Abstract
The gut microbiota has acquired significant attention in recent years for its potential as a diagnostic biomarker for colorectal cancer (CRC). In this literature review, we looked at the studies exploring alterations in gut microbiota composition associated with CRC, the potential mechanisms linking gut dysbiosis to CRC development, and the diagnostic approaches utilizing gut microbiota analysis. Our research has led to the conclusion that individuals with CRC often display alterations in their gut microbiota composition compared to healthy individuals. These alterations can include changes in the diversity, abundance, and type of bacteria present in the gut. While the use of gut microbiota as a diagnostic biomarker for CRC holds promise, further research is needed to validate its effectiveness and standardize testing protocols. Additionally, considerations such as variability in the microbiota composition among individuals and potential factors must be addressed before microbiota-based tests can be widely implemented in clinical practice.
1. Introduction
Colorectal cancer is a malignant tumor that develops in the colon or rectum, which are parts of the large intestine. It arises from the uncontrolled growth of abnormal cells in the lining of the colon or rectum and can spread to other parts of the body if not detected and treated early [1]. CRC is one of the most common cancers worldwide with significant morbidity and mortality [2].
CRC is the third most commonly diagnosed cancer in both men and women globally [3]. The incidence varies geographically with higher rates in developed countries compared to developing nations [4]. However, incidence rates are rising in many regions, including parts of Asia and Africa, due to changes in lifestyle, diet, and aging populations [5]. CRC is also the third leading cause of cancer-related deaths worldwide. Mortality rates have been declining in some high-income countries due to improved screening programs, early detection, and advances in treatment [6]. However, in low- and middle-income countries, where access to healthcare and screening is limited, mortality rates remain high [7].
Several risk factors contribute to the development of CRC, including age (risk increases with age), family history of CRC or polyps, inherited genetic syndromes (e.g., Lynch syndrome, familial adenomatous polyposis), personal history of inflammatory bowel disease (e.g., Crohn’s disease, ulcerative colitis), sedentary lifestyle, obesity, smoking, heavy alcohol consumption, and a diet high in red and processed meats and low in fruits, vegetables, and fiber [8].
Early detection of CRC through screening can significantly improve outcomes. Common screening methods include fecal occult blood tests, colonoscopy, sigmoidoscopy, and stool DNA tests [9]. Lifestyle modifications such as maintaining a healthy weight, regular physical activity, avoiding smoking and excessive alcohol consumption, and adopting a diet rich in fruits, vegetables, and whole grains can help reduce the risk of developing CRC [10].
1.1. Gut Microbiota
The gut microbiota is a complex community of trillions of microorganisms, including bacteria, viruses, fungi, and other single-celled organisms, which inhabit the gastrointestinal tract [11]. This ecosystem plays a crucial role in human health and disease through various mechanisms. It helps break down dietary components that the human digestive system cannot process alone, such as complex carbohydrates and fibers [12]. These microorganisms produce enzymes that facilitate the digestion of these substances, releasing nutrients that can then be absorbed by the host [13]. It also plays a vital role in regulating the immune system by helping the immune system distinguish between harmless substances and pathogens, thereby preventing inappropriate immune responses that can lead to inflammation or autoimmune diseases [14].
The microbiome contributes to maintaining the integrity of the intestinal barrier, which acts as a physical and immunological barrier between the gut lumen and the bloodstream [15]. A healthy gut microbiota helps prevent the translocation of harmful bacteria and toxins from the gut into systemic circulation, which can trigger inflammatory responses and contribute to various diseases [16]. Certain members are capable of synthesizing vitamins, such as vitamin K and some B vitamins, which are essential for human health. Additionally, gut microbes produce various metabolites, including short-chain fatty acids (SCFAs), which play important roles in energy metabolism, gut motility, and immune regulation [17].
Emerging research suggests a bidirectional communication system, known as the gut–brain axis, through which the gut microbiota can influence neurological and psychological function [18]. Alterations in the gut microbiota composition have been linked to conditions such as anxiety, depression, and neurodegenerative diseases [19].
Dysbiosis, or imbalance in the gut microbiota composition, has been implicated in the pathogenesis of numerous diseases, including inflammatory bowel diseases (e.g., Crohn’s disease, ulcerative colitis), obesity, type 2 diabetes, allergies, and certain cancers [20]. Understanding the role of the gut microbiota in these conditions may lead to the development of novel therapeutic approaches targeting the microbiome [21].
1.2. Gut Microbiota Dysbiosis in Colorectal Cancer
Gut microbiota dysbiosis, characterized by alterations in the composition and function of the gut microbiota, has been implicated in the development and progression of colorectal cancer [22].
Dysbiosis in the gut microbiota can lead to chronic inflammation and immune dysregulation within the intestinal mucosa, creating an environment conducive to CRC development. Certain bacterial species, such as Fusobacterium nucleatum and enterotoxigenic Bacteroides fragilis, have been associated with pro-inflammatory responses and promotion of CRC progression [23].
Dysbiosis in gut microbiota may produce metabolites that are carcinogenic or promote tumorigenesis. For example, some bacteria produce genotoxic metabolites such as hydrogen sulfide, secondary bile acids, and certain enzymes that can damage DNA and contribute to CRC initiation [24]. It can also compromise the integrity of the intestinal barrier, allowing the translocation of microbial products and toxins into the systemic circulation. This can trigger inflammation and immune responses both locally in the gut and systemically, which may contribute to CRC development and progression [25].
Metabolic dysregulation, such as obesity and insulin resistance, is a known risk factor for CRC. It interacts with the host immune system, affecting immune surveillance mechanisms against tumor cells [26]. Certain microbial species may modulate immune responses in a way that promotes tumor growth and the evasion of immune detection [27].
Understanding the role of gut microbiota dysbiosis in CRC development and progression is an active area of research. Therapeutic strategies aimed at restoring a healthy gut microbiota composition, such as probiotics, prebiotics, dietary interventions, and fecal microbiota transplantation, are being investigated as potential adjunctive treatments for CRC prevention and management [28].
2. Materials and Methods
We conducted a comprehensive literature search on PubMed using the specific keywords “microbiota and colorectal cancer”, “bacteria in colorectal cancer”, and “colorectal cancer and biomarkers”. We then performed a manual search of all eligible papers by using the references from the first search results, reviews, and other relevant publications. As this study is a literature review, an ethical approval was not needed.
The selection criteria was limited to free complete texts in English and randomized clinical trials involving people aged 18 and older. Only articles that were published throughout the timeframe of January 2013 to December 2023 were taken into account, and this review excluded articles that were restricted to abstracts, posters, editorials, and comments.
The exclusion criteria included age (under 18 years old), as well as non-peer-reviewed research. Case studies were also excluded. Studies without sufficient data and those without quantifiable results for outcomes were likewise removed.
For the final studies that we included in our analysis, we used a systematic review technique using the Patient, Intervention, Comparison, Outcome (PICO) framework, as it was defined by Eriksen and Frandsen in 2018 [29].
Population: patients aged 18 and above diagnosed with CRC.
Intervention: methods of analysis of microbiota.
Comparison: gut microbiota of CRC patients in comparison with the healthy controls.
Outcome: to establish the relationship between microbiome and CRC.
