Pea Seed Priming with Pluronic P85-Grafted Single-Walled Carbon Nanotubes Affects Photosynthetic Gas Exchange but Not Photosynthetic Light Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Seed Germination of Soil Gardening Pea Plants
2.2. Leaf Pigments and Photosynthetic Performance
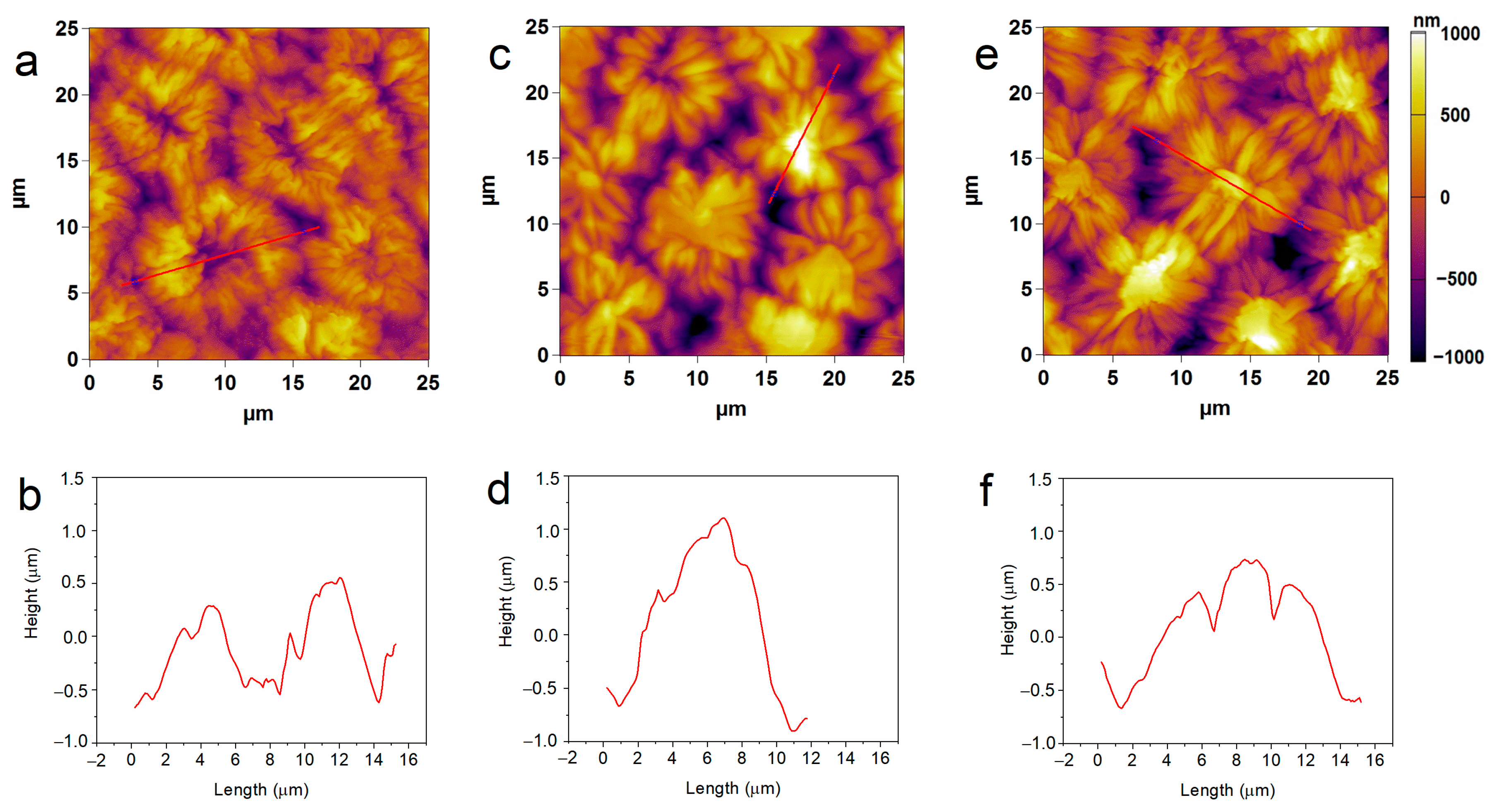
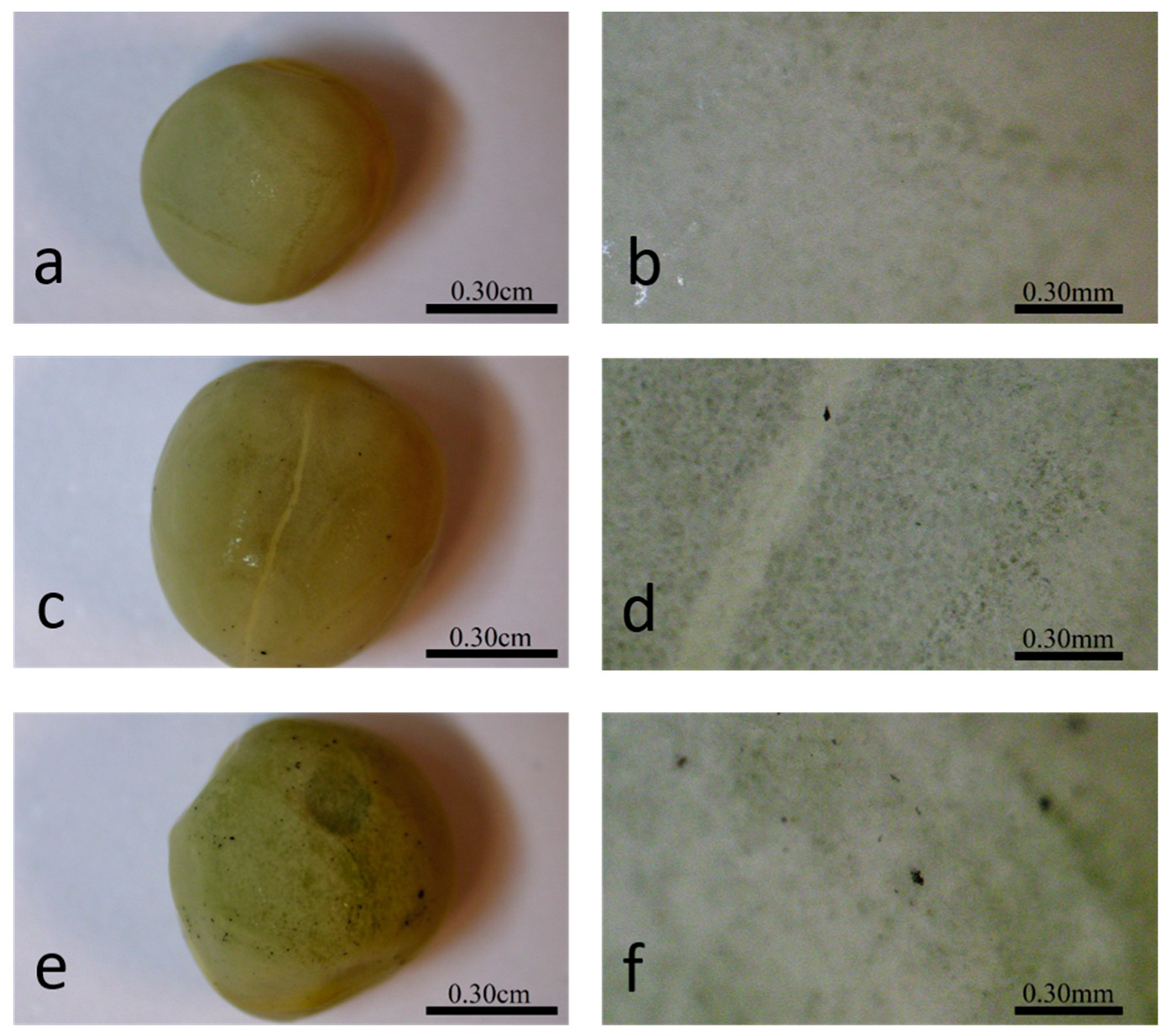
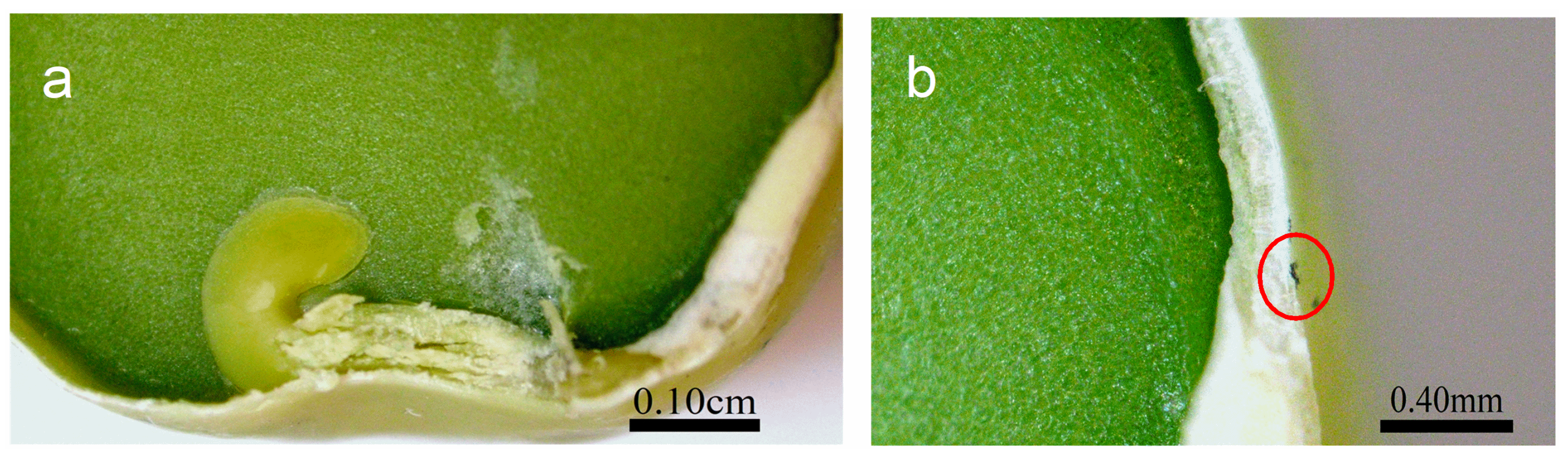
2.3. Seeds Topology Studied by AFM
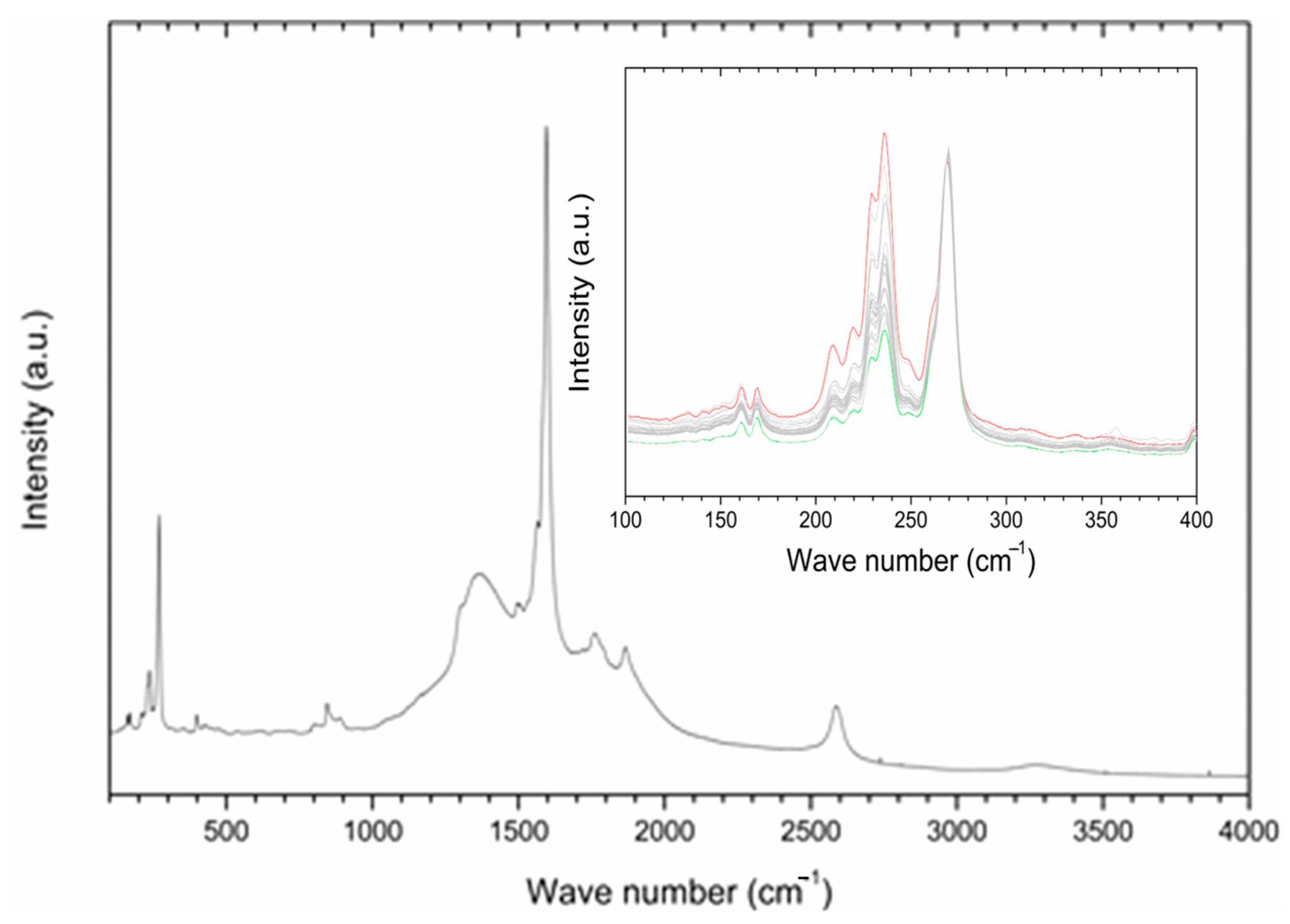
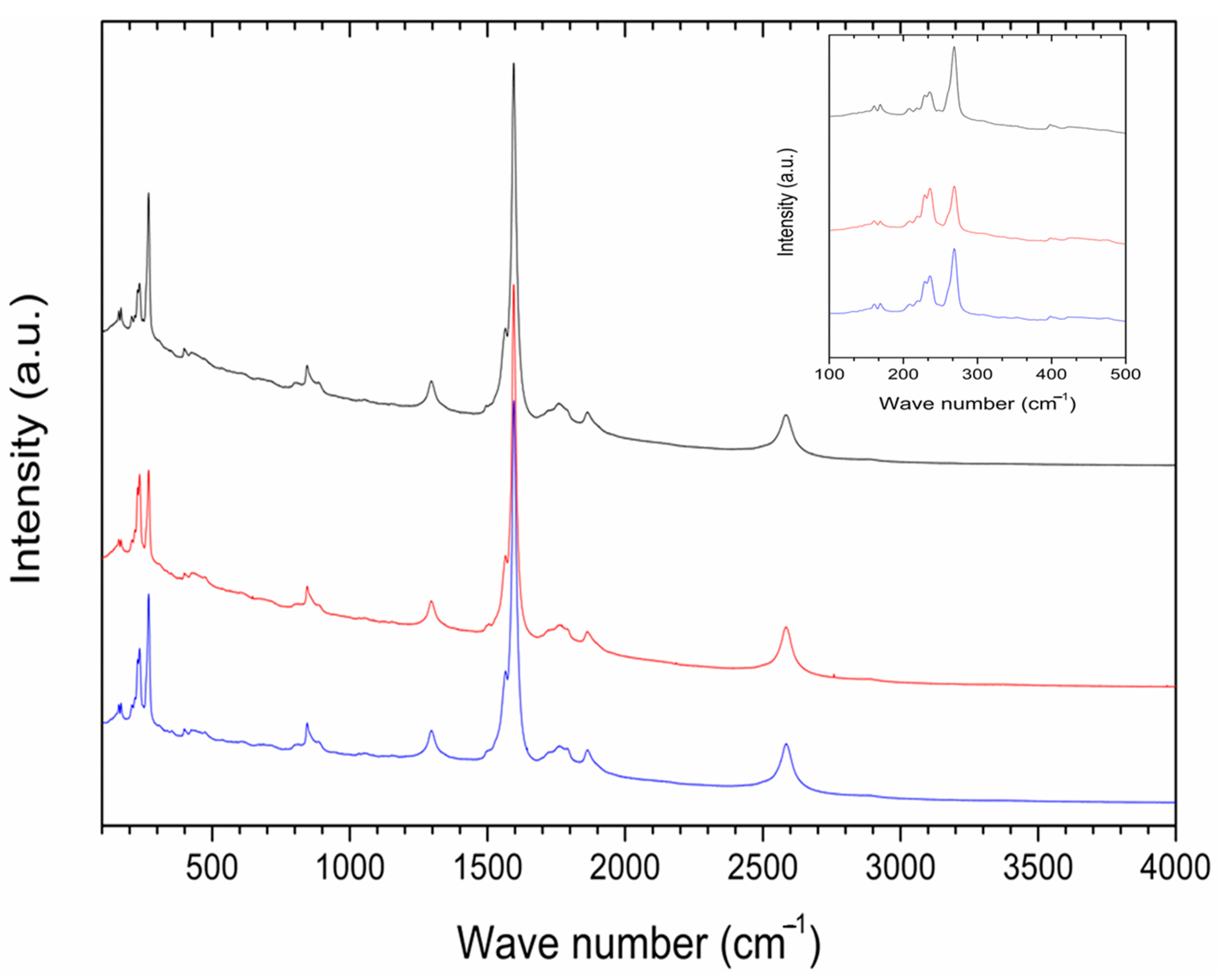
2.4. P85-SWCNT–Seed Interaction Probed by Raman Spectroscopy
3. Materials and Methods
3.1. Seed Priming and Growth Conditions
3.2. Characterization of Seeds and Early Plant Growth
3.2.1. Seed Germination
3.2.2. Atomic Force Microscopy on Seeds
3.2.3. Micro-Raman Spectroscopy on Seeds
3.3. Physiological Measurements
3.3.1. Leaf Pigments
3.3.2. Photosynthetic Gas Exchange
3.3.3. Chlorophyll Fluorescence
3.4. Thylakoid Membrane Studies
3.4.1. Preparation and Fractionation
3.4.2. Lipid Peroxidation Evaluation
3.5. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.O.; Kim, J. Engineering plants with carbon nanotubes: A sustainable agriculture approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Pourkhaloee, A.; Haghighi, M.; Saharkhiz, M.J.; Jouzi, H.; Doroodmand, M.M. Carbon Nanotubes Can Promote Seed Germination via Seed Coat Penetration. Seed Technol. 2011, 33, 155–169. [Google Scholar]
