The Effect of Maternal High-Fat or High-Carbohydrate Diet during Pregnancy and Lactation on Cytochrome P450 2D (CYP2D) in the Liver and Brain of Rat Offspring
Abstract
:1. Introduction
2. Results
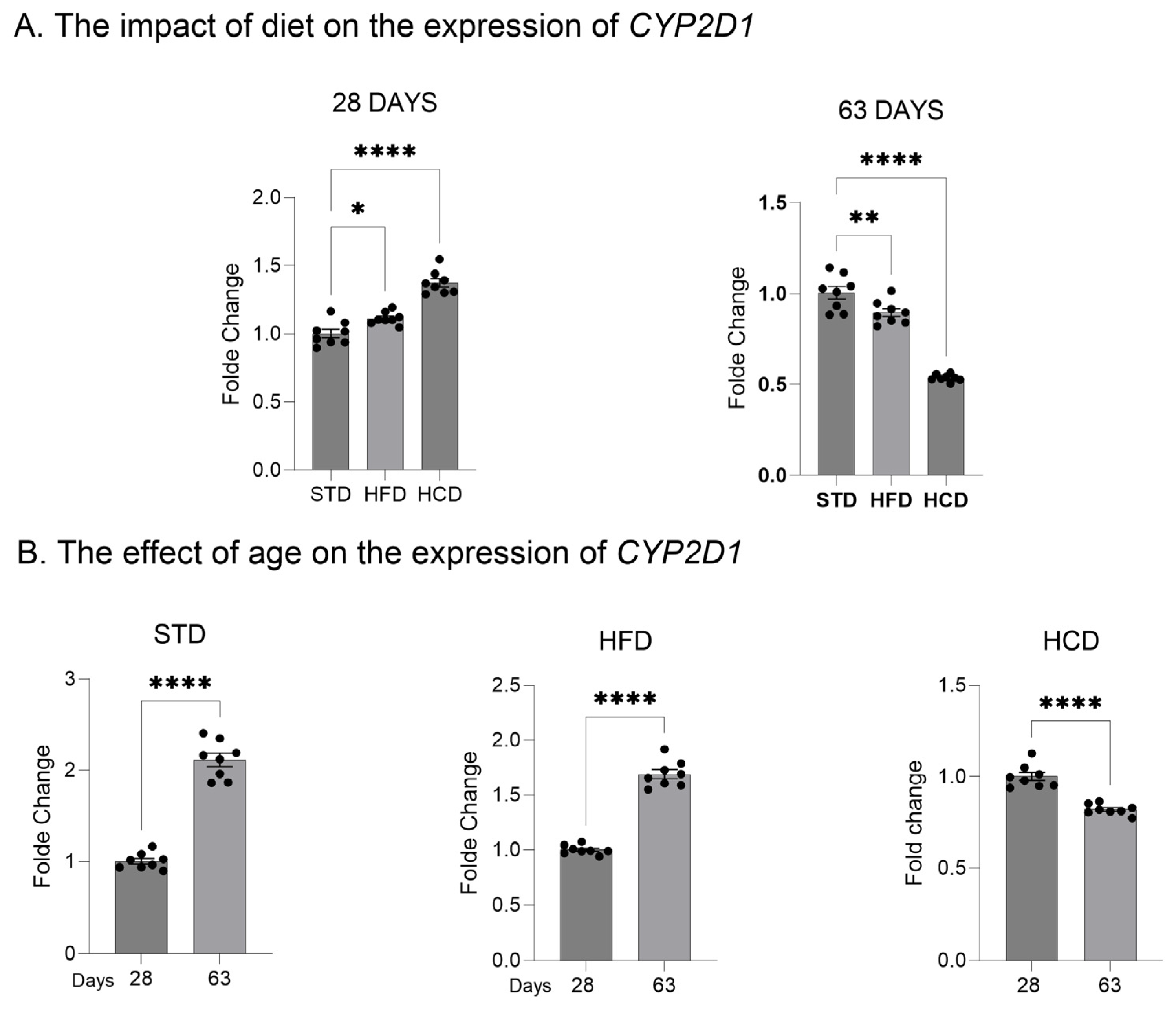
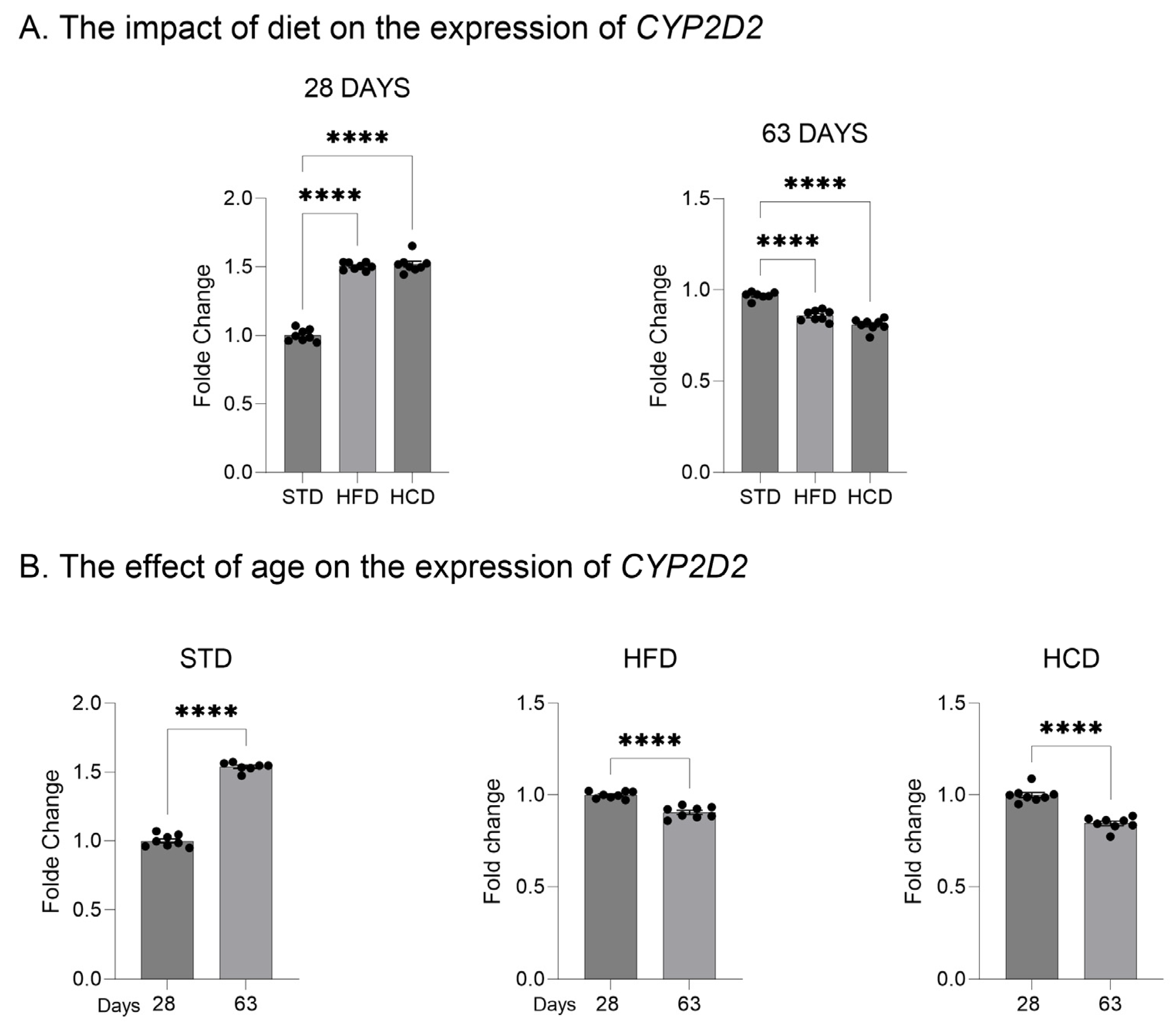
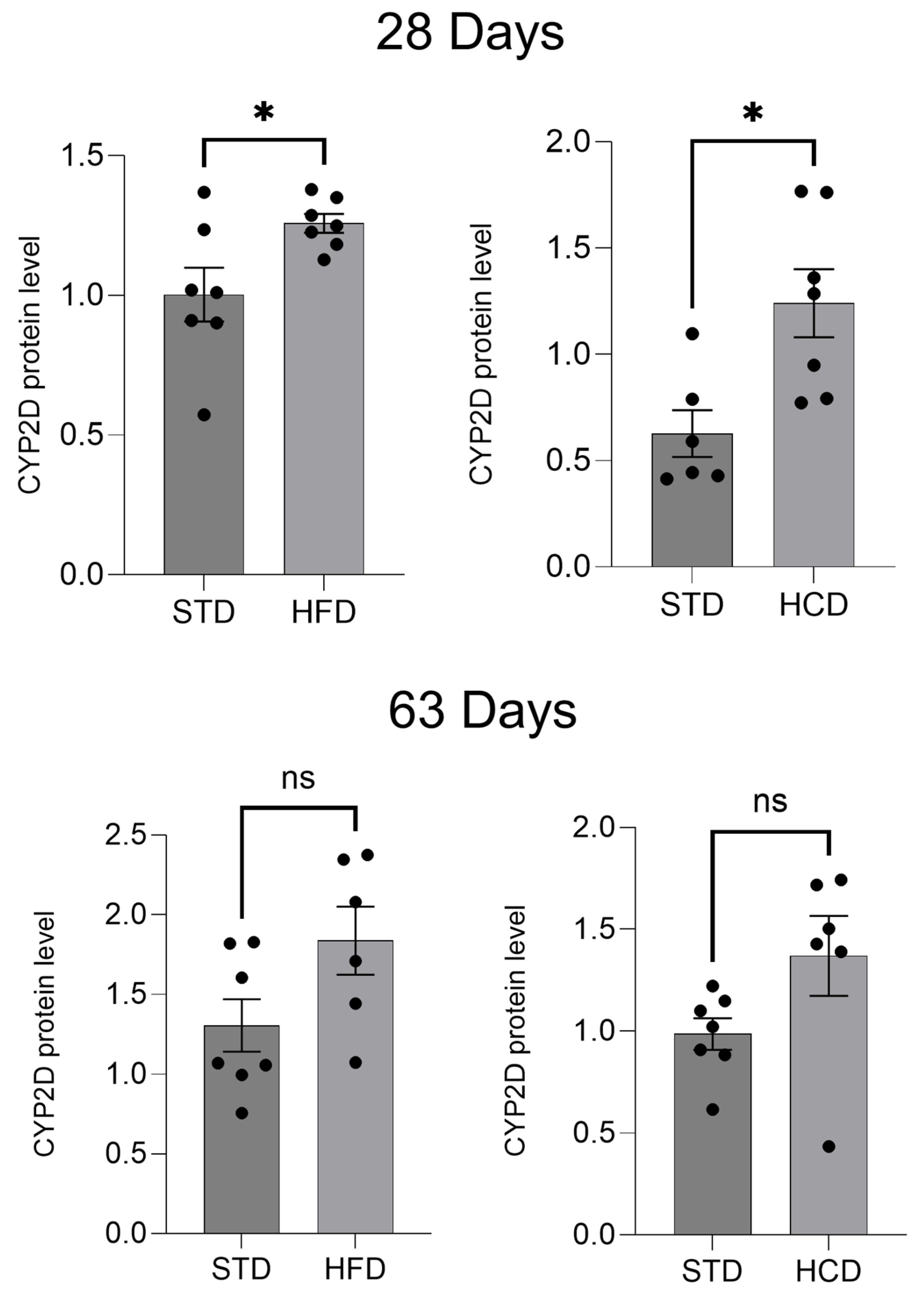
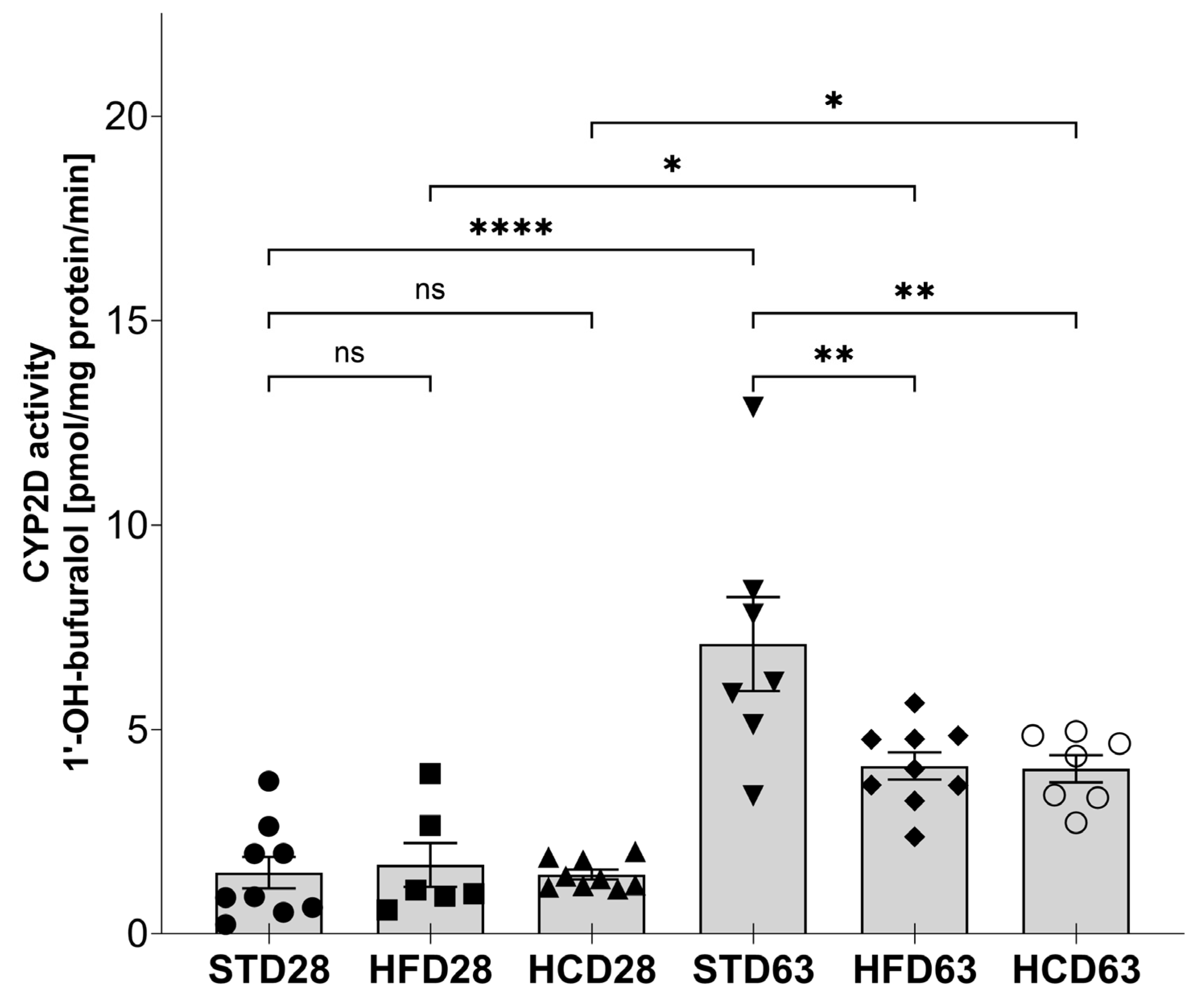
2.1. The Influence of Maternal Diet on the Expression and Activity of CYP2D in the Liver of Adolescent and Adult Male Offspring
2.2. The Influence of Maternal Diet on the Expression of CYP2D Genes in the Brain of Adolescent and Adult Male Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Tissue Preparation and Determination of CYP2D Activity
4.4. Evaluation of CYP2D Protein in Liver Microsomes
4.5. Examination of the Expression of Gene Coding for CYP2D Enzymes in the Liver and Brain
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal Dietary Patterns Are Associated with Susceptibility to a Depressive-like Phenotype in Rat Offspring. Dev. Cogn. Neurosci. 2021, 47, 100879. [Google Scholar] [CrossRef]
- Gawliński, D.; Gawlińska, K.; Frankowska, M.; Filip, M. Maternal High-Sugar Diet Changes Offspring Vulnerability to Reinstatement of Cocaine-Seeking Behavior: Role of Melanocortin-4 Receptors. FASEB J. 2020, 34, 9192–9206. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The Impact of Maternal High-Fat Diet on Offspring Neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Witek, K.; Wydra, K.; Suder, A.; Filip, M. Maternal Monosaccharide Diets Evoke Cognitive, Locomotor, and Emotional Disturbances in Adolescent and Young Adult Offspring Rats. Front. Nutr. 2023, 10, 1176213. [Google Scholar] [CrossRef]