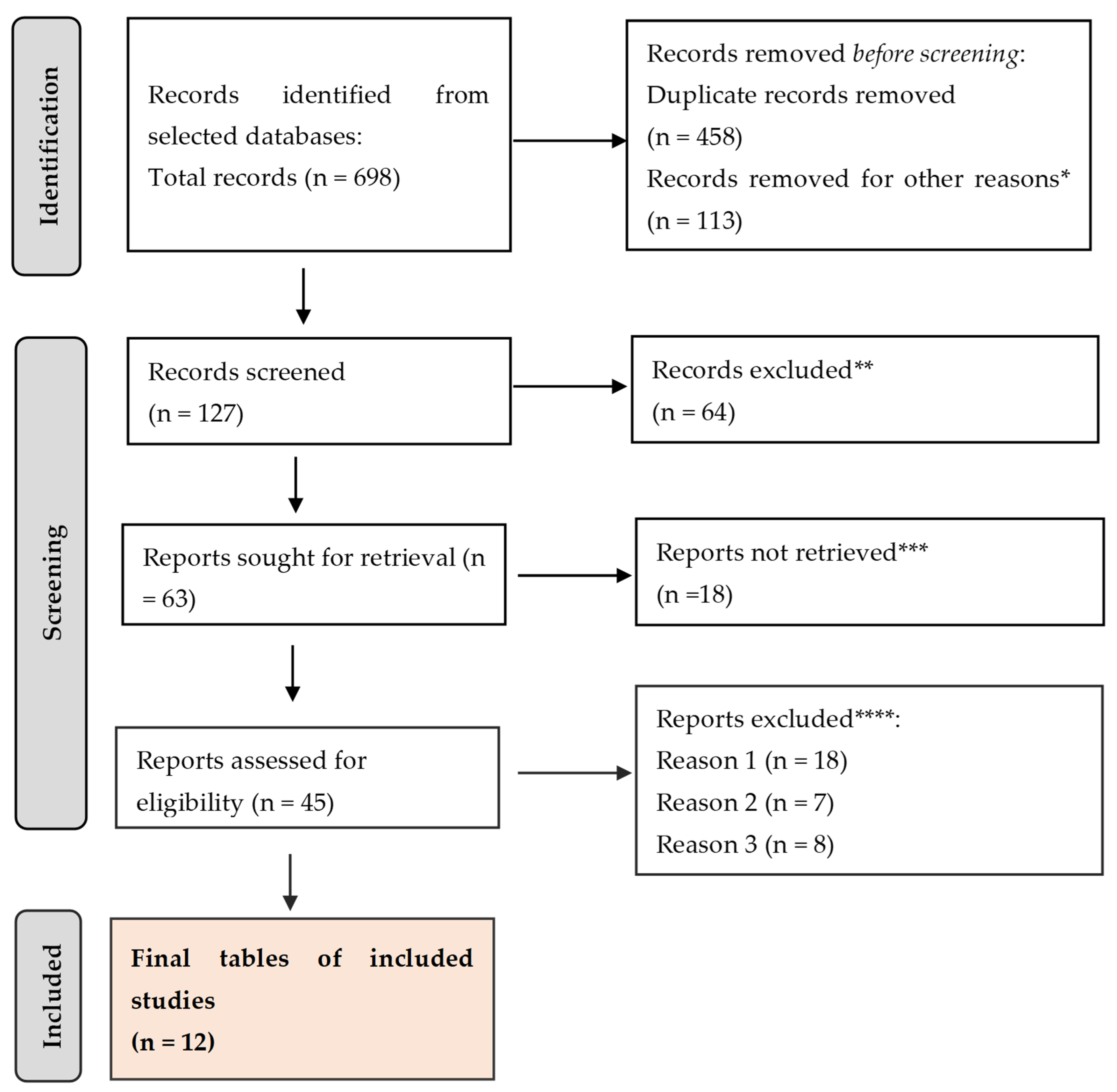
The review was outlined here using the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Figure 1).
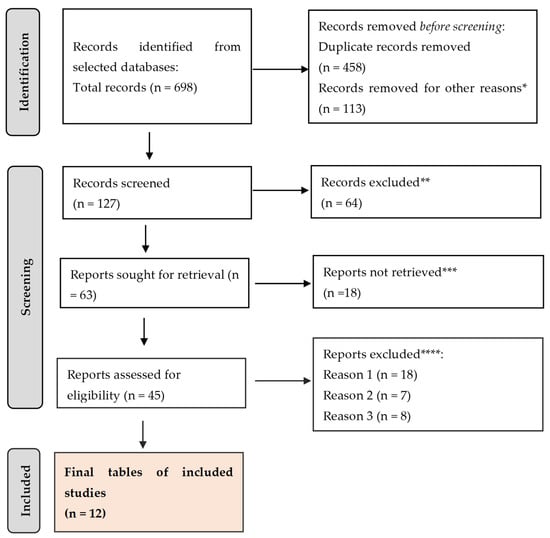
Figure 1.
PRISMA framework. * studies are not relevant for the present review. ** studies do not help us to provide an answer to the current research. *** unable to find the full text of the study. **** Reason 1—study on animals/Reason 2—wrong setting/Reason 3—research question not relevant.
A total of 698 citations were retrieved after scanning the aforementioned databases. After eliminating duplicate entries and excluding 113 items that did not satisfy the search parameters, the list was reduced to 127 remaining articles. A total of 64 studies were excluded from consideration as they did not meet the requirements of our research based on their abstracts. Additionally, 18 papers were further eliminated because they did not address the specific question of this study. Furthermore, 18 studies were excluded due to the unavailability of the full text. Another 7 studies were omitted because they focused on the wrong age group. Lastly, 8 articles were disregarded as they were written in a language other than English. Thus, we based our final analysis on a total of 12 search results that met the criteria for our investigation.
The studies met the standards for inclusion, and we organized the data from these publications in Table 1.

Table 1.
Results of clinical studies investigating the manifestations of gut microbiota in relation with CRC.
The search resulted in a total of 44 citations for “microbiota and colorectal cancer”.
The search for “bacteria in colorectal cancer” revealed a total of 72 citations.
The search for “colorectal cancer and biomarkers” resulted in 582 articles.
The search filters we used on PubMed included the following criteria: availability of free full text, randomized controlled trials, English language, adults 18 years or older, from January 2013 to December 2023.
3. Results
For this study, we selected 20 studies that were analyzed and included in Table 1. We presented the main conclusions of each study and, using the PICO framework, we answered a clear and focused research question, which is whether CRC is associated with gut microbiota. The main data are presented in Table 1.
3.1. Statistical Analysis
The papers included in this meta-analysis provide valuable insights into the connection between microbiota and CRC and, in our opinion, give crucial context for the advancement of future therapies. Descriptive characteristics of study population included in this meta-analysis are included in Table 2 below.

Table 2.
Population characteristics included in the selected studies.
3.1.1. Forest Plot
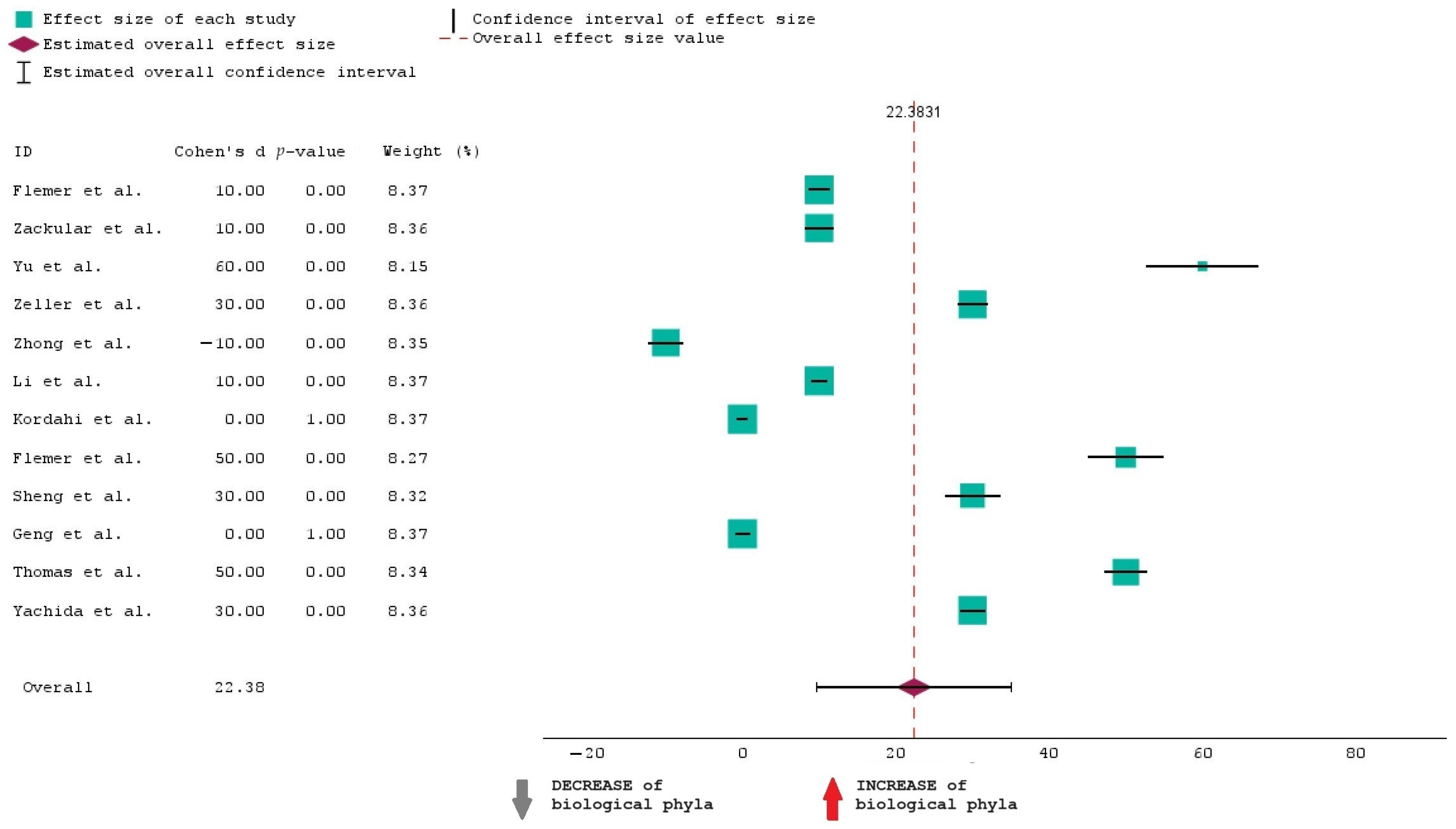
To analyze the data in Table 1, we have opted for a forest plot as a graphical representation. Meta-analyses and systematic reviews often use this method to present the findings of several research on a certain subject. The purpose of this map is to visually summarize the estimated effect sizes and their confidence intervals from the 12 chosen studies, which allows for an assessment of the general trend and variability in the data.
