Eco-Friendly g-C3N4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants
Abstract
:1. Introduction
2. Results and Discussion
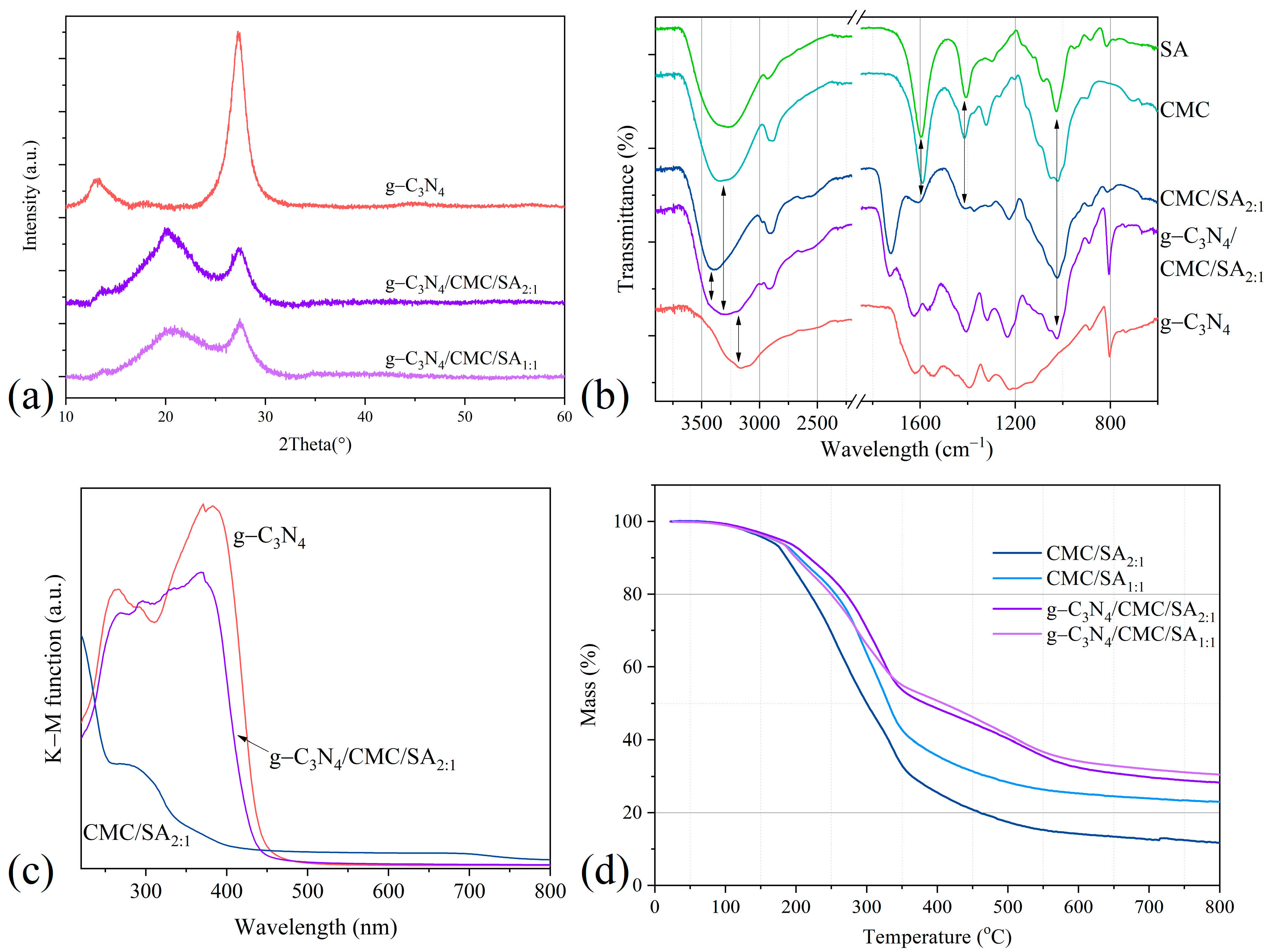
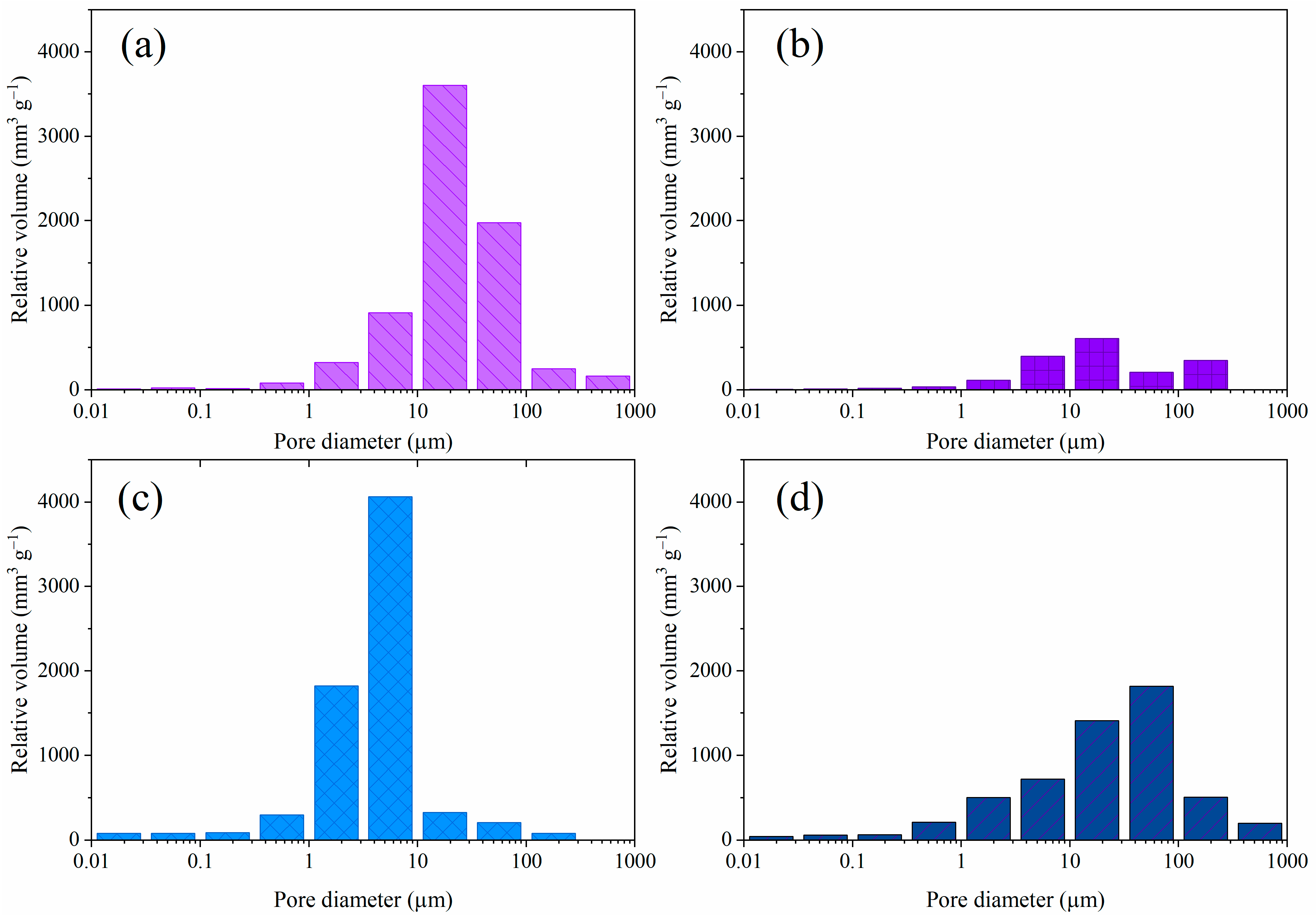
2.1. Composite Hydrogel Characterization
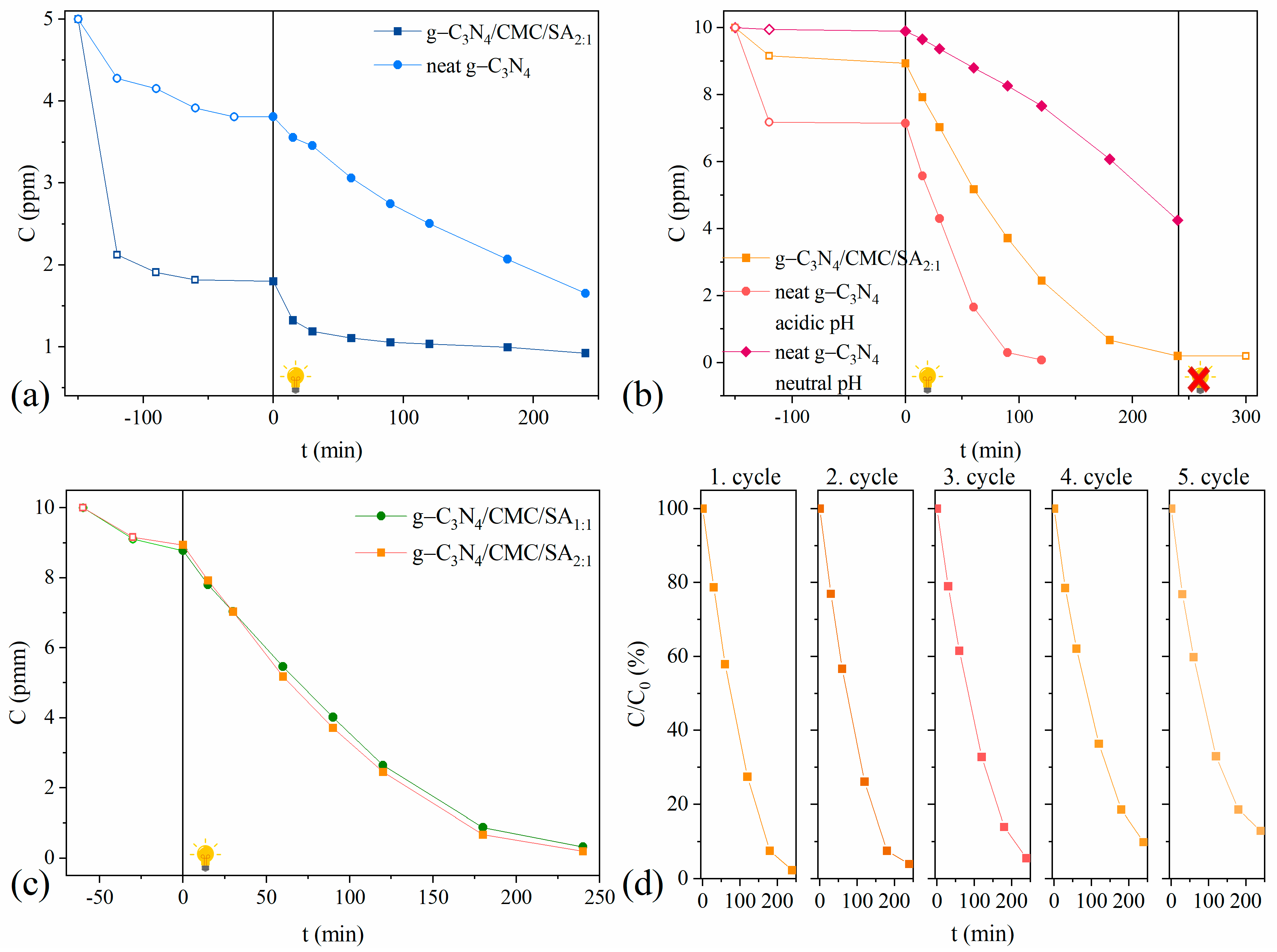
2.2. Photocatalytic Activity of g-C3N4/CMC/SA2:1 Beads
2.2.1. Single-Dye Photocatalytic Systems
2.2.2. Leaching and Stability Test
2.2.3. Radical Scavenger Study
- photocatalyst + hν → eCB− + hVB+;
- O2 + e− → O2•−;
- h+ + OG → intermediates and by-products;
- O2 + 2e− + 2H+ → H2O2;
- H2O2 + e− + H+ → •OH + H2O;
- h+ + OH− → •OH;
- HO•/O2•− + OG → intermediates and by-products.
2.2.4. Binary-Dye Photocatalytic Systems
3. Materials and Methods
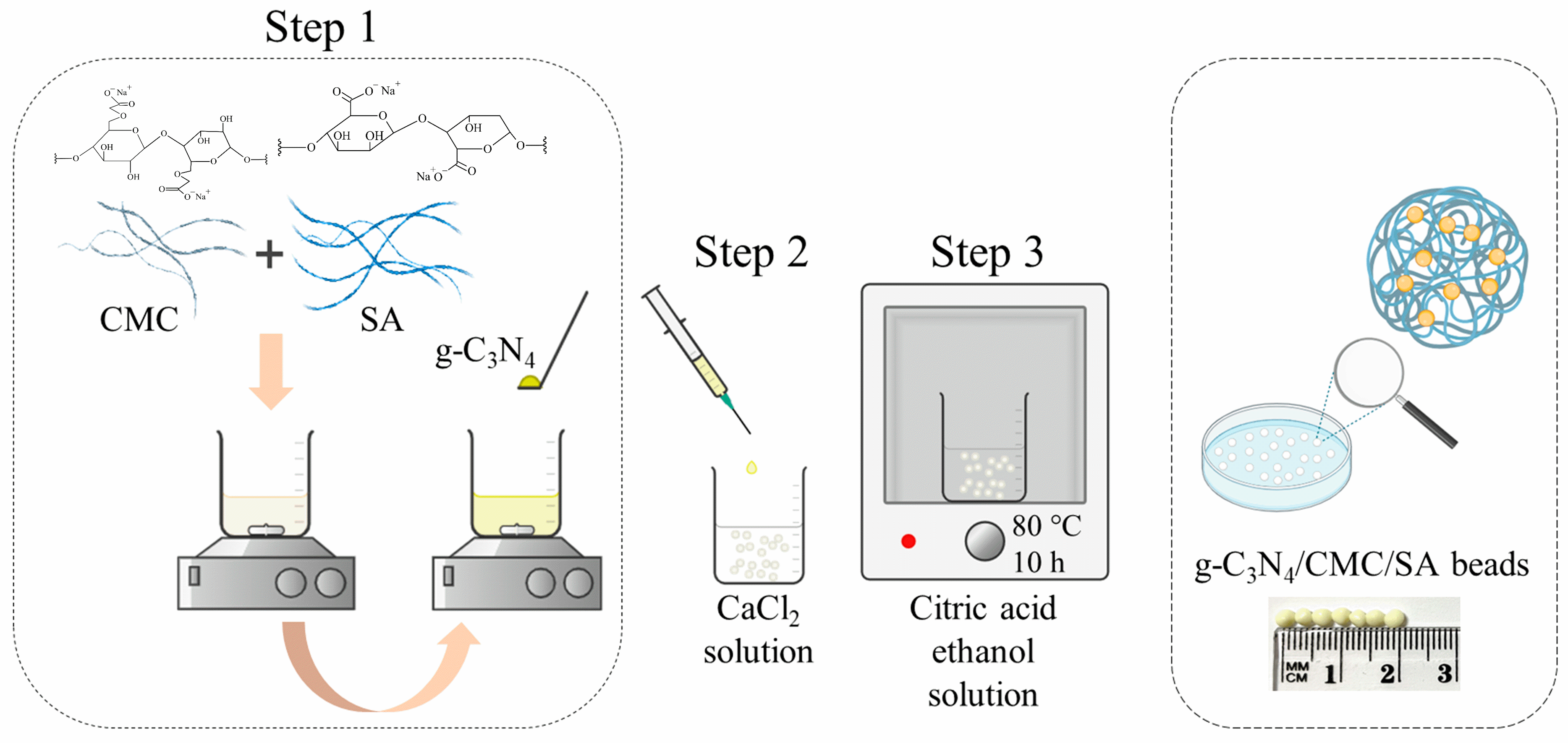
3.1. Synthesis of Composite Hydrogel
3.2. Characterization of Composite Hydrogel
3.3. Photocatalytic Activities and Radical Scavenger Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- El-sayed, M.E.A. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Fakhri, V.; Jafari, A.; Layaei Vahed, F.; Su, C.-H.; Pirouzfar, V. Polysaccharides as Eco-Friendly Bio-Adsorbents for Wastewater Remediation: Current State and Future Perspective. J. Water Process Eng. 2023, 54, 103980. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A Review on Heterogeneous Photocatalysis for Environmental Remediation: From Semiconductors to Modification Strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Raaja Rajeshwari, M.; Kokilavani, S.; Sudheer Khan, S. Recent Developments in Architecturing the G-C3N4 Based Nanostructured Photocatalysts: Synthesis, Modifications and Applications in Water Treatment. Chemosphere 2022, 291, 132735. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Okab, A.A.; Issa, M.A.; Ammar, S.H.; Khadim, H.J.; Shafiq, Y.A. A Review Study Summarizes the Main Characterization Techniques of Nano-Composite Photocatalysts and Their Applications in Photodegradation of Organic Pollutants. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100765. [Google Scholar] [CrossRef]