- Frankowska, M.; Surówka, P.; Gawlińska, K.; Borczyk, M.; Korostyński, M.; Filip, M.; Smaga, I. A Maternal High-Fat Diet during Pregnancy and Lactation Induced Depression-like Behavior in Offspring and Myelin-Related Changes in the Rat Prefrontal Cortex. Front. Mol. Neurosci. 2023, 16, 1303718. [Google Scholar] [CrossRef]
- Bekdash, R.A. Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection. Int. J. Mol. Sci. 2023, 24, 2346. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, B.; Scheers, N.; Rasmussen, M.K.; Young, J.F.; Ross, A.B.; Landberg, R. Brain Foods—The Role of Diet in Brain Performance and Health. Nutr. Rev. 2021, 79, 693–708. [Google Scholar] [CrossRef]
- Kolb, B.; Harker, A.; Gibb, R. Principles of Plasticity in the Developing Brain. Dev. Med. Child. Neurol. 2017, 59, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-Y.; Yin, X.-X.; Guan, Q.-W.; Xia, Q.-X.; Yang, N.; Zhou, H.-H.; Liu, Z.-Q.; Jin, W.-L. Dietary Nutrition for Neurological Disease Therapy: Current Status and Future Directions. Pharmacol. Ther. 2021, 226, 107861. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and Depression: Exploring the Biological Mechanisms of Action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Epigenetic Aberrations in Major Psychiatric Diseases Related to Diet and Gut Microbiome Alterations. Genes 2023, 14, 1506. [Google Scholar] [CrossRef]
- Sánchez-Lafuente, C.L.; Reive, B.S.; Kalynchuk, L.E.; Caruncho, H.J. A Scoping Review of Rodent Studies Investigating the Epigenetic Mechanisms in the Brain Underlying the Effects of Diet on Depressive-like Behaviour. Biomedicines 2022, 10, 3213. [Google Scholar] [CrossRef] [PubMed]