The forest plot presented showcases the results of this meta-analysis examining the effect sizes of the selected studies on the increase or decrease in biological phyla. Each study’s effect size is represented by a square with larger squares indicating studies that carry more weight in the analysis. The horizontal lines extending from these squares depict the 95% confidence intervals (CI) for each effect size. A positive effect size, shown to the right of the zero line, suggests an increase in biological phyla, while a negative effect size, shown to the left, suggests a decrease. The data indicate that most studies report a positive effect size, with the majority having a p-value of 0.00, signifying statistically significant results. Only one study, conducted by Zhong et al. [34], shows a negative effect size, indicating a decrease in biological phyla. This suggests that there is substantial evidence across most studies supporting an increase in biological phyla.
Figure 2 shoes that the overall effect size is 22.38, and this suggests that when all studies are combined, the estimated effect is 22.38. This means that the “INCREASE in biological phyla” in CRC is 22.38% more than the “DECREASE in biological phyla”.
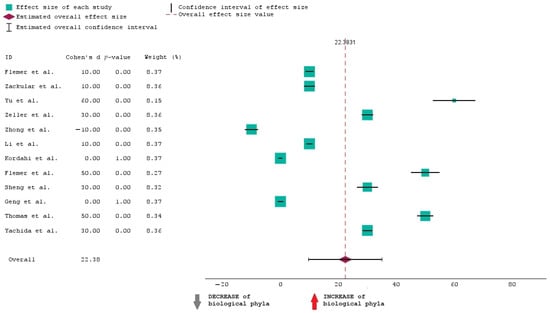
Figure 2.
Forest plot of the 12 studies included in the research [30,31,32,33,34,35,36,37,38,39,40,41].
The average CI is from approximately 10 to 35. This means we are 95% confident that the true increase in phyla lies between 10% and 35%.
It can also be seen that when it comes to the weight of studies, larger studies contribute more to the overall effect size.
The overall effect size of 22.38 is a significant and substantial finding, indicating the effectiveness of the intervention of the studies selected for this analysis.
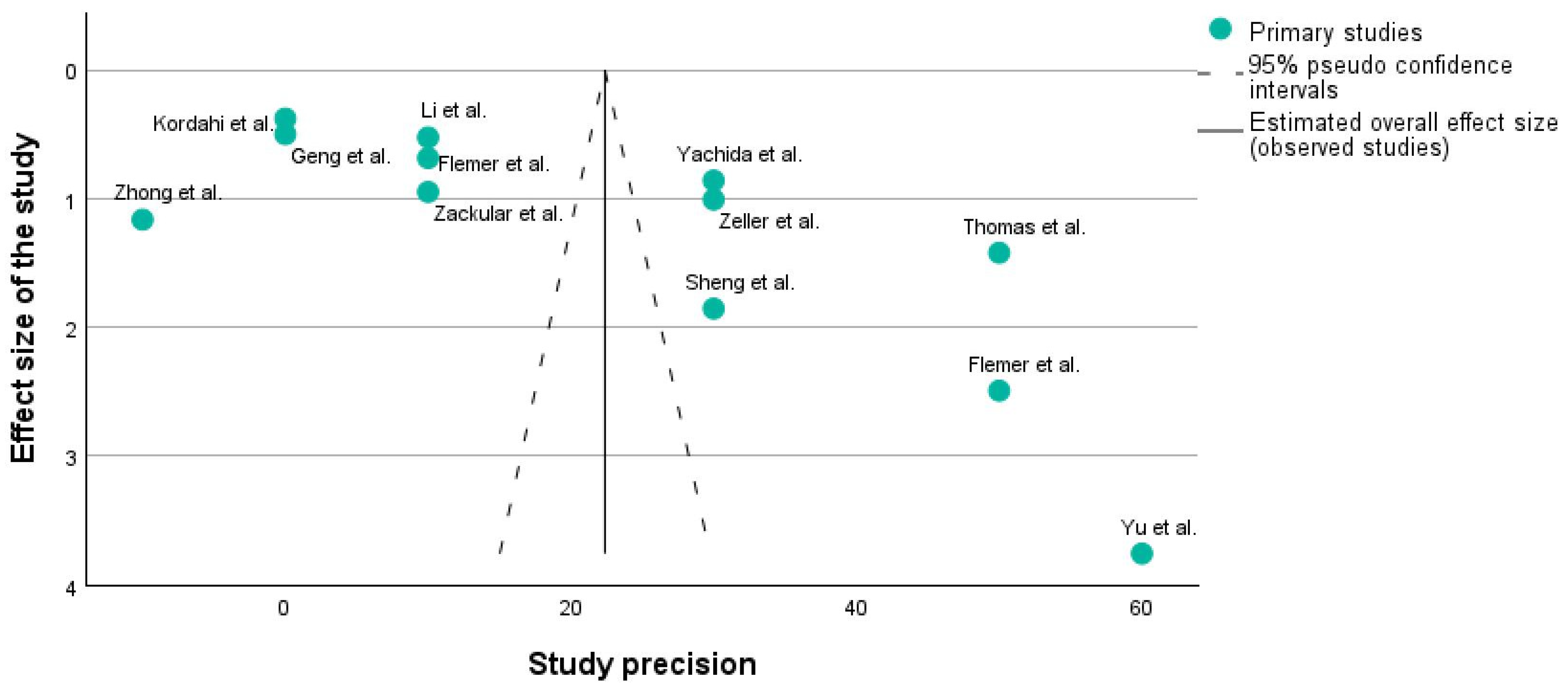
3.1.2. Funnel Plot
Figure 3 shows a funnel plot of the studies which are described in Table 1 using the PICO framework. A funnel plot is typically used to assess publication bias. It is a scatter plot of the effect estimates from individual studies against a measure of each study’s size or precision (e.g., standard error or sample size). Studies with higher precision are plotted toward the top of the graph, closer to the vertical line indicating the estimated overall effect size. The dashed lines represent the 95% pseudo-confidence intervals, creating a funnel shape.
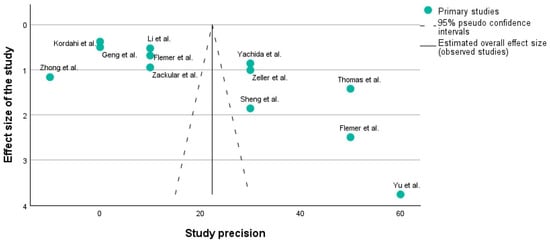
Figure 3.
