Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury
Abstract
:1. Introduction
2. Overview of Peripheral Nerve Injury
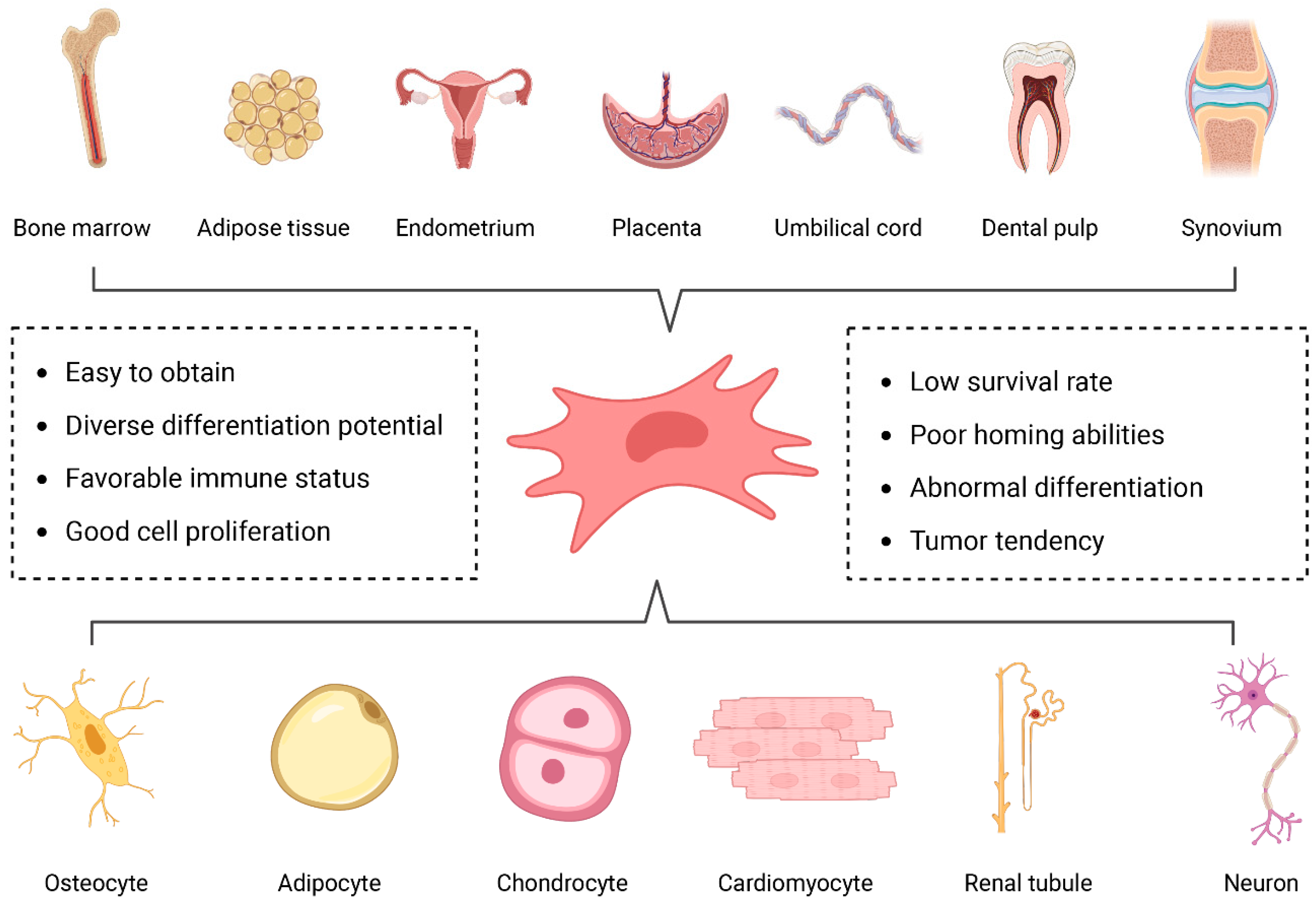
3. Mesenchymal Stem Cells
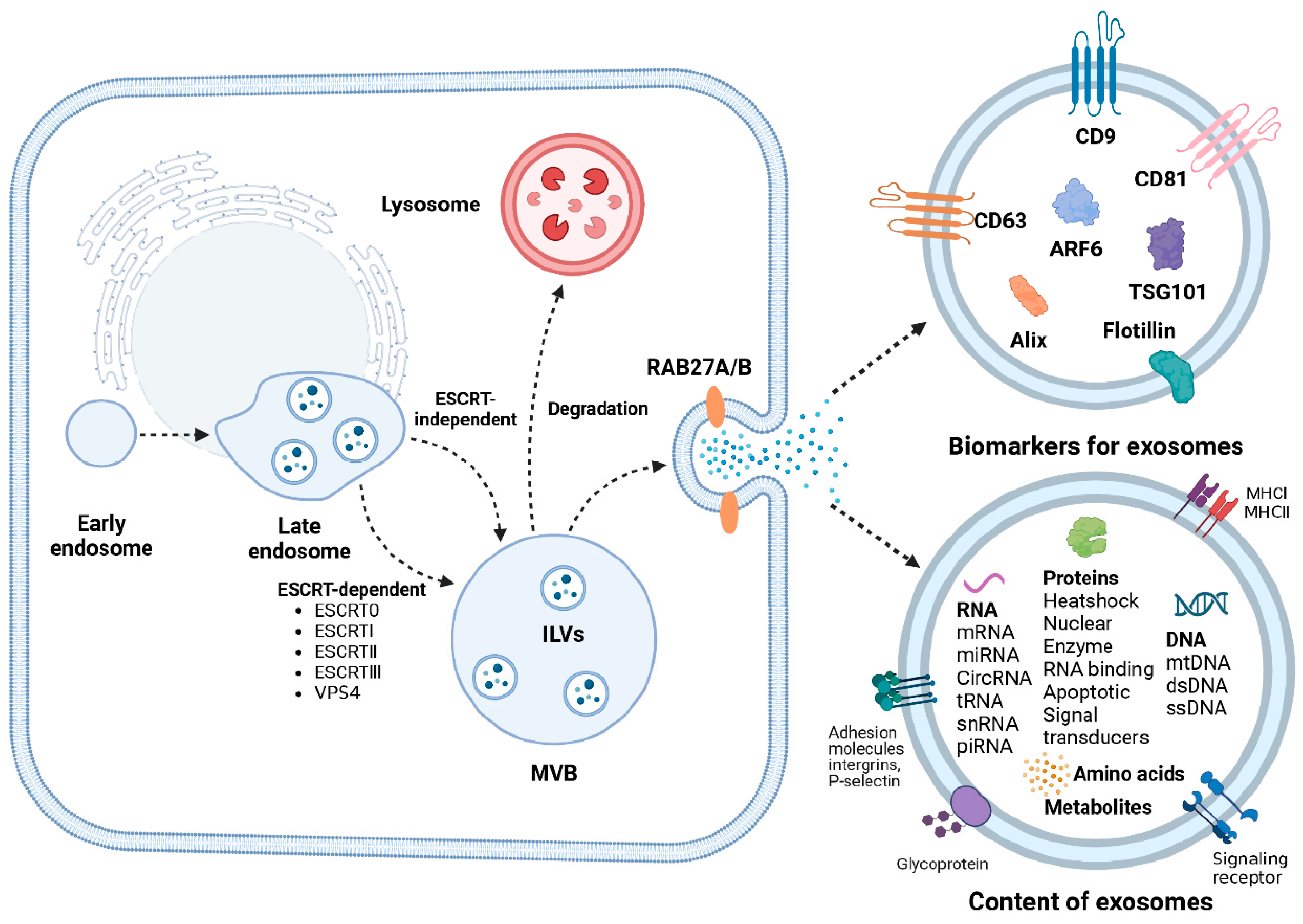
4. Biogenesis of Exosomes
5. Isolation of Exosomes
6. Effects of MSC-Derived Exosomes on Regeneration of PNI
6.1. MSC Exosomes Promote Angiogenesis
6.2. MSC Exosomes Mediate Axonal Regeneration
6.3. MSC Exosomes Modulate Neuroinflammation
6.4. MSC Exosomes Alleviate Neuropathic Pain
| Source Cell | Exosomal Cargo | Signaling Pathway | Effects | References |
|---|---|---|---|---|
| ADMSCs | miRN-132-3p, miRNA-199b-5p | N/A | Protected SCs from oxidative stress, enhanced angiogenesis, promoted axonal growth | [69] |
| ADMSCs | N/A | PI3K/AKT signaling pathway | Promoted PC12 cell proliferation and migration, inhibited apoptosis | [74] |
| GMSCs | N/A | Expression of c-JUN key transcription factor | Promoted axonal regeneration and functional recovery, activated repair phenotype of SCs | [75] |
| MenSCs | N/A | PI3K/AKT signaling pathway | Against neuron injury induced by glutamate | [89] |
| BMSCs | miR-17-92 cluster | PI3K/protein kinase B/mechanistic target of rapamycin/glycogen synthase kinase 3β | Increased neural plasticity and functional recovery | [79] |
| BMSCs | Let-7a, miR-23a and miR-125b | TLR/NF-κB signaling pathway | Increased angiogenesis and nerve regeneration, suppressed proinflammatory cytokines | [85] |
7. Strategies to Improve Therapeutic Function of MSC Exosomes
7.1. Pretreatment Approaches of Parental Cells
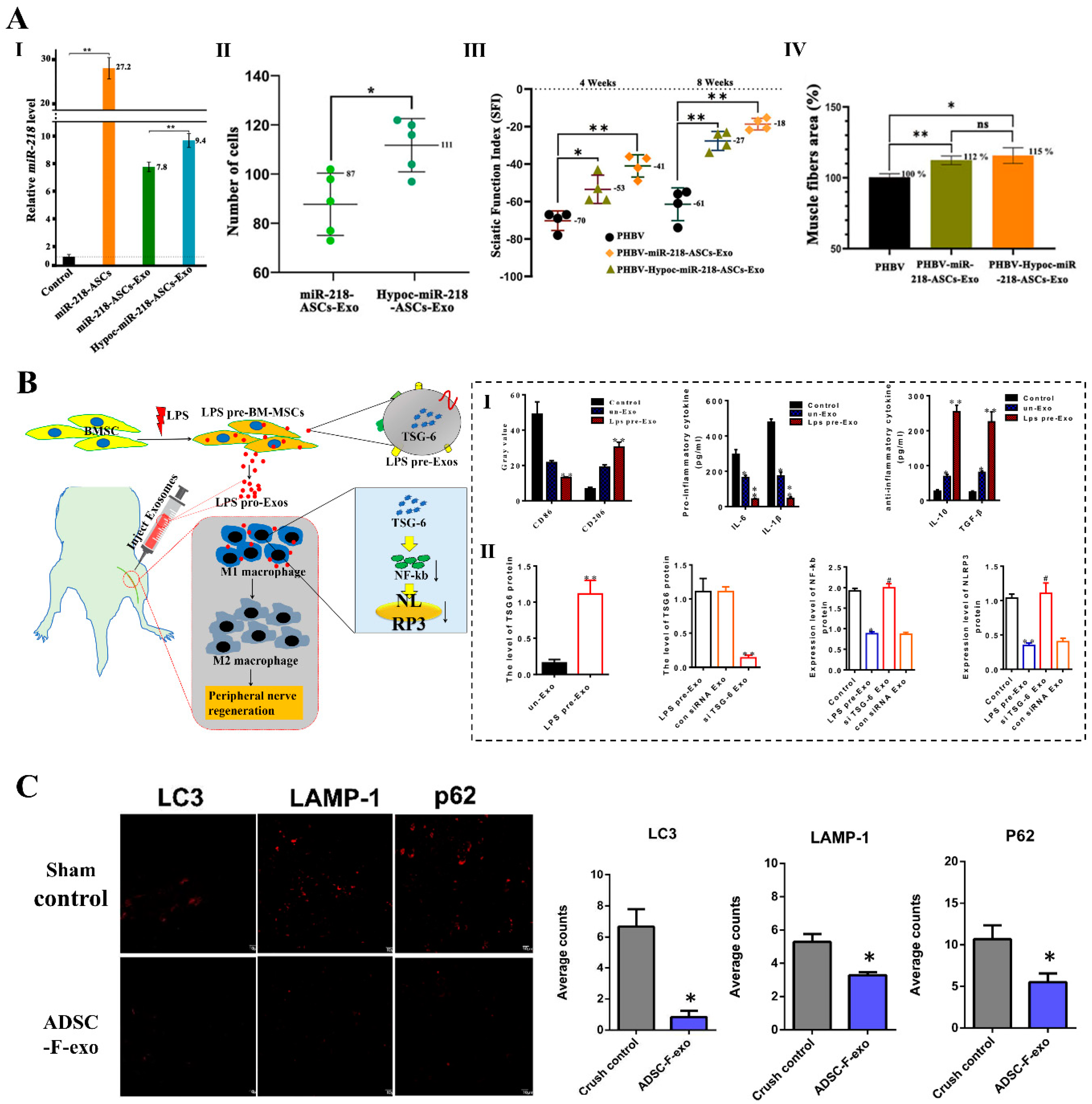
7.2. Direct Engineering Approaches of Exosomes
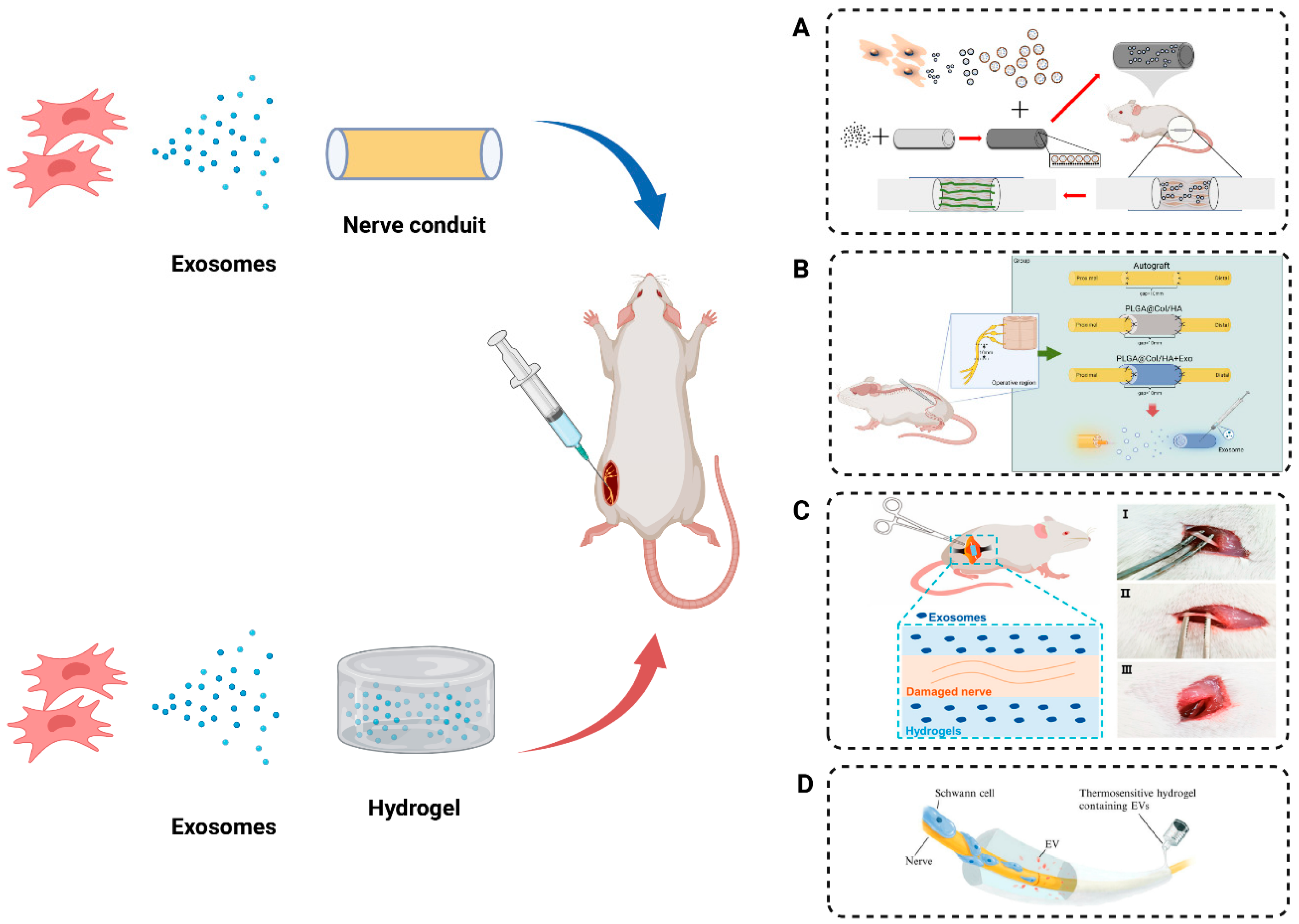
8. MSC Exosomes Combined with Biomaterials Promote Peripheral Nerve Regeneration
8.1. Nerve Conduit Incorporating Exosomes
8.2. Hydrogels Incorporating Exosomes
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.H.; Tang, Q.; Liu, X.X.; Qi, J.; Zeng, R.X.; Zhu, Z.W.; He, B.; Xu, Y.B. Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages. Neural Regen. Res. 2018, 13, 2182–2190. [Google Scholar] [CrossRef]
- Sullivan, T.B.; Robert, L.C.; Teebagy, P.A.; Morgan, S.E.; Beatty, E.W.; Cicuto, B.J.; Nowd, P.K.; Rieger-Christ, K.M.; Bryan, D.J. Spatiotemporal microRNA profile in peripheral nerve regeneration: miR-138 targets vimentin and inhibits Schwann cell migration and proliferation. Neural Regen. Res. 2018, 13, 1253–1262. [Google Scholar] [CrossRef]
- Hopf, A.; Al-Bayati, L.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Optimized Decellularization Protocol for Large Peripheral Nerve Segments: Towards Personalized Nerve Bioengineering. Bioengineering 2022, 9, 412. [Google Scholar] [CrossRef]
- Aigner, T.B.; Haynl, C.; Salehi, S.; O’Connor, A.; Scheibel, T. Nerve guidance conduit design based on self-rolling tubes. Mater. Today Bio 2020, 5, 100042. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M.; Panneerselvam, A.; Chinnaiyan, M. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci. 2023, 327, 121785. [Google Scholar] [CrossRef]