- Haghighi, B.J.; Alizadeh, O.; Firoozabadi, A.H. The role of Plant Growth Promoting Rhizobacteria (PGPR) in sustainable Agriculture. Adv. Environ. Biol. 2011, 5, 3079–3083. [Google Scholar] [CrossRef]
- Haghighi, M.; Teixeira da Silva, J.A. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J. Hazard. Mater 2013, 246–247, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard Mater. 2017, 324 Pt B, 306–320. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, M.; Zheng, X.; Xie, C.; Zhou, H.; Li, L. Physiological Effects of Single- and Multi-Walled Carbon Nanotubes on Rice Seedlings. IEEE Trans. NanoBiosci. 2017, 16, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Exposure to single-walled carbon nanotubes differentially affect in vitro germination, biochemical and antioxidant properties of Thymus daenensis celak. seedlings. BMC Plant Biol. 2023, 23, 579. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Kwak, S.-Y.; Jain, R.M.; Wong, M.H.; Iverson, N.M.; Ben-Naim, M.; Strano, M.S. A Ratiometric Sensor Using Single Chirality Near-Infrared Fluorescent Carbon Nanotubes: Application to in Vivo Monitoring. Small 2014, 11, 3973–3984. [Google Scholar] [CrossRef]
- Wiwatowski, K.; Dużyńska, A.; Swiniarski, M.; Szalkowski, M.; Zdrojek, M.; Judek, J.; Mackowski, S.; Kaminska, I. Energy transfer from natural photosynthetic complexes to single-wall carbon nanotubes. J. Lumin. 2016, 170, 855–859. [Google Scholar] [CrossRef]