- Ghose, R.; Omoluabi, O.; Gandhi, A.; Shah, P.; Strohacker, K.; Carpenter, K.C.; McFarlin, B.; Guo, T. Role of High-Fat Diet in Regulation of Gene Expression of Drug Metabolizing Enzymes and Transporters. Life Sci. 2011, 89, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, J.; Sadamitsu, S.; Wada, T.; Yamazaki, Y.; Ikari, A.; Miwa, M. Effects of Dietary Inulin, Statin, and Their Co-Treatment on Hyperlipidemia, Hepatic Steatosis and Changes in Drug-Metabolizing Enzymes in Rats Fed a High-Fat and High-Sucrose Diet. Nutr. Metab. 2012, 9, 23. [Google Scholar] [CrossRef]
- Tajima, M.; Ikarashi, N.; Okaniwa, T.; Imahori, Y.; Saruta, K.; Toda, T.; Ishii, M.; Tanaka, Y.; Machida, Y.; Ochiai, W.; et al. Consumption of a High-Fat Diet during Pregnancy Changes the Expression of Cytochrome P450 in the Livers of Infant Male Mice. Biol. Pharm. Bull. 2013, 36, 649–657. [Google Scholar] [CrossRef]
- Sadler, N.C.; Webb-Robertson, B.-J.M.; Clauss, T.R.; Pounds, J.G.; Corley, R.; Wright, A.T. High-Fat Diets Alter the Modulatory Effects of Xenobiotics on Cytochrome P450 Activities. Chem. Res. Toxicol. 2018, 31, 308–318. [Google Scholar] [CrossRef]
- Al Nebaihi, H.M.; Al Batran, R.; Ussher, J.R.; Maayah, Z.H.; El-Kadi, A.O.S.; Brocks, D.R. Dietary-Induced Obesity, Hepatic Cytochrome P450, and Lidocaine Metabolism: Comparative Effects of High-Fat Diets in Mice and Rats and Reversibility of Effects With Normalization of Diet. J. Pharm. Sci. 2020, 109, 1199–1210. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Saedisomeolia, A.; Shekarabi, M.; Khorshidi, M.; Emami, M.R.; Müller, D.J. The Effect of Obesity, Macronutrients, Fasting and Nutritional Status on Drug-Metabolizing Cytochrome P450s: A Systematic Review of Current Evidence on Human Studies. Eur. J. Nutr. 2021, 60, 2905–2921. [Google Scholar] [CrossRef] [PubMed]
