Novel Piperazine Derivatives of Vindoline as Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
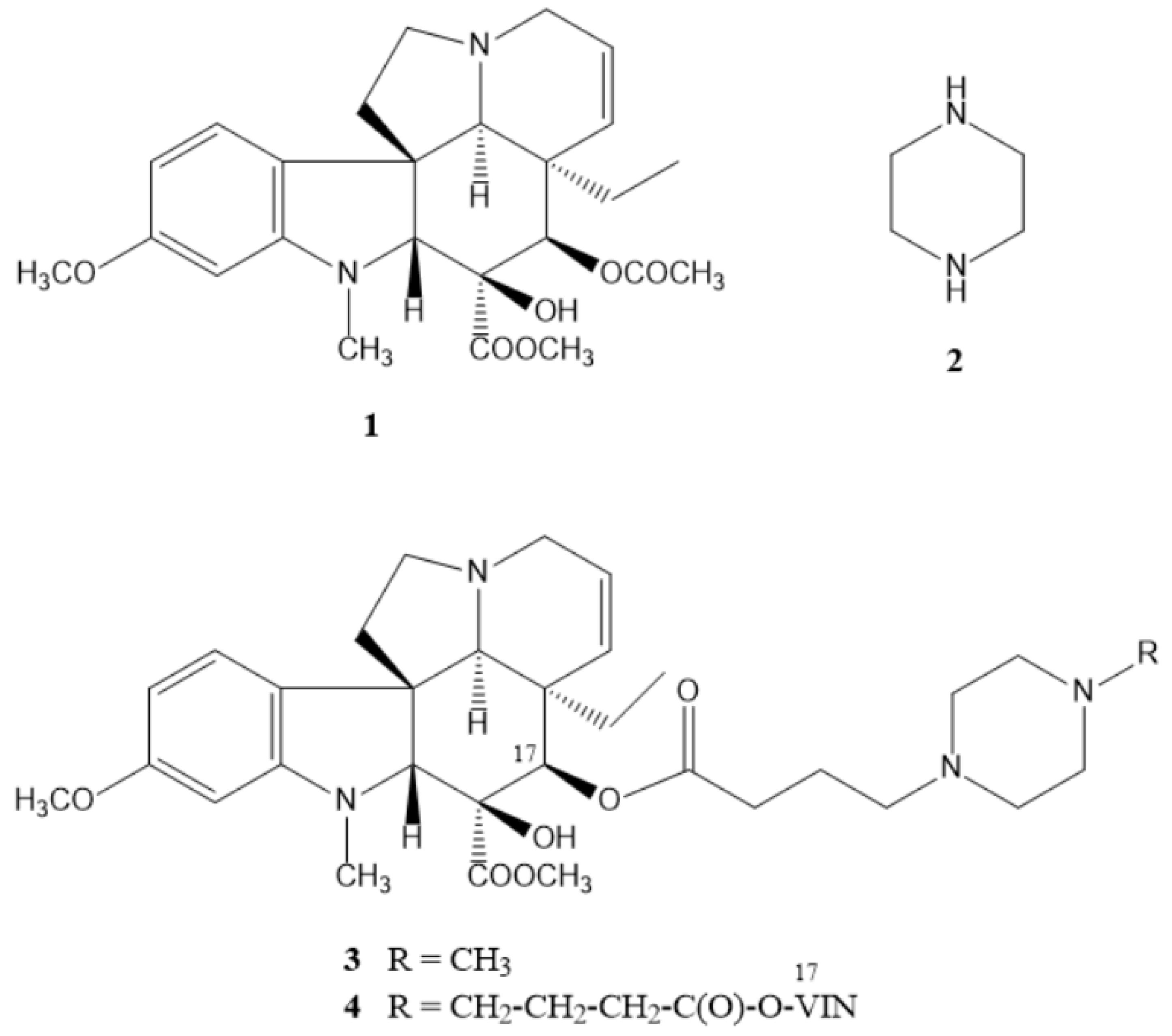
2.1. Chemistry
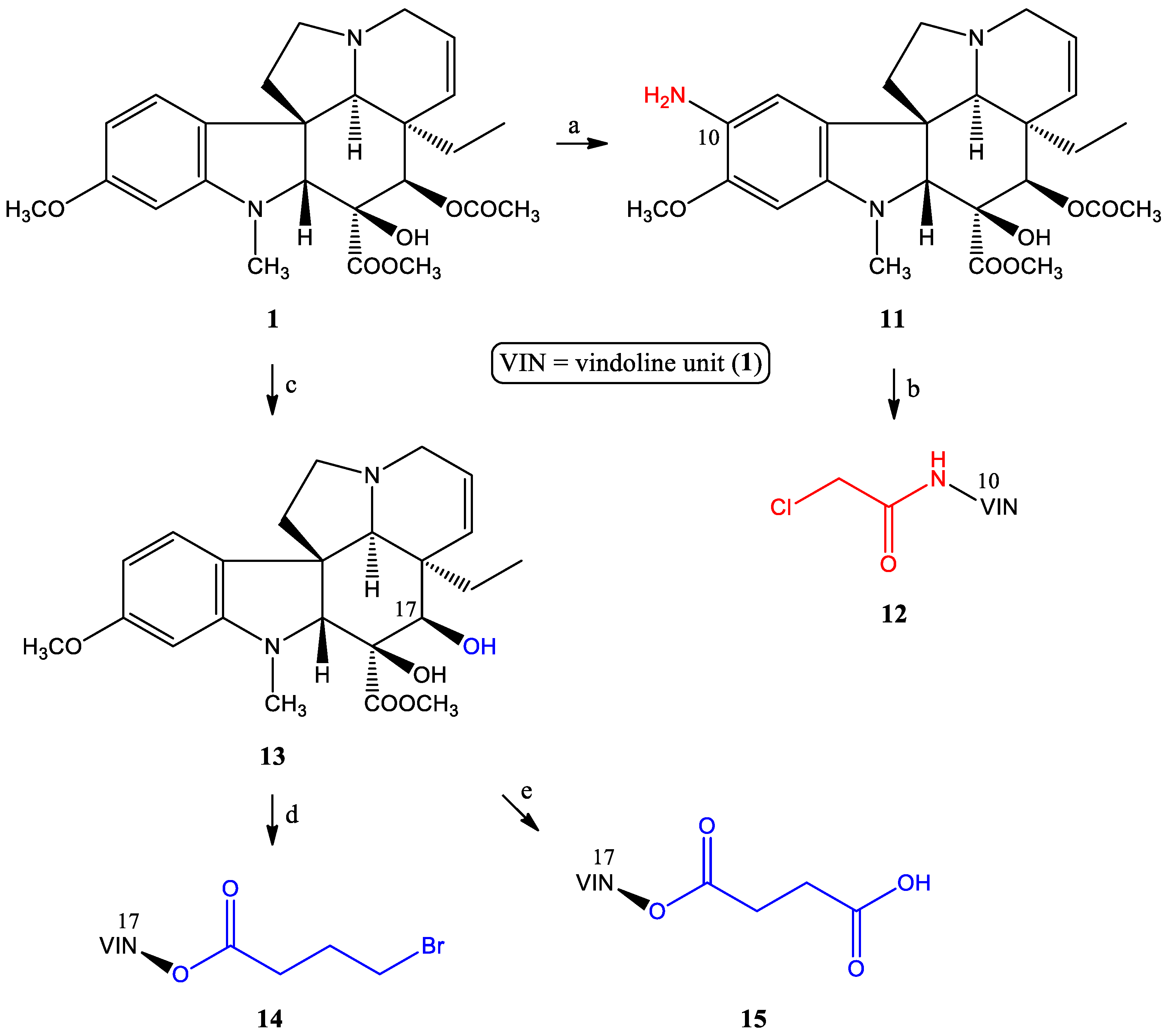
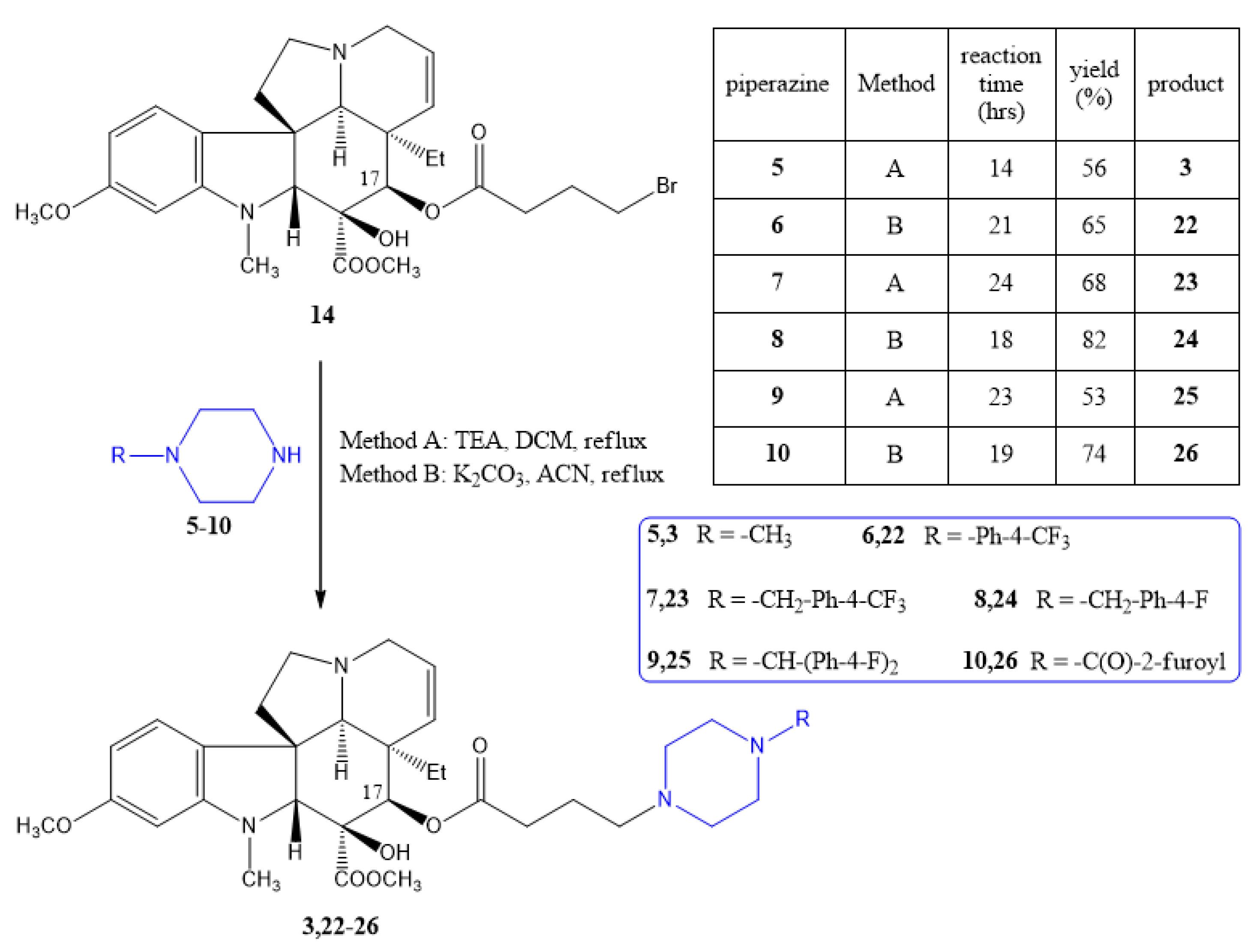
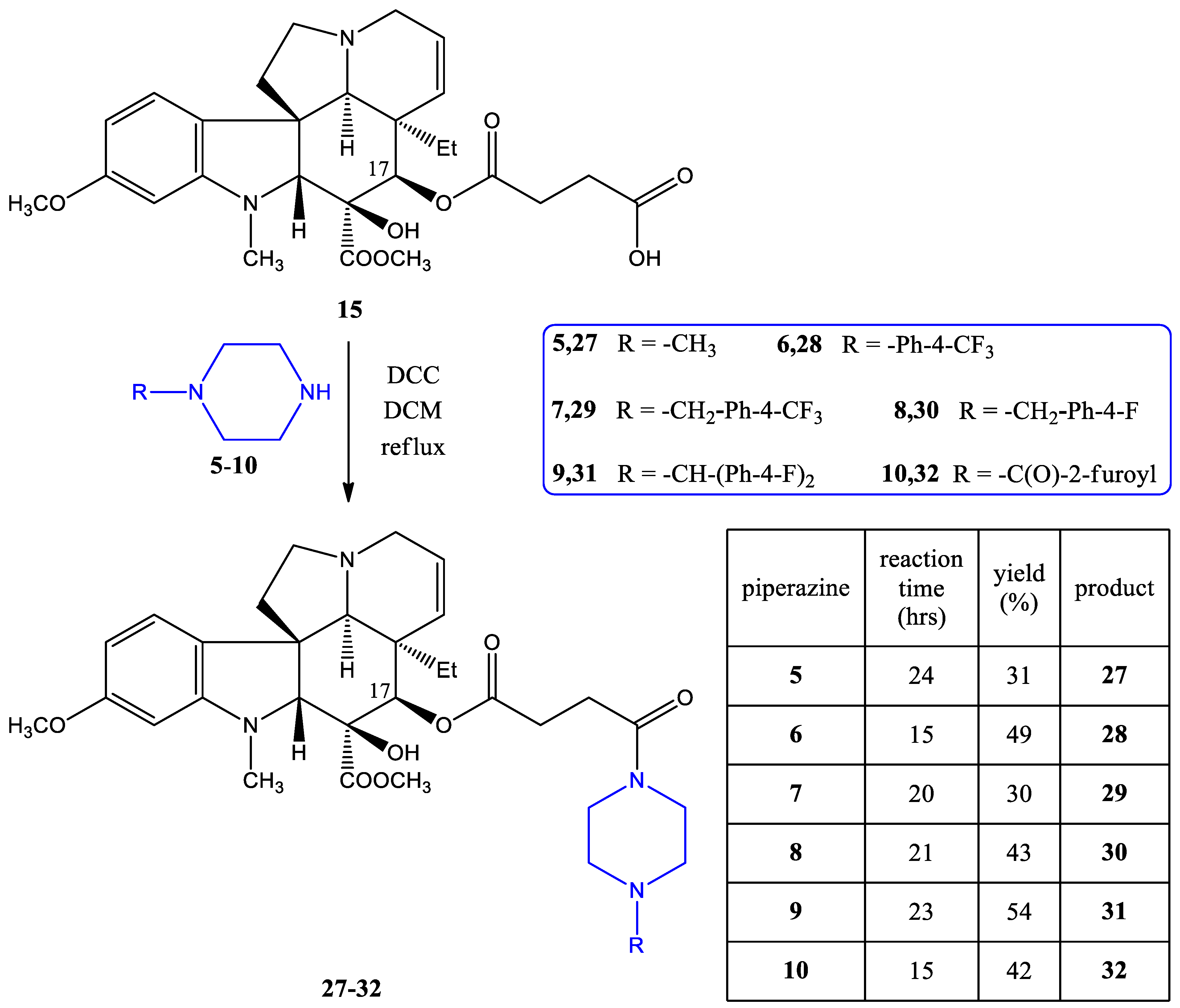
2.1.1. Preparation of the Linker-Containing Vindoline Derivatives
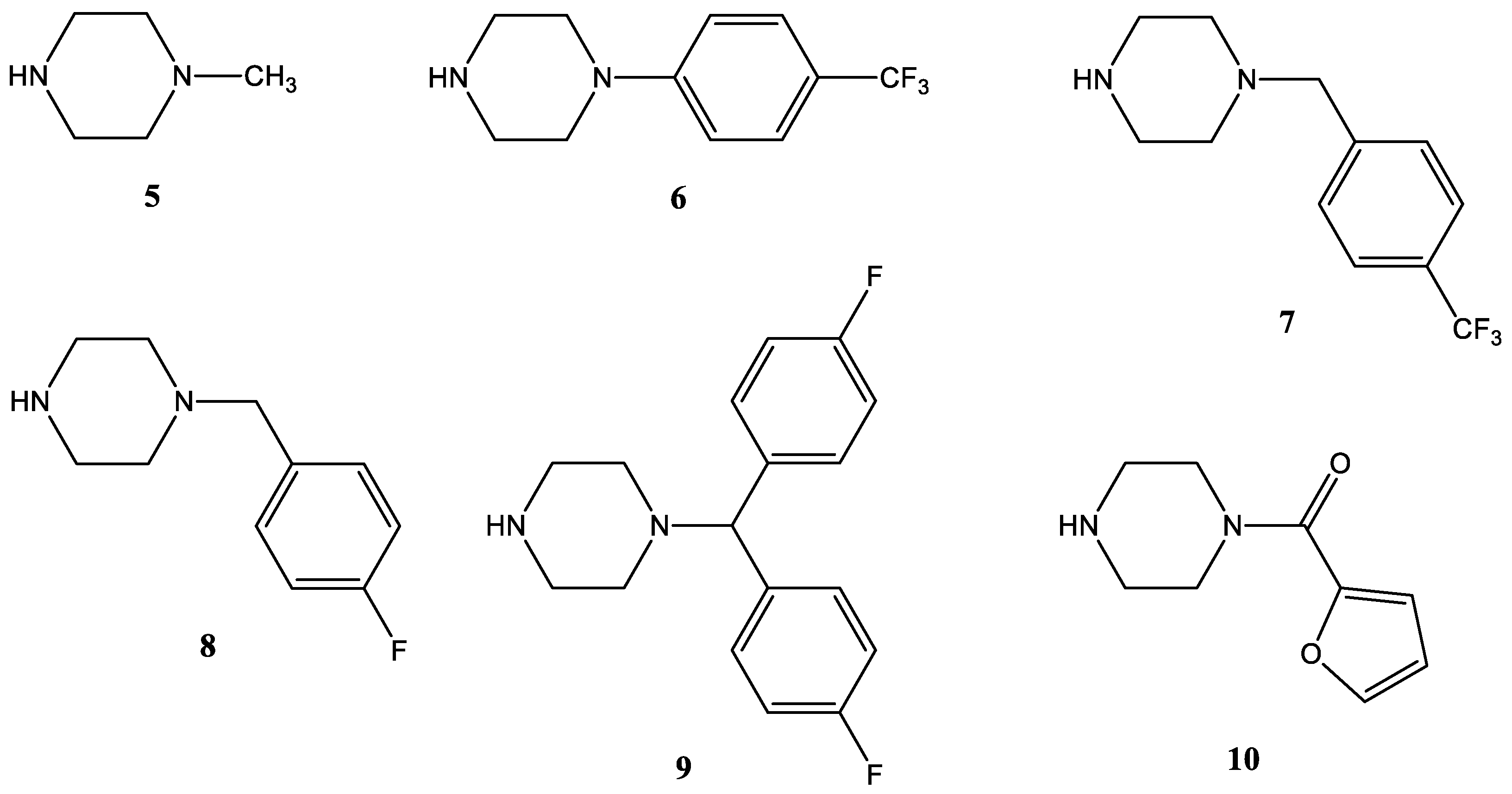
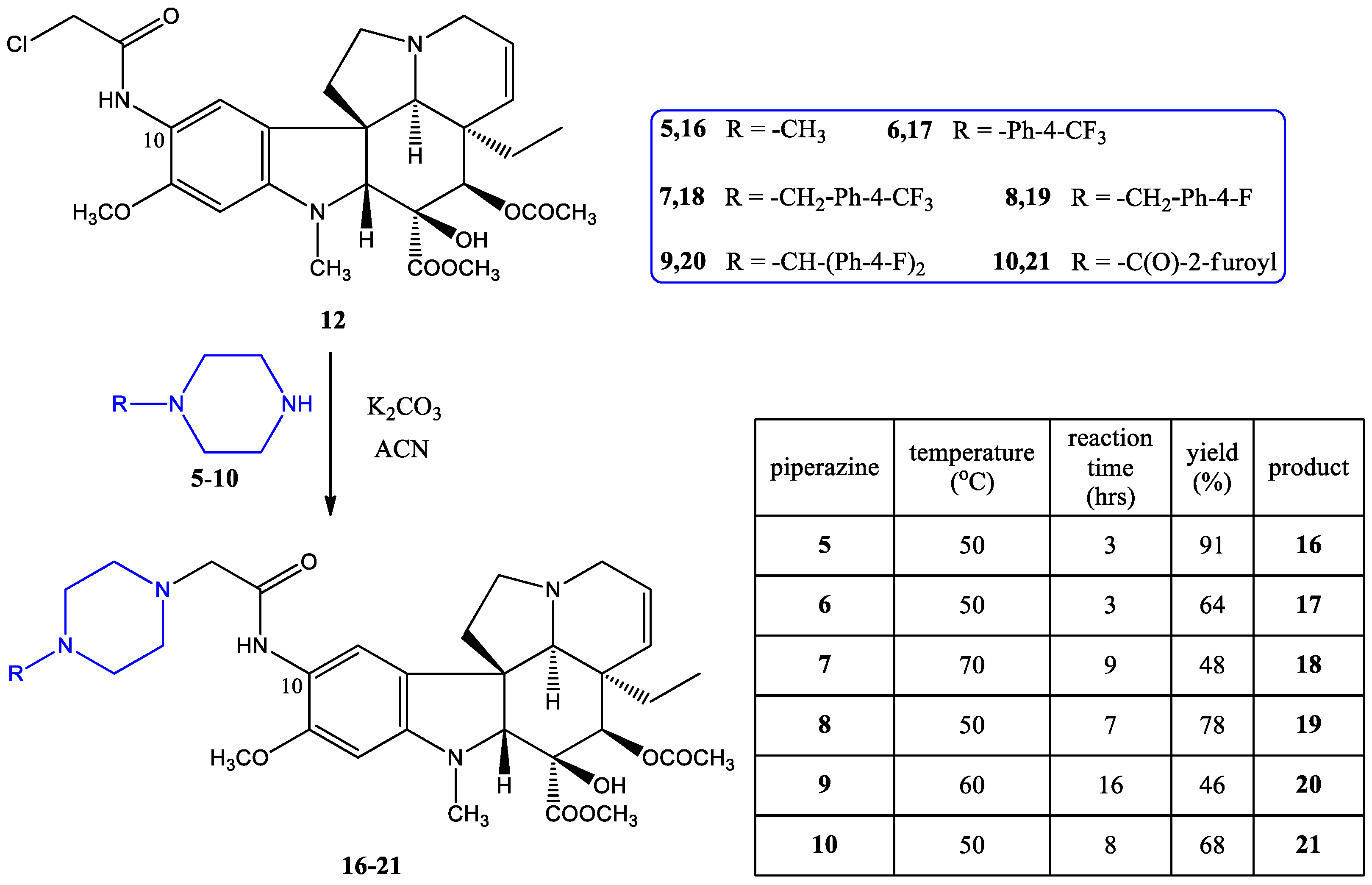
2.1.2. Coupling of the Linker-Containing Vindoline Derivatives With Piperazines
2.2. Evaluation of the Biological Activities
2.2.1. NCI60 Screening
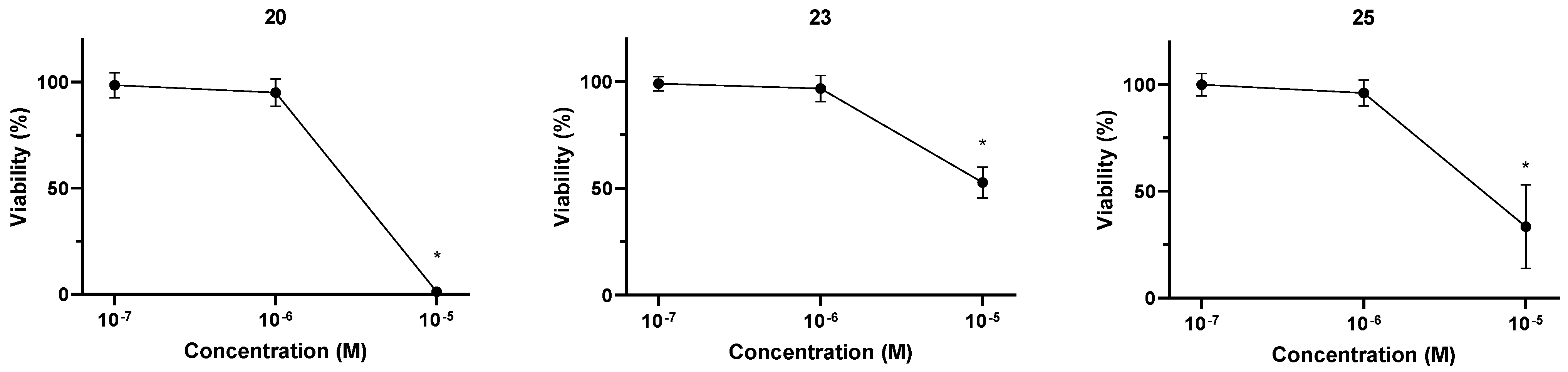
2.2.2. Effect of Selected Conjugates on Cell Viability of Non-Tumor Chinese Hamster Ovary (CHO) Cell Lines
3. Materials and Methods
3.1. General
3.2. Chemistry
3.3. Biological Evaluation
3.3.1. NCI60 Screening
3.3.2. CellTiter-Glo Luminescent Cell Viability Assay on Non-Tumor CHO Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Modifications on the Basic Skeletons of Vinblastine and Vincristine. Molecules 2012, 17, 5893–5914. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R. New Prospects for Vinblastine Analogues as Anticancer Agents. J. Med. Chem. 2013, 56, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Sears, J.E.; Boger, D.L. Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure–Function Properties. Acc. Chem. Res. 2015, 48, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Rahman, M.A.; Faizi, M.S.H.; Khan, M.S. Next Generation Antineoplastic Agents: A Review on Structurally Modified Vinblastine (VBL) Analogues. Curr. Med. Chem. 2018, 25, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.; Gotoh, H.; Robertson, W.M.; Boger, D.L. Catharanthine C16 substituent effects on the biomimetic coupling with vindoline: Preparation and evaluation of a key series of vinblastine analogues. Bioorg. Med. Chem. Lett. 2010, 20, 6408–6410. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Hu, L.; Meng, Y.; Ma, L.; Guo, D.; Liu, X.; Hu, L. The effect of vindoline C-16 substituents on the biomimetic coupling reaction: Synthesis and cytotoxicity evaluation of the corresponding vinorelbine analogues. Bioorg. Med. Chem. Lett. 2012, 22, 3485–3487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Zu, Y.; Chen, X.; Lu, Q.; Ma, Y.; Yang, L. Preparation and Physicochemical Properties of Vinblastine Microparticles by Supercritical Antisolvent Process. Int. J. Mol. Sci. 2012, 13, 12598–12607. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, A.; Sepsey Für, C.; Bölcskei, H.; Ilkei, V.; Keglevich, P.; Hazai, L. Results in Chemistry of Natural Organic Compounds. Synthesis of New Anticancer Vinca Alkaloids and Flavone Alkaloids. Chemistry 2020, 2, 714–726. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Dubrovay, Z.; Dékány, M.; Szántay, C., Jr.; Kalaus, G.; Szántay, C. Bisindole Alkaloids Condensed with a Cyclopropane Ring, Part 1. 14,15-Cyclopropano-vinblastine and -vincristine. Heterocycles 2014, 89, 653–668. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, C.; Wang, P.; Li, A.; Zhang, H.; Xu, S. Structural Basis and Mechanism for Vindoline Dimers Interacting with α,β-Tubulin. ACS Omega 2019, 4, 11938–11948. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hénon, E.; Leroy, R.; Massiot, G. Addition of Vindoline to p-Benzoquinone: Regiochemistry, Stereochemistry and Symmetry Considerations. Molecules 2021, 26, 6395. [Google Scholar] [CrossRef] [PubMed]
- Asia; Sammer, Y.; Vendier, L.; Massiot, G. Structure and Synthesis of Vindolicine and Derivatives. Chem. Biodivers. 2024, 21, e202301928. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Braconi, L.; Gabellini, A.; Manetti, D.; Marotta, G.; Teodori, E. Synthetic Approaches to Piperazine-Containing Drugs Approved by FDA in the Period of 2011–2023. Molecules 2024, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-H.; Guo, H.-Y.; Deng, H.; Li, J.; Quan, Z.-S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzym. Inhib. Med. Chem. 2021, 36, 1165–1197. [Google Scholar] [CrossRef]