- Dorogi, M.; Balint, Z.; Mikó, C.; Vileno, B.; Milas, M.; Hernadi, K.; Forró, L.; Varó, G.; Nagy, L. Stabilization effect of single-walled carbon nanotubes on the functioning of photosynthetic reaction centers. J. Phys. Chem. B 2006, 110, 21473–21479. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, K.; Szabó, T.; Magyar, M.; Bencsik, G.; Németh, Z.; Nagy, K.; Magrez, A.; Forró, L.; Váró, G.; Hernádi, K.; et al. Photosynthetic reaction center protein in nanostructures. Phys. Stat. Sol. B 2011, 248, 2700–2703. [Google Scholar] [CrossRef]
- Nagy, L.; Magyar, M.; Szabó, T.; Hajdu, K.; Giotta, L.; Dorogi, M.; Milano, F. Photosynthetic machineries in nano-systems. Curr. Protein Pept. Sci. 2014, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah Verma, K.K. Nano-Enabled Products: Challenges and Opportunities for Sustainable Agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Petrova, N.; Kovács, L.; Petrova, A.; Koleva, D.; Tsonev, T.; Taneva, S.; Petrov, P.; Krumova, S. Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants. Int. J. Mol. Sci. 2021, 22, 4878. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Paunov, M.; Petrov, P.; Velikova, V.; Goltsev, V.; Krumova, S. Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants. Molecules 2021, 26, 5958. [Google Scholar] [CrossRef]
- Petrova, N.; Todinova, S.; Petrov, P.; Velikova, V.; Krumova, S. Foliar application of Pluronic P85-grafted single-walled carbon nanotubes induces thylakoid membrane structural remodeling. Acta Physiol. Plant. 2023, 45, 133. [Google Scholar] [CrossRef]