- Yousefi, F.; Lavi Arab, F.; Nikkhah, K.; Amiri, H.; Mahmoudi, M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019, 221, 99–108. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, D.N.; Pinto, G.B.A.; Cartarozzi, L.P.; de Oliveira, A.L.R.; Bovolato, A.L.C.; de Carvalho, M.; da Silva, J.V.L.; Dernowsek, J.A.; Golim, M.; Barraviera, B.; et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res. Ther. 2021, 12, 303. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, P.; Chen, F.; Zhao, Y.; Li, Y.; He, X.; Huselstein, C.; Ye, Q.; Tong, Z.; Chen, Y. Brain Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor-Transfected Bone Mesenchymal Stem Cells for the Repair of Periphery Nerve Injury. Front. Bioeng. Biotechnol. 2020, 8, 874. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast. Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef]
- Xie, N.; Li, Z.; Adesanya, T.M.; Guo, W.; Liu, Y.; Fu, M.; Kilic, A.; Tan, T.; Zhu, H.; Xie, X. Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J. Cell. Mol. Med. 2016, 20, 29–37. [Google Scholar] [CrossRef]
- Li, K.L.; Li, J.Y.; Xie, G.L.; Ma, X.Y. Exosomes Released From Human Bone Marrow-Derived Mesenchymal Stem Cell Attenuate Acute Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation in Mice. Front. Cell Dev. Biol. 2021, 9, 617589. [Google Scholar] [CrossRef]
- Xiao, Q.; Lin, C.; Peng, M.; Ren, J.; Jing, Y.; Lei, L.; Tao, Y.; Huang, J.; Yang, J.; Sun, M.; et al. Circulating plasma exosomal long non-coding RNAs LINC00265, LINC00467, UCA1, and SNHG1 as biomarkers for diagnosis and treatment monitoring of acute myeloid leukemia. Front. Oncol. 2022, 12, 1033143. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Mei, S.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell. Mol. Med. 2017, 21, 1228–1236. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, J.; Zhu, L.; Peng, Y. Exosomal noncoding RNAs in Glioma: Biological functions and potential clinical applications. Mol. Cancer 2020, 19, 66. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, Y.; He, R.; Huang, K.; Liu, L.; Jiang, C.; Liu, Z.; Wang, Y.; Yan, X.; Cao, F.; et al. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res. Ther. 2020, 11, 360. [Google Scholar] [CrossRef]
- Soares Martins, T.; Marçalo, R.; da Cruz, E.S.C.B.; Trindade, D.; Catita, J.; Amado, F.; Melo, T.; Rosa, I.M.; Vogelgsang, J.; Wiltfang, J.; et al. Novel Exosome Biomarker Candidates for Alzheimer’s Disease Unravelled through Mass Spectrometry Analysis. Mol. Neurobiol. 2022, 59, 2838–2854. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Y.; Chen, Q.; Chen, H.; Zhu, X.; Li, Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020, 11, 290. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef]
- Matos Cruz, A.J.; De Jesus, O. Neurotmesis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Y.; Shan, Q.; Meng, Y.; Pan, J.; Yi, S. Mrpl10 and Tbp Are Suitable Reference Genes for Peripheral Nerve Crush Injury. Int. J. Mol. Sci. 2017, 18, 263. [Google Scholar] [CrossRef]