- İbiş, K.; Nalbat, E.; Çalışkan, B.; Kahraman, D.C.; Cetin-Atalay, R.; Banoglu, E. Synthesis and biological evaluation of novel isoxazol-piperazone hybrids as potential anti-cancer agents with inhibitory effect on liver cancer stem cells. Eur. J. Med. Chem. 2021, 221, 113489. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghorbani, M.; Gouda, M.A.; Baashen, M.; Alharbi, O.; Almalki, F.A.; Ranganatha, L.V. Piperazine Heterocycles as Potential Anticancer Agents: A Review. Pharm. Chem. J. 2022, 56, 29–37. [Google Scholar] [CrossRef]
- Sharma, V.; Rina, D.; Sharma, D.; Mujwar, S.; Mehta, D.K. Green chemistry approach towards Piperazine: Anticancer agents. J. Mol. Struct. 2023, 1292, 136089. [Google Scholar] [CrossRef]
- Brossard, D.; Zhang, Y.; Haider, S.M.; Sgobba, M.; Khalid, M.; Legay, R.; Duterque-Coquillaud, M.; Galera, P.; Rault, S.; Dallermagne, P.; et al. N-substituted Piperazinopyridylsteroid Derivatives as Abiraterone Analogues Inhibit Growth and Induce Pro-apoptosis in Human Hormone-independent Prostate Cancer Cell Lines. Chem. Biol. Drug Des. 2013, 82, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Mistry, B.; Keum, Y.S.; Pandurangan, M.; Patel, R.V.; Kim, D.H. Synthesis of berberine-piperazine conjugates as potential antioxidant and cytotoxic agents. Med. Chem. Res. 2016, 25, 2461–2470. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigne, B.A. Pentacyclic Triterpenoids with Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Goto, M.; Chen, X.; Morris-Natschke, S.L.; Lee, K.-H. Lead Optimization: Synthesis and Biological Evaluation of PBT-1 Derivatives as Novel Antitumor Agents. ACS Med. Chem. Lett. 2021, 12, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Han, G.; Ren, H.; Li, X.; Li, X.; Yue, L.; Xu, J.; Feng, J.; Guo, L. Synthesis, biological evaluation and preliminary mechanisms of 6-amino substituted harmine derivatives as potential antitumor agents. Fitoterapia 2022, 163, 105329. [Google Scholar] [CrossRef] [PubMed]
- Kilbile, J.T.; Tamboli, Y.; Gadekar, S.S.; Islam, I.; Supuran, C.T.; Sapkal, S.B. An insight into the biological activity and structure-based drug design attributes of sulfonylpiperazine derivatives. J. Mol. Struct. 2023, 1278, 134971. [Google Scholar] [CrossRef]