- Tajima, M.; Ikarashi, N.; Imahori, Y.; Okaniwa, T.; Saruta, K.; Ishii, M.; Kusunoki, Y.; Kon, R.; Toda, T.; Ochiai, W.; et al. Consumption of a High-Fat Diet during Pregnancy Decreases the Activity of Cytochrome P450 3a in the Livers of Offspring. Eur. J. Pharm. Sci. 2012, 47, 108–116. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic Polymorphisms of Cytochrome P450 2D6 (CYP2D6): Clinical Consequences, Evolutionary Aspects and Functional Diversity. Pharmacogenomics J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Nelson, D.R.; Zeldin, D.C.; Hoffman, S.M.; Maltais, L.J.; Wain, H.M.; Nebert, D.W. Comparison of Cytochrome P450 (CYP) Genes from the Mouse and Human Genomes, Including Nomenclature Recommendations for Genes, Pseudogenes and Alternative-Splice Variants. Pharmacogenetics 2004, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Chow, T.; Imaoka, S.; Funae, Y. Catalytic Specificity of CYP2D Isoforms in Rat and Human. Drug Metab. Dispos. 2002, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Imaoka, S.; Chow, T.; Funae, Y. Tissue Distributions of CYP2D1, 2D2, 2D3 and 2D4 mRNA in Rats Detected by RT-PCR. Biochim. Biophys. Acta 1998, 1380, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the Brain. Drug Metab. Pharmacokinet. 2003, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Rao, Y.; Sellers, E.M.; Kwan, M.; Mendis, D.; Tyndale, R.F. Regional and Cellular Distribution of CYP2D Subfamily Members in Rat Brain. Xenobiotica 2000, 30, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Bromek, E.; Haduch, A.; Daniel, W.A. The Ability of Cytochrome P450 2D Isoforms to Synthesize Dopamine in the Brain: An in Vitro Study. Eur. J. Pharmacol. 2010, 626, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Miksys, S.L.; Gaedigk, A.; Kish, S.J.; Mash, D.C.; Tyndale, R.F. The Neuroprotective Enzyme CYP2D6 Increases in the Brain with Age and Is Lower in Parkinson’s Disease Patients. Neurobiol. Aging 2012, 33, 2160–2171. [Google Scholar] [CrossRef]