- Iqbal, O.; Ali, H.; Li, N.; Al-Sulami, A.I.; Alshammari, K.F.; Abd-Rabboh, H.S.M.; Al-Hadeethi, Y.; Din, I.U.; Alharthi, A.I.; Altamimi, R.; et al. A Review on the Synthesis, Properties, and Characterizations of Graphitic Carbon Nitride (g-C3N4) for Energy Conversion and Storage Applications. Mater. Today Phys. 2023, 34, 101080. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and Modification Strategies of G-C3N4 Nanosheets for Photocatalytic Applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Preethi, J.; Meenakshi, S. Removal of Chlorpyrifos, an Insecticide Using Metal Free Heterogeneous Graphitic Carbon Nitride (g-C3N4) Incorporated Chitosan as Catalyst: Photocatalytic and Adsorption Studies. Int. J. Biol. Macromol. 2019, 132, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-H.; Dong, M.; Li, R.; Cui, Y.-Q.; Xie, G.-X.; Wang, X.-X.; Long, Y.-Z. Enhancement of Cr(VI) Removal Efficiency via Adsorption/Photocatalysis Synergy Using Electrospun Chitosan/g-C3N4/TiO2 Nanofibers. Carbohydr. Polym. 2021, 253, 117200. [Google Scholar] [CrossRef] [PubMed]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization Techniques of a Photocatalyst into and onto a Polymer Membrane for Photocatalytic Activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Sudhaik, A.; Raizada, P.; Nguyen, V.-H.; Xia, C.; Parwaz Khan, A.A.; Thakur, S.; Nguyen-Tri, P.; Nguyen, C.C.; Kim, S.Y.; et al. Graphitic Carbon Nitride Based Immobilized and Non-Immobilized Floating Photocatalysts for Environmental Remediation. Chemosphere 2022, 297, 134229. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Kamali, M.; Zhang, X.; Askari, N.; De Preter, C.; Appels, L.; Dewil, R. Immobilization of Photocatalytic Materials for (Waste)Water Treatment Using 3D Printing Technology—Advances and Challenges. Environ. Pollut. 2023, 316, 120549. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An Overview of Photocatalyst Immobilization Methods for Air Pollution Remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Le, A.T.; Le, T.D.H.; Cheong, K.