- Keglevich, A.; Dányi, L.; Rieder, A.; Horváth, D.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Latif, A.D.; Hunyadi, A.; Zupkó, I.; et al. Synthesis and Cytotoxic Activity of New Vindoline Derivatives Coupled to Natural and Synthetic Pharmacophores. Molecules 2020, 25, 1010. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Nagy, N.; Keglevich, P.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Hazai, L. Synthesis of Novel Vindoline-Chrysin Hybrids. Chem. Biodivers. 2022, 19, e2100725. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shen, X.; Jiang, H.; Lei, M.; Chen, J.; Chen, F.; Yu, L.; Li, C. Medicinal application of Vindoline. CN Patent 103304565A, 11 May 2016. [Google Scholar]
- Passarella, D.; Giardini, A.; Peretto, B.; Fontana, G.; Sacchetti, A.; Silvani, A.; Ronchi, C.; Cappelletti, G.; Cartelli, D.; Borlak, J.; et al. Inhibitors of tubulin polymerization: Synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III. Bioorg. Med. Chem. 2008, 16, 6269–6285. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, A.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Keglevich, P.; Hazai, L. Synthesis and in vitro Antitumor Effect of New Vindoline Derivatives Coupled with Triphenylphosphine. Curr. Org. Chem. 2019, 23, 852–858. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.H.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, A.M.; Hursey, L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Shoemaker, R.H.; Monks, A.; Alley, M.C.; Scudiero, D.A.; Fine, D.L.; McLemore, T.L.; Abbott, B.J.; Paull, K.D.; Mayo, J.G.; Boyd, M.R. Development of Human Tumor Cell Line Panels for Use in Disease-Oriented Drug Screening. Prog. Clin. Biol. Res. 1988, 276, 265–286. [Google Scholar] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesh, H.; Kennedy, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- NCI-60 Screening Methodology. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 15 July 2024).
