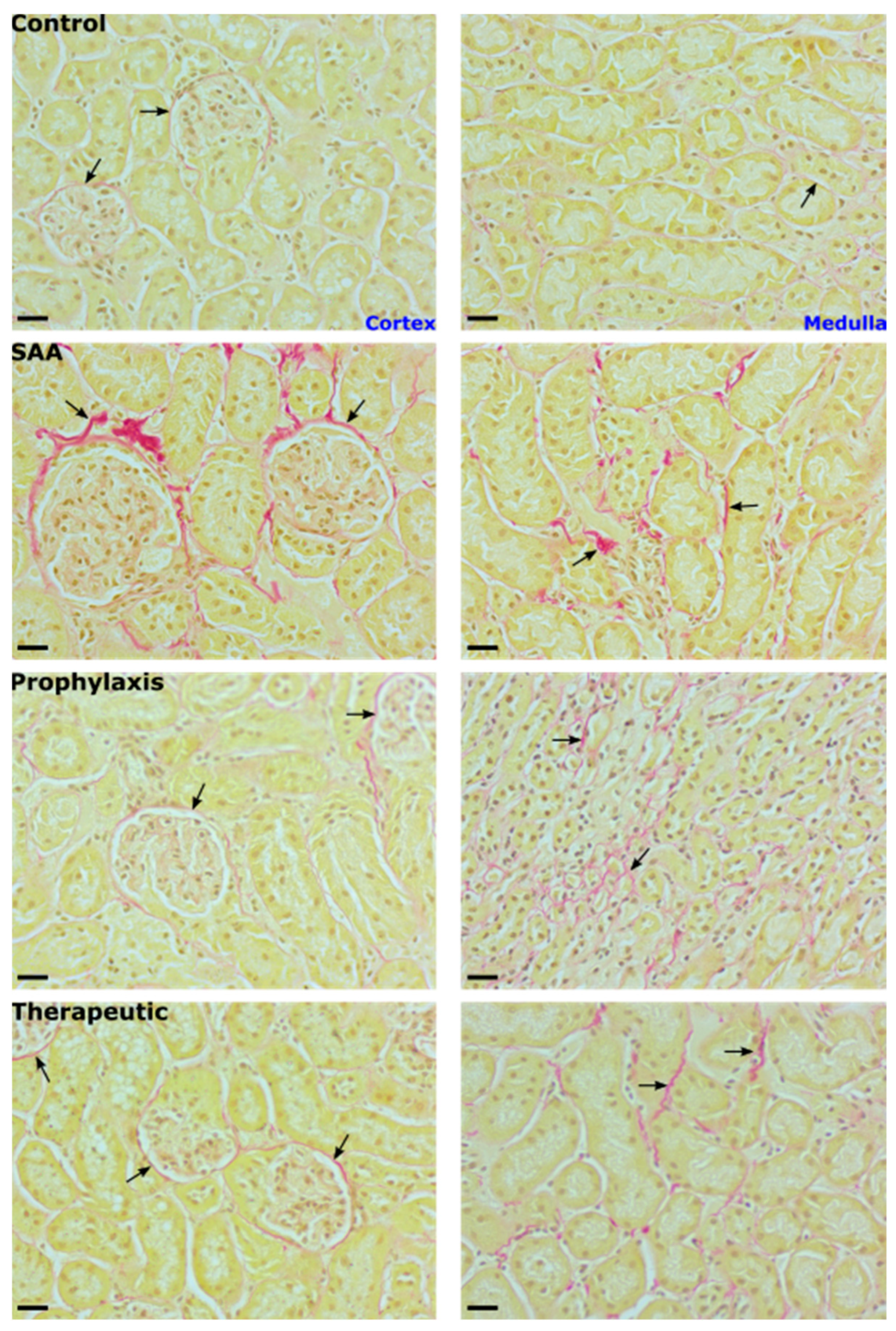
2.11. SAA Administration Leads to Fibrotic Changes to the Mouse Kidney That Are Ameliorated in the Presence of 4-MetT
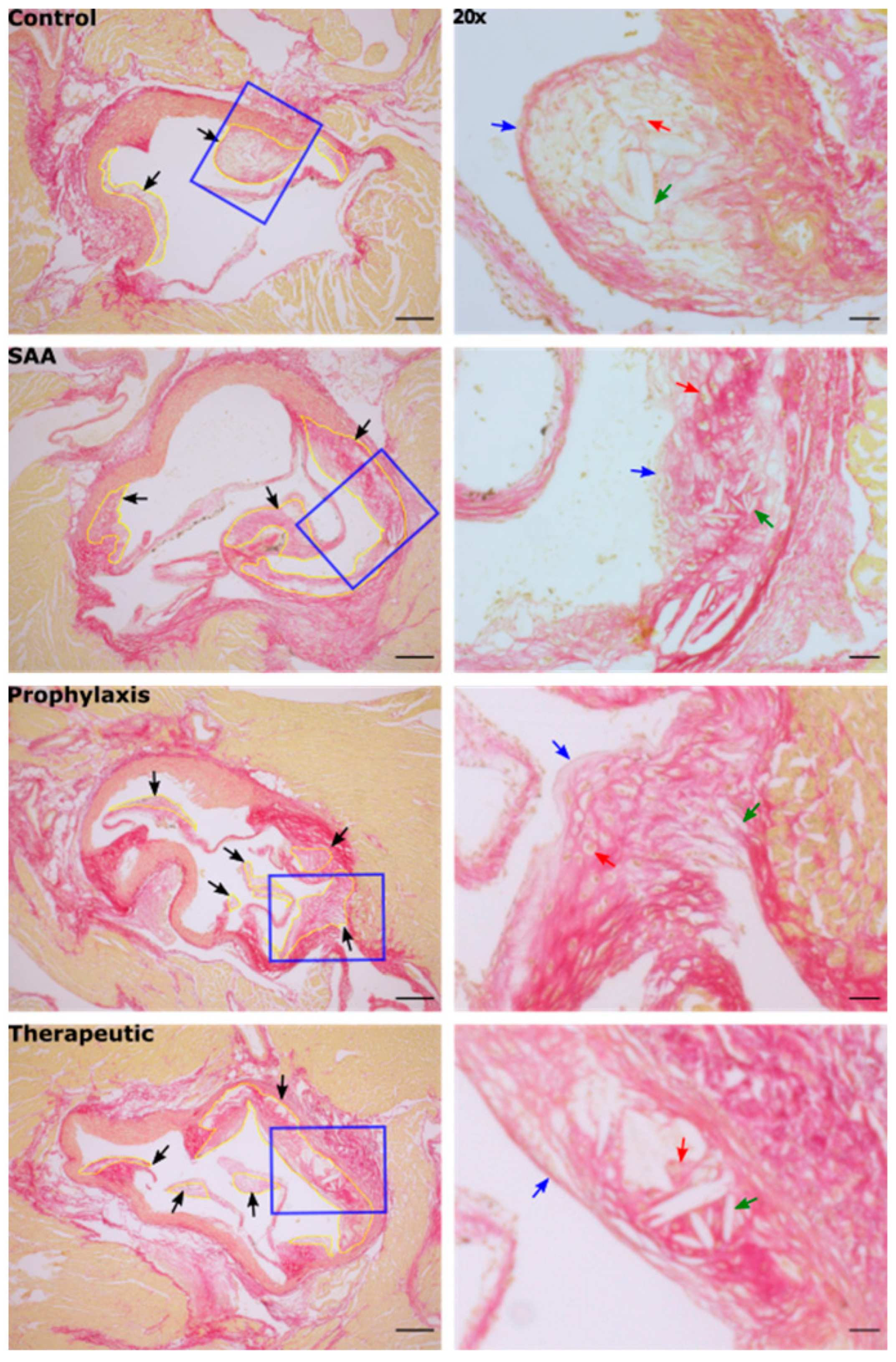
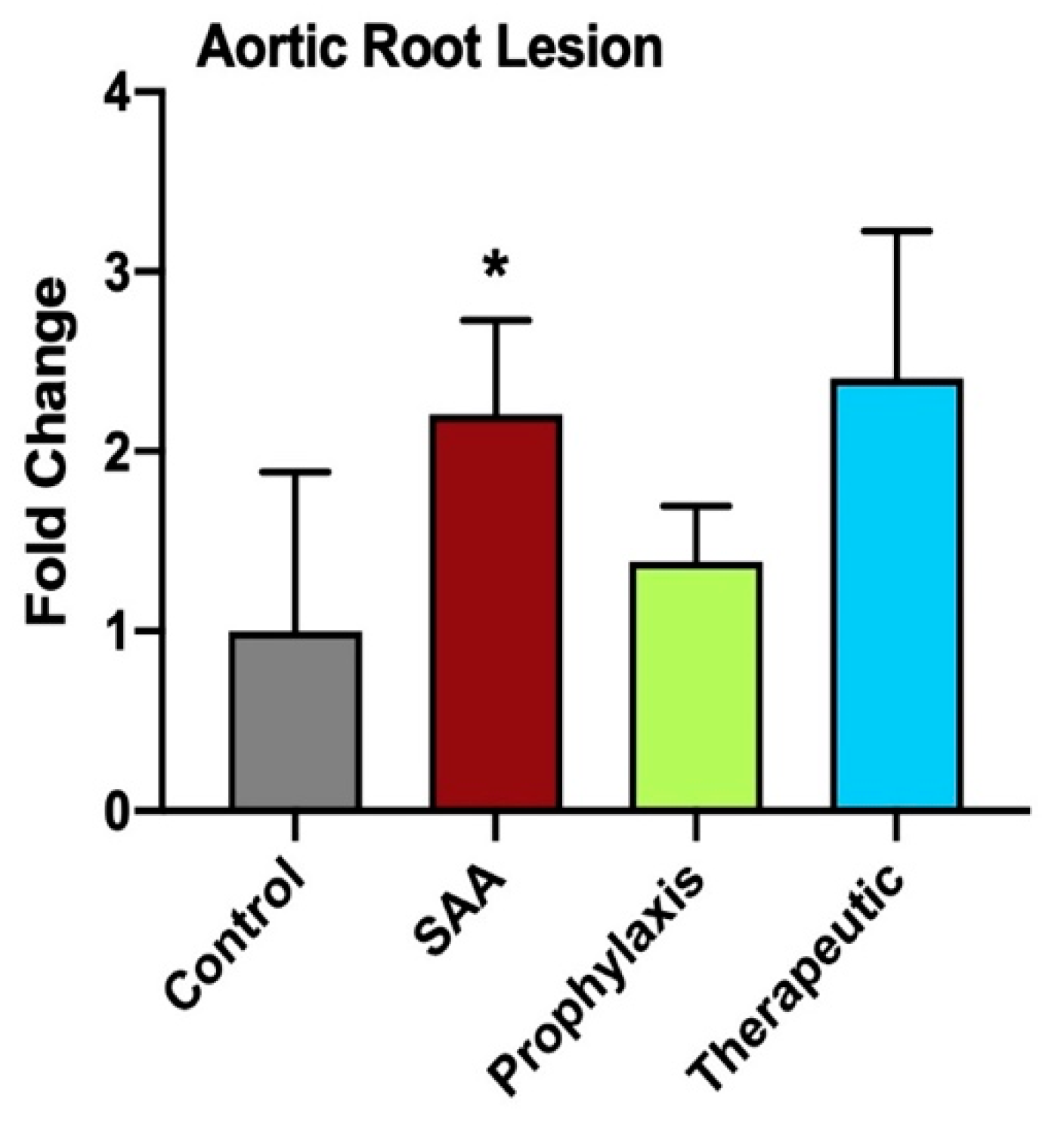
2.12. SAA Enhances Atherosclerotic Lesion Size While 4-MetT Alters the Thickness and Composition of the Aortic Lesion
3. Discussion
4. Methods and Materials
4.1. Vascular Function Studies
4.1.1. Animals
4.1.2. Vascular Reactivity
4.2. Murine Model
4.3. Experimental Design
- (i)
- Vehicle control: ApoE−/− mice were administered 100 µL of phosphate-buffered saline (PBS) via intraperitoneal (i.p.) injection every 3 days for 14 days.
- (ii)
- SAA group: ApoE−/− mice were administered 100 µL of filter-sterilised recombinant human SAA (120 µg/mL) via i.p. injection every 3 days for 14 days. This dose was determined to be equivalent to a short-term moderate level of SAA within the circulation which can further rise in an acute-phase response.