Funnel plot of the 12 studies included in the research [31,32,33,34,35,36,37,38,39,40,41].
X-axis: Usually represents the effect size (e.g., risk ratio, odds ratio, mean difference). In our case, it corresponds to the effect size.
Y-axis: Represents a measure of the study’s precision, often the standard error or inverse of the standard error (1/SE).
A symmetrical funnel plot suggests that there is no publication bias. In Figure 3, it can be seen that on each side of the main line, there are 6 studies, which means that our research does not have any publication bias.
Funnel plots are crucial because they help understand whether the results of a meta-analysis might be skewed by the selective publication of studies.
In this plot, the distribution of studies appears somewhat asymmetrical. For instance, the study by Yu et al. [32] is an outlier with high precision but a large effect size, which might indicate a particularly strong effect or possibly a methodological difference. Additionally, studies such as those by Zhong et al. [34], Kordahi et al. [36], and Geng et al. [39] are clustered at the lower precision end, which might indicate smaller sample sizes or higher variability.
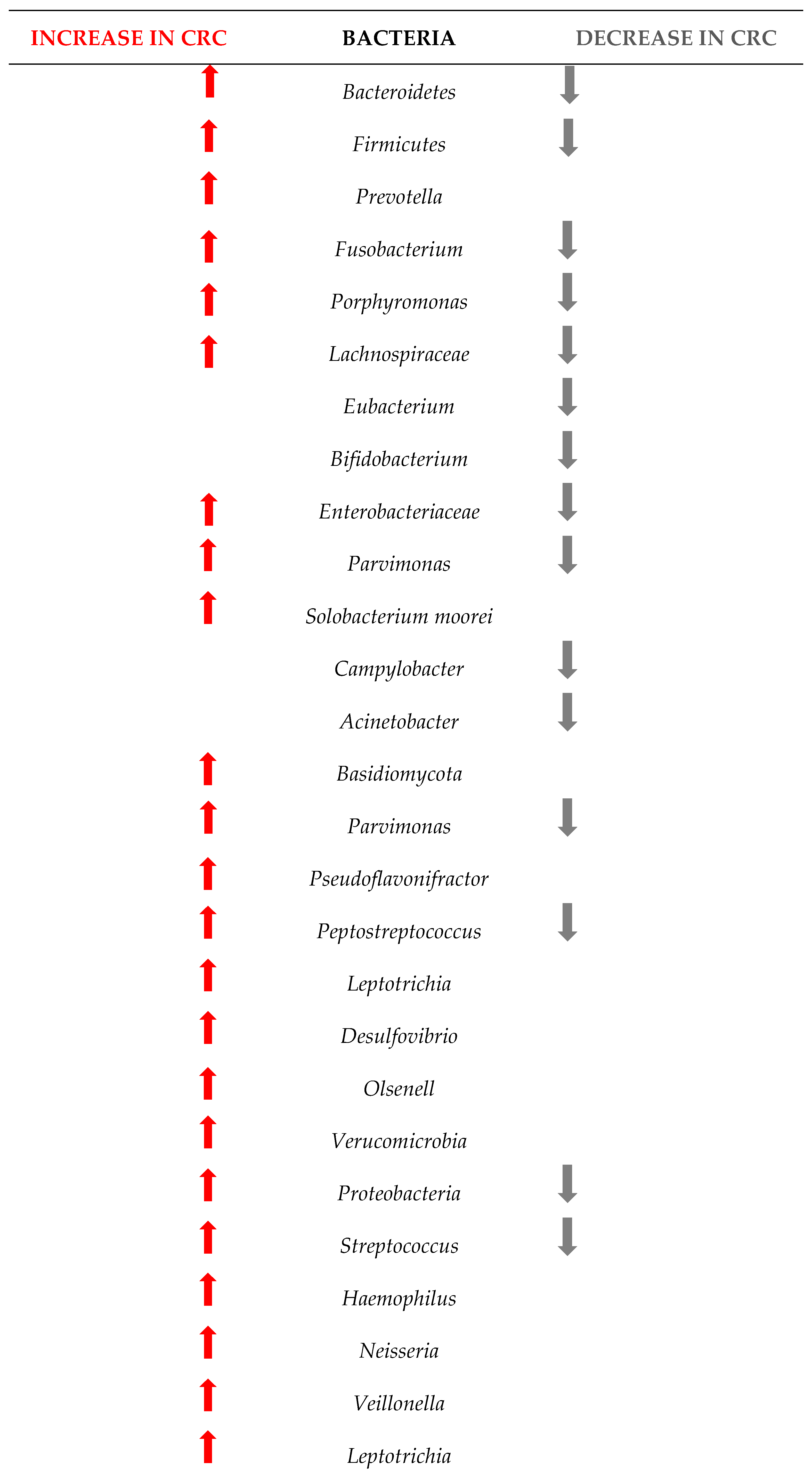
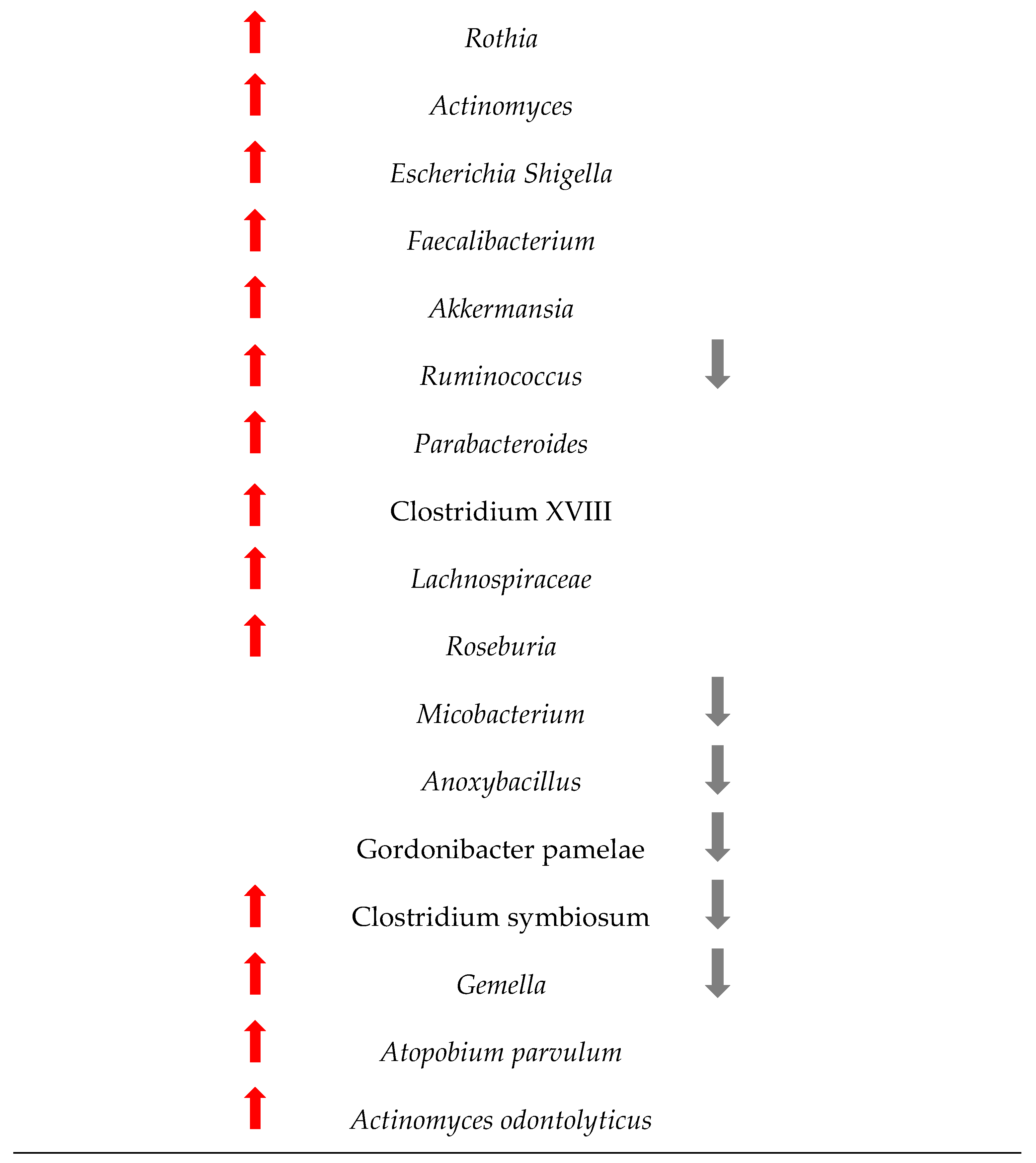
Figure 4 shows the changes in the microbiome among the 1363 patients from the 12 studies included in this research. Understanding these changes in bacterial populations has significant implications for CRC diagnosis, prevention, and treatment.
