The Link between Inflammation, Lipid Derivatives, and Microbiota Metabolites in COVID-19 Patients: Implications on Eating Behaviors and Nutritional Status
Abstract
1. Introduction
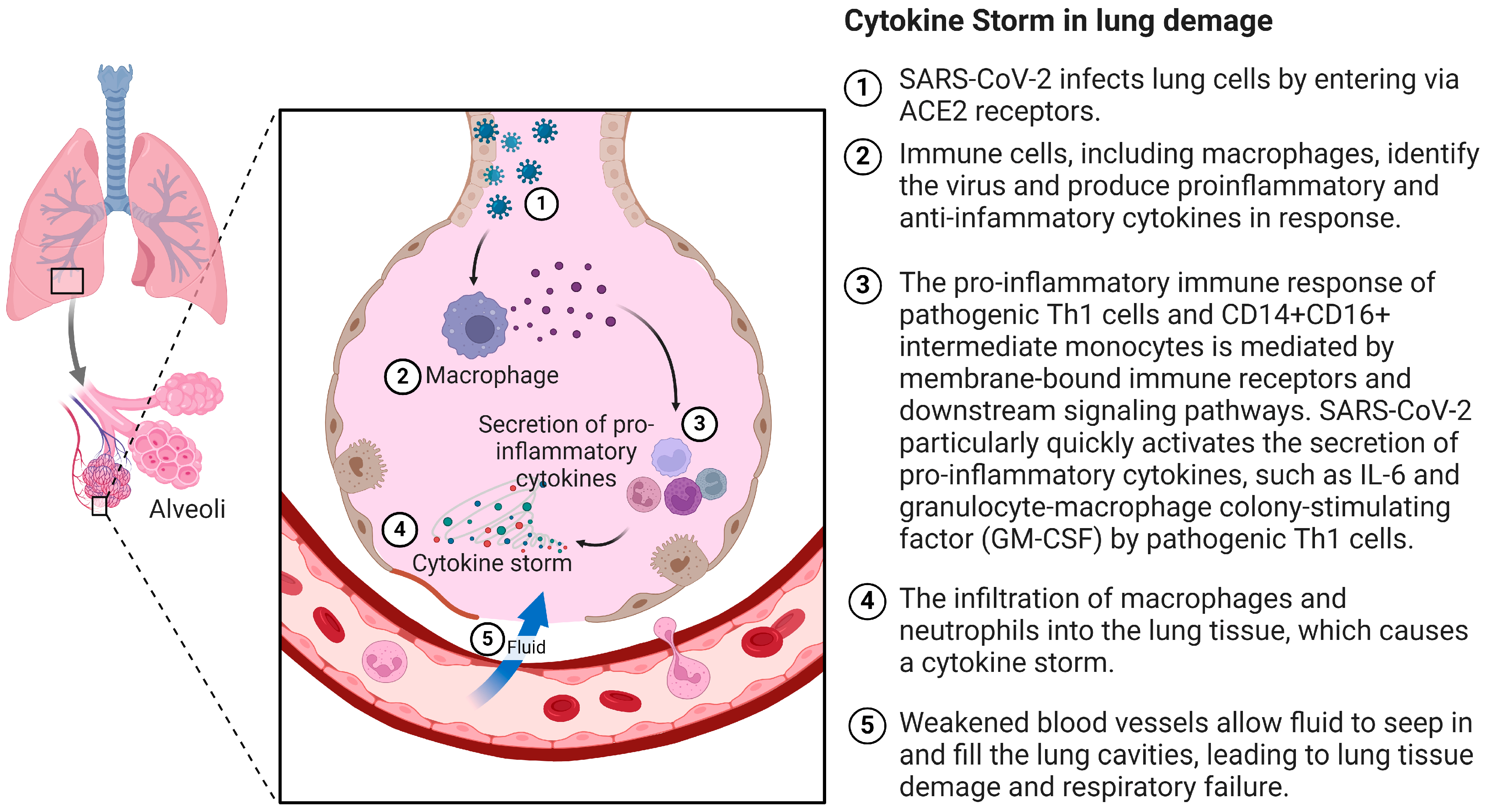
2. Pathogenesis of the Cytokine Storm in COVID-19
3. Cytokine Storm and Comorbidities
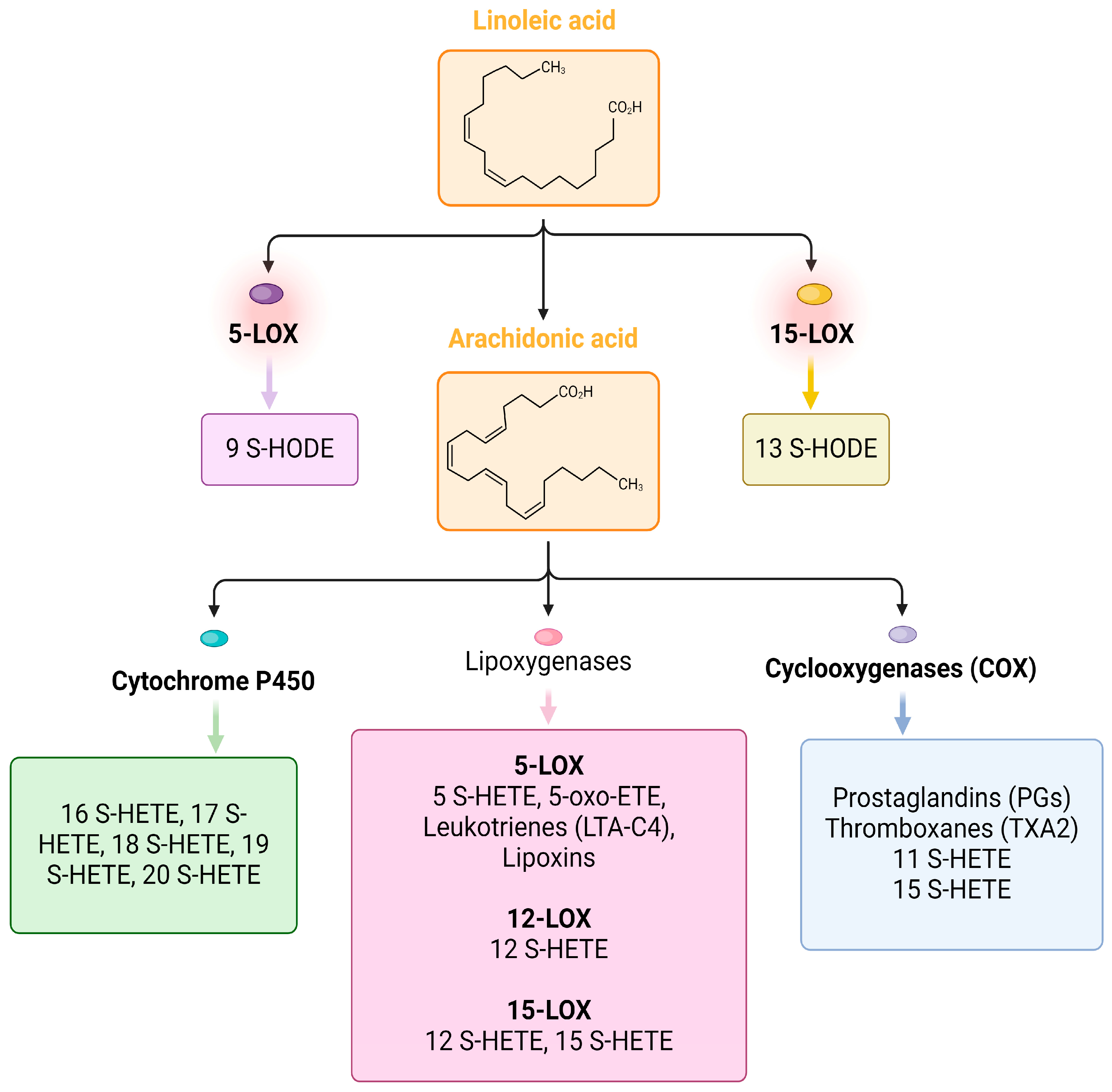
4. Lipid Markers of Inflammation in COVID-19
5. Cytokine Storm and Microbiota Metabolites
5.1. Short-Chain Fatty Acids (SCFAs)
5.2. Deoxycholic Acid (DCA) and Lithocholic Acid (LCA)
5.3. Antimicrobial Peptides (AMPs)
5.4. Tryptophan Derivatives
5.5. Desaminotyrosine
6. Eating Behavior in the Pandemic: Implications on the Immune System
7. Bioactive Food Compounds, Immune System, and COVID-19
8. Obese Patients with COVID-19: Risk Factor for Worse Prognosis
9. Malnutrition in Patients with COVID-19: A Result of Hospitalization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Eurosurveillance 2020, 25, 200131e. [Google Scholar] [CrossRef]
- Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 19 March 2023).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 7 July 2023).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef]
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. The Pathogenesis of End-Stage Renal Disease from the Standpoint of the Theory of General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Qu, J. Coronavirus disease 2019 (COVID-19): A clinical update. Front. Med. 2020, 14, 126–135. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, D.; Szabla, R.; Zheng, M.; Li, G.; Du, P.; Zheng, S.; Li, X.; Song, C.; Li, R.; et al. Broad and Differential Animal Angiotensin-Converting Enzyme 2 Receptor Usage by SARS-CoV-2. J. Virol. 2020, 94, e00940-20. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Murakami, M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. Biorxiv 2020. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Ong, E.Z.; Chan, Y.F.Z.; Leong, W.Y.; Lee, N.M.Y.; Kalimuddin, S.; Haja Mohideen, S.M.; Chan, K.S.; Tan, A.T.; Bertoletti, A.; Ooi, E.E.; et al. A Dynamic Immune Response Shapes COVID-19 Progression. Cell Host Microbe 2020, 27, 879–882.e2. [Google Scholar] [CrossRef]
- Kesmez Can, F.; Özkurt, Z.; Öztürk, N.; Sezen, S. Effect of IL-6, IL-8/CXCL8, IP-10/CXCL 10 levels on the severity in COVID-19 infection. Int. J. Clin. Pract. 2021, 75, e14970. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Marchingo, J.M.; Sinclair, L.V.; Howden, A.J.; Cantrell, D.A. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. eLife 2020, 9, e53725. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease. J. Clin. Investig. 