- (iii)
- Prophylaxis group: ApoE−/− mice were administered 100 µL of 4-MetT (15 mg/kg) via i.p. injection daily for 2 weeks followed by 100 µL of SAA (as described above).
- (iv)
- Therapeutic group: ApoE−/− mice were administered 100 µL of SAA (as above) followed by 100 µL of 4-MetT (15 mg/kg) via i.p. injection daily for 2 weeks.
4.4. Live Animal Imaging
4.5. Urine Collection
4.6. Collection of Tissue Specimens
4.7. Tissue Homogenisation
4.8. Biochemical Assays
4.8.1. Vasodilating cGMP
4.8.2. Biomarkers of Renal Injury
4.8.3. Interferon-Gamma (IFN-γ)
4.8.4. Western Blot Studies for p-p38 MAPK
4.9. Immunofluorescence Studies
4.9.1. Immunohistochemistry Studies
4.9.2. Assessing Tissue Fibrosis with Picro-Sirius Red Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef]
- Wanner, C.; Amann, K.; Shoji, T. The heart and vascular system in dialysis. Lancet 2016, 388, 276–284. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Gorevic, P.D. Amyloid and inflammation. Proc. Natl. Acad. Sci. USA 2013, 110, 16291–16292. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; Gabriels, R.Y.; Blokzijl, T.; Buist-Homan, M.; Heegsma, J.; Jansen, B.H.; van Dullemen, H.M.; Festen, E.A.M.; Ter Steege, R.W.F.; et al. A Combined Set of Four Serum Inflammatory Biomarkers Reliably Predicts Endoscopic Disease Activity in Inflammatory Bowel Disease. Front Med. 2019, 6, 251. [Google Scholar] [CrossRef]
- Shen, C.; Sun, X.G.; Liu, N.; Mu, Y.; Hong, C.C.; Wei, W.; Zheng, F. Increased serum amyloid A and its association with autoantibodies, acute phase reactants and disease activity in patients with rheumatoid arthritis. Mol. Med. Rep. 2015, 11, 1528–1534. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; May, K.S.; Zhang, X.S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: Evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Heng, C.K. Impact of serum amyloid A on tissue factor and tissue factor pathway inhibitor expression and activity in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1645–1650. [Google Scholar] [CrossRef]
- Wang, X.; Chai, H.; Wang, Z.; Lin, P.H.; Yao, Q.; Chen, C. Serum amyloid A induces endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2399–H2408. [Google Scholar] [CrossRef]
- De Buck, M.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Opdenakker, G.; Struyf, S.; Van Damme, J. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions During Host Insults. Curr. Med. Chem. 2016, 23, 1725–1755. [Google Scholar] [CrossRef]
- Hayat, S.; Raynes, J.G. Acute phase serum amyloid A protein increases high density lipoprotein binding to human peripheral blood mononuclear cells and an endothelial cell line. Scand. J. Immunol. 2000, 51, 141–146. [Google Scholar] [CrossRef]
- Zhang, X.C.; Chen, J.Q.; Wang, S.X. Serum Amyloid A Induces a Vascular Smooth Muscle Cell Phenotype Switch through the p38 MAPK Signaling Pathway. Biomed. Res. Int. 2017, 2017, 4941379. [Google Scholar] [CrossRef]
- Thompson, J.C.; Jayne, C.; Thompson, J.; Wilson, P.G.; Yoder, M.H.; Webb, N.; Tannock, L.R. A brief elevation of serum amyloid A is sufficient to increase atherosclerosis. J. Lipid Res. 2015, 56, 286–293. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Z.; Wang, Y.; Li, G.; Bai, X. Serum amyloid A and interleukin -1beta facilitate LDL transcytosis across endothelial cells and atherosclerosis via NF-kappaB/caveolin-1/cavin-1 pathway. Atherosclerosis 2023, 375, 87–97. [Google Scholar] [CrossRef]