- Haduch, A.; Daniel, W.A. The Engagement of Brain Cytochrome P450 in the Metabolism of Endogenous Neuroactive Substrates: A Possible Role in Mental Disorders. Drug Metab. Rev. 2018, 50, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Ceceli, A.O.; Bradberry, C.W.; Goldstein, R.Z. The Neurobiology of Drug Addiction: Cross-Species Insights into the Dysfunction and Recovery of the Prefrontal Cortex. Neuropsychopharmacology 2022, 47, 276–291. [Google Scholar] [CrossRef]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the Prefrontal Cortex: An Integrative Review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.K.P.; Uchendu, S.; Arnsten, A.F.T. Stress and Inflammation Target Dorsolateral Prefrontal Cortex Function: Neural Mechanisms Underlying Weakened Cognitive Control. Biol. Psychiatry, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Levy, R. The Prefrontal Cortex: From Monkey to Man. Brain 2024, 147, 794–815. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal Cortex and Depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Smucny, J.; Dienel, S.J.; Lewis, D.A.; Carter, C.S. Mechanisms Underlying Dorsolateral Prefrontal Cortex Contributions to Cognitive Dysfunction in Schizophrenia. Neuropsychopharmacology 2022, 47, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of Maternal High-Fat Diet during Pregnancy and Lactation to Offspring Health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Kuban, W.; Daniel, W.A. Cytochrome P450 Expression and Regulation in the Brain. Drug Metab. Rev. 2020, 53, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.; Gustafsson, J.A.; Warner, M. Cytochrome P450 in the Brain; a Review. Curr. Drug Metab. 