- Wang, W.; Gao, J.; Na, L.; Jiang, H.; Xue, J.; Yang, Z.; Wang, P. Craniocerebral injury promotes the repair of peripheral nerve injury. Neural Regen. Res. 2014, 9, 1703–1708. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.J.; Sun, R.B. Effect of the combination of high-frequency repetitive magnetic stimulation and neurotropin on injured sciatic nerve regeneration in rats. Neural Regen. Res. 2020, 15, 145–151. [Google Scholar] [CrossRef]
- Fu, C.; Yin, Z.; Yu, D.; Yang, Z. Substance P and calcitonin gene-related peptide expression in dorsal root ganglia in sciatic nerve injury rats. Neural Regen. Res. 2013, 8, 3124–3130. [Google Scholar] [CrossRef]
- Xue, J.; Yang, J.; O’Connor, D.M.; Zhu, C.; Huo, D.; Boulis, N.M.; Xia, Y. Differentiation of Bone Marrow Stem Cells into Schwann Cells for the Promotion of Neurite Outgrowth on Electrospun Fibers. ACS Appl. Mater. Interfaces 2017, 9, 12299–12310. [Google Scholar] [CrossRef]
- Man, L.L.; Liu, F.; Wang, Y.J.; Song, H.H.; Xu, H.B.; Zhu, Z.W.; Zhang, Q.; Wang, Y.J. The HMGB1 signaling pathway activates the inflammatory response in Schwann cells. Neural Regen. Res. 2015, 10, 1706–1712. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, K.H.; Kwon, O.H.; Kwon, O.K.; Uyama, H.; Kim, Y.J. Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1035. [Google Scholar] [CrossRef]
- Zhang, P.X.; Han, N.; Kou, Y.H.; Zhu, Q.T.; Liu, X.L.; Quan, D.P.; Chen, J.G.; Jiang, B.G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51–58. [Google Scholar] [CrossRef]
- Bahrehbar, K.; Rezazadeh Valojerdi, M.; Esfandiari, F.; Fathi, R.; Hassani, S.N.; Baharvand, H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J. Stem Cells 2020, 12, 857–878. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Q.; Zhang, Y.; Bian, Q.; Hong, Y.; Shen, Z.; Xu, H.; Rui, K.; Yin, K.; Wang, S. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Experimental Colitis via Modulating Th1/Th17 and Treg Cell Responses. Front. Immunol. 2020, 11, 598322. [Google Scholar] [CrossRef]
- Zhou, S.; Lei, Y.; Wang, P.; Chen, J.; Zeng, L.; Qu, T.; Maldonado, M.; Huang, J.; Han, T.; Wen, Z.; et al. Human Umbilical Cord Mesenchymal Stem Cells Encapsulated with Pluronic F-127 Enhance the Regeneration and Angiogenesis of Thin Endometrium in Rat via Local IL-1β Stimulation. Stem Cells Int. 2022, 2022, 7819234. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wei, P.; Hao, L.; Zhong, L.; Zhong, K.; Liu, C.; Liu, P.; Feng, Q.; Wang, S.; et al. 3D-printed collagen/silk fibroin/secretome derived from bFGF-pretreated HUCMSCs scaffolds enhanced therapeutic ability in canines traumatic brain injury model. Front. Bioeng. Biotechnol. 2022, 10, 995099. [Google Scholar] [CrossRef]
- Ren, C.; Yin, P.; Ren, N.; Wang, Z.; Wang, J.; Zhang, C.; Ge, W.; Geng, D.; Wang, X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res. Ther. 2018, 9, 66. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Liu, M.; Wang, N. Distal segment extracts of the degenerated rat sciatic nerve induce bone marrow stromal cells to express Schwann cell markers in vitro. Neurosci. Lett. 2013, 544, 89–93. [Google Scholar] [CrossRef]
- Ren, Z.; Qi, Y.; Sun, S.; Tao, Y.; Shi, R. Mesenchymal Stem Cell-Derived Exosomes: Hope for Spinal Cord Injury Repair. Stem Cells Dev. 2020, 29, 1467–1478. [Google Scholar] [CrossRef]