2019, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, 761–775. [CrossRef] [PubMed]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Caci, G.; Albini, A.; Malerba, M.; Noonan, D.M.; Pochetti, P.; Polosa, R. COVID-19 and Obesity: Dangerous Liaisons. J. Clin. Med. 2020, 9, 2511. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S. Nutritional status and COVID-19: An opportunity for lasting change? Clin. Med. 2020, 20, 270–273. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Pugliese, G.; Barrea, L.; Savastano, S.; Colao, A. Commentary: Obesity: The “Achilles heel” for COVID-19? Metabolism 2020, 108, 154251. [Google Scholar] [CrossRef]
- Misumi, I.; Starmer, J.; Uchimura, T.; Beck, M.A.; Magnuson, T.; Whitmire, J.K. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep. 2019, 27, 514–524.e5. [Google Scholar] [CrossRef]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Umpierrez, G.E. Letter to the Editor: COVID-19 in patients with diabetes: Risk factors that increase morbidity. Metabolism 2020, 108, 154224. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Meng, H.; Sengupta, A.; Ricciotti, E.; Mrčela, A.; Mathew, D.; Mazaleuskaya, L.L.; Ghosh, S.; Brooks, T.G.; Turner, A.P.; Schanoski, A.S.; et al. Deep phenotyping of the lipidomic response in COVID-19 and non-COVID-19 sepsis. Clin. Transl. Med. 2023, 13, e1440. [Google Scholar] [CrossRef]
- Ravindran, R.; O’Connor, E.; Gupta, A.; Luciw, P.A.; Khan, A.I.; Dorreh, N.; Chiang, K.; Ikram, A.; Reddy, S. Lipid Mediators and Cytokines/Chemokines Display Differential Profiles in Severe versus Mild/Moderate COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 13054. [Google Scholar] [CrossRef] [PubMed]
- Borras, E.; McCartney, M.M.; Rojas, D.E.; Hicks, T.L.; Tran, N.K.; Tham, T.; Juarez, M.M.; Franzi, L.; Harper, R.W.; Davis, C.E.; et al. Oxylipin concentration shift in exhaled breath condensate (EBC) of SARS-CoV-2 infected patients. J. Breath Res. 2023, 17, 047103. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Reuben, R.C.; Beugnon, R.; Jurburg, S.D. COVID-19 alters human microbiomes: A meta-analysis. Front. Cell Infect. Microbiol. 2023, 13, 1211348. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Lotan-Pompan, M.; Weinberger, A.; Segal, E. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: A diet intervention in pre-diabetes. Gut 2023, 72, 1486–1496. [Google Scholar] [CrossRef]
- Hoque, M.N.; Sarkar, M.M.H.; Rahman, M.S.; Akter, S.; Banu, T.A.; Goswami, B.; Jahan, I.; Hossain, M.S.; Shamsuzzaman, A.K.M.; Nafisa, T.; et al. SARS-CoV-2 infection reduces human nasopharyngeal commensal microbiome with inclusion of pathobionts. Sci. Rep. 2021, 11, 24042. [Google Scholar] [CrossRef]