- Krumova, S.; Petrova, A.; Petrova, N.; Stoichev, S.; Ilkov, D.; Tsonev, T.; Petrov, P.; Koleva, D.; Velikova, V. Seed Priming with Single-Walled Carbon Nanotubes Grafted with Pluronic P85 Preserves the Functional and Structural Characteristics of Pea Plants. Nanomaterials 2023, 13, 1332. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some Emerging opportunities of nanotechnology development for soilless and microgreen farming. Agronomy 2021, 11, 1213. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Silva, K.D.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.; Zharov, V.P. Complex Genetic, Photothermal, and Photoacoustic Analysis of Nanotube–Plant Interactions. Proc. Nat. Acad. Sci. USA 2011, 109, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2021, 31, 167–176. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Wei, H.; Jing, Y.; Zhang, L.; Kong, D. Phytohormones and their crosstalk in regulating stomatal development and patterning. J. Exp. Bot. 2021, 72, 2356–2370. [Google Scholar] [CrossRef] [PubMed]
- Raschke, K. Stomatal action. An Rev. Plant Physiol. 1975, 26, 309–340. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Müller, C.; Ghirardo, A.; Rock, T.M.; Aichler, M.; Walch, A.; Schmitt-Kopplin, P.; Schnitzler, J.P. Knocking Down Isoprene Emission Modifies the Lipid Matrix of Thylakoid Membranes and Influences the Chloroplast Ultrastructure in Poplar. Plant Physiol. 2015, 168, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Elliott-Kingston, C.; McElwain, C. Stomatal control as a driver of plant evolution. J. Exp. Bot. 2011, 62, 2419–2423. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Liang, L.; Wong, S.C.; Lisak, G. Effects of plastic-derived carbon dots on germination and growth of pea (Pisum sativum) via seed nano-priming. Chemosphere 2023, 316, 137868. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Santoro, G. Espectroscopía Raman de nanotubos de carbono. Opt. Pura Apl. 2007, 40, 175–186. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Davijani, A.A.B.; Chang, H.; Liu, H.C.; Luo, J.; Kumar, S. Stress transfer in nanocomposites enabled by poly (methyl methacrylate) wrapping of carbon nanotubes. Polymer 2017, 130, 191–198. [Google Scholar] [CrossRef]
- Ranal, M.A.; de Santana, D.G.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Rev. Bras. Botânica 2009, 32, 849–855. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef]
- Petrova, N.; Todinova, S.; Paunov, M.; Kovács, L.; Taneva, S.; Krumova, S. Thylakoid membrane unstacking increases LHCII thermal stability and lipid phase fluidity. J. Bioenerg. Biomembr. 2018, 50, 425–435. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Chow, W.S.; Thorne, S.W.; Duniec, J.T.; Sculley, M.J.; Boardman, N.K. The stacking of chloroplast thylakoids: Effects of cation screening and binding, studied by the digitonin method. Arch. Biochem. Biophys. 