- Yu, M.H.; Li, X.; Li, Q.; Mo, S.J.; Ni, Y.; Han, F.; Wang, Y.B.; Tu, Y.X. SAA1 increases NOX4/ROS production to promote LPS-induced inflammation in vascular smooth muscle cells through activating p38MAPK/NF-kappaB pathway. BMC Mol. Cell Biol. 2019, 20, 15. [Google Scholar] [CrossRef]
- Oh, H.; Ghosh, S. NF-kappaB: Roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 2013, 252, 41–51. [Google Scholar] [CrossRef]
- Siegmund, S.V.; Schlosser, M.; Schildberg, F.A.; Seki, E.; De Minicis, S.; Uchinami, H.; Kuntzen, C.; Knolle, P.A.; Strassburg, C.P.; Schwabe, R.F. Serum Amyloid A Induces Inflammation, Proliferation and Cell Death in Activated Hepatic Stellate Cells. PLoS ONE 2016, 11, e0150893. [Google Scholar] [CrossRef]
- Fang, Y.W.; Yang, L.; He, J.W. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef]
- Davis, T.A.; Conradie, D.; Shridas, P.; de Beer, F.C.; Engelbrecht, A.M.; de Villiers, W.J.S. Serum Amyloid A Promotes Inflammation-Associated Damage and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1329–1341. [Google Scholar] [CrossRef]
- Furlaneto, C.J.; Campa, A. A novel function of serum amyloid A: A potent stimulus for the release of tumor necrosis factor-alpha, interleukin-1beta, and interleukin-8 by human blood neutrophil. Biochem. Biophys. Res. Commun. 2000, 268, 405–408. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Kaufman, A.; Berkman, N. The nitroxide/antioxidant 3-carbamoyl proxyl attenuates disease severity in murine models of severe asthma. Free Radic. Biol. Med. 2021, 177, 181–188. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Berkman, N. 3-Carbamoyl-proxyl nitroxide radicals attenuate bleomycin-induced pulmonary fibrosis in mice. Free Radic. Biol. Med. 2021, 171, 135–142. [Google Scholar] [CrossRef]
- Huang, S.; Xu, M.; Da, Q.; Jing, L.; Wang, H. Mitochondria-Targeted Nitronyl Nitroxide Radical Nanoparticles for Protection against Radiation-Induced Damage with Antioxidant Effects. Cancers 2024, 16, 351. [Google Scholar] [CrossRef]
- Mitchell, J.B.; DeGraff, W.; Kaufman, D.; Krishna, M.C.; Samuni, A.; Finkelstein, E.; Ahn, M.S.; Hahn, S.M.; Gamson, J.; Russo, A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys. 1991, 289, 62–70. [Google Scholar] [CrossRef]
- Hahn, S.M.; Krishna, M.C.; DeLuca, A.M.; Coffin, D.; Mitchell, J.B. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med. 2000, 28, 953–958. [Google Scholar] [CrossRef]
- Ali, B.M.; Velavan, B.; Sudhandiran, G.; Sridevi, J.; Nasar, A.S. Radical dendrimers: Synthesis, anti-tumor activity and enhanced cytoprotective performance of TEMPO free radical functionalized polyurethane dendrimers. Eur. Polym. J. 2020, 122, 109354. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Bu, W.; Wei, M.Y.; Li, W.W.; Yao, M.N.; Ma, Z.Y.; Lu, C.T.; Li, H.H.; Hu, N.P.; et al. Synthesis of a novel adamantyl nitroxide derivative with potent anti-hepatoma activity in vitro and in vivo. Am. J. Cancer Res. 2016, 6, 1271–1286. [Google Scholar]
- Kaewpila, S.; Venkataraman, S.; Buettner, G.R.; Oberley, L.W. Manganese superoxide dismutase modulates hypoxia-inducible factor-1 alpha induction via superoxide. Cancer Res. 2008, 68, 2781–2788. [Google Scholar] [CrossRef]
- El Kazzi, M.; Shi, H.; Vuong, S.; Wang, X.; Chami, B.; Liu, Y.; Rayner, B.S.; Witting, P.K. Nitroxides Mitigate Neutrophil-Mediated Damage to the Myocardium after Experimental Myocardial Infarction in Rats. Int. J. Mol. Sci. 2020, 21, 7650. [Google Scholar] [CrossRef]