- Wang, D.; Shen, M.; Kitamura, N.; Sennari, Y.; Morita, K.; Tsukada, J.; Kanazawa, T.; Yoshida, Y. Mitogen-activated protein kinases are involved in cucurbitacin D-induced antitumor effects on adult T-cell leukemia cells. Investig. New Drugs 2021, 39, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.J.; Castro, K.E.; Xu, B.; Li, J.; Dinh, N.B.; Thompson, J.M.; Woytash, J.; Kipp, K.R.; Razorenova, O.V. Synthetic lethality of cyclin-dependent kinase inhibitor Dinaciclib with VHL-deficiency allows for selective targeting of clear cell renal cell carcinoma. Cell Cycle 2022, 21, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Nehr-Majoros, A.K.; Erostyák, J.; Fenyvesi, É.; Szabó-Meleg, E.; Szőcs, L.; Sétáló, G., Jr.; Helyes, Z.; Szőke, É. Cyclodextrin derivatives decrease Transient Receptor Potential vanilloid 1 and Ankyrin 1 ion channel activation via altering the surrounding membrane microenvironment by cholesterol depletion. Front. Cell Dev. Biol. 2024, 12, 1334130. [Google Scholar] [CrossRef] [PubMed]
| Panel | Cell Line | 17 | 20 | 22 | 23 | 24 | 25 | 28 | 29 | 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | GI50 | Mean | ||
| Leukemia | CCRF-CEM | 3.24 | 2.75 | 2.88 | 2.38 | 1.86 | 1.77 | 2.04 | 1.71 | 4.07 | 3.19 | 2.04 | 1.76 | 1.66 | 1.81 | 11.75 | 9.07 | 2.11 | 1.89 |
| HL-60(TB) | 2.14 | 2.19 | 1.66 | 1.74 | 3.31 | 1.55 | 1.45 | 12.88 | 1.93 | ||||||||||
| K-562 | 3.02 | 3.16 | 1.41 | 1.70 | 2.40 | 1.91 | 1.70 | 3.80 | 2.92 | ||||||||||
| MOLT-4 | 2.09 | 2.14 | 1.95 | 1.15 | 1.74 | 1.45 | 1.17 | 5.25 | 1.35 | ||||||||||
| RPMI-8226 | 3.09 | 1.91 | 2.04 | 2.00 | 4.17 | 1.95 | 2.57 | 6.92 | 1.42 | ||||||||||
| SR | 2.95 | 2.00 | 1.70 | 1.66 | 3.47 | 1.70 | 2.29 | 13.80 | 1.63 | ||||||||||
| Non-small CellLung Cancer | A549/ATCC | 4.07 | 7.29 | 4.37 | 15.67 | 2.95 | 2.88 | 1.78 | 1.80 | 10.23 | 9.83 | 2.63 | 1.85 | 2.75 | 4.23 | 8.32 | 10.40 | 2.67 | 2.59 |
| EKVX | 6.03 | 4.07 | 3.09 | 1.70 | 12.02 | 1.70 | 3.63 | 7.59 | n.d. | ||||||||||
| HOP-62 | 3.72 | 89.13 | 3.02 | 1.74 | 10.23 | 1.62 | 5.50 | 14.13 | 2.55 | ||||||||||