2001, 2, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Tyndale, R.F. The Unique Regulation of Brain Cytochrome P450 2 (CYP2) Family Enzymes by Drugs and Genetics. Drug Metab. Rev. 2004, 36, 313–333. [Google Scholar] [CrossRef]
- Yue, J.; Miksys, S.; Hoffmann, E.; Tyndale, R.F. Chronic Nicotine Treatment Induces Rat CYP2D in the Brain but Not in the Liver: An Investigation of Induction and Time Course. J. Psychiatry Neurosci. 2008, 33, 54–63. [Google Scholar]
- Li, J.; Xie, M.; Wang, X.; Ouyang, X.; Wan, Y.; Dong, G.; Yang, Z.; Yang, J.; Yue, J. Sex Hormones Regulate Cerebral Drug Metabolism via Brain miRNAs: Down-Regulation of Brain CYP2D by Androgens Reduces the Analgesic Effects of Tramadol: Regulation of Brain CYP2D by Androgens via miRNAs. Br. J. Pharmacol. 2015, 172, 4639–4654. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.M.; Tyndale, R.F. CYP-Mediated Drug Metabolism in the Brain Impacts Drug Response. Pharmacol. Ther. 2018, 184, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, S.; Wang, H.; Xie, W. Regulation of Brain Drug Metabolizing Enzymes and Transporters by Nuclear Receptors. Drug Metab. Rev. 2018, 50, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Na, S.; Wu, J.; Yang, Z.; Xie, X.; Wan, Y.; Li, K.; Yue, J. The Involvement of PPARs in the Selective Regulation of Brain CYP2D by Growth Hormone. Neuroscience 2018, 379, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.; Imaoka, S.; Hiroi, T.; Funae, Y. Developmental Changes in the Catalytic Activity and Expression of CYP2D Isoforms in the Rat Liver. Drug Metab. Dispos. 1999, 27, 188–192. [Google Scholar] [PubMed]