- Keysberg, C.; Hertel, O.; Schelletter, L.; Busche, T.; Sochart, C.; Kalinowski, J.; Hoffrogge, R.; Otte, K.; Noll, T. Exploring the molecular content of CHO exosomes during bioprocessing. Appl. Microbiol. Biotechnol. 2021, 105, 3673–3689. [Google Scholar] [CrossRef]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2018, 32, 765–776. [Google Scholar] [CrossRef]
- Li, Q.C.; Li, C.; Zhang, W.; Pi, W.; Han, N. Potential Effects of Exosomes and their MicroRNA Carrier on Osteoporosis. Curr. Pharm. Des. 2022, 28, 899–909. [Google Scholar] [CrossRef]
- Nepal, B.; Sepehri, A.; Lazaridis, T. Mechanisms of negative membrane curvature sensing and generation by ESCRT III subunit Snf7. Protein Sci. 2020, 29, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Bache, K.G.; Gillooly, D.J.; Madshus, I.H.; Stang, E.; Stenmark, H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002, 4, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Corvera, J.; Gallick, G.E.; Lin, S.H.; Kuang, J. ALG-2 activates the MVB sorting function of ALIX through relieving its intramolecular interaction. Cell Discov. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, L.; Jeske, R.; Nkosi, D.; York, S.B.; Liu, Y.; Grant, S.C.; Meckes, D.G., Jr.; Li, Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12235. [Google Scholar] [CrossRef]
- Sun, B.; Peng, J.; Wang, S.; Liu, X.; Zhang, K.; Zhang, Z.; Wang, C.; Jing, X.; Zhou, C.; Wang, Y. Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev. Neurosci. 2018, 29, 531–546. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Dash, M.; Palaniyandi, K.; Ramalingam, S.; Sahabudeen, S.; Raja, N.S. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183490. [Google Scholar] [CrossRef]
- Shi, L.; Rana, A.; Esfandiari, L. A low voltage nanopipette dielectrophoretic device for rapid entrapment of nanoparticles and exosomes extracted from plasma of healthy donors. Sci. Rep. 2018, 8, 6751. [Google Scholar] [CrossRef]
- Hassanpour Tamrin, S.; Sanati Nezhad, A.; Sen, A. Label-Free Isolation of Exosomes Using Microfluidic Technologies. ACS Nano 2021, 15, 17047–17079. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Lin, S.; Xu, J.; Yang, G.; Chen, H.; Jiang, X. Dominoes with interlocking consequences triggered by zinc: Involvement of microelement-stimulated MSC-derived exosomes in senile osteogenesis and osteoclast dialogue. J. Nanobiotechnol. 2023, 21, 346. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F.; et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. CCS 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Martin, H.C.; Huang, H.; Luheshi, G.; Rivest, S.; Zhang, J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 2014, 155, 954–967. [Google Scholar] [CrossRef]
- Thibodeau, A.; Galbraith, T.; Fauvel, C.M.; Khuong, H.T.; Berthod, F. Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials 2022, 280, 121269. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Lu, P.; Cai, X.; Wang, G.; Xu, X.; Liu, Y.; Huang, T.; Li, M.; Qian, T.; et al. Neural tissue-engineered prevascularization in vivo enhances peripheral neuroregeneration via rapid vascular inosculation. Mater. Today. Bio 2023, 21, 100718. [Google Scholar] [CrossRef]