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Chang, Y.; Kim, H.-N.; Ryu, S.; Kwon, M.-J.; Cho, Y.K.; Kim, H.-L.; Cheong, H.S.; Joo, E.-J. Alterations of the Gut Microbiome in Chronic Hepatitis B Virus Infection Associated with Alanine Aminotransferase Level. J. Clin. Med. 2019, 8, 173. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Hasani-Ranjbar, S.; Siadat, S.-D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung–gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal. Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Talavera-Rodríguez, A.; Gosalbes, M.J.; Madrid, N.; Pérez-Molina, J.A.; Elliott, R.J.; Navia, B.; Lanza, V.F.; Vallejo, A.; Osman, M.; et al. Fecal microbiota transplantation in HIV: A pilot placebo-controlled study. Nat. Commun. 2021, 12, 1139. [Google Scholar] [CrossRef]
- Xu, R.; Lu, R.; Zhang, T.; Wu, Q.; Cai, W.; Han, X.; Wan, Z.; Jin, X.; Zhang, Z.; Zhang, C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun. Biol. 2021, 4, 240. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.-Y.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.P.; Ferreira, D.M.; Smits, H.H. Microbiota and compartment matter in the COVID-19 response. Nat. Immunol. 2021, 22, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.A.; McGeoch, L.J.; Hay, A.D.; Hammond, A. Respiratory tract infections and gut microbiome modifications: A systematic review. PLoS ONE 2022, 17, e0262057. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57 (Suppl. S1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, M.; Cao, S.; Sun, Y.; Zhao, L.; Mao, X.; Chen, P.; Tong, X.; Ou, Z.; Zhu, H.; et al. Distinct gut microbiota and health outcomes in asymptomatic infection, viral nucleic acid test re-positive, and convalescent COVID-19 cases. mLife 2022, 1, 183–197. [Google Scholar] [CrossRef]
- Sokol, H.; Contreras, V.; Maisonnasse, P.; Desmons, A.; Delache, B.; Sencio, V.; Machelart, A.; Brisebarre, A.; Humbert, L.; Deryuter, L.; et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 2021, 13, 1893113. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Chemudupati, M.; Kenney, A.D.; Smith, A.C.; Fillinger, R.J.; Zhang, L.; Zani, A.; Liu, S.-L.; Anderson, M.Z.; Sharma, A.; Yount, J.S. Butyrate Reprograms Expression of Specific Interferon-Stimulated Genes. J. Virol. 2020, 94, e00326-20. [Google Scholar] [CrossRef]
- Friedland, R.P.; Haribabu, B. The role for the metagenome in the pathogenesis of COVID-19. EBioMedicine 2020, 61, 103019. [Google Scholar] [CrossRef]
- Jardou, M.; Lawson, R. Supportive therapy during COVID-19: The proposed mechanism of short-chain fatty acids to prevent cytokine storm and multi-organ failure. Med. Hypotheses 2021, 154, 110661. [Google Scholar] [CrossRef]