1980, 201, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Singhal, G.S. Function of photosynthetic apparatus of intact wheat leaves under high light and heat stress and its relationship with peroxidation of thylakoid lipids. Plant Physiol. 1992, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Priming Treatment | G (%) | MT (Days) | Z | MR (Germinated Seeds/Day) |
|---|---|---|---|---|
| H2O | 75.4 ± 5.5 | 2.47 ± 0.15 | 0.35 ± 0.05 | 0.42 ± 0.03 |
| 10 mg/L P85-SWCNT | 68.1 ± 4.3 * | 3.20 ± 0.12 * | 0.40 ± 0.05 * | 0.32 ± 0.01 * |
| 50 mg/L P85-SWCNT | 61.2 ± 3.5 * | 3.31 ± 0.07 * | 0.36 ± 0.06 * | 0.30 ± 0.01 * |
| Priming Treatment | Chl | Flavonoid | NBI |
|---|---|---|---|
| H2O | 28.4 ± 0.5 | 0.511 ± 0.041 | 55.8 ± 0.9 |
| 10 mg/L P85-SWCNT | 28.0 ± 0.5 | 0.522 ± 0.052 | 54.0 ± 1.0 * |
| 50 mg/L P85-SWCNT | 27.3 ± 0.4 | 0.515 ± 0.005 | 53.3 ± 0.9 * |
| Priming Treatment | AN (μmol m−2 s−1) | gs (mol m−2 s−1) | iWUE (μmol mol−1) |
|---|---|---|---|
| H2O | 15.3 ± 0.3 | 0.32 ± 0.02 | 48.7 ± 3.0 |
| 10 mg/L P85-SWCNT | 14.5 ± 0.3 * | 0.26 ± 0.01 * | 57.4 ± 2.8 * |
| 50 mg/L P85-SWCNT | 14.2 ± 0.3 * | 0.27 ± 0.02 * | 56.7 ± 4.9 * |
| Priming | H2O | 10 mg/L P85-SWCNT | 50 mg/L P85-SWCNT | |
|---|---|---|---|---|
| Parameter | ||||
| Fv/Fm | 0.794 ± 0.002 | 0.785 ± 0.003 * | 0.791 ± 0.002 | |
| ΦPSII | 0.520 ± 0.004 | 0.528 ± 0.003 | 0.526 ± 0.003 | |
| NPQ | 1.089 ± 0.016 | 1.047 ± 0.017 | 1.132 ± 0.013 | |
| qL | 0.589 ± 0.007 | 0.595 ± 0.006 | 0.614 ± 0.006 * | |
| Priming Treatment | Chl a/b in Thylakoids | Chl a/b in Grana | Chl in Grana (%) | MDA (µmol) |