- Du, W.; Zhang, K.; Zhang, S.; Wang, R.; Nie, Y.; Tao, H.; Han, Z.; Liang, L.; Wang, D.; Liu, J.; et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 2017, 133, 70–81. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl. Med. 2017, 6, 51–59. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J. Cell. Mol. Med. 2021, 25, 2148–2162. [Google Scholar] [CrossRef]
- Hu, H.; Hu, X.; Li, L.; Fang, Y.; Yang, Y.; Gu, J.; Xu, J.; Chu, L. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules 2022, 12, 883. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Cai, W.; Yang, Y.; Guo, T.; Li, J.; Dai, H. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal microRNA-29b-3p Promotes Angiogenesis and Ventricular Remodeling in Rats with Myocardial Infarction by Targeting ADAMTS16. Cardiovasc. Toxicol. 2022, 22, 689–700. [Google Scholar] [CrossRef]
- Liu, B.; Kong, Y.; Shi, W.; Kuss, M.; Liao, K.; Hu, G.; Xiao, P.; Sankarasubramanian, J.; Guda, C.; Wang, X.; et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact. Mater. 2022, 14, 61–75. [Google Scholar] [CrossRef]
- López-Leal, R.; Díaz-Viraqué, F.; Catalán, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Liu, S.; Yang, P.; Liang, Y.; Ma, J.; Mao, S.; Sun, C.; Yang, Y. Fibroblast exosomal TFAP2C induced by chitosan oligosaccharides promotes peripheral axon regeneration via the miR-132-5p/CAMKK1 axis. Bioact. Mater. 2023, 26, 249–263. [Google Scholar] [CrossRef]
- Tang, B.L. Promoting axonal regeneration through exosomes: An update of recent findings on exosomal PTEN and mTOR modifiers. Brain Res. Bull. 2018, 143, 123–131. [Google Scholar] [CrossRef]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.; Zhu, Y.; Chen, X.; Lin, T.; Zhou, D. Adipose Mesenchymal Stem Cell-Derived Exosomes Enhance PC12 Cell Function through the Activation of the PI3K/AKT Pathway. Stem Cells Int. 2021, 2021, 2229477. [Google Scholar] [CrossRef]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z.; He, L.; Liu, C.; Wang, N.; Rong, L.; Liu, B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res. Ther. 2021, 12, 224. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery after Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Qu, W.R.; Zhu, Z.; Liu, J.; Song, D.B.; Tian, H.; Chen, B.P.; Li, R.; Deng, L.X. Interaction between Schwann cells and other cells during repair of peripheral nerve injury. Neural Regen. Res. 2021, 16, 93–98. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Yang, H.K.; Son, W.S.; Lim, K.S.; Kim, G.H.; Lim, E.J.; Gadhe, C.G.; Lee, J.Y.; Jeong, K.S.; Lim, S.M.; Pae, A.N. Synthesis and biological evaluation of pyrrolidine-based T-type calcium channel inhibitors for the treatment of neuropathic pain. J. Enzym. Inhib. Med. Chem. 2018, 33, 1460–1471. [Google Scholar] [CrossRef]
- Zhang, Y.U.; Ye, G.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An. Acad. Bras. Cienc. 2022, 94, e20210564. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Shiue, S.J.; Rau, R.H.; Shiue, H.S.; Hung, Y.W.; Li, Z.X.; Yang, K.D.; Cheng, J.K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef]
