Abstract
A growing number of studies indicate that mitochondrial dysfunction serves as a pathological mechanism for periodontitis. Therefore, this two-sample Mendelian randomization (MR) study was carried out to explore the causal associations between mitochondrial biological function and periodontitis, because the specific nature of this causal relationship remains inconclusive in existing MR studies. Inverse variance weighting, Mendelian randomization-Egger, weighted mode, simple mode, and weighted median analyses were performed to assess the causal relationships between the exposure factors and periodontitis. The results of the present study revealed a causal association between periodontitis and medium-chain specific acyl-CoA dehydrogenase (MCAD), malonyl-CoA decarboxylase (MLYCD), glutaredoxin 2 (Grx2), oligoribonuclease (ORN), and pyruvate carboxylase (PC). Notably, MCAD and MLYCD are causally linked to periodontitis, and serve as protective factors. However, Grx2, ORN, and PC function as risk factors for periodontitis. Our study established a causal relationship between mitochondrial biological function and periodontitis, and such insights may provide a promising approach for treating periodontitis via mitochondrial regulation.
1. Introduction
Periodontitis is a prevalent infectious disease characterized by biofilm-induced and host mediated inflammation that progressively leads to the destruction of connective tissues, tooth loss and, eventually, jawbone resorption and edentulism. It represents the sixth most prevalent disease worldwide and affects up to 90% of the global population [1,2,3]. Additionally, periodontitis is epidemiologically related to various chronic disorders, including cardiovascular disease, Alzheimer’s disease, type 2 diabetes mellitus, nonalcoholic fatty liver disease, inflammatory bowel disease, rheumatoid arthritis, and certain cancers, which seriously affect the quality of life and account for a vast burden of healthcare cost [4,5]. Thus, the prevention and treatment of periodontitis are difficult problems that society as a whole needs to address [6].
At present, common clinical periodontal treatment strategies, including supragingival scaling, subgingival scaling and root planing, bone transplantation, and guided tissue regeneration, are mainly focused on controlling inflammation and terminating periodontal tissue damage [7]. Although these methods, to a certain degree, can control the clinical progression of periodontitis, reconstruction of the damaged periodontal tissues has remained the ultimate goal of periodontal treatment, and it is also a difficult task in clinic. In this context, further research into the etiology of periodontitis and its progression mechanisms, the identification of novel therapeutic targets, and the design of efficacious treatment measures are of great significance.
Mitochondria are the primary site for the synthesis of adenosine triphosphate (ATP), the main energy source for physiological and biochemical processes. Accumulating research has indicated the vital role of mitochondrial dysfunction during the initiation and progression of periodontitis; more specifically, the excessive production of reactive oxygen species (ROS), alterations in mitochondrial biogenesis and dynamics, mitophagy and mitochondrial DNA damage, can strongly affect the development and progression of periodontitis [8,9]. Mitochondria are the main site for ROS production, and studies have demonstrated that the excessive generation of ROS causes abnormalities in the mitochondrial electron transfer chain and much more ROS release; consequently, excessive ROS can cause oxidative stress, resulting in various disorders, including periodontal tissue damage [10,11,12]. A previous study demonstrated that a hybrid of indole and dithiocarbamate protected against periodontitis by restoring osteoclast mitochondrial function, in terms of mitochondrial ROS, mitochondrial membrane potential, and ATP production [13]. In addition, recent studies have verified that mitochondrial dysfunction might be a vital driver of the reciprocal comorbidity between periodontitis and type 2 diabetes mellitus [14]. Despite continuous research, a comprehensive theoretical framework illustrating the causal link between mitochondrial biological function and periodontitis has yet to be established.
Mendelian randomization (MR) studies are an epidemiological approach that leverages genome-wide association study (GWAS) data and employs genetic variations as instrumental variables (IVs) to assess causal relationships between exposures and outcomes. MR can mitigate the effect of confounding factors and address reverse causation and inference, and its basis on large sample studies facilitates more robust results [15,16]. Hence, the present study was performed, employing MR to assess whether a causal link exists between mitochondrial biological function and periodontitis, with the aim of providing valuable insights into the treatment of periodontitis by regulating mitochondrial function.