- Wang, S.; Huang, M.; You, X.; Zhao, J.; Chen, L.; Wang, L.; Luo, Y.; Chen, Y. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice. Sci. Rep. 2018, 8, 13037. [Google Scholar] [CrossRef]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Bezençon, J.; Sjöstedt, N.; Fallon, J.K.; Brouwer, K.L.R. Role of Organic Solute Transporter Alpha/Beta in Hepatotoxic Bile Acid Transport and Drug Interactions. Toxicol. Sci. 2020, 176, 34–45. [Google Scholar] [CrossRef]
- Huang, D.; Xiong, M.; Xu, X.; Wu, X.; Xu, J.; Cai, X.; Lu, L.; Zhou, H. Bile acids elevated by high-fat feeding induce endoplasmic reticulum stress in intestinal stem cells and contribute to mucosal barrier damage. Biochem. Biophys. Res. Commun. 2020, 529, 289–295. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Lopes, F.E.S.; Amaral, J.L.; Freitas, C.D.T.; Oliveira, J.T.A. A molecular docking study revealed that synthetic peptides induced conformational changes in the structure of SARS-CoV-2 spike glycoprotein, disrupting the interaction with human ACE2 receptor. Int. J. Biol. Macromol. 2020, 164, 66–76. [Google Scholar] [CrossRef]
- Solanki, S.S.; Singh, P.; Kashyap, P.; Sansi, M.S.; Ali, S.A. Promising role of defensins peptides as therapeutics to combat against viral infection. Microb. Pathog. 2021, 155, 104930. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Camargo, S.M.R.; Vuille-Dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and gut amino acid transport. Clin. Sci. 2020, 134, 2823–2833. [Google Scholar] [CrossRef]
- Nataf, S.; Pays, L. Molecular Insights into SARS-CoV2-Induced Alterations of the Gut/Brain Axis. Int. J. Mol. Sci. 2021, 22, 10440. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; de Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Kaur, G.; Ji, X.; Rahman, I. SARS-CoV2 Infection Alters Tryptophan Catabolism and Phospholipid Metabolism. Metabolites 2021, 11, 659. [Google Scholar] [CrossRef]
- de Hernández-Flores, T.J.; Pedraza-Brindis, E.J.; Cárdenas-Bedoya, J.; Ruíz-Carrillo, J.D.; Méndez-Clemente, A.S.; Martínez-Guzmán, M.A.; Iñiguez-Gutiérrez, L. Role of Micronutrients and Gut Microbiota-Derived Metabolites in COVID-19 Recovery. Int. J. Mol. Sci. 2022, 23, 12324. [Google Scholar] [CrossRef]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Johnson, A.N.; Clockston, R.L.M.; Fremling, L.; Clark, E.; Lundeberg, P.; Mueller, M.; Graham, D.J. Changes in Adults’ Eating Behaviors During the Initial Months of the COVID-19 Pandemic: A Narrative Review. J. Acad. Nutr. Diet 2023, 123, 144–194.e30. [Google Scholar] [CrossRef]
- Grant, F.; Scalvedi, M.L.; Scognamiglio, U.; Turrini, A.; Rossi, L. Eating Habits during the COVID-19 Lockdown in Italy: The Nutritional and Lifestyle Side Effects of the Pandemic. Nutrients 2021, 13, 2279. [Google Scholar] [CrossRef]
- Rodriguez-Besteiro, S.; Tornero-Aguilera, J.F.; Fernández-Lucas, J.; Clemente-Suárez, V.J. Gender Differences in the COVID-19 Pandemic Risk Perception, Psychology, and Behaviors of Spanish University Students. Int. J. Environ. Res. Public Health 2021, 18, 3908. [Google Scholar] [CrossRef]