- Oesterheld, J.R. A Review of Developmental Aspects of Cytochrome P450. J. Child. Adolesc. Psychopharmacol. 1998, 8, 161–174. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.; Guchelaar, H.-J.; Swen, J.J. Substrate Specificity of CYP2D6 Genetic Variants. Pharmacogenomics 2021, 22, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Ushirozako, G.; Murayama, N.; Tsukiyama-Kohara, K.; Yamazaki, H.; Uno, Y. Novel Tree Shrew Cytochrome P450 2Ds (CYP2D8a and CYP2D8b) Are Functional Drug-Metabolizing Enzymes That Metabolize Bufuralol and Dextromethorphan. Drug Metab. Dispos. 2024, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.; Yamazaki, H.; Sugahara, C.; Hiroi, T.; Funae, Y.; Shimada, T. Differential Roles of Cytochromes P450 2D1, 2C11, and 1A1/2 in the Hydroxylation of Bufuralol by Rat Liver Microsomes. Biochem. Pharmacol. 1994, 47, 1957–1963. [Google Scholar] [CrossRef]
- Kobayashi, K.; Urashima, K.; Shimada, N.; Chiba, K. Substrate Specificity for Rat Cytochrome P450 (CYP) Isoforms: Screening with cDNA-Expressed Systems of the Rat. Biochem. Pharmacol. 2002, 63, 889–896. [Google Scholar] [CrossRef]
- Yamazaki, H.; Guo, Z.; Persmark, M.; Mimura, M.; Inoue, K.; Guengerich, F.P.; Shimada, T. Bufuralol Hydroxylation by Cytochrome P450 2D6 and 1A2 Enzymes in Human Liver Microsomes. Mol. Pharmacol. 1994, 46, 568–577. [Google Scholar] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-12-374121-9. [Google Scholar]
- Haduch, A.; Rysz, M.; Papp, M.; Daniel, W.A. The Activity of Brain and Liver Cytochrome P450 2D (CYP2D) Is Differently Affected by Antidepressants in the Chronic Mild Stress (CMS) Model of Depression in the Rat. Biochem. Pharmacol. 2018, 156, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

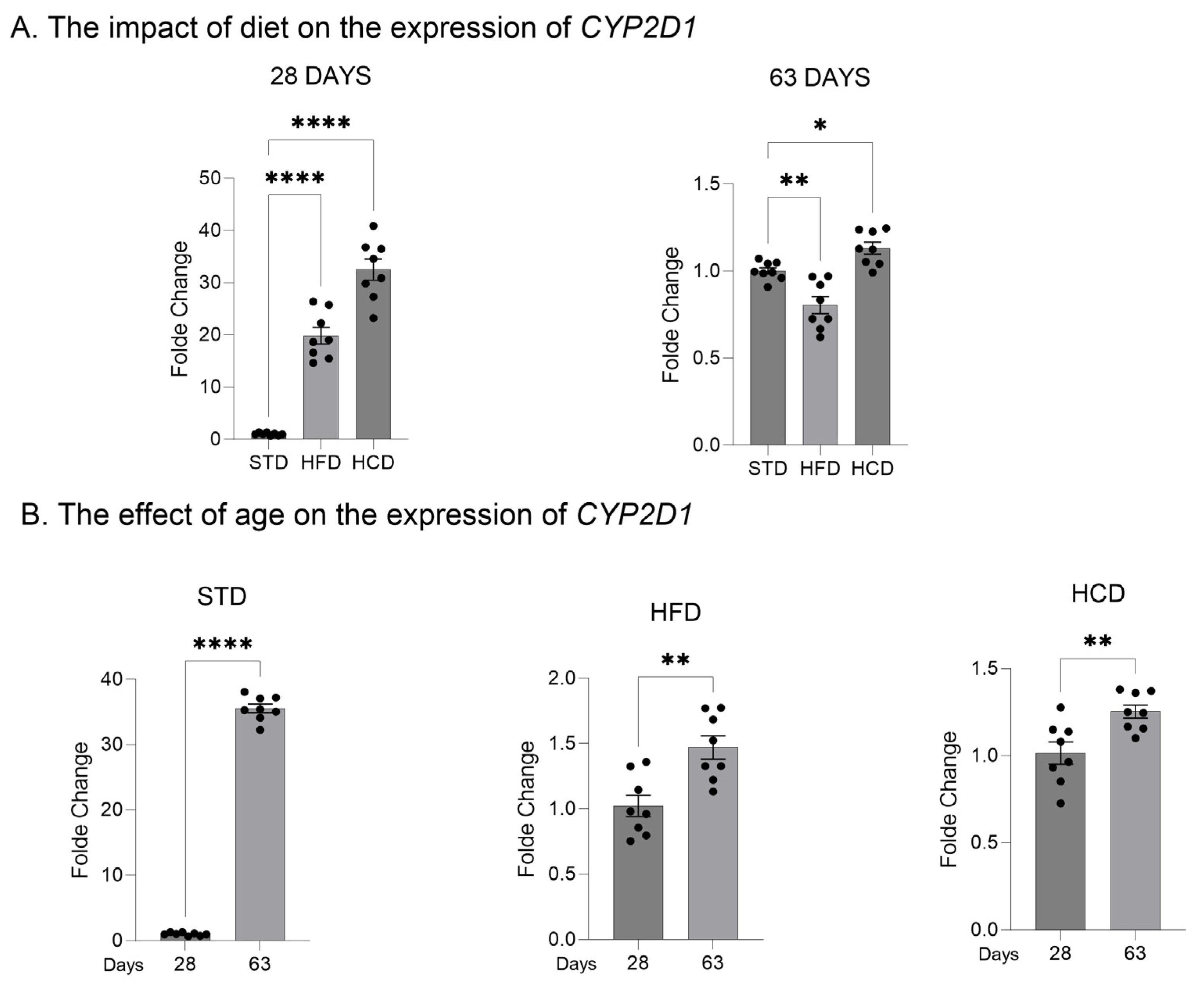
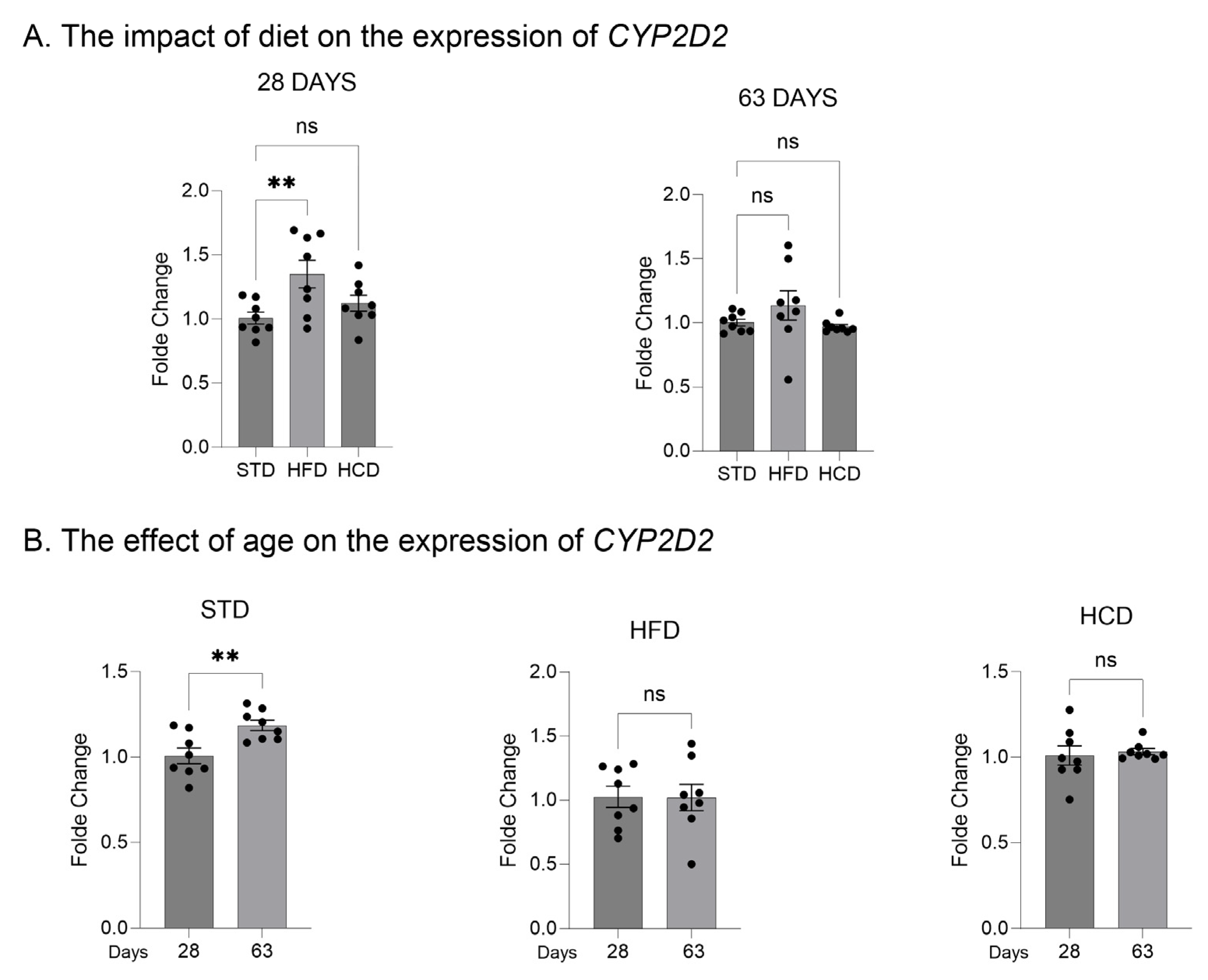