- Hsu, J.M.; Shiue, S.J.; Yang, K.D.; Shiue, H.S.; Hung, Y.W.; Pannuru, P.; Poongodi, R.; Lin, H.Y.; Cheng, J.K. Locally Applied Stem Cell Exosome-Scaffold Attenuates Nerve Injury-Induced Pain in Rats. J. Pain Res. 2020, 13, 3257–3268. [Google Scholar] [CrossRef]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotechnol. 2022, 20, 324. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Zhao, J.; Liu, X.; Liu, X.; Cai, Y.; Wehbe, A.; Ding, Y.; Yu, S.; Wei, L.; et al. Hypoxia-Preconditioned Bone Marrow Mesenchymal Stem Cells Improved Cerebral Collateral Circulation and Stroke Outcome in Mice. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1281–1294. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, T.; Hu, F. Hypocapnia Stimuli-Responsive Engineered Exosomes Delivering miR-218 Facilitate Sciatic Nerve Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 825146. [Google Scholar] [CrossRef]
- Abdollahi, S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol. Bioeng. 2021, 118, 1029–1049. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Shi, Z.; Wu, P.; Fu, J.; Tang, J.; Qing, L. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp. Neurol. 2022, 356, 114139. [Google Scholar] [CrossRef]
- Watanabe, H.; Tsuchiya, T.; Shimoyama, K.; Shimizu, A.; Akita, S.; Yukawa, H.; Baba, Y.; Nagayasu, T. Adipose-derived mesenchymal stem cells attenuate rejection in a rat lung transplantation model. J. Surg. Res. 2018, 227, 17–27. [Google Scholar] [CrossRef]
- Schweizer, R.; Taddeo, A.; Waldner, M.; Klein, H.J.; Fuchs, N.; Kamat, P.; Targosinski, S.; Barth, A.A.; Drach, M.C.; Gorantla, V.S.; et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am. J. Transplant. 2020, 20, 1272–1284. [Google Scholar] [CrossRef]
- Kuo, P.J.; Rau, C.S.; Wu, S.C.; Lin, C.W.; Huang, L.H.; Lu, T.H.; Wu, Y.C.; Wu, C.J.; Tsai, C.W.; Hsieh, C.H. Exosomes Secreted by Adipose-Derived Stem Cells Following FK506 Stimulation Reduce Autophagy of Macrophages in Spine after Nerve Crush Injury. Int. J. Mol. Sci. 2021, 22, 9628. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, T.; Liu, R.; Jin, X. Potential in exosome-based targeted nano-drugs and delivery vehicles for posterior ocular disease treatment: From barriers to therapeutic application. Mol. Cell. Biochem. 2023, 479, 1319–1333. [Google Scholar] [CrossRef]
- Singh, A.; Raghav, A.; Shiekh, P.A.; Kumar, A. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact. Mater. 2021, 6, 2231–2249. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xu, Y.; Jiang, W.; Shao, Y.; Xing, J.; Chen, Y.; Han, Y. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res. Ther. 2021, 12, 442. [Google Scholar] [CrossRef]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P.; et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell. Physiol. 2019, 234, 23097–23110. [Google Scholar] [CrossRef]