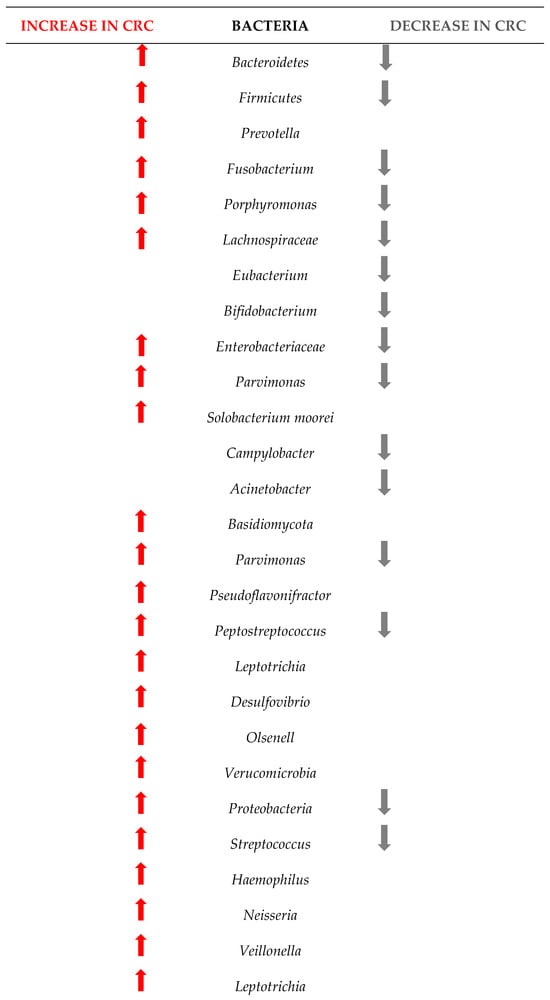
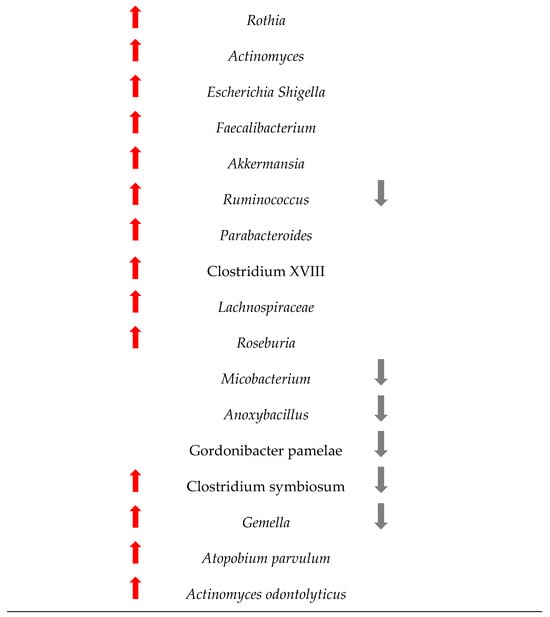
Figure 4.
Changes in the bacteria populations among patients with CRC. The red arrow reflets an increase in the bacteria, and the grey arrow a decrease.
Our research has pinpointed several bacterial species whose presence or abundance in the gut microbiota can be indicative of early-stage CRC.
The comparison between the two sets of taxa underscores a significant shift in the gut microbiome composition in CRC patients. The increased presence of certain bacteria associated with inflammation, infection, and dysbiosis, coupled with the decreased presence of beneficial bacteria, highlights the complex interplay between the microbiome and colorectal cancer. This information is critical for developing microbiome-targeted therapies and preventive strategies, and targeting specific harmful taxa, it may be possible to mitigate the risk or progression of CRC.
3.2. Potential Biomarkers for the CRC
The gut microbiota has garnered significant attention as a potential biomarker for colorectal cancer with various bacterial species demonstrating strong associations with the disease. Notably, certain bacterial populations increase in abundance in CRC patients, making them prime candidates for early detection biomarkers. For instance, Yu et al. [32], Thomas et al. [40] and Yachida et al. [41] identified Fusobacterium nucleatum consistently in higher concentrations in CRC. This bacterium is known to promote inflammation and tumorigenesis, contributing to the carcinogenic environment in the colon [42]. Similarly, Bacteroides fragilis, particularly the enterotoxigenic strains, produce toxins that induce colonic inflammation and have been linked to carcinogenesis [43]. Flemer et al. [30], Kordahi et al. [36], Sheng et al. [38], Geng et al. [39] showed an increase in this bacteria in CRC patients.
Peptostreptococcus anaerobius was increased in CRC patients, as shown by Yu et al. [32] and Zeller et al. [33]. Yu et al. [32] further confirmed the same patterns in Parvimonas micra and Solobacterium moorei, both showing a higher abundance.
These studies underscore the potential of these bacterial species not only as biomarkers for CRC detection but also as targets for therapeutic interventions aimed at modulating the gut microbiota to prevent or manage colorectal cancer.
4. Discussion
This extensive metagenomic dataset examined in this study enabled us to analyze the gut microbial virulence believed to contribute to the development of colorectal cancer.
The link between gut dysbiosis and colorectal cancer involves several complex mechanisms that influence CRC initiation, promotion, and progression [44].
Dysbiosis can lead to chronic inflammation in the gut mucosa, which is characterized by increased levels of pro-inflammatory cytokines and immune cell infiltration [45]. Chronic inflammation creates a microenvironment conducive to carcinogenesis by promoting cell proliferation, inhibiting apoptosis, and stimulating angiogenesis [23,27]. Moreover, dysbiosis-induced inflammation can contribute to the production of reactive oxygen and nitrogen species, causing DNA damage and genomic instability, which are early events in CRC development [46,47].
Our study strengthens the fact that microbial metabolism in the gut plays a critical role in human health and disease, including colorectal carcinogenesis [48]. The gut microbiota metabolizes various dietary components and produces a wide array of metabolites, including short-chain fatty acids (SCFAs) and secondary bile acids, which can impact colorectal carcinogenesis through diverse mechanisms [49,50]. SCFAs, such as acetate, propionate, and butyrate, are produced by the fermentation of dietary fibres and resistant starches by gut bacteria, particularly Firmicutes and Bacteroidetes [51]. Butyrate, in particular, serves as a major energy source for colonic epithelial cells and plays a crucial role in maintaining gut barrier function and modulating immune responses [52]. SCFAs have anti-inflammatory properties and can inhibit the growth of cancer cells by inducing apoptosis, promoting cell cycle arrest, and suppressing tumor cell proliferation [53]. Additionally, SCFAs can regulate gene expression patterns in colonic epithelial cells, modulating various signaling pathways involved in cell growth, differentiation, and apoptosis [51].
