A Natural Bioactive Peptide from Pinctada fucata Pearls Can Be Used as a Potential Inhibitor of the Interaction between SARS-CoV-2 and ACE2 against COVID-19
Abstract
:1. Introduction
2. Results
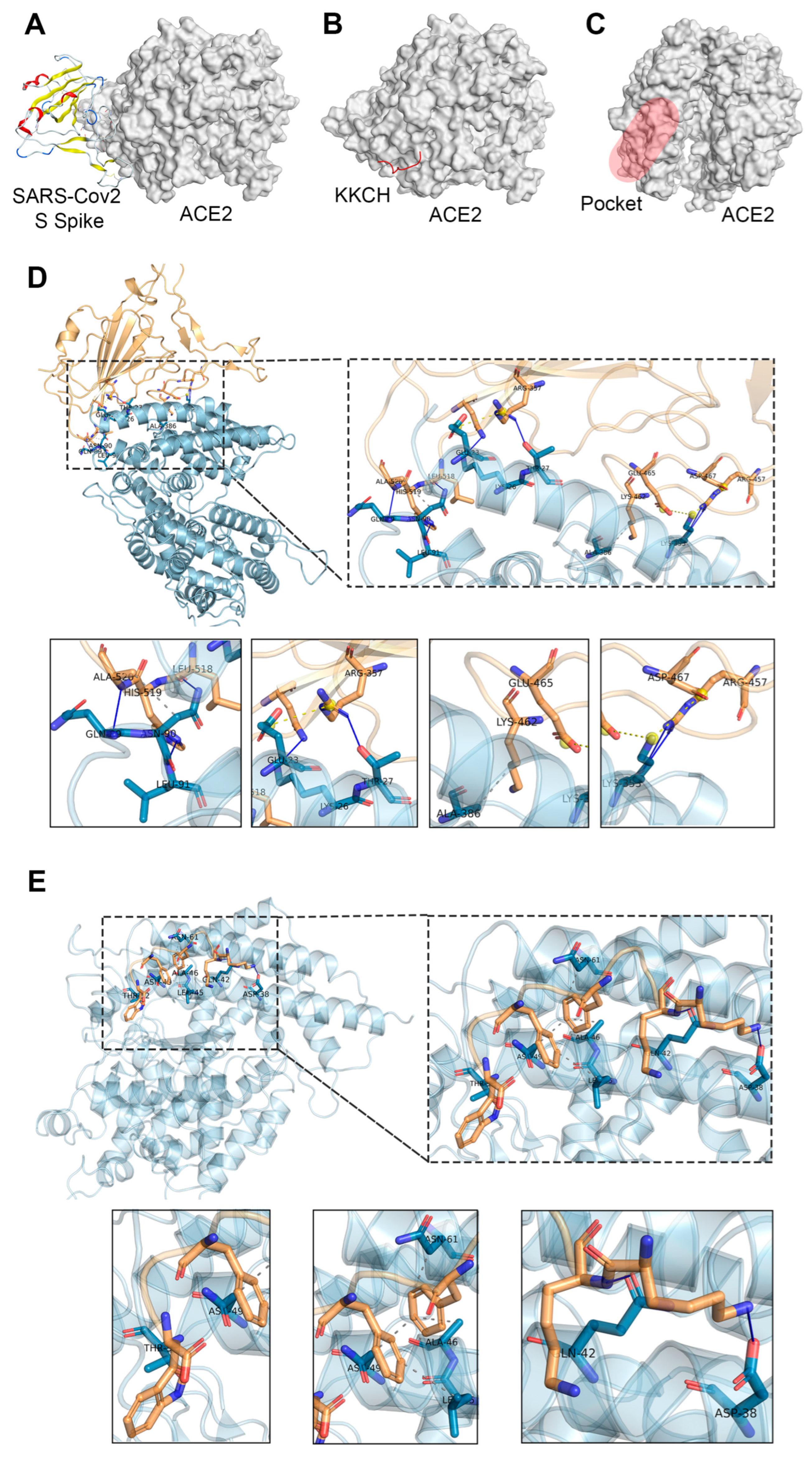
2.1. Docking Studies
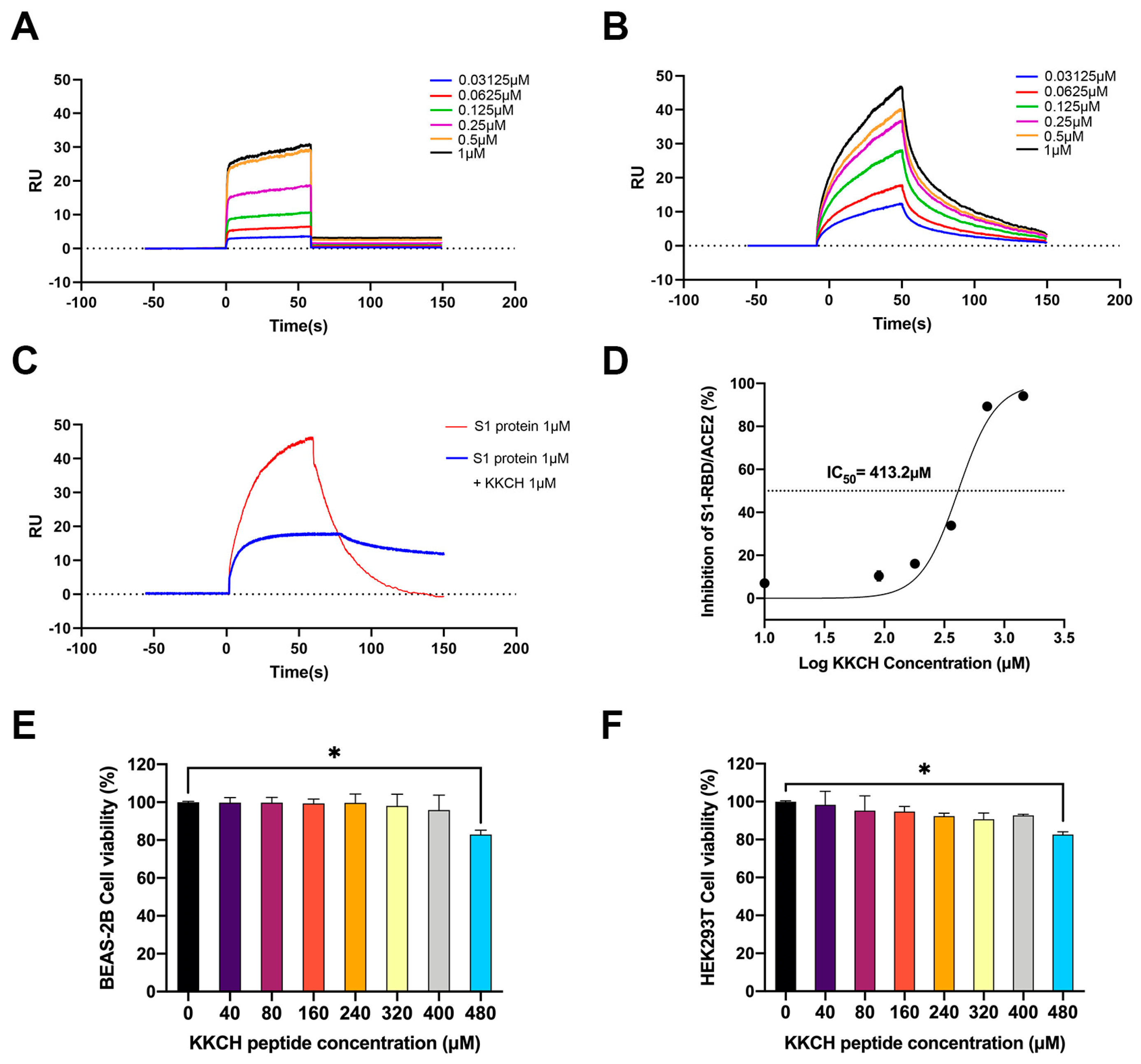
2.2. Surface Plasmon Resonance (SPR) Analysis of the Affinity between KKCH/SARS-CoV-2 S1 Protein and ACE2
2.3. KKCH Inhibits Binding of the SARS-CoV-2 S1 Protein RBD to ACE2
2.4. Cytotoxicity Analysis of KKCH Peptide
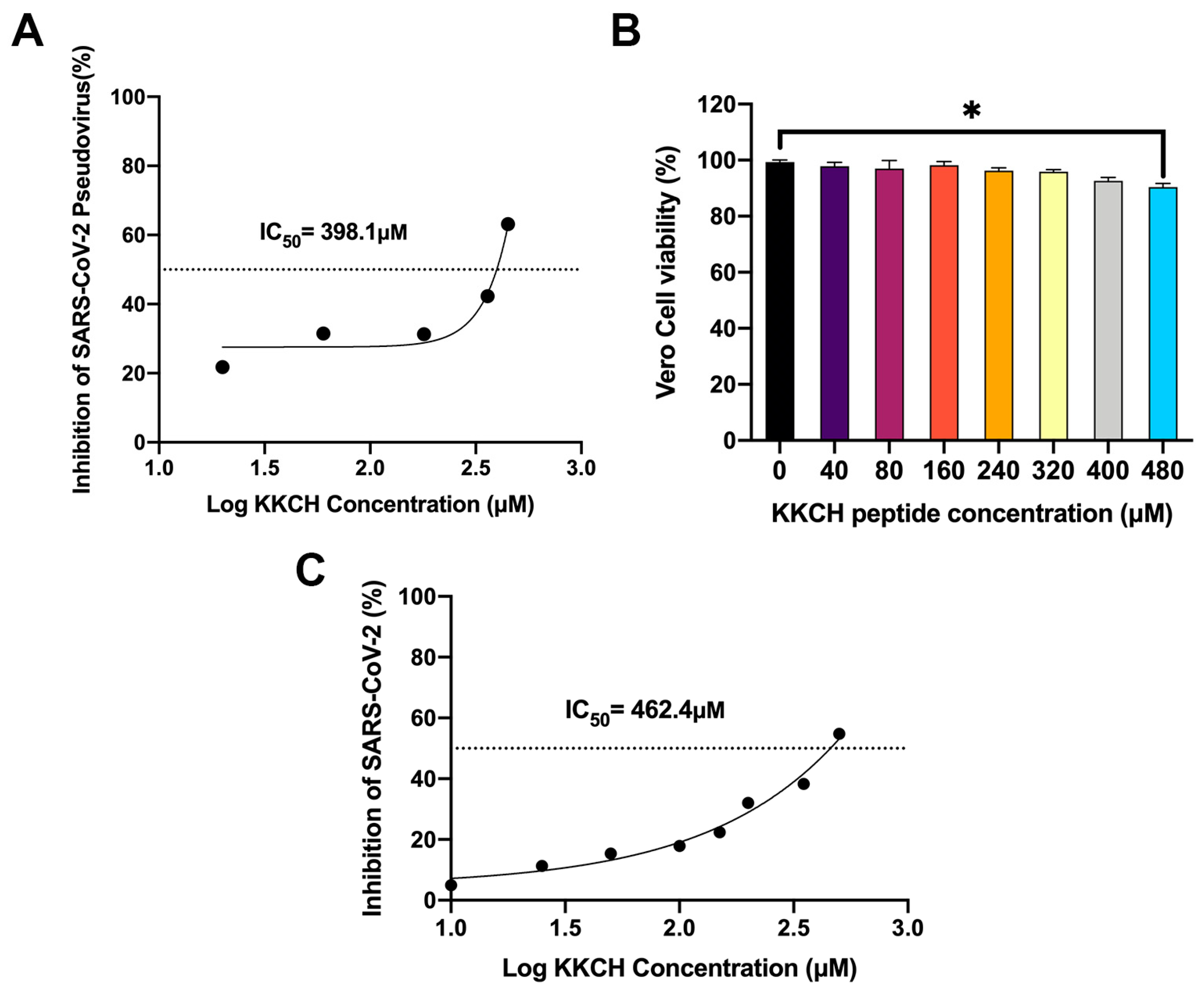
2.5. KKCH Inhibits the Binding of the S1 Protein to Cell Surfaces
2.6. In Vitro Antiviral Activity of KKCH Peptide
3. Discussion
4. Materials and Methods
4.1. Source of Peptide Materials
4.2. Molecular Docking
4.3. SPR Analysis
4.4. ELISA Analysis
4.5. Cell Lines and Cultures
4.6. Cell Activity Analysis
4.7. Biotin/Avidin-Labeled Immunoassay
4.8. SARS-CoV-2 Pseudovirus Analysis
4.9. SARS-CoV-2 Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Angiotensin-converting Enzyme II | ACE2 |
| Cell Counting Kit-8 | CCK8 |
| Enzyme-Linked Immunosorbent Assay | ELISA |
| Fetal Bovine Serum | FBS |
| Half-Maximal Inhibitory Concentration | IC50 |
| High-Performance Liquid Chromatography | HPLC |
| Human Defensin 5 | HD5 |
| Optical Density | OD |
| Receptor Binding Domain | RBD |
| Reaction Value | RU |
| Severe Acute Respiratory Syndrome Coronavirus 2 | SARS-CoV-2 |
| Spike Protein | S protein |
| Surface Plasmon Resonance | SPR |
| Transmembrane Protease Serine 2 | TMPRSS2 |
| Wild-Type | WT |
References
- Yamasoba, D.; Kimura, I.; Nasser, H.; Morioka, Y.; Nao, N.; Ito, J.; Uriu, K.; Tsuda, M.; Zahradnik, J.; Shirakawa, K. Virological Characteristics of the SARS-CoV-2 Omicron BA.2 Spike. Cell 2022, 185, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, B.; Li, Y.; Yuan, S.; Zhuang, Z.; Li, G.; Wang, D.; Ma, L.; Zhu, J.; Zhao, J.; et al. P21-Activated Kinase 1 (PAK1)-Mediated