-Y.; Pung, S.-Y. Immobilization of Zinc Oxide-Based Photocatalysts for Organic Pollutant Degradation: A Review. J. Environ. Chem. Eng. 2022, 10, 108505. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Li, X.; Lin, X.; Du, B.; Luo, J.; Zhang, X. Mussel-Inspired Immobilization of Photocatalysts with Synergistic Photocatalytic–Photothermal Performance for Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 31066–31076. [Google Scholar] [CrossRef] [PubMed]
- Tennakone, K.; Tilakaratne, C.T.K.; Kottegoda, I.R.M. Photocatalytic Degradation of Organic Contaminants in Water with TiO2 Supported on Polythene Films. J. Photochem. Photobiol. A Chem. 1995, 87, 177–179. [Google Scholar] [CrossRef]
- Wu, D.; Yi, M.; Duan, H.; Xu, J.; Wang, Q. Tough TiO2-RGO-PDMAA Nanocomposite Hydrogel via One-Pot UV Polymerization and Reduction for Photodegradation of Methylene Blue. Carbon N. Y. 2016, 108, 394–403. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules 2017, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Sawut, A.; Wu, T.; Simayi, R.; Wu, T.; Gong, X.; Wang, Z. Reduced Graphene Oxide-TiO2/Sodium Alginate/Polyacrylamide Composite Hydrogel for Enhanced Adsorption-Photocatalytic Degradation of Methylene Blue. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 678, 132531. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Zheng, Q.; Zhang, Y.; Lv, S.-W. The Calcium Alginate-Immobilized Co-g-C3N4 Composite Microspheres as an Efficient Mediator to Activate Peroxymonosulfate for Degrading Organic Pollutants. Environ. Res. 2022, 215, 114414. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K. 3D Kaolinite/g-C3N4-Alginate Beads as an Affordable and Sustainable Photocatalyst for Wastewater Remediation. Carbohydr. Polym. 2024, 323, 121420. [Google Scholar] [CrossRef] [PubMed]