2. Results
2.1. Dataset Screening
Five SNP datasets were strongly associated with periodontitis: ebi-a-GCST90019404 (medium-chain specific acyl-CoA dehydrogenase (MCAD); mitochondrial measurement) [17], prot-a-1907(malonyl-CoA decarboxylase (MLYCD), mitochondrial measurement), prot-a-1220 (glutaredoxin-2 (Grx2); mitochondrial measurement), prot-a-2526 (oligoribonuclease, (ORN); mitochondrial measurement) and prot-a-2190 (pyruvate carboxylase (PC); mitochondrial measurement) [18]. In addition, mitochondrial DNA copy number (mtDNA-CN) was demonstrated to reflect the ratio of mitochondrial to nuclear DNA copy number and can function as a rough substitute to reflect mitochondrial biological function [19,20]. MR analysis of mtDNA-CN and periodontitis was also performed (ebi-a-GCST90026372, mtDNA-CN, mitochondrial measurement), although there was no statistically significant difference [21].
2.2. Selection of Instrumental Variables
The “TwoSampleMR” package was used to explore SNPs showing robust relationship between mitochondrial biological function and periodontitis. Specifically, “ebi-a-GCST90019404” exhibited 18 SNPs with robust periodontitis associations, “prot-a-1907” featured 9 SNPs exhibiting robust associations with