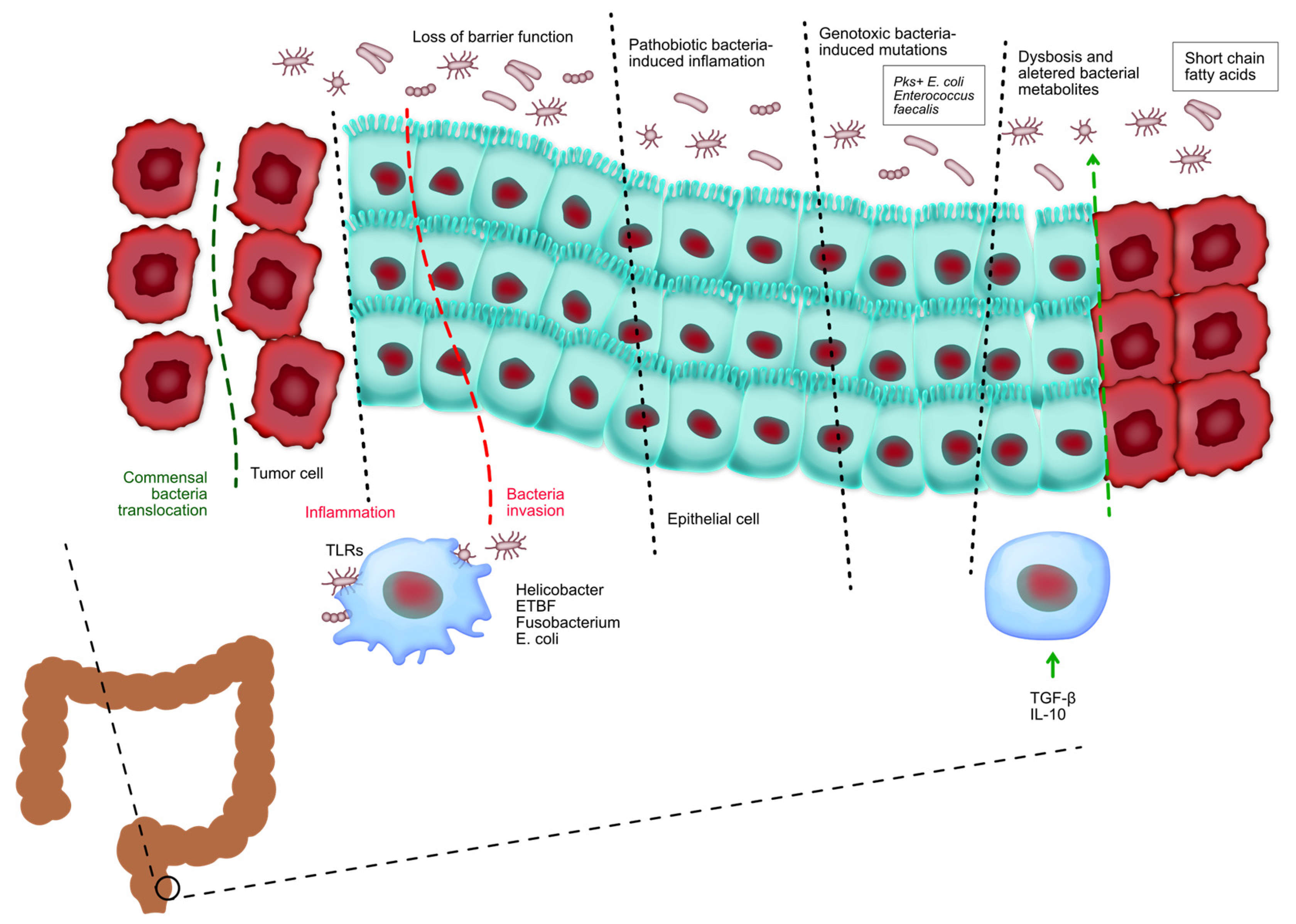
Bile acids are synthesized in the liver from cholesterol and secreted into the small intestine, where they aid in the digestion and absorption of dietary fats. Primary bile acids are subsequently metabolized by gut bacteria into secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA) [54]. Figure 5 shows the relationship between microbiota and colorectal cancer. It can be seen that the gut microbiota has a crucial role in controlling the onset and advancement of colorectal cancer. The loss of the protective function on the surface of colorectal tumors allows the entry of beneficial microorganisms and their by-products, which subsequently trigger activation.
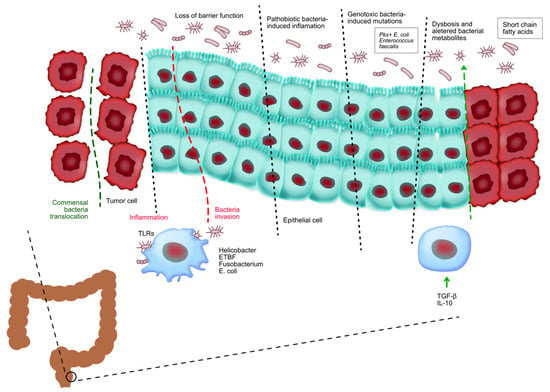
Figure 5.
Tumor-associated myeloid cells are stimulated by the presence of tumors and contribute to the promotion of inflammation that supports tumor growth. Pathogenic bacteria infiltrate healthy colorectal tissues and facilitate the initiation of inflammation and cancer. Bacteria harboring genotoxic markers facilitate the accumulation of genetic abnormalities in the cells lining the intestines, hence initiating the development of CRC. Chronic inflammation in inflammatory bowel disease and CRC changes the makeup of beneficial bacteria and leads to an imbalance known as dysbiosis, which in turn worsens tissue inflammation. The development of colorectal cancer is regulated by food metabolites produced by commensal bacteria. These metabolites affect the growth and survival of tumor cells as well as the ability of immune cells to eliminate tumors.
The results of this study are in line with those of other researchers. For example, Wu et al. showed that at the phylum level, the gut microbiota in healthy controls, adenomas, and CRC was mostly composed of Firmicutes and Bacteroidetes, with Proteobacteria, Actinobacteria, Verrucomicrobia, Tenericutes, and Fusobacteria also present [55]. Figure 4 confirms some of these results, as our meta-analysis proved that Firmicutes, Bacteroidetes and Verrucomicrobia are indeed increasing, but Proteobacteria and Fusobacteria can also decrease in some patients with CRC, while Acinetobacteria mostly decreases. Wirbel et al. [56] also concluded that colorectal carcinogenesis is influenced by Fusobacterium nucleatum, Bacteroides fragilis, some Escherichia coli strains, and intestinal Clostridium spp. Our results show similar results, as we found evidence that the above phyla increases in abundance but also decreases in some patients.
One of the most well-documented bacterial species associated with CRC is Fusobacterium nucleatum. Research indicates that this bacteria can adhere to and invade colonic epithelial cells, facilitating a pro-inflammatory environment that supports cancer development. For instance, a study by Ou et al. [57] detailed how this bacterium interacts with the immune system, promoting a local inflammatory response that can lead to tumor progression. Moreover, Escherichia coli strains, particularly those producing colibactin, are implicated in CRC due to their genotoxic effects, which can induce DNA damage and contribute to carcinogenesis [58]. Studies have shown that Escherichia coli can adhere to the intestinal mucosa and release toxins that disrupt cellular processes, further linking these bacteria to CRC [59,60].
Secondary bile acids can disrupt the intestinal epithelial barrier and promote inflammation, further contributing to colorectal carcinogenesis [61]. In addition to SCFAs and secondary bile acids, gut microbiota produce a wide range of other metabolites that can impact colorectal carcinogenesis, including indole derivatives, polyamines, and trimethylamine N-oxide (TMAO) [62]. Indole derivatives, such as indole-3-propionic acid (IPA), have been shown to exhibit anti-inflammatory and anti-tumorigenic effects in the colon by modulating immune responses and inhibiting cell proliferation [63]. Polyamines, such as putrescine and spermidine, are involved in cell growth and proliferation and may contribute to CRC progression when dysregulated [64].
Studies investigating the diagnostic potential of gut microbiota analysis for colorectal cancer have gained significant attention in recent years due to the potential for non-invasive and accurate screening methods.
In our study, we show that Zeller et al. [33] identified microbial signatures associated with CRC and developed a diagnostic model based on microbial biomarkers. The model showed high sensitivity and specificity for detecting CRC, suggesting the potential of gut microbiota analysis for non-invasive CRC screening [33].
In another study, Yu et al. [32] investigated the fecal microbiota composition of CRC patients and healthy controls using 16S rRNA gene sequencing. They identified specific bacterial taxa that were significantly associated with CRC and developed a microbial-based diagnostic classifier. The classifier demonstrated high accuracy in distinguishing CRC patients from healthy individuals, highlighting the potential of gut microbiota analysis for CRC screening. Baxter et al. utilized metagenomic shotgun sequencing to characterize the gut microbiota of CRC patients, adenoma patients, and healthy controls. The researchers identified microbial biomarkers associated with CRC and developed a diagnostic model incorporating these biomarkers. The model accurately differentiated CRC patients from healthy controls and adenoma patients, indicating the potential utility of gut microbiota analysis for CRC detection [32].