- Stobel Christy, E.J.; Pius, A. Performance of Metal Free G-C3N4 Reinforced Graphene Oxide Bio-Composite for the Removal of Persistent Dyes. Environ. Chem. Ecotoxicol. 2021, 3, 220–233. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Shokri, Z.; Seidi, F.; Karami, S.; Li, C.; Saeb, M.R.; Xiao, H. Laccase Immobilization onto Natural Polysaccharides for Biosensing and Biodegradation. Carbohydr. Polym. 2021, 262, 117963. [Google Scholar] [CrossRef]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional Applications of Natural Polysaccharide Starch and Cellulose: An Update on Recent Advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Hart, P.; Thakur, V.K. Cellulose-Alginate Hydrogels and Their Nanocomposites for Water Remediation and Biomedical Applications. Environ. Res. 2024, 243, 117889. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lv, Y.; Zhao, W.; Dong, Y.; Wang, L. Sodium Alginate Intercalated 2D G-C3N4 Membrane: Efficient Dye Removal and Photocatalytic Self-Cleaning. Sep. Purif. Technol. 2023, 320, 124177. [Google Scholar] [CrossRef]
- Li, W.-H.; Yang, N. Green Fabrication of AuNPs/CMTKP/g-C3N4 Nanocomposites with Enhanced Photocatalytic Activity for the Removal of Nitric Oxide under Visible-Light Irradiation. J. Clean. Prod. 2020, 256, 120257. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, Y.; Cai, P. Sugarcane Cellulose-Based Composite Hydrogel Enhanced by g-C3N4 Nanosheet for Selective Removal of Organic Dyes from Water. Int. J. Biol. Macromol. 2022, 205, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Nisbet, D.R.; Pfeffer, F.M.; Rifai, A.; Williams, R.; Agrawal, R. Waste to High-Value Products: The Performance and Potential of Carboxymethylcellulose Hydrogels via the Circular Economy. Cellulose 2023, 30, 2713–2730. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent Advances in Cellulose- and Alginate-Based Hydrogels for Water and Wastewater Treatment: A Review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Cai, C.; Gao, L.; Xiong, Y. Green Recyclable Chitosan/Carboxymethyl Cellulose/Sodium Alginate Magnetic Double-Network Composite Hydrogels for Adsorption of MB and Cu2+. J. Water Process Eng. 2024, 63, 105420. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.