- Namini, M.S.; Ebrahimi-Barough, S.; Ai, J.; Jahromi, H.K.; Mikaeiliagah, E.; Azami, M.; Bahrami, N.; Lotfibakhshaiesh, N.; Saremi, J.; Shirian, S. Tissue-Engineered Core-Shell Silk-Fibroin/Poly-l-Lactic Acid Nerve Guidance Conduit Containing Encapsulated Exosomes of Human Endometrial Stem Cells Promotes Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 3496–3511. [Google Scholar] [CrossRef]
- Cui, T.W.; Lu, L.F.; Cao, X.D.; Zhang, Q.P.; He, Y.B.; Wang, Y.R.; Ren, R.; Ben, X.Y.; Ni, P.L.; Ma, Z.J.; et al. Exosomes combined with biosynthesized cellulose conduits improve peripheral nerve regeneration. IBRO Neurosci. Rep. 2023, 15, 262–269. [Google Scholar] [CrossRef]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 2546367. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, X.X.; Li, Q.C.; Pi, W.; Han, N. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration. Neural Regen. Res. 2023, 18, 200–206. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.Y.; Zhang, M.; Pi, W.; Wang, B.; Li, Q.C.; Lu, C.F.; Zhang, P.X. Sustained release of exosomes loaded into polydopamine-modified chitin conduits promotes peripheral nerve regeneration in rats. Neural Regen. Res. 2022, 17, 2050–2057. [Google Scholar] [CrossRef]
- Tang, H.; Li, J.; Wang, H.; Ren, J.; Ding, H.; Shang, J.; Wang, M.; Wei, Z.; Feng, S. Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats. Neural Regen. Res. 2024, 19, 900–907. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Mihajlovic, M.; Maas-Bakker, R.F.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels-Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, H.; Li, J.; Wang, L.; Fan, X.; Song, H.; Yang, M.; Wang, H.; Jiang, X.; Zhou, X.; et al. Low-Stiffness Hydrogels Promote Peripheral Nerve Regeneration through the Rapid Release of Exosomes. Front. Bioeng. Biotechnol. 2022, 10, 922570. [Google Scholar] [CrossRef]
- Yang, Q.; Su, S.; Liu, S.; Yang, S.; Xu, J.; Zhong, Y.; Yang, Y.; Tian, L.; Tan, Z.; Wang, J.; et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact. Mater. 2023, 26, 194–215. [Google Scholar] [CrossRef]
- Chen, S.H.; Kao, H.K.; Wun, J.R.; Chou, P.Y.; Chen, Z.Y.; Chen, S.H.; Hsieh, S.T.; Fang, H.W.; Lin, F.H. Thermosensitive hydrogel carrying extracellular vesicles from adipose-derived stem cells promotes peripheral nerve regeneration after microsurgical repair. APL Bioeng. 2022, 6, 046103. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Dou, K.; Li, K.; Zhou, Q.; Fu, Q. Injectable thermo-sensitive hydrogel containing ADSC-derived exosomes for the treatment of cavernous nerve injury. Carbohydr. Polym. 2023, 300, 120226. [Google Scholar] [CrossRef] [PubMed]
- Civelek, E.; Kabatas, S.; Savrunlu, E.C.; Diren, F.; Kaplan, N.; Ofluoğlu, D.; Karaöz, E. Effects of exosomes from mesenchymal stem cells on functional recovery of a patient with total radial nerve injury: A pilot study. World J. Stem Cells 2024, 16, 19–32. [Google Scholar] [CrossRef] [PubMed]


| Isolation Technique | Isolation Principle | Potential Advantage | Potential Disadvantage |
|---|---|---|---|
| Ultracentrifugation | Density, Size |
|
|
| Ultrafiltration | Molecular weight, Size difference |
|
|
| Immunoaffinity capture | Affinity |
|
|