Wirbel et al. [65] conducted a large-scale metagenomic analysis of fecal samples from CRC patients and healthy individuals across multiple cohorts. They identified a microbial signature associated with CRC and developed a diagnostic model based on microbial species abundance. The model demonstrated high sensitivity and specificity for CRC detection, suggesting the potential of gut microbiota analysis as a non-invasive screening tool for CRC. Moreover, Zackular et al. [31] investigated the fecal microbiota composition of CRC patients and healthy controls using 16S rRNA gene sequencing. The researchers identified microbial signatures associated with CRC and developed a microbial-based diagnostic test. The test accurately distinguished CRC patients from healthy controls, indicating the potential of gut microbiota analysis for CRC screening [31].
These studies highlight the growing interest in gut microbiota analysis for CRC diagnosis and provide valuable insights into the development of non-invasive screening methods for CRC. Further research and clinical validation are needed to optimize the performance and utility of gut microbiota-based tests in clinical practice.
5. Limitations
Studies included in the literature review may vary in terms of study design, sample size, methodology for gut microbiota analysis, and criteria used to define early- and late-onset CRC. This heterogeneity can make it challenging to draw definitive conclusions and may introduce bias or inconsistencies in the results. Different studies may utilize different techniques for gut microbiota analysis, such as 16S rRNA gene sequencing, metagenomic shotgun sequencing, or functional metagenomics. Variability in methodologies and bioinformatics pipelines can lead to differences in the identification and characterization of microbial taxa, potentially affecting the comparability of results across studies.
Numerous confounding factors, such as diet, lifestyle, medication use, comorbidities, and geographical location, can influence the gut microbiota composition in CRC patients. Failure to adequately account for these confounders in the reviewed studies may limit the ability to attribute observed differences in gut microbiota profiles specifically to age of onset of CRC. Also, studies with statistically significant findings may be more likely to be published than those with null or nonsignificant results, leading to publication bias. This bias can skew the overall interpretation of the literature and may overemphasize certain findings while underrepresenting others.
Despite advances in gut microbiota research, our understanding of the functional roles of specific microbial taxa and their interactions with the host and the tumor microenvironment in CRC is still evolving. This incomplete understanding may limit the interpretation of gut microbiota profiles in the context of CRC pathogenesis.
6. Conclusions
Conclusions drawn from studies comparing gut microbiota profiles in colorectal cancer can provide insights into the potential role of the gut microbiota in CRC pathogenesis and the differences between these two subtypes of the disease. Studies have reported differences in the gut microbiota composition between early- and late-onset CRC patients. While there is some variability among studies, certain microbial taxa have been consistently associated with CRC regardless of age of onset. However, specific differences in microbial composition in CRC may exist, suggesting potential differences in the underlying pathogenic mechanisms.
Factors such as age-related changes in the gut environment, host immune function, and lifestyle factors may influence the composition and activity of the gut microbiota in CRC patients. These age-related factors could contribute to differences in microbial profiles observed in CRC. Gut microbiota profiles in CRC patients have been linked to tumor molecular subtypes, such as microsatellite instability status and CpG island methylator phenotype. Differences in the prevalence of these molecular subtypes between early- and late-onset CRC may influence the gut microbiota composition and contribute to observed differences.
Understanding the differences in gut microbiota profiles of CRC could have clinical implications for disease management and treatment. Gut microbiota-based biomarkers may aid in CRC risk stratification, early detection, and personalized treatment approaches based on age of onset and microbial profiles.
Further research is needed to elucidate the underlying mechanisms driving differences in microbial composition between these two subtypes of CRC and to explore the clinical implications and therapeutic potential of gut microbiota-based interventions for CRC management.
Author Contributions
Conceptualization, L.-F.H., A.H. and R.D.; methodology, A.S., S.I. and R.S.; software, L.-F.H. and L.A.; validation, R.S., A.H. and A.N.-T.; formal analysis, A.H. and L.A.; investigation, L.A.; resources, R.D.; data curation, A.S., L.A. and R.D.; writing—original draft preparation, L.-F.H., S.I., A.N.-T. and L.A.; writing—review and editing, S.I., A.N.-T. and R.D.; visualization, S.I. and R.D.; supervision, L.-F.H., A.S. and A.N.-T.; project administration, A.S., R.S., A.H. and R.D.; funding acquisition, L.-F.H., R.S., A.H. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
The article processing charge was supported by the Victor Babes University of Medicine and Pharmacy Timisoara.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Social determinants of colorectal cancer risk, stage, and survival: A systematic review. Int. J. Colorectal Dis. 2020, 35, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardamean, C.I.; Sudigyo, D.; Budiarto, A.; Mahesworo, B.; Hidayat, A.A.; Baurley, J.W.; Pardamean, B. Changing Colorectal Cancer Trends in Asians: Epidemiology and Risk Factors. Oncol. Rev. 2023, 17, 10576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maki, G.; Zervos, M. Health Care-Acquired Infections in Low- and Middle-Income Countries and the Role of Infection Prevention and Control. Infect. Dis. Clin. N. Am. 2021, 35, 827–839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toma, M.; Beluşică, L.; Stavarachi, M.; Apostol, P.; Spandole, S.; Radu, I.; Cimponeriu, D. Rating the environmental and genetic risk factors for colorectal cancer. J. Med. Life 2012, 5, 152–159. [Google Scholar] [PubMed] [PubMed Central]
- Tocia, C.; Dumitru, A.; Mateescu, B.; Negreanu, L.; State, M.; Cozaru, G.C.; Mitroi, A.F.; Brinzan, C.; Popescu, R.; Leopa, N.; et al. Tissue and Circulating MicroRNA-31, MicroRNA-200b, and MicroRNA-200c Reflects Disease Activity in Crohn’s Disease Patients: Results from the BIOMIR Study. J. Gastrointest. Liver Dis. 2023, 32, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Durko, L.; Malecka-Panas, E. Lifestyle Modifications and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mindrescu, N.M.; Guja, C.; Jinga, V.; Ispas, S.; Curici, A.; Nelson Twakor, A.; Pantea Stoian, A.M. Interactions between Gut Microbiota and Oral Antihyperglycemic Drugs: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicolae, M.; Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Frecus, C.E.; Mihai, L.; Lupu, V.V.; Ion, I.; Pantazi, A.C.; Nelson Twakor, A.; et al. Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients 2023, 15, 3430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minhajat, R.; Harjianti, T.; Rasyid, H.; Bukhari, A.; Chaidir Islam, I.; Zainal, A.T.F.; Khaliq Gunawan, A.M.A.; Ramadhan, A.C.; Hatta, H.; Syamsu Alam, N.I.; et al. Colorectal cancer patients’ outcome in correlation with dietary and nutritional status: A systematic review. Ann. Med. 2023, 55, 2281662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhimani, N.; Wong, G.Y.M.; Molloy, C.; Pavlakis, N.; Diakos, C.I.; Clarke, S.J.; Dieng, M.; Hugh, T.J. Cost of treating metastatic colorectal cancer: A systematic review. Public Health 2022, 211, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Syvyk, S.; Roberts, S.E.; Finn, C.B.; Wirtalla, C.; Kelz, R. Colorectal cancer disparities across the continuum of cancer care: A systematic review and meta-analysis. Am. J. Surg. 2022, 224, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Kumar, V. Epigenetic Biomarkers in Colorectal Cancer. Mol. Diagn. Ther. 2017, 21, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ispas, S.; Tuta, L.A.; Botnarciuc, M.; Ispas, V.; Staicovici, S.; Ali, S.; Nelson-Twakor, A.; Cojocaru, C.; Herlo, A.; Petcu, A. Metabolic Disorders, the Microbiome as an Endocrine Organ, and Their Relations with Obesity: A Literature Review. J. Pers. Med. 2023, 13, 1602. [Google Scholar] [CrossRef]