Cytoskeleton Rearrangement Promotes SARS-CoV-2 Entry and ACE2 Autophagic Degradation. Signal Transduct. Target. Ther. 2023, 8, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Risk of Death in Patients Hospitalized for COVID-19 vs Seasonal Influenza in Fall-Winter 2022–2023. Jama 2023, 329, 1697–1699. [Google Scholar] [CrossRef]
- Dhama, K.; Nainu, F.; Frediansyah, A.; Yatoo, M.I.; Mohapatra, R.K.; Chakraborty, S.; Zhou, H.; Islam, M.R.; Mamada, S.S.; Kusuma, H.I.; et al. Global Emerging Omicron Variant of SARS-CoV-2: Impacts, Challenges and Strategies. J. Infect. Public Health 2023, 16, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Gorabi, A.M.; Talaei, S.; Beiraghdar, F.; Akbarzadeh, A.; Tarhriz, V.; Mellatyar, H. An Overview on the Treatments and Prevention against COVID-19. Virol. J. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Ding, Y.; Xu, Y.; Li, M.; Zheng, R.; Cao, Z.; Wang, W.; Bi, Y.; Ning, G.; Xu, Y.; et al. Small-Molecule Anti-COVID-19 Drugs and a Focus on China’s Homegrown Mindeudesivir (VV116). Front. Med. 2023, 17, 1068–1079. [Google Scholar] [CrossRef]
- Corbitt, K.; Izmirly, P.; Saxena, A. Molnupiravir and Nirmatelvir/Ritonavir in the Treatment of Patients With Systemic Autoimmune Rheumatic Disease With SARS-CoV-2. J. Rheumatol. 2023, 50, 974–975. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. An Update on COVID-19: SARS-CoV-2 Variants, Antiviral Drugs, and Vaccines. Heliyon 2023, 9, e13952. [Google Scholar] [CrossRef]
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted Managements and Vaccine Development. Cytokine Growth Factor Rev. 2021, 58, 16–29. [Google Scholar] [CrossRef]
- Bernardi, S.; Michelli, A.; Zuolo, G.; Candido, R.; Fabris, B. Update on RAAS Modulation for the Treatment of Diabetic Cardiovascular Disease. J. Diabetes Res. 2016, 2016, 8917578. [Google Scholar] [CrossRef]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Li, L.; Yang, X.; Wang, J.; Hu, X. Purification and Identification of an Antioxidant Peptide from Pinctada Fucata Muscle. CyTA-J. Food 2018, 16, 11–19. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Kong, J.; Liu, Y.; Wang, T.; Xie, L.; Zhang, R. In-Depth Proteomic Analysis of Shell Matrix Proteins of Pinctada Fucata. Sci. Rep. 2015, 5, 17269. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Isolation, Purification and Activity of Oligopeptides before and after Preparation of Mother of Pearl [D]. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2010. [Google Scholar]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada Fucata Martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Lavandero, S.; Xie, X.; Sabharwal, B.; Zheng, Y.-Y.; Correa, A.; Narula, J.; Levy, P. Manipulation of ACE2 Expression in COVID-19. Open Heart 2020, 7, e001424. [Google Scholar] [CrossRef]
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Zununi Vahed, S. COVID-19 and Kidney Injury: Pathophysiology and Molecular Mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Głowacka, M.; Lipka, S.; Młynarska, E.; Franczyk, B.; Rysz, J. Acute Kidney Injury in COVID-19. Int. J. Mol. Sci. 2021, 22, 8081. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, X.; Jin, Y.; Hao, H.; Yang, F.; Yin, D.; Chen, X.; Xue, Y.; Han, L.; Zhang, M. Factors Associated with the Expression of ACE2 in Human Lung Tissue: Pathological Evidence from Patients with Normal FEV(1) and FEV(1)/FVC. J. Inflamm. Res. 2021, 14, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhu, J.; Chen, W.; Zhu, G.; Wang, W.; Chen, C.; Jia, Z.; Zha, Y.; Xu, P.; Wang, Z.; et al. Expression Profiles Revealed Potential Kidney Injury Caused by SARS-CoV-2: A Systematic Analysis of ACE2 and Clinical Lessons Learned from This Discovery. Aging 2020, 13, 10821–10832. [Google Scholar] [CrossRef]