periodontitis, “prot-a-1220” presented 13 SNPs showing robust associations with periodontitis, 11 SNPs in “prot-a-2526” displayed strongly associations with periodontitis, and 9 SNPs within “prot-a-2190” demonstrated robust associations with periodontitis. For mtDNA-CN, 101 independent periodontitis-related SNPs were screened from the ebi-a-GCST90026372 database (Table 1).

Table 1.
Mitochondrial dataset tool variation scale.
2.3. Results of MR Analysis
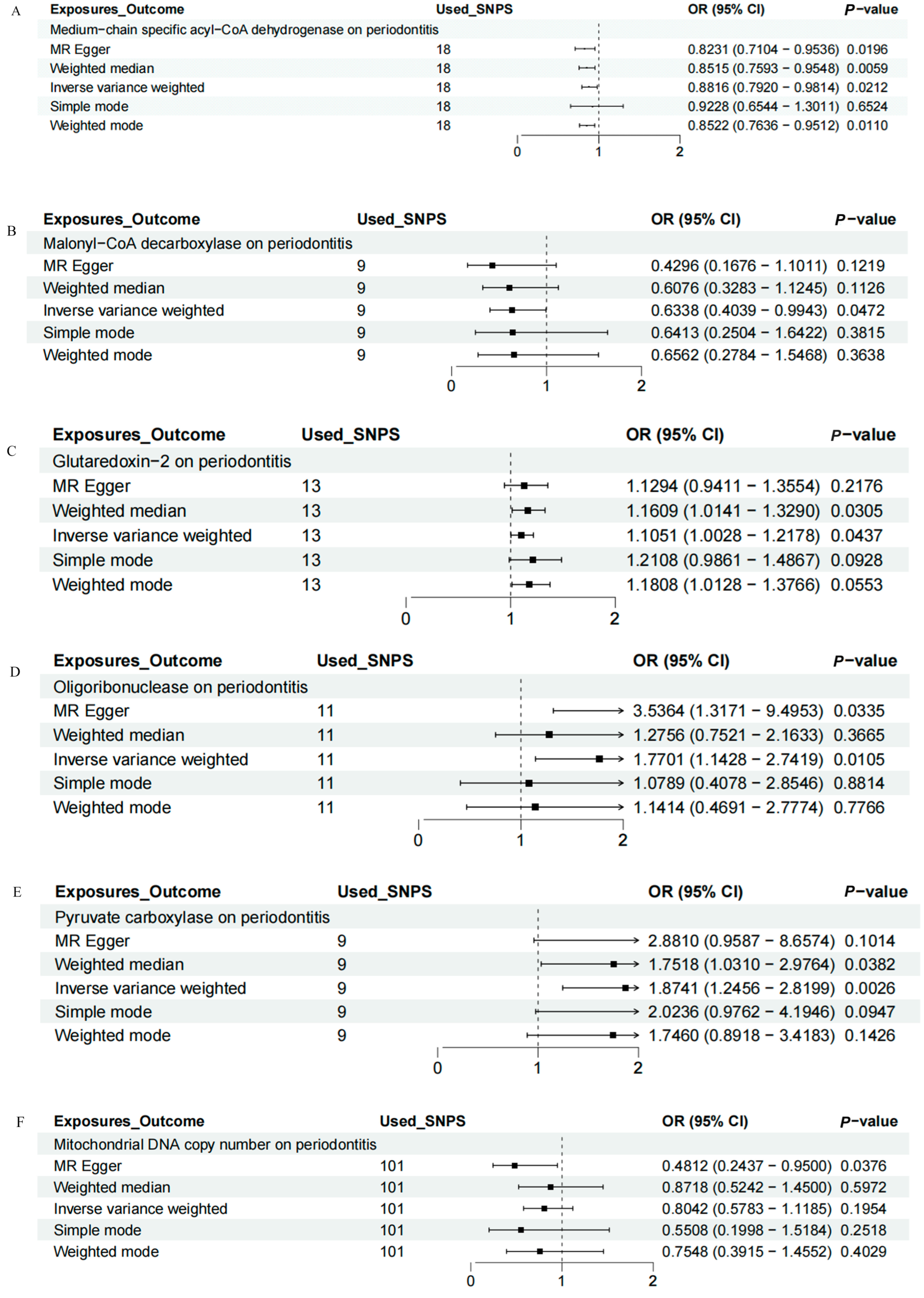
IVW, MR-Egger, weighted median, weighted mode, and simple mode analyses were performed to evaluate the causal relationship between mitochondrial function and periodontitis. The MR results were conducted and visualized via the “TwoSampleMR” package in R software (version 4.3.1), as shown in the scatter plots (Figure 1) and forest plots (Figure 2). Based on the IVW results, MCAD (OR = 0.8816, 95% CI = 0.7920–0.9814, p = 0.0212) and MLYCD (OR = 0.6338, 95% CI = 0.4039–0.9943, p = 0.0472) significantly reduced the risk of periodontitis, acting as protective factors. Grx2 (OR = 1.1051, 95% CI = 1.0028–1.2178, p = 0.0437), ORN (OR = 1.7701, 95% CI = 1.1428–2.7419, p = 0.0105) and PC (OR = 1.8741, 95% CI = 1.2456–2.8199, p = 0.0026) functioned as risk factors for periodontitis, increasing its incidence. There was no causal relationship between mtDNA-CN and periodontitis (OR = 0.8042, 95% CI = 0.5783–1.1185, p = 0.1954).