|---|---|---|---|---|
| H2O | 2.85 ± 0.02 | 1.97 ± 0.12 | 36.5 ± 6.7 | 0.343 ± 0.020 |
| 10 mg/L P85-SWCNT | 2.85 ± 0.02 | 1.95 ± 0.12 | 35.4 ± 6.6 | 0.319 ± 0.017 |
| 50 mg/L P85-SWCNT | 2.86 ± 0.02 | 1.90 ± 0.10 | 31.7 ± 7.0 | 0.400 ± 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumova, S.; Stoichev, S.; Ilkov, D.; Strijkova, V.; Katrova, V.; Crespo, A.; Álvarez, J.; Martínez, E.; Martínez-Ramírez, S.; Tsonev, T.; et al. Pea Seed Priming with Pluronic P85-Grafted Single-Walled Carbon Nanotubes Affects Photosynthetic Gas Exchange but Not Photosynthetic Light Reactions. Int. J. Mol. Sci. 2024, 25, 7901. https://doi.org/10.3390/ijms25147901
Krumova S, Stoichev S, Ilkov D, Strijkova V, Katrova V, Crespo A, Álvarez J, Martínez E, Martínez-Ramírez S, Tsonev T, et al. Pea Seed Priming with Pluronic P85-Grafted Single-Walled Carbon Nanotubes Affects Photosynthetic Gas Exchange but Not Photosynthetic Light Reactions. International Journal of Molecular Sciences. 2024; 25(14):7901. https://doi.org/10.3390/ijms25147901
Chicago/Turabian StyleKrumova, Sashka, Svetozar Stoichev, Daniel Ilkov, Velichka Strijkova, Vesela Katrova, Ana Crespo, José Álvarez, Elvira Martínez, Sagrario Martínez-Ramírez, Tsonko Tsonev, and et al. 2024. "Pea Seed Priming with Pluronic P85-Grafted Single-Walled Carbon Nanotubes Affects Photosynthetic Gas Exchange but Not Photosynthetic Light Reactions" International Journal of Molecular Sciences 25, no. 14: 7901. https://doi.org/10.3390/ijms25147901