| Polymer precipitation | Solubility or dispersibility, Surface charge |
|
|
| Size exclusion chromatography | Molecular weight, Size difference |
|
|
| Ion exchange chromatography | Charge |
|
|
| Microfluidics-based techniques | Acoustic, density, electrophoretic, electromagnetic immunoaffinity, size |
|
|
| Membrane-based isolation | Surface properties |
|
|
| Nerve Conduit | Source Cell | Exosome Isolation Methods | Exosome Concentration | Effects | References |
|---|---|---|---|---|---|
| Chitin conduit | hGMSC | Ultracentrifugation | 100 μg/mL (in vitro), 10 μg exosomes in 10 μL PBS (in vivo) | Increased the number and diameter of nerve fibers and promoted myelin formation | [104] |
| Polydopamine-modified chitin conduit | BMSCs | Ultracentrifugation | N/A | Promoted the functional recovery of injured peripheral nerves | [106] |
| rGO-GelMA-PCL nerve conduit | BMSCs | Ultracentrifugation | 20 μg exosomes in 20 μL PBS (in vitro and in vivo) | Increased the number of newly formed vessels and axonal sprouts | [105] |
| Silicone tube | ADMSCs | Ultracentrifugation | 20 μg/mL (in vitro), 1 mg/mL in alginate solution (in vivo) | Improved the function recovery of gastrocenemius muscles and promoted nerve regeneration | [100] |
| Silicone tube | ADMSCs | Ultracentrifugation | 5, 10, 20 μg/mL (in vitro), 20 μg/mL (in vivo) | Improved axon regeneration, myelination, and restoration of denervation muscle atrophy. | [101] |
| Electrospun hollow poly (lactic-co-glycolic acid) tube with collagen/hyaluronic acid | hUCMSCs | Ultracentrifugation | PBS containing 10 μL of exosomes (10 mg/mL in PBS) | Promoted peripheral nerve regeneration and restoration of motor function. | [107] |
| Core-shell silk-fibroin/poly-l-Lactic acid nerve conduit | Human endometrial stem cells | Ultracentrifugation | N/A | Enhanced the regeneration process of axons and improved the functional recovery of rat sciatic nerve defects | [102] |
| Biosynthetic cellulose conduit | ADMSCs | Ultracentrifugation | 4 μL exosome solution (1.9 × 108 exosomes/μL) | Produced comprehensive and durable repair of peripheral nerve defects | [103] |
| PHBV nanofibrous scaffold | ADMSC | Ultracentrifugation | 5, 10, 20 μg/mL (in vitro), 2 mg for each rat (in vivo) | Facilitated the regeneration of injured sciatic nerves | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, F.; Fu, X.; Han, N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. Int. J. Mol. Sci. 2024, 25, 7882. https://doi.org/10.3390/ijms25147882
Li Q, Zhang F, Fu X, Han N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. International Journal of Molecular Sciences. 2024; 25(14):7882. https://doi.org/10.3390/ijms25147882
Chicago/Turabian StyleLi, Qicheng, Fengshi Zhang, Xiaoyang Fu, and Na Han. 2024. "Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury" International Journal of Molecular Sciences 25, no. 14: 7882. https://doi.org/10.3390/ijms25147882
APA StyleLi, Q., Zhang, F., Fu, X., & Han, N. (2024). Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. International Journal of Molecular Sciences, 25(14), 7882. https://doi.org/10.3390/ijms25147882