| HOP-92 | 2.57 | 5.01 | 1.95 | 1.51 | 1.66 | 1.35 | 2.09 | 4.27 | 1.99 | ||||||||||
| NCI-H226 | 6.46 | 6.31 | 2.88 | 2.04 | 10.47 | 2.04 | 3.16 | 9.77 | 1.84 | ||||||||||
| NCI-H23 | 10.72 | 6.76 | 4.57 | 1.91 | 13.18 | 1.91 | 4.37 | 12.02 | 2.70 | ||||||||||
| NCI-H322M | 15.14 | 12.02 | 3.72 | 2.24 | 8.32 | 1.95 | 10.00 | 14.45 | 4.99 | ||||||||||
| NCI-H460 | 6.46 | 3.16 | 1.86 | 1.55 | 8.51 | 1.78 | 3.31 | 10.47 | 1.93 | ||||||||||
| NCI-H522 | 10.47 | 10.23 | 1.91 | 1.70 | 13.80 | 1.70 | 3.24 | 12.59 | 2.04 | ||||||||||
| Colon Cancer | COLO 205 | 3.24 | 4.31 | 2.45 | 3.40 | 1.78 | 2.02 | 1.58 | 1.64 | 1.95 | 6.09 | 1.66 | 1.73 | 1.91 | 3.16 | 7.08 | 9.71 | 2.82 | 2.23 |
| HCC-2998 | 7.76 | 3.89 | 1.82 | 1.51 | 2.19 | 1.66 | 2.40 | 12.88 | 1.24 | ||||||||||
| HCT-116 | 3.72 | 3.09 | 1.91 | 1.70 | 5.62 | 1.78 | 3.31 | 7.76 | 2.72 | ||||||||||
| HCT-15 | 3.89 | 3.55 | 1.82 | 1.74 | 5.13 | 1.70 | 3.16 | 10.23 | 2.45 | ||||||||||
| HT29 | 2.09 | 2.88 | 1.74 | 1.66 | 3.80 | 1.91 | 2.57 | 6.76 | 1.76 | ||||||||||
| KM12 | 3.72 | 3.98 | 2.14 | 1.74 | 10.47 | 1.78 | 4.79 | 11.75 | 2.36 | ||||||||||
| SW-620 | 5.75 | 3.98 | 2.95 | 1.55 | 13.49 | 1.62 | 3.98 | 11.48 | 2.27 | ||||||||||
| CNS Cancer | SF-268 | 3.02 | 4.01 | 5.13 | 4.40 | 3.63 | 2.47 | 1.35 | 1.57 | 5.25 | 8.08 | 1.70 | 2.21 | 4.07 | 3.69 | 5.75 | 11.07 | 2.98 | 2.60 |
| SF-295 | 3.55 | 3.47 | 1.95 | 1.78 | 12.59 | 1.70 | 3.16 | 9.33 | 2.11 | ||||||||||
| SF-539 | 4.68 | 4.07 | 1.78 | 1.58 | 10.47 | 1.66 | 5.25 | 12.88 | 2.02 | ||||||||||
| SNB-19 | 5.37 | 5.89 | 3.55 | 1.58 | 11.48 | 1.66 | 4.37 | 11.75 | 3.58 | ||||||||||
| SNB-75 | 1.45 | 1.82 | 1.66 | 1.45 | 1.12 | 5.01 | 2.00 | 15.49 | 2.11 | ||||||||||
| U251 | 6.03 | 6.03 | 2.24 | 1.66 | 7.59 | 1.55 | 3.31 | 11.22 | 2.78 | ||||||||||
| Melano-ma | LOX IMVI | 4.27 | 5.89 | 3.24 | 5.60 | 1.70 | 1.98 | 1.78 | 1.62 | 5.01 | 5.92 | 1.74 | 1.78 | 3.24 | 3.63 | 13.80 | 9.42 | 1.80 | 2.40 |
| MALME-3M | 14.79 | 8.13 | 1.58 | 1.58 | 2.29 | 1.51 | 5.37 | 11.48 | 2.20 | ||||||||||
| M14 | 3.63 | 4.07 | 1.86 | 1.55 | 5.50 | 1.91 | 2.40 | 8.13 | 3.55 | ||||||||||