-J. A Reusable, Separation-Free and Biodegradable Calcium Alginate/g-C3N4 Microsphere for Sustainable Photocatalytic Wastewater Treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Narita, Y.; Nishi, K.; Matsuyama, T.; Ida, J. Reusable Isotype Heterojunction GC 3 N 4/Alginate Hydrogel Spheres for Photocatalytic Wastewater Treatment. RSC Adv. 2024, 14, 20898–20907. [Google Scholar] [CrossRef]
- Valiyathur, M.D.F.; Kottur, A.B.; Sakvai, M.S. Alginate Reinforced Composite of CuO-GC3N4: Synthesis, Characterization and Photocatalytic Activity. Mater. Lett. 2024, 371, 136901. [Google Scholar] [CrossRef]
- Sheoran, K.; Kaur, H.; Siwal, S.S.; Saini, A.K.; Vo, D.-V.N.; Thakur, V.K. Recent Advances of Carbon-Based Nanomaterials (CBNMs) for Wastewater Treatment: Synthesis and Application. Chemosphere 2022, 299, 134364. [Google Scholar] [CrossRef] [PubMed]
- El-Bana, A.A.; Barakat, N.M.; Abdelghany, A.M.; Meikhail, M.S. Effect of Surfactants Addition on Physical, Structure and Antimicrobial Activity of (Na-CMC/Na–Alg) Biofilms. Polym. Bull. 2023, 80, 2883–2909. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, M.S.; Islam, M.D.; Rahman, M.M.; Ratna, A.S.; Mukhopadhyay, S. Nontoxic Cross-Linked Graphitic Carbon Nitride-Alginate-Based Biodegradable Transparent Films for UV and High-Energy Blue Light Shielding. ACS Appl. Mater. Interfaces 2023, 15, 32011–32023. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Daneshvar, H.; Seyed Dorraji, M.S.; Ghasempour, Z.; Panahi-Azar, V.; Ehsani, A. Starch/Alginate/ Cu-g-C3N4 Nanocomposite Film for Food Packaging. Mater. Chem. Phys. 2021, 267, 124583. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.; Yang, Z.; Ye, G. A Reappraisal of the Ink-Bottle Effect and Pore Structure of Cementitious Materials Using Intrusion-Extrusion Cyclic Mercury Porosimetry. Cem. Concr. Res. 2022, 161, 106942. [Google Scholar] [CrossRef]