- Martin, N.J.; Chami, B.; Vallejo, A.; Mojadadi, A.A.; Witting, P.K.; Ahmad, G. Efficacy of the Piperidine Nitroxide 4-MethoxyTEMPO in Ameliorating Serum Amyloid A-Mediated Vascular Inflammation. Int. J. Mol. Sci. 2021, 22, 4549. [Google Scholar] [CrossRef]
- Gao, A.; Gupta, S.; Shi, H.; Liu, Y.; Schroder, A.L.; Witting, P.K.; Ahmad, G. Pro-Inflammatory Serum Amyloid a Stimulates Renal Dysfunction and Enhances Atherosclerosis in Apo E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 12582. [Google Scholar] [CrossRef]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. Apoe-Deficient Mice Develop Lesions of All Phases of Atherosclerosis Throughout the Arterial Tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef]
- Belmokhtar, K.; Robert, T.; Ortillon, J.; Braconnier, A.; Vuiblet, V.; Boulagnon-Rombi, C.; Diebold, M.D.; Pietrement, C.; Schmidt, A.M.; Rieu, P.; et al. Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 800–809. [Google Scholar] [CrossRef]
- Christophersen, D.V.; Moller, P.; Thomsen, M.B.; Lykkesfeldt, J.; Loft, S.; Wallin, H.; Vogel, U.; Jacobsen, N.R. Accelerated atherosclerosis caused by serum amyloid A response in lungs of ApoE mice. Faseb J. 2021, 35, e21307. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, T.; Qin, W.; An, C.; Wang, Z.; Zhang, M.; Zhang, Y.; Zhang, C.; An, F. Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol. Med. 2011, 17, 1357–1364. [Google Scholar] [CrossRef]
- Cai, X.; Ahmad, G.; Hossain, F.; Liu, Y.; Wang, X.; Dennis, J.; Freedman, B.; Witting, P.K. High-Density Lipoprotein (HDL) Inhibits Serum Amyloid A (SAA)-Induced Vascular and Renal Dysfunctions in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2020, 21, 1316. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, X.; Duan, L.; Fang, H.; Rao, S.; Liu, W.; Guo, B.; Zhang, X. Lysyl Hydroxylase Inhibition by Minoxidil Blocks Collagen Deposition and Prevents Pulmonary Fibrosis via TGF-beta(1)/Smad3 Signaling Pathway. Med. Sci. Monit. 2018, 24, 8592–8601. [Google Scholar] [CrossRef]
- Parish, J.L.; Hughes, M.A.; Cherry, G.W.; Ferguson, D.J. The effect of minoxidil analogues and metabolites on the contraction of collagen lattices by human skin fibroblasts. Br. J. Plast. Surg. 1995, 48, 154–160. [Google Scholar] [CrossRef]
- Zuurmond, A.M.; van der Slot-Verhoeven, A.J.; van Dura, E.A.; De Groot, J.; Bank, R.A. Minoxidil exerts different inhibitory effects on gene expression of lysyl hydroxylase 1, 2, and 3: Implications for collagen cross-linking and treatment of fibrosis. Matrix Biol. 2005, 24, 261–270. [Google Scholar] [CrossRef]
- Wissing, T.B.; Van der Heiden, K.; Serra, S.M.; Smits, A.; Bouten, C.V.C.; Gijsen, F.J.H. Tissue-engineered collagenous fibrous cap models to systematically elucidate atherosclerotic plaque rupture. Sci. Rep. 2022, 12, 5434. [Google Scholar] [CrossRef]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic. Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]
- Halvorsen, B.; Otterdal, K.; Dahl, T.B.; Skjelland, M.; Gullestad, L.; Oie, E.; Aukrust, P. Atherosclerotic plaque stability--what determines the fate of a plaque? Prog. Cardiovasc. Dis. 2008, 51, 183–194. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Vergis, A.L.; Ye, H.; Bajwa, A.; Narayan, V.; Strieter, R.M.; Rosin, D.L.; Okusa, M.D. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Investig. 2010, 120, 331–342. [Google Scholar] [CrossRef]