- Vanslambrouck, J.M.; Neil, J.A.; Rudraraju, R.; Mah, S.; Tan, K.S.; Groenewegen, E.; Forbes, T.A.; Karavendzas, K.; Elliott, D.A.; Porrello, E.R.; et al. Kidney Organoids Reveal Redundancy in Viral Entry Pathways during ACE2-Dependent SARS-CoV-2 Infection. J. Virol. 2024, 98, e0180223. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A Crucial Role of Angiotensin Converting Enzyme 2 (ACE2) in SARS Coronavirus-Induced Lung Injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Host Cell Proteases: Critical Determinants of Coronavirus Tropism and Pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P.; et al. Small Molecules Blocking the Entry of Severe Acute Respiratory Syndrome Coronavirus into Host Cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef] [PubMed]
- Fuzimoto, A.D.; Isidoro, C. The Antiviral and Coronavirus-Host Protein Pathways Inhibiting Properties of Herbs and Natural Compounds-Additional Weapons in the Fight against the COVID-19 Pandemic? J. Tradit. Complement. Med. 2020, 10, 405–419. [Google Scholar] [CrossRef]
- Rolta, R.; Salaria, D.; Sharma, P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum Emodi, Thymus Serpyllum, and Artemisia Annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Curr. Pharmacol. Rep. 2021, 7, 135–149. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Huang, X.; Ma, Q.; Song, J.; Wu, X.; Zhou, H.; Weng, Y.; Yang, Z.; Wang, X. Liu Shen Wan Inhibits Influenza Virus-Induced Secondary Staphylococcus Aureus Infection in Vivo and in Vitro. J. Ethnopharmacol. 2021, 277, 114066. [Google Scholar] [CrossRef] [PubMed]
- Haga, J.H.; Ichikawa, K.; Date, S. Virtual Screening Techniques and Current Computational Infrastructures. Curr. Pharm. Des. 2016, 22, 3576–3584. [Google Scholar] [CrossRef]
- Vardhan, S.; Sahoo, S.K. In Silico ADMET and Molecular Docking Study on Searching Potential Inhibitors from Limonoids and Triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Khalid, S.; Sufyan, M.; Ashfaq, U.A. In-Silico Elucidation Reveals Potential Phytochemicals against Angiotensin-Converting Enzyme 2 (ACE-2) Receptor to Fight Coronavirus Disease 2019 (COVID-19). Z. Naturforsch. C 2022, 77, 473–482. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Yu, R.; Li, P. Screening of Potential Spike Glycoprotein/ACE2 Dual Antagonists against COVID-19 in Silico Molecular Docking. J. Virol. Methods 2022, 301, 114424. [Google Scholar] [CrossRef] [PubMed]
- Treebupachatsakul, T.; Shinnakerdchoke, S.; Pechprasarn, S. Sensing Mechanisms of Rough Plasmonic Surfaces for Protein Binding of Surface Plasmon Resonance Detection. Sensors 2023, 23, 3377. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, N.J.; Wootla, B.; Jordan, L.R.; Denic, A.; Warrington, A.E.; Oh, S.H.; Rodriguez, M. Applications of SPR for the Characterization of Molecules Important in the Pathogenesis and Treatment of Neurodegenerative Diseases. Expert Rev. Neurother. 2014, 14, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lin, X.; Chen, Z.; Yang, F.; Lin, S.; Yang, J.; Chen, H.; Sun, H.; Wang, L.; Wen, A.; et al. S19W, T27W, and N330Y Mutations in ACE2 Enhance SARS-CoV-2 S-RBD Binding toward Both Wild-Type and Antibody-Resistant Viruses and Its Molecular Basis. Signal Transduct. Target. Ther. 2021, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Li, Y.; Wang, Z.; Chu, M.; Wang, Y. Tuftsin: A Natural Molecule Against SARS-CoV-2 Infection. Front. Mol. Biosci. 2022, 9, 859162. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Fang, S.; Zhang, J.; Pan, Y.; Liu, H.; Wang, Y.; Li, M.; Liu, L. Chinese Herbal Compounds against SARS-CoV-2: Puerarin and Quercetin Impair the Binding of Viral S-Protein to ACE2 Receptor. Comput. Struct. Biotechnol. J. 2020, 18, 3518–3527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, S.; Wang, J.; Xue, Z.; Wang, C.; Wang, N. Dexamethasone Inhibits SARS-CoV-2 Spike Pseudotyped Virus Viropexis by Binding to ACE2. Virology 2021, 554, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, Y.; Wang, K.; Hettinghouse, A.; Hu, W.; Wang, J.Q.; Lei, Z.N.; Chen, Z.S.; Stapleford, K.A.; Liu, C. ju Repurposing FDA-Approved Drugs for SARS-CoV-2 through an ELISA-Based Screening for the Inhibition of RBD/ACE2 Interaction. Protein Cell 2021, 12, 586–591. [Google Scholar] [CrossRef]