- Svoboda, L.; Licciardello, N.; Dvorský, R.; Bednář, J.; Henych, J.; Cuniberti, G. Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes. Polymers 2020, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Han, S.; Wang, N.; Yin, W.; Yin, X.; Gao, B.; Wang, X.; Wang, J. Carboxymethyl Cellulose Stabilized ZnO/Biochar Nanocomposites: Enhanced Adsorption and Inhibited Photocatalytic Degradation of Methylene Blue. Chemosphere 2018, 197, 20–25. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Sun, J.; Sun, R.; Sun, S.; Qiao, L. Photocatalytic Degradation and Kinetics of Orange G Using Nano-Sized Sn(IV)/TiO2/AC Photocatalyst. J. Mol. Catal. A Chem. 2006, 260, 241–246. [Google Scholar] [CrossRef]
- Heidarpour, H.; Golizadeh, M.; Padervand, M.; Karimi, A.; Vossoughi, M.; Tavakoli, M.H. In-Situ Formation and Entrapment of Ag/AgCl Photocatalyst inside Cross-Linked Carboxymethyl Cellulose Beads: A Novel Photoactive Hydrogel for Visible-Light-Induced Photocatalysis. J. Photochem. Photobiol. A Chem. 2020, 398, 112559. [Google Scholar] [CrossRef]
- Benhalima, T.; Mokhtari, M.; Ferfera-Harrar, H. Synergistic Adsorption/Photodegradation Effect for Effective Removal of Crystal Violet Dye and Acetamiprid Pesticide Using Fe3+ Cross-Linked Ternary Carboxymethyl Cellulose/Polyaniline/TiO2 Photocomposites. J. Water Process Eng. 2024, 57, 104670. [Google Scholar] [CrossRef]
- Xue, Y.; Lu, Y.; Feng, K.; Zhang, C.; Feng, X.; Zhao, Y.; Chen, L. Preparation of the Self-Accelerating Photocatalytic Self-Cleaning Carboxymethyl Cellulose Sodium-Based Hydrogel for Removing Cationic Dyes. Int. J. Biol. Macromol. 2023, 250, 125891. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous Catalysts for Liquid-Phase Oxidations: Philosophers’ Stones or Trojan Horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Han, Y.; Ye, H.; Liu, R.; Lan, Y.; Xue, M.; Xie, X.; Yu, S.; Zhang, L.; et al. G-C3N4-Based Photocatalysts for Organic Pollutant Removal: A Critical Review. Carbon Res. 2023, 2, 14. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, C.; Su, Z. High-Efficiency and Conveniently Recyclable g-C3N4/Diatomite/Calcium Alginate Composite Gel Bead Photocatalysts. J. Phys. Conf. Ser. 2024, 2691, 12062. [Google Scholar] [CrossRef]