- Hubackova, S.; Kucerova, A.; Michlits, G.; Kyjacova, L.; Reinis, M.; Korolov, O.; Bartek, J.; Hodny, Z. IFNgamma induces oxidative stress, DNA damage and tumor cell senescence via TGFbeta/SMAD signaling-dependent induction of Nox4 and suppression of ANT2. Oncogene 2016, 35, 1236–1249. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-alpha and IFN-gamma Activation on the Dynamics of iNOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef]
- Amore, A.; Emancipator, S.N.; Cirina, P.; Conti, G.; Ricotti, E.; Bagheri, N.; Coppo, R. Nitric oxide mediates cyclosporine-induced apoptosis in cultured renal cells. Kidney Int. 2000, 57, 1549–1559. [Google Scholar] [CrossRef]
- Olechnowicz-Tietz, S.; Gluba, A.; Paradowska, A.; Banach, M.; Rysz, J. The risk of atherosclerosis in patients with chronic kidney disease. Int. Urol. Nephrol. 2013, 45, 1605–1612. [Google Scholar] [CrossRef]
- Chade, A.R.; Rodriguez-Porcel, M.; Grande, J.P.; Krier, J.D.; Lerman, A.; Romero, J.C.; Napoli, C.; Lerman, L.O. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 2002, 106, 1165–1171. [Google Scholar] [CrossRef]
- Chade, A.R.; Lerman, A.; Lerman, L.O. Kidney in early atherosclerosis. Hypertension 2005, 45, 1042–1049. [Google Scholar] [CrossRef]
- Chami, B.; Hossain, F.; Hambly, T.W.; Cai, X.; Aran, R.; Fong, G.; Vellajo, A.; Martin, N.J.J.; Wang, X.; Dennis, J.M.; et al. Serum Amyloid A Stimulates Vascular and Renal Dysfunction in Apolipoprotein E-Deficient Mice Fed a Normal Chow Diet. Front. Immunol. 2019, 10, 380. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Korkmaz, L.; Adar, A.; Korkmaz, A.A.; Erkan, H.; Agac, M.T.; Acar, Z.; Kurt, I.H.; Akyuz, A.R.; Celik, S. Atherosclerosis burden and coronary artery lesion complexity in acute coronary syndrome patients. Cardiol. J. 2012, 19, 295–300. [Google Scholar] [CrossRef]
- Vallejo, A.; Chami, B.; Dennis, J.M.; Simone, M.; Ahmad, G.; Abdo, A.I.; Sharma, A.; Shihata, W.A.; Martin, N.; Chin-Dusting, J.P.F.; et al. NFkappaB Inhibition Mitigates Serum Amyloid A-Induced Pro-Atherogenic Responses in Endothelial Cells and Leukocyte Adhesion and Adverse Changes to Endothelium Function in Isolated Aorta. Int. J. Mol. Sci. 2018, 20, 105. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, A.; Xie, K.; Gupta, S.; Ahmad, G.; Witting, P.K. Cyclic Nitroxide 4-Methoxy-Tempo May Decrease Serum Amyloid A-Mediated Renal Fibrosis and Reorganise Collagen Networks in Aortic Plaque. Int. J. Mol. Sci. 2024, 25, 7863. https://doi.org/10.3390/ijms25147863
Gao A, Xie K, Gupta S, Ahmad G, Witting PK. Cyclic Nitroxide 4-Methoxy-Tempo May Decrease Serum Amyloid A-Mediated Renal Fibrosis and Reorganise Collagen Networks in Aortic Plaque. International Journal of Molecular Sciences. 2024; 25(14):7863. https://doi.org/10.3390/ijms25147863
Chicago/Turabian StyleGao, Antony, Kangzhe Xie, Sameesh Gupta, Gulfam Ahmad, and Paul K. Witting. 2024. "Cyclic Nitroxide 4-Methoxy-Tempo May Decrease Serum Amyloid A-Mediated Renal Fibrosis and Reorganise Collagen Networks in Aortic Plaque" International Journal of Molecular Sciences 25, no. 14: 7863. https://doi.org/10.3390/ijms25147863
APA StyleGao, A., Xie, K., Gupta, S., Ahmad, G., & Witting, P. K. (2024). Cyclic Nitroxide 4-Methoxy-Tempo May Decrease Serum Amyloid A-Mediated Renal Fibrosis and Reorganise Collagen Networks in Aortic Plaque. International Journal of Molecular Sciences, 25(14), 7863. https://doi.org/10.3390/ijms25147863