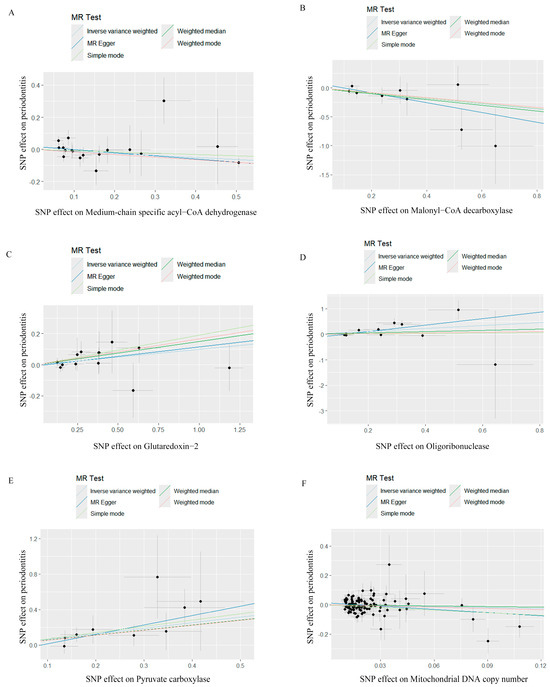
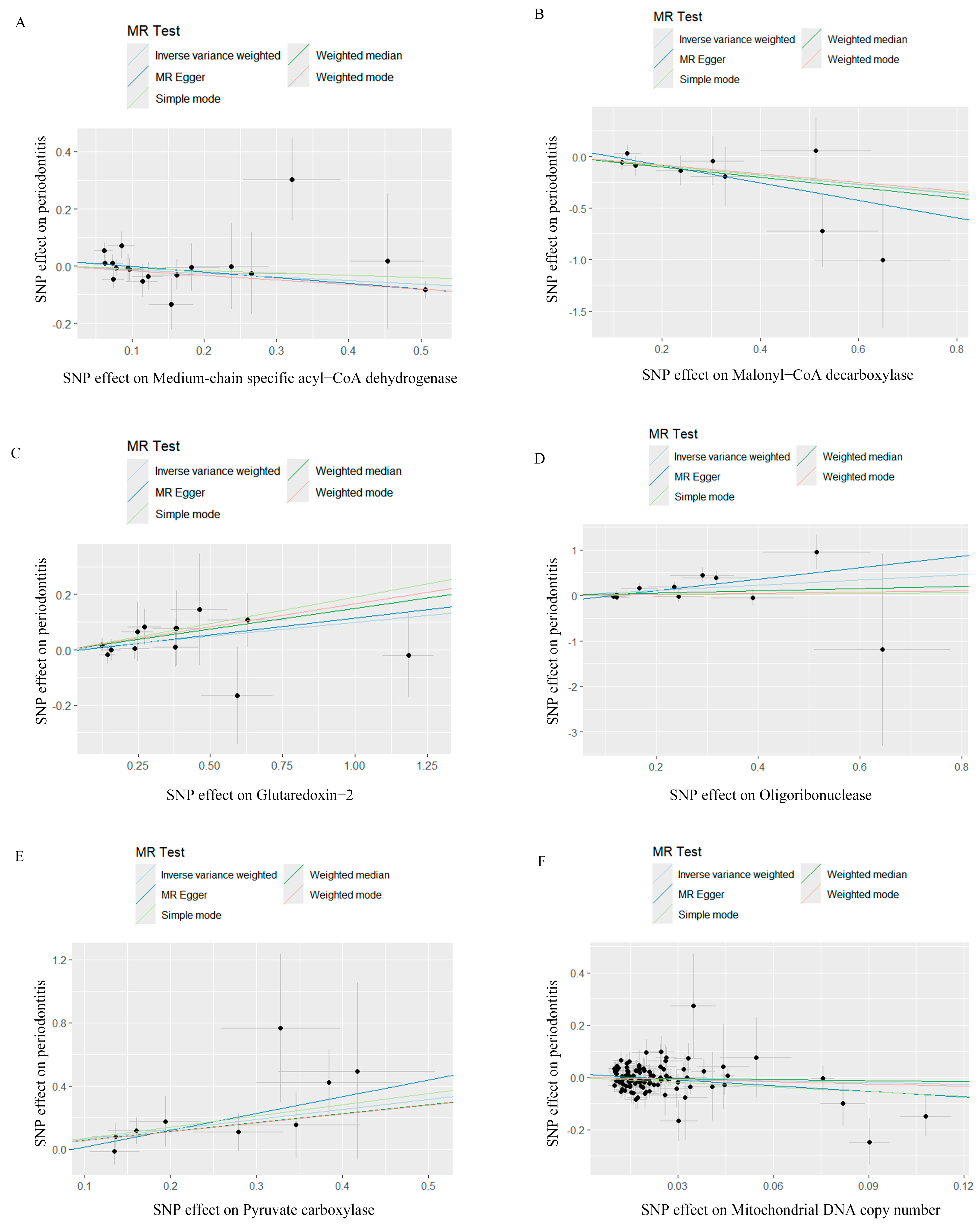
Figure 1.
Scatter plots for Mendelian randomization analyses of the causal effect of mitochondrial biological function on periodontitis. (A) Scatter plot of the causal relationship between medium−chain specific acyl−CoA dehydrogenase and periodontitis, evaluated by the IVW method. (B) Scatter plot of the causal relationship between malonyl−CoA decarboxylase and periodontitis, primarily evaluated via the IVW method. (C) Scatter plot of the causal relationship between glutaredoxin−2, mitochondrial and periodontitis, primarily evaluated using the IVW method. (D) Scatter plot of the causal relationship between oligoribonuclease and periodontitis using the IVW method. (E) Scatter plot of the causal relationship between pyruvate carboxylase and periodontitis, primarily evaluated with the IVW method. (F) Scatter plot of the causal relationship between mitochondrial DNA copy number and periodontitis, evaluated using the IVW method. IVW, inverse variance weighting.
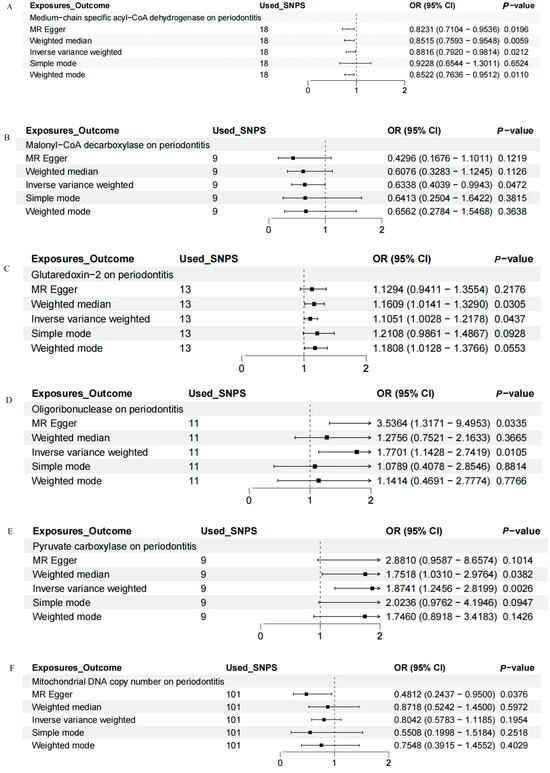
Figure 2.