- Araujo, F.P.; Trigueiro, P.; Honório, L.M.C.; Furtini, M.B.; Oliveira, D.M.; Almeida, L.C.; Garcia, R.R.P.; Viana, B.C.; Silva-Filho, E.C.; Osajima, J.A. A Novel Green Approach Based on ZnO Nanoparticles and Polysaccharides for Photocatalytic Performance. Dalt. Trans. 2020, 49, 16394–16403. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Li, C.; Wang, H.; Hu, H.; Wang, W.; Zhang, X. Photocatalytic Degradation, Toxicological Assessment and Degradation Pathway of C.I. Reactive Blue 19 Dye. Chem. Eng. Res. Des. 2018, 129, 384–390. [Google Scholar] [CrossRef]

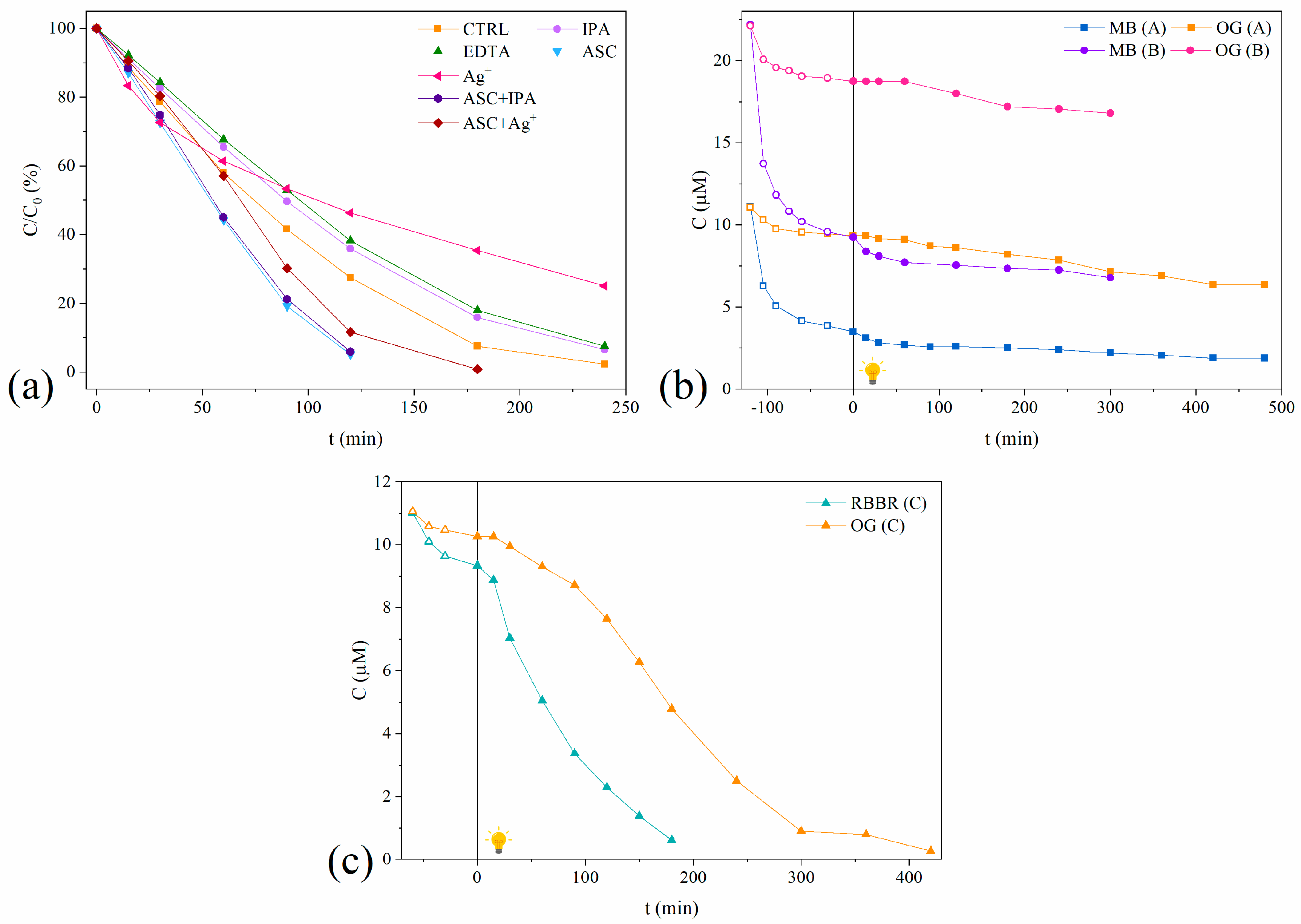
| Sample | T5%, °C | T30%, °C | THRI, °C |
|---|---|---|---|
| CMC/SA1:1 | 168 | 259 | 109 |
| CMC/SA2:1 | 159 | 248 | 104 |
| g-C3N4/CMC/SA1:1 | 169 | 287 | 117 |
| g-C3N4/CMC/SA2:1 | 178 | 301 | 123 |
| Sample | a σ, N/mm2 | b MSS, % |
|---|---|---|
| CMC/SA1:1 | 0.1548 | 59.94 |
| CMC/SA2:1 | 0.1735 | 39.48 |
| g-C3N4/CMC/SA1:1 | 0.1539 | 62.91 |
| g-C3N4/CMC/SA2:1 | 0.0988 | 47.98 |
| Sample | a Vp (mm3 g−1) | b Dp (µm) |
|---|---|---|
| CMC/SA1:1 | 7489 | 23 |
| CMC/SA2:1 | 2036 | 23 |
| g-C3N4/CMC/SA1:1 | 7181 | 4 |
| g-C3N4/CMC/SA2:1 | 5663 | 24 |
| Photocatalyst Material | Pollutant | Pollutant Concentration, ppm | Removal Efficiency | Ref. |
|---|---|---|---|---|
| Ag/AgCl/Al-CMC | Rhodamine B | 10 | 98% in 60 min | [52] |
| Ag/AgCl/Fe-CMC | Rhodamine B | 10 | 87% in 60 min | [52] |
| CMC/polyanilyne/TiO2 | Cristal Violet | 50 | 99% in 80 min | [53] |
| g-C3N4/calcium alginate microsphere | Rhodamine B | 5 | 97% in 34 h | [39] |
| g-C3N4/calcium alginate microsphere | MB | 10 | 81% in 42 h | [39] |
| g-C3N4/calcium alginate beads | Rhodamine B | 10 | 98% in 60 min | [40] |
| CuO/g-C3N4/calcium alginate | Tetracycline | 13 | 75% in 60 min | [41] |
| nitrogen-doped graphene oxide/ZnO/ZnO2/CMC | Methyl Orange | 10 | 99% in 200 min | [54] |
| nitrogen-doped graphene oxide/ZnO/ZnO2/CMC | Rhodamine B | 50 | 99% in 160 min | [54] |
| g-C3N4/CMC/SA2:1 | OG | 10 | 98% in 180 min | Present work |
| Scavengers | Limiting Species | a k [μmol dm−3 min−1] × 102 | b kscavenger/kcontrol, % | c t1/2, min |