- Feng, Y.L.; Shu, L.; Zheng, P.F.; Zhang, X.Y.; Si, C.J.; Yu, X.L.; Gao, W.; Zhang, L. Dietary patterns and colorectal cancer risk: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Mountjoy, L.J.; Firwana, B.; Liu, A.J.; Almader-Douglas, D.; Mody, K.; Hubbard, J.; Borad, M.; Ahn, D.H.; Murad, M.H.; et al. The Role of Maintenance Strategies in Metastatic Colorectal Cancer: A Systematic Review and Network Meta-analysis of Randomized Clinical Trials. JAMA Oncol. 2020, 6, e194489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tocia, C.; Alexandrescu, L.; Dumitru, E. Sa1892—Nutritional Status Correlates with Quality of Life in Active Crohn’s Disease. Gastroenterology 2019, 156, S-444. [Google Scholar] [CrossRef]
- Puli, A.V.; Lussiez, A.; MacEachern, M.; Hayward, L.; Dualeh, S.; Richburg, C.E.; Capellari, E.; Kwakye, G. Barriers to Colorectal Cancer Screening in US Immigrants: A Scoping Review. J. Surg. Res. 2023, 282, 53–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bopanna, S.; Ananthakrishnan, A.N.; Kedia, S.; Yajnik, V.; Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 269–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tocia, C.; Iordache, M.M.; Manea, M.; Cozaru, G.C.; Chisoi, A.; Dumitru, A.; Rafti, R.; Cornea, V.; Dina, E.; Alexandrescu, L.; et al. Sa1623: THE LINK BETWEEN PSYCHOLOGICAL STATE AND INTESTINAL PERMEABILITY IN PATIENTS WITH IBD IN REMISSION. Gastroenterology 2022, 162, S-443–S-444. [Google Scholar] [CrossRef]
- Christodoulides, N.; Lami, M.; Malietzis, G.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. Sporadic colorectal cancer in adolescents and young adults: A scoping review of a growing healthcare concern. Int. J. Colorectal Dis. 2020, 35, 1413–1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nenkov, M.; Ma, Y.; Gaßler, N.; Chen, Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2021, 22, 6262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winawer, S.J.; Krabshuis, J.; Lambert, R.; O’Brien, M.; Fried, M.; World Gastroenterology Organization Guidelines Committee. Cascade colorectal cancer screening guidelines: A global conceptual model. J. Clin. Gastroenterol. 2011, 45, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O.′Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Rogers, M.A.; Ruffin, M.T.; Schloss, P.D., 4th. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Böhm, J.; Brunetti, F.; Habermann, N.; et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, X.; Wang, Y.; Xu, J.; Cao, H.; Zhang, F.; Wang, X. Gut microbiota signatures in tissues of the colorectal polyp and normal colorectal mucosa, and faeces. Front. Cell. Infect. Microbiol. 2023, 12, 1054808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Cao, H.; Fei, B.; Gao, Q.; Yi, W.; Han, W.; Bao, C.; Xu, J.; Zhao, W.; Zhang, F. Gut Microbiota Signatures in Tumor, Para-Cancerous, Normal Mucosa, and Feces in Colorectal Cancer Patients. Front. Cell Dev. Biol. 2022, 10, 916961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kordahi, M.C.; Stanaway, I.B.; Avril, M.; Chac, D.; Blanc, M.P.; Ross, B.; Diener, C.; Jain, S.; McCleary, P.; Parker, A.; et al. Genomic and functional characterization of a mucosal symbiont involved in early-stage colorectal cancer. Cell Host Microbe 2021, 29, 1589–1598.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, Q.S.; He, K.X.; Li, J.J.; Zhong, Z.F.; Wang, F.X.; Pan, L.L.; Lin, J.J. Comparison of Gut Microbiome in Human Colorectal Cancer in Paired Tumor and Adjacent Normal Tissues. OncoTargets Ther. 2020, 13, 635–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geng, J.; Fan, H.; Tang, X.; Zhai, H.; Zhang, Z. Diversified pattern of the human colorectal cancer microbiome. Gut Pathog. 2013, 5, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678, Erratum in Nat. Med. 2019, 25, 1948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nouri, R.; Hasani, A.; Masnadi Shirazi, K.; Alivand, M.R.; Sepehri, B.; Sotoudeh, S.; Hemmati, F.; Fattahzadeh, A.; Abdinia, B.; Ahangarzadeh Rezaee, M. Mucosa-Associated Escherichia coli in Colorectal Cancer Patients and Control Subjects: Variations in the Prevalence and Attributing Features. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 2131787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. 2020, 27, 9–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coleman, O.; Haller, D. Dysbiosis of the Intestinal Microbiota and Colorectal Cancer; Elsevier eBooks; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Z.; Wei, J.; Zhao, L. Effects of gut microbiota on immune responses and immunotherapy in colorectal cancer. Front. Immunol. 2022, 13, 1030745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tocia, C.; Dumitru, I.M.; Alexandrescu, L.; Petcu, L.C.; Dumitru, E. Does rifaximin offer any promise in Crohn’s disease in remission and concurrent irritable bowel syndrome-like symptoms? Medicine 2021, 100, e24059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobiela, J.; Spychalski, P.; Marvaso, G.; Ciardo, D.; Dell′Acqua, V.; Kraja, F.; Błażyńska-Spychalska, A.; Łachiński, A.J.; Surgo, A.; Glynne-Jones, R.; et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: Systematic review. Crit. Rev. Oncol. Hematol. 2018, 129, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B. Ethical issues with colorectal cancer screening-a systematic review. J. Eval. Clin. Pract. 2017, 23, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Santarpia, L.; Rispo, A.; Marra, M.; Testa, A.; Contaldo, F.; Castiglione, F.; Pasanisi, F. Association between Health-Related Quality of Life and Nutritional Status in Adult Patients with Crohn’s Disease. Nutrients 2020, 12, 746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; He, M.; Zhang, M.; Sun, Q.; Zeng, S.; Chen, L.; Yang, H.; Liu, M.; Ren, S.; Meng, X.; et al. Colorectal Cancer, Gut Microbiota and Traditional Chinese Medicine: A Systematic Review. Am. J. Chin. Med. 2021, 49, 805–828. [Google Scholar] [CrossRef] [PubMed]
- Jaspan, V.; Lin, K.; Popov, V. The impact of anthropometric parameters on colorectal cancer prognosis: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 159, 103232. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, E.; Alexandrescu, L.; Suceveanu, A.I.; Dumitru, I.M.; Tofolean, I.T. M1255 Fecal Calprotectin in Diagnosis of Complicated Colonic Diverticular Disease. Gastroenterology 2010, 138, S365. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Jiao, N.; Zhu, R.; Zhang, Y.; Wu, D.; Wang, A.J.; Fang, S.; Tao, L.; Li, Y.; Cheng, S.; et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat. Commun. 2021, 12, 3063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Xu, P.; Zhu, R.; Gao, W.; Yin, W.; Lan, P.; Zhu, L.; Jiao, N. Multi-kingdom microbial signatures in excess body weight colorectal cancer based on global metagenomic analysis. Commun. Biol. 2024, 7, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell. Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Fu, K. Genotoxins: The Mechanistic Links between Escherichia coli and Colorectal Cancer. Cancers 2023, 15, 1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadeghi, M.; Mestivier, D.; Sobhani, I. Contribution of pks+ Escherichia coli (E. coli) to Colon Carcinogenesis. Microorganisms 2024, 12, 1111. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duizer, C.; de Zoete, M.R. The Role of Microbiota-Derived Metabolites in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 8024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismael, S.; Rodrigues, C.; Santos, G.M.; Castela, I.; Barreiros-Mota, I.; Almeida, M.J.; Calhau, C.; Faria, A.; Araújo, J.R. IPA and its precursors differently modulate the proliferation, differentiation, and integrity of intestinal epithelial cells. Nutr. Res. Pract. 2023, 17, 616–630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snezhkina, A.V.; Krasnov, G.S.; Lipatova, A.V.; Sadritdinova, A.F.; Kardymon, O.L.; Fedorova, M.S.; Melnikova, N.V.; Stepanov, O.A.; Zaretsky, A.R.; Kaprin, A.D.; et al. The Dysregulation of Polyamine Metabolism in Colorectal Cancer Is Associated with Overexpression of c-Myc and C/EBPβ rather than Enterotoxigenic Bacteroides fragilis Infection. Oxid. Med. Cell Longev. 2016, 2016, 2353560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).