- Shalash, A.O.; Azuar, A.; Madge, H.Y.R.; Modhiran, N.; Amarilla, A.A.; Liang, B.; Khromykh, A.A.; Watterson, D.; Young, P.R.; Toth, I.; et al. Detection and Quantification of SARS-CoV-2 Receptor Binding Domain Neutralization by a Sensitive Competitive ELISA Assay. Vaccines 2021, 9, 1493. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Ye, F.; Guo, Y.; Xia, L.; Zhong, X.; Chi, X.; Zhou, Q. Structural Basis for the Different States of the Spike Protein of SARS-CoV-2 in Complex with ACE2. Cell Res. 2021, 31, 717–719. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, D.; Wei, D.-Q.; Zhao, J.; Wang, J. Human Intestinal Defensin 5 Inhibits SARS-CoV-2 Invasion by Cloaking ACE2. Gastroenterology 2020, 159, 1145–1147. [Google Scholar] [CrossRef]
- Swithenbank, L.; Cox, P.; Harris, L.G.; Dudley, E.; Sinclair, K.; Lewis, P.; Cappiello, F.; Morgan, C. Temporin A and Bombinin H2 Antimicrobial Peptides Exhibit Selective Cytotoxicity to Lung Cancer Cells. Scientifica 2020, 2020, 3526286. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Azghandi, M.; Mousavi, Z.; Javadmanesh, A. Expression of Thanatin in HEK293 Cells and Investigation of Its Antibacterial Effects on Some Human Pathogens. Protein Pept. Lett. 2020, 27, 41–47. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and Validation of a Pseudovirus Neutralization Assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.E. Construction and Applications of Sars-Cov-2 Pseudoviruses: A Mini Review. Int. J. Biol. Sci. 2021, 17, 1574–1580. [Google Scholar] [CrossRef]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.H.; Michailidis, E.; Lorenzi, J.C.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 Neutralizing Antibody Activity Using Pseudotyped and Chimeric Viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef]