|---|---|---|---|---|
| CONTROL | / | 12.0 | / | 82 |
| IPA | •OH | 11.8 | 98.3 | 89 |
| EDTA | h+ | 10.9 | 90.8 | 95 |
| ASC | O2•−/h+ | 18.6 | 155 | 55 |
| Ag+ | e− | 6.6 | 55.0 | 129 |
| IPA/ASC | •OH and O2•−/h+ | 18.3 | 152 | 56 |
| ASC/Ag+ | O2•−/h+ and e− | 15.4 | 128 | 64 |
| Solution | Dye | Total Initial Dye Concentration, μmol dm−3 | Dye Concentration, μmol dm−3 | a R, % | b qe, mg g−1 | c k [μmol dm−3 min−1] × 102 |
|---|---|---|---|---|---|---|
| Binary solutions, MB-OG | ||||||
| A | MB | 22.2 | 11.1 | 68.6 | 0.042 | 1.3 |
| OG | 11.1 | 15.3 | 0.011 | 0.73 | ||
| B | MB | 44.2 | 22.1 | 58.4 | 0.071 | 3.8 |
| OG | 22.1 | 15.2 | 0.022 | k1 = 0.014; k2 = 0.8 | ||
| Binary solutions, RBBR-OG | ||||||
| C | RBBR | 22.2 | 11.1 | 15 | 0.015 | 7.0 |
| OG | 11.1 | 7 | 0.0053 | k1 = 2.0; k2 = 4.0 | ||
| Single solutions | ||||||
| MB | 13.3 | 13.3 | 64 | 0.031 | 2.0 | |
| OG | 22.1 | 22.1 | 11 | 0.016 | 12.0 | |
| RBBR | 22.1 | 22.1 | 21 | 0.042 | 14.0 | |
| Sample Codes | CMC Content, mg | SA Content, mg | g-C3N4 Content, mg |
|---|---|---|---|
| CMC/SA1:1 | 187.5 | 187.5 | / |
| CMC/SA2:1 | 250 | 125 | / |
| g-C3N4/CMC/SA1:1 | 187.5 | 187.5 | 75 |
| g-C3N4/CMC/SA2:1 | 250 | 125 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milošević, K.; Lončarević, D.; Kalagasidis Krušić, M.; Hadnađev-Kostić, M.; Dostanić, J. Eco-Friendly g-C3N4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants. Int. J. Mol. Sci. 2024, 25, 7896. https://doi.org/10.3390/ijms25147896
Milošević K, Lončarević D, Kalagasidis Krušić M, Hadnađev-Kostić M, Dostanić J. Eco-Friendly g-C3N4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants. International Journal of Molecular Sciences. 2024; 25(14):7896. https://doi.org/10.3390/ijms25147896
Chicago/Turabian StyleMilošević, Ksenija, Davor Lončarević, Melina Kalagasidis Krušić, Milica Hadnađev-Kostić, and Jasmina Dostanić. 2024. "Eco-Friendly g-C3N4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants" International Journal of Molecular Sciences 25, no. 14: 7896. https://doi.org/10.3390/ijms25147896