- Jia, P.; Liu, L.; Xie, X.; Yuan, C.; Chen, H.; Guo, B.; Zhou, J.; Yang, S. Changes in dietary patterns among youths in China during COVID-19 epidemic: The COVID-19 impact on lifestyle change survey (COINLICS). Appetite 2021, 158, 105015. [Google Scholar] [CrossRef]
- Batlle-Bayer, L.; Aldaco, R.; Bala, A.; Puig, R.; Laso, J.; Margallo, M.; Vázquez-Rowe, I.; Antó, J.M.; Fullana-i-Palmer, P. Environmental and nutritional impacts of dietary changes in Spain during the COVID-19 lockdown. Sci. Total Environ. 2020, 748, 141410. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Rundle, A.G.; Park, Y.; Herbstman, J.B.; Kinsey, E.W.; Wang, Y.C. COVID-19 Related School Closings and Risk of Weight Gain Among Children. Obesity 2020, 28, 1008–1009. [Google Scholar] [CrossRef]
- Opichka, K.; Smith, C.; Levine, A.S. Problematic Eating Behaviors Are More Prevalent in African American Women Who Are Overweight or Obese Than African American Women Who Are Lean or Normal Weight. Fam. Community Health 2019, 42, 81. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk-Bębenek, E.; Jagielski, P.; Bolesławska, I.; Jagielska, A.; Nitsch-Osuch, A.; Kawalec, P. Nutrition Behaviors in Polish Adults before and during COVID-19 Lockdown. Nutrients 2020, 12, 3084. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Gasmi Benahmed, A.; Bjørklund, G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Raheem, D.; Ramos, F.; Carrascosa, C.; Saraiva, A.; Raposo, A. Highlights of Current Dietary Guidelines in Five Continents. Int. J. Environ. Res. Public Health 2021, 18, 2814. [Google Scholar] [CrossRef]
- Bhaskaram, P. Micronutrient malnutrition, infection, and immunity: An overview. Nutr. Rev. 2002, 60, S40–S45. [Google Scholar] [CrossRef]
- Cava, E.; Carbone, S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 229–235. [Google Scholar] [CrossRef]
- Nehme, M.; Braillard, O.; Rodondi, P.-Y.; Guessous, I.; CoviCare Study Team. Use of complementary medicine and its association with SARS-CoV-2 vaccination during the COVID-19 pandemic: A longitudinal cohort study. Swiss. Med. Wkly. 2023, 153, 3505. [Google Scholar] [CrossRef]
- Seely, D.; Legacy, M.; Conte, E.; Keates, C.; Psihogios, A.; Ramsay, T.; Fergusson, D.A.; Kanji, S.; Simmons, J.-G.; Wilson, K. Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: A double-blind randomised controlled trial. BMJ Open 2023, 13, e073761. [Google Scholar] [CrossRef]
- Mahjoub, L.; Youssef, R.; Yaakoubi, H.; Salah, H.B.; Jaballah, R.; Mejri, M.; Sekma, A.; Trabelsi, I.; Nouira, S.; Khrouf, M.; et al. Melatonin, vitamins and minerals supplements for the treatment of COVID-19 and COVID-like illness: A prospective, randomized, double-blind multicenter study. Explore 2023, 20, 95–100. [Google Scholar] [CrossRef]