- Yamada, H.; Sasaki, S.-I.; Tani, H.; Somekawa, M.; Kawasuji, H.; Saga, Y.; Yoshida, Y.; Yamamoto, Y.; Hayakawa, Y.; Morinaga, Y. A Novel Hamster Model of SARS-CoV-2 Respiratory Infection Using a Pseudotyped Virus. Sci. Rep. 2022, 12, 11125. [Google Scholar] [CrossRef]
- Tamin, A.; Harcourt, B.H.; Lo, M.K.; Roth, J.A.; Wolf, M.C.; Lee, B.; Weingartl, H.; Audonnet, J.-C.; Bellini, W.J.; Rota, P.A. Development of a Neutralization Assay for Nipah Virus Using Pseudotype Particles. J. Virol. Methods 2009, 160, 1–6. [Google Scholar] [CrossRef]
- Wu, C.; Yin, Z.; Wang, Y.; Chen, X.; Li, B.; Wang, Q.; Yao, L.; Zhang, Z.; Liu, X.; Zhang, R. The First Bioactive (Angiotensin-Converting Enzyme-Inhibitory) Peptide Isolated from Pearl Matrix Protein. Heliyon 2024, 10, e28060. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Q.; Chen, X.; Li, B.; Zhang, Z.; Yao, L.; Liu, X.; Zhang, R. A Natural Bioactive Peptide from Pinctada fucata Pearls Can Be Used as a Potential Inhibitor of the Interaction between SARS-CoV-2 and ACE2 against COVID-19. Int. J. Mol. Sci. 2024, 25, 7902. https://doi.org/10.3390/ijms25147902
Wang Y, Wang Q, Chen X, Li B, Zhang Z, Yao L, Liu X, Zhang R. A Natural Bioactive Peptide from Pinctada fucata Pearls Can Be Used as a Potential Inhibitor of the Interaction between SARS-CoV-2 and ACE2 against COVID-19. International Journal of Molecular Sciences. 2024; 25(14):7902. https://doi.org/10.3390/ijms25147902
Chicago/Turabian StyleWang, Yayu, Qin Wang, Xinjiani Chen, Bailei Li, Zhen Zhang, Liping Yao, Xiaojun Liu, and Rongqing Zhang. 2024. "A Natural Bioactive Peptide from Pinctada fucata Pearls Can Be Used as a Potential Inhibitor of the Interaction between SARS-CoV-2 and ACE2 against COVID-19" International Journal of Molecular Sciences 25, no. 14: 7902. https://doi.org/10.3390/ijms25147902