Forest map of the causal relationship between mitochondria and periodontitis (IVW method). (A) Forest map of the causal relationship between medium−chain specific acyl−CoA dehydrogenase and periodontitis. (B) Forest map of the causal relationship between malonyl−CoA decarboxylase and periodontitis. (C) Forest map of the causal relationship between glutaredoxin − 2 and periodontitis. (D) Forest map of the causal relationship between oligoribonuclease and periodontitis. (E) Forest map of the causal relationship between pyruvate carboxylase and periodontitis. (F) Forest map of the causal relationship between mitochondrial DNA copy number and periodontitis. IVW, inverse variance weighting.
2.4. Sensitivity Analysis
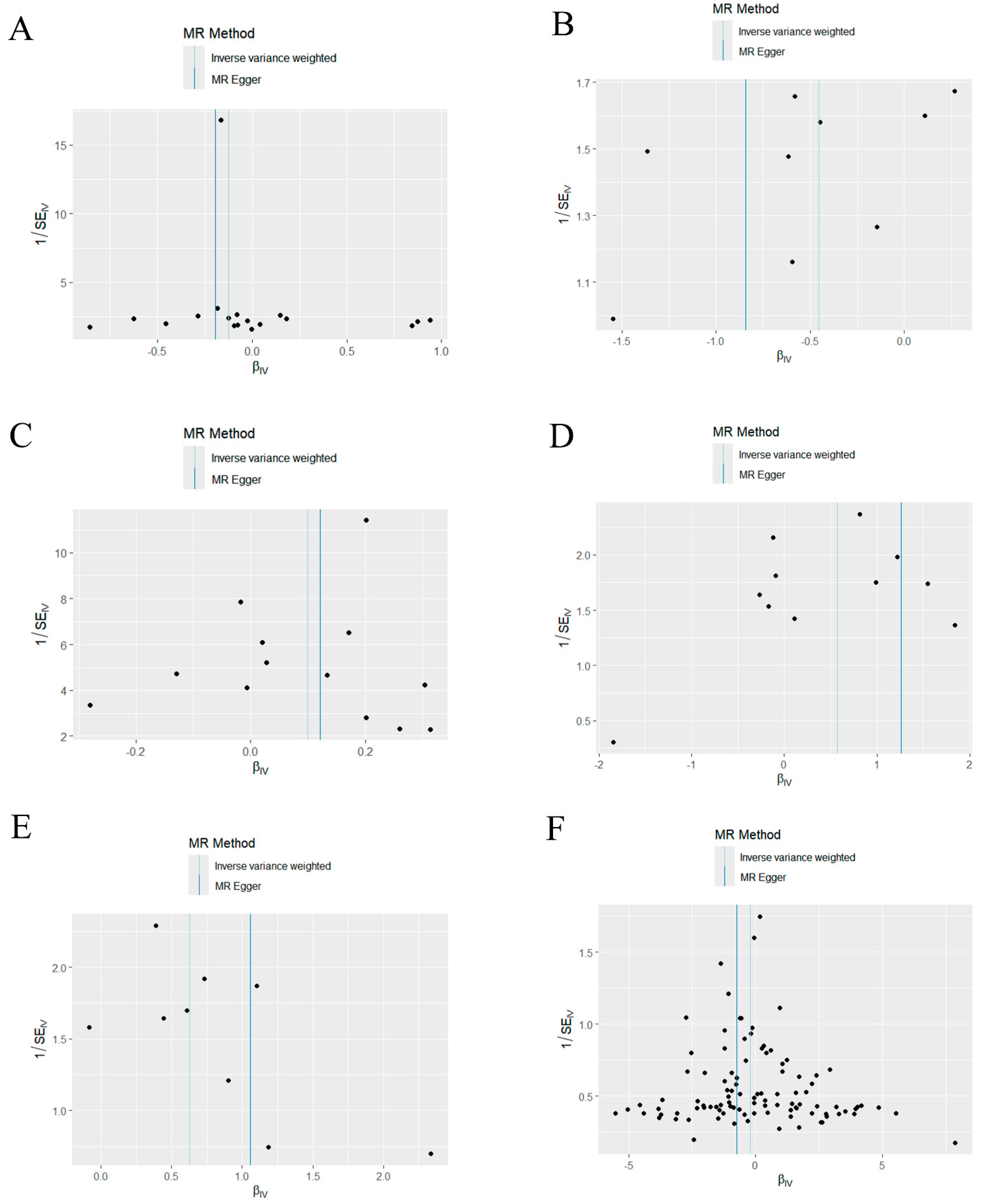