- Nawaiseh, H.K.; Abdelrahim, D.N.; Al-Domi, H.; AL-Assaf, M.S.; AL-Nawaiseh, F.K. The impact of vitamin D, vitamin C, and zinc supplements on immune status among Jordanian adults during COVID-19: Cross-sectional study findings. BMC Public Health 2023, 23, 2251. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Pokharna, P.; Shetty, S.R. Unwinding the potentials of vitamin C in COVID-19 and other diseases: An updated review. Nutr. Health 2022, 29, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S.; Ramachandram, D.S. The effect of vitamin C on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2023, 31, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Sciurti, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The Efficacy of Multivitamin, Vitamin A, Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention and Management of COVID-19 and Long-COVID: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 1345. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Shahidi, R.; Mojdeganlou, H.; Akhtaran, F.K.; Maroufi, S.F.; Maroufi, S.P.; Mirzapour, P.; Karimi, A.; Khodaei, S.; Pour, M.M.; et al. The effect of macronutrient and micronutrient supplements on COVID-19: An umbrella review. J. Health Popul. Nutr. 2024, 43, 16. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-Hydroxyvitamin D3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Hemilä, H.; Carr, A.; Chalker, E. Vitamin C May Increase the Recovery Rate of Outpatient Cases of SARS-CoV-2 Infection by 70%: Reanalysis of the COVID A to Z Randomized Clinical Trial. Front. Immunol. 2021, 12, 674681. [Google Scholar] [CrossRef] [PubMed]
- Bychinin, M.V.; Klypa, T.V.; Mandel, I.A.; Yusubalieva, G.M.; Baklaushev, V.P.; Kolyshkina, N.A.; Troitsky, A.V. Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. Sci. Rep. 2022, 12, 18604. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Alemzadeh, E.; Alemzadeh, E.; Salehiniya, H. The effect of polyunsaturated fatty acids on the severity and mortality of COVID patients: A systematic review. Life Sci. 2022, 299, 120489. [Google Scholar] [CrossRef] [PubMed]
- Cuerda, C.; Sánchez López, I.; Gil Martínez, C.; Merino Viveros, M.; Velasco, C.; Cevallos Peñafiel, V.; Maíz Jiménez, M.; Gonzalo, I.; González-Sánchez, V.; Ramos Carrasco, A.; et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin. Nutr. 2022, 41, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Muollo, V.; Dalla Valle, Z.; Urbani, S.; Pellegrini, M.; El Ghoch, M.; Mazzali, G. The Role of Obesity, Body Composition, and Nutrition in COVID-19 Pandemia: A Narrative Review. Nutrients 2022, 14, 3493. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Besutti, G.; Pellegrini, M.; Ottone, M.; Cantini, M.; Milic, J.; Bonelli, E.; Dolci, G.; Cassone, G.; Ligabue, G.; Spaggiari, L.; et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS ONE 2021, 16, e0251768. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Wang, Q. Sarcopenia: An underlying treatment target during the COVID-19 pandemic. Nutrition 2021, 84, 111104. [Google Scholar] [CrossRef] [PubMed]
- Wierdsma, N.J.; Kruizenga, H.M.; Konings, L.A.; Krebbers, D.; Jorissen, J.R.; Joosten, M.-H.I.; van Aken, L.H.; Tan, F.M.; van Bodegraven, A.A.; Soeters, M.R.; et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN 2021, 43, 369–376. [Google Scholar] [CrossRef]
- Rossi, A.P.; Gottin, L.; Donadello, K.; Schweiger, V.; Brandimarte, P.; Zamboni, G.A.; Florio, A.; Boetti, R.; Pavan, G.; Zamboni, M.; et al. Intermuscular Adipose Tissue as a Risk Factor for Mortality and Muscle Injury in Critically Ill Patients Affected by COVID-19. Front. Physiol. 2021, 12, 651167. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Z.; He, X.; Wang, X.; Yuan, C.; Huang, L.; Song, R.; Wu, Y. Risk of Malnutrition in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5267. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition-A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Itoh, T.; Yabe, A.; Imai, S.; Nakamura, Y.; Mizokami, Y.; Okouchi, Y.; Ikeshita, A.; Kominato, H. Polypharmacy is associated with malnutrition and activities of daily living disability among daycare facility users: A cross-sectional study. Medicine 2021, 100, e27073. [Google Scholar] [CrossRef]
- Thomas, S.; Alexander, C.; Cassady, B.A. Nutrition risk prevalence and nutrition care recommendations for hospitalized and critically-ill patients with COVID-19. Clin. Nutr. ESPEN 2021, 44, 38–49. [Google Scholar] [CrossRef]
- Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. [Google Scholar] [CrossRef]
- Mohammadi, P.; Varpaei, H.A.; Khafaee Pour Khamseh, A.; Mohammadi, M.; Rahimi, M.; Orandi, A. Evaluation of the Relationship between Nutritional Status of COVID-19 Patients Admitted to the ICU and Patients’ Prognosis: A Cohort Study. J. Nutr. Metab. 2022, 2022, 5016649. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.; Bauer, J.M. Malnutrition and Sarcopenia in COVID-19 Survivors. Clin. Geriatr. Med. 2022, 38, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hinkelmann, J.V.; de Oliveira, N.A.; Marcato, D.F.; Costa, A.R.R.O.; Ferreira, A.M.; Tomaz, M.; Rodrigues, T.J.; Mendes, A.P. Nutritional support protocol for patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 544–550. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Ali, Z.; Armitage, A.E.; Bonell, A.; Cerami, C.; Drakesmith, H.; Jobe, M.; Jones, K.S.; Liew, Z.; Moore, S.E.; et al. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J. Nutr. 2021, 151, 1854–1878. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Feng, Q.; Boateng, J.; Wang, X.; Brooke, J.; Adegboye, A.R.A. The Effects of Enteral Nutrition in Critically Ill Patients with COVID-19: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Bodolea, C.; Nemes, A.; Avram, L.; Craciun, R.; Coman, M.; Ene-Cocis, M.; Ciobanu, C.; Crisan, D. Nutritional Risk Assessment Scores Effectively Predict Mortality in Critically Ill Patients with Severe COVID-19. Nutrients 2022, 14, 2105. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.W.; Roberts, A.P.; Frugé, A.D. Negative Association Between Mediterranean Diet Adherence and COVID-19 Cases and Related Deaths in Spain and 23 OECD Countries: An Ecological Study. Front. Nutr. 2021, 8, 591964. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Read, S.A.; Shackel, N.A.; Hebbard, L.; George, J.; Ahlenstiel, G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells 2019, 8, 603. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawryłkowicz, V.; Stasiewicz, B.; Maciejewska, D.; Sołek-Pastuszka, J.; Komorniak, N.; Skonieczna-Żydecka, K.; Martynova-Van Kley, A.; Stachowska, E. The Link between Inflammation, Lipid Derivatives, and Microbiota Metabolites in COVID-19 Patients: Implications on Eating Behaviors and Nutritional Status. Int. J. Mol. Sci. 2024, 25, 7899. https://doi.org/10.3390/ijms25147899
Hawryłkowicz V, Stasiewicz B, Maciejewska D, Sołek-Pastuszka J, Komorniak N, Skonieczna-Żydecka K, Martynova-Van Kley A, Stachowska E. The Link between Inflammation, Lipid Derivatives, and Microbiota Metabolites in COVID-19 Patients: Implications on Eating Behaviors and Nutritional Status. International Journal of Molecular Sciences. 2024; 25(14):7899. https://doi.org/10.3390/ijms25147899
Chicago/Turabian StyleHawryłkowicz, Viktoria, Beata Stasiewicz, Dominika Maciejewska, Joanna Sołek-Pastuszka, Natalia Komorniak, Karolina Skonieczna-Żydecka, Alexandra Martynova-Van Kley, and Ewa Stachowska. 2024. "The Link between Inflammation, Lipid Derivatives, and Microbiota Metabolites in COVID-19 Patients: Implications on Eating Behaviors and Nutritional Status" International Journal of Molecular Sciences 25, no. 14: 7899. https://doi.org/10.3390/ijms25147899
APA StyleHawryłkowicz, V., Stasiewicz, B., Maciejewska, D., Sołek-Pastuszka, J., Komorniak, N., Skonieczna-Żydecka, K., Martynova-Van Kley, A., & Stachowska, E. (2024). The Link between Inflammation, Lipid Derivatives, and Microbiota Metabolites in COVID-19 Patients: Implications on Eating Behaviors and Nutritional Status. International Journal of Molecular Sciences, 25(14), 7899. https://doi.org/10.3390/ijms25147899