| MDA-MB-435 | 1.95 | 3.89 | 1.70 | 1.48 | 5.50 | 1.66 | 3.89 | 10.47 | 2.70 | ||||||||||
| SK-MEL-2 | 4.37 | 3.02 | 2.19 | 1.78 | 15.14 | 2.40 | 2.75 | 2.82 | 1.62 | ||||||||||
| SK-MEL-28 | 5.89 | 12.30 | 1.95 | 1.62 | 4.68 | 1.74 | 5.01 | 11.75 | 3.26 | ||||||||||
| SK-MEL-5 | 2.40 | 2.34 | 1.70 | 1.35 | 1.66 | 1.55 | 3.24 | 3.89 | 1.84 | ||||||||||
| UACC-257 | 5.50 | 5.25 | 1.82 | 1.74 | 2.00 | 1.74 | 3.89 | 11.48 | 2.70 | ||||||||||
| UACC-62 | 10.23 | 8.13 | 3.31 | 1.66 | 11.48 | 1.78 | 2.88 | 10.96 | 1.92 | ||||||||||
| Ovarian Cancer | IGROV1 | 10.00 | 6.48 | 7.94 | 5.30 | 2.00 | 3.05 | 1.78 | 1.77 | 2.51 | 10.09 | 1.38 | 1.73 | 3.31 | 3.92 | 10.72 | 10.59 | 2.50 | 2.15 |
| OVCAR-3 | 2.88 | 4.37 | 2.45 | 1.55 | 10.72 | 1.74 | 3.72 | 6.46 | 1.60 | ||||||||||
| OVCAR-4 | 3.89 | 3.31 | 3.80 | 2.04 | 5.37 | 1.95 | 4.27 | 5.62 | 1.62 | ||||||||||
| OVCAR-5 | 10.72 | 3.55 | 4.07 | 1.62 | 13.80 | 1.66 | 5.25 | 14.45 | 2.18 | ||||||||||
| OVCAR-8 | 8.51 | 7.59 | 3.02 | 1.82 | 14.45 | 2.09 | 3.72 | 12.02 | 3.18 | ||||||||||
| NCI/ADR-RES | 6.03 | 4.47 | 2.29 | 1.58 | 11.75 | 1.74 | 3.31 | 12.30 | 1.95 | ||||||||||
| SK-OV-3 | 3.31 | 5.89 | 3.72 | 2.00 | 12.02 | 1.55 | 3.89 | 12.59 | 2.02 | ||||||||||
| Renal Cancer | 786–0 | 6.31 | 6.01 | 7.76 | 5.23 | 2.57 | 2.59 | 1.48 | 1.63 | 6.31 | 5.58 | 1.58 | 2.18 | 5.01 | 3.67 | 12.30 | 9.90 | 2.47 | 2.33 |
| A498 | 10.72 | 5.62 | 3.72 | 1.62 | 12.88 | 5.25 | 5.75 | 12.30 | 2.38 | ||||||||||
| ACHN | 5.62 | 4.90 | 3.24 | 1.78 | 5.89 | 2.09 | 3.80 | 10.72 | 2.06 | ||||||||||
| CAKI-1 | 2.63 | 3.16 | 2.24 | 1.48 | 2.24 | 1.48 | 2.00 | 8.71 | 3.07 | ||||||||||
| RXF 393 | 5.37 | 2.69 | 1.55 | 1.35 | 1.00 | n.d. | 2.29 | 4.47 | n.d. | ||||||||||
| SN12C | 7.76 | 4.90 | 2.63 | 2.09 | 8.32 | 1.66 | 4.17 | 11.48 | 1.47 | ||||||||||
| TK-10 | 4.57 | 7.59 | 2.88 | 1.66 | 4.90 | 1.51 | 4.07 | 12.30 | 2.57 | ||||||||||
| UO-31 | 5.13 | 5.25 | 1.86 | 1.58 | 3.09 | 1.66 | 2.29 | 6.92 | 2.32 | ||||||||||
| Prostate Cancer | PC-3 | 3.31 | 4.88 | 4.47 | 5.39 | 2.45 | 2.67 | 1.58 | 1.58 | 4.17 | 7.32 | 1.51 | 1.65 | 2.45 | 3.85 | 3.39 | 7.30 | 1.86 | 2.27 |