| Diets/Postnatal Day | Maternal High Fat Diet (HFD) vs. Control Standard Diet (STD) | Maternal High-Carbohydrate Diet (HCD) vs. Control Standard Diet (STD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28-Day Offspring | 63-Day Offspring | 28-Day Offspring | 63-Day Offspring | |||||||||
| CYP2Ds | 2D1 | 2D2 | 2D4 | 2D1 | 2D2 | 2D4 | 2D1 | 2D2 | 2D4 | 2D1 | 2D2 | 2D4 |
| Prefrontal cortex mRNA | ↑ | ↑ | ↑ | ↓ | n.s. | ↓ | ↑ | n.s. | n.s. | ↑ | n.s. | ↓ |
| Liver mRNA | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ |
| Liver protein | ↑ | n.s. | ↑ | n.s. | ||||||||
| Liver activity | n.s. | ↓ | n.s. | ↓ | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuban, W.; Haduch, A.; Bromek, E.; Basińska-Ziobroń, A.; Gawlińska, K.; Gawliński, D.; Filip, M.; Daniel, W.A. The Effect of Maternal High-Fat or High-Carbohydrate Diet during Pregnancy and Lactation on Cytochrome P450 2D (CYP2D) in the Liver and Brain of Rat Offspring. Int. J. Mol. Sci. 2024, 25, 7904. https://doi.org/10.3390/ijms25147904
Kuban W, Haduch A, Bromek E, Basińska-Ziobroń A, Gawlińska K, Gawliński D, Filip M, Daniel WA. The Effect of Maternal High-Fat or High-Carbohydrate Diet during Pregnancy and Lactation on Cytochrome P450 2D (CYP2D) in the Liver and Brain of Rat Offspring. International Journal of Molecular Sciences. 2024; 25(14):7904. https://doi.org/10.3390/ijms25147904
Chicago/Turabian StyleKuban, Wojciech, Anna Haduch, Ewa Bromek, Agnieszka Basińska-Ziobroń, Kinga Gawlińska, Dawid Gawliński, Małgorzata Filip, and Władysława A. Daniel. 2024. "The Effect of Maternal High-Fat or High-Carbohydrate Diet during Pregnancy and Lactation on Cytochrome P450 2D (CYP2D) in the Liver and Brain of Rat Offspring" International Journal of Molecular Sciences 25, no. 14: 7904. https://doi.org/10.3390/ijms25147904