| DU-145 | 6.46 | 6.31 | 2.88 | 1.58 | 10.47 | 1.78 | 5.25 | 11.22 | 2.67 | ||||||||||
| Breast Cancer | MCF7 | 3.72 | 6.11 | 2.95 | 4.52 | 2.00 | 2.51 | 1.55 | 1.53 | 3.98 | 4.25 | 1.70 | 1.67 | 2.95 | 3.44 | 5.62 | 8.72 | 1.74 | 2.00 |
| MDA-MB231ATCC | 11.22 | 9.12 | 2.82 | 1.74 | 2.82 | 1.58 | 4.17 | 14.13 | 3.02 | ||||||||||
| HS 578T | 14.79 | 5.89 | 2.82 | 1.51 | 4.07 | 1.78 | 4.79 | 10.23 | 1.74 | ||||||||||
| BT-549 | 2.34 | 2.57 | 3.02 | 1.66 | 7.24 | 1.78 | 3.31 | 15.14 | 1.81 | ||||||||||
| T-47D | 2.19 | 3.55 | 3.02 | 1.74 | 5.62 | 1.66 | 3.55 | 5.25 | 2.47 | ||||||||||
| MDA-MB-468 | 2.40 | 3.02 | 1.38 | 1.00 | 1.74 | 1.51 | 1.86 | 1.95 | 1.20 | ||||||||||
| Mean | 5.53 | 6.27 | 2.44 | 1.65 | 6.84 | 1.85 | 3.55 | 9.76 | 2.29 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsoldos, B.; Nagy, N.; Donkó-Tóth, V.; Keglevich, P.; Weber, M.; Dékány, M.; Nehr-Majoros, A.; Szőke, É.; Helyes, Z.; Hazai, L. Novel Piperazine Derivatives of Vindoline as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7929. https://doi.org/10.3390/ijms25147929
Zsoldos B, Nagy N, Donkó-Tóth V, Keglevich P, Weber M, Dékány M, Nehr-Majoros A, Szőke É, Helyes Z, Hazai L. Novel Piperazine Derivatives of Vindoline as Anticancer Agents. International Journal of Molecular Sciences. 2024; 25(14):7929. https://doi.org/10.3390/ijms25147929
Chicago/Turabian StyleZsoldos, Bernadett, Nóra Nagy, Viktória Donkó-Tóth, Péter Keglevich, Márton Weber, Miklós Dékány, Andrea Nehr-Majoros, Éva Szőke, Zsuzsanna Helyes, and László Hazai. 2024. "Novel Piperazine Derivatives of Vindoline as Anticancer Agents" International Journal of Molecular Sciences 25, no. 14: 7929. https://doi.org/10.3390/ijms25147929
APA StyleZsoldos, B., Nagy, N., Donkó-Tóth, V., Keglevich, P., Weber, M., Dékány, M., Nehr-Majoros, A., Szőke, É., Helyes, Z., & Hazai, L. (2024). Novel Piperazine Derivatives of Vindoline as Anticancer Agents. International Journal of Molecular Sciences, 25(14), 7929. https://doi.org/10.3390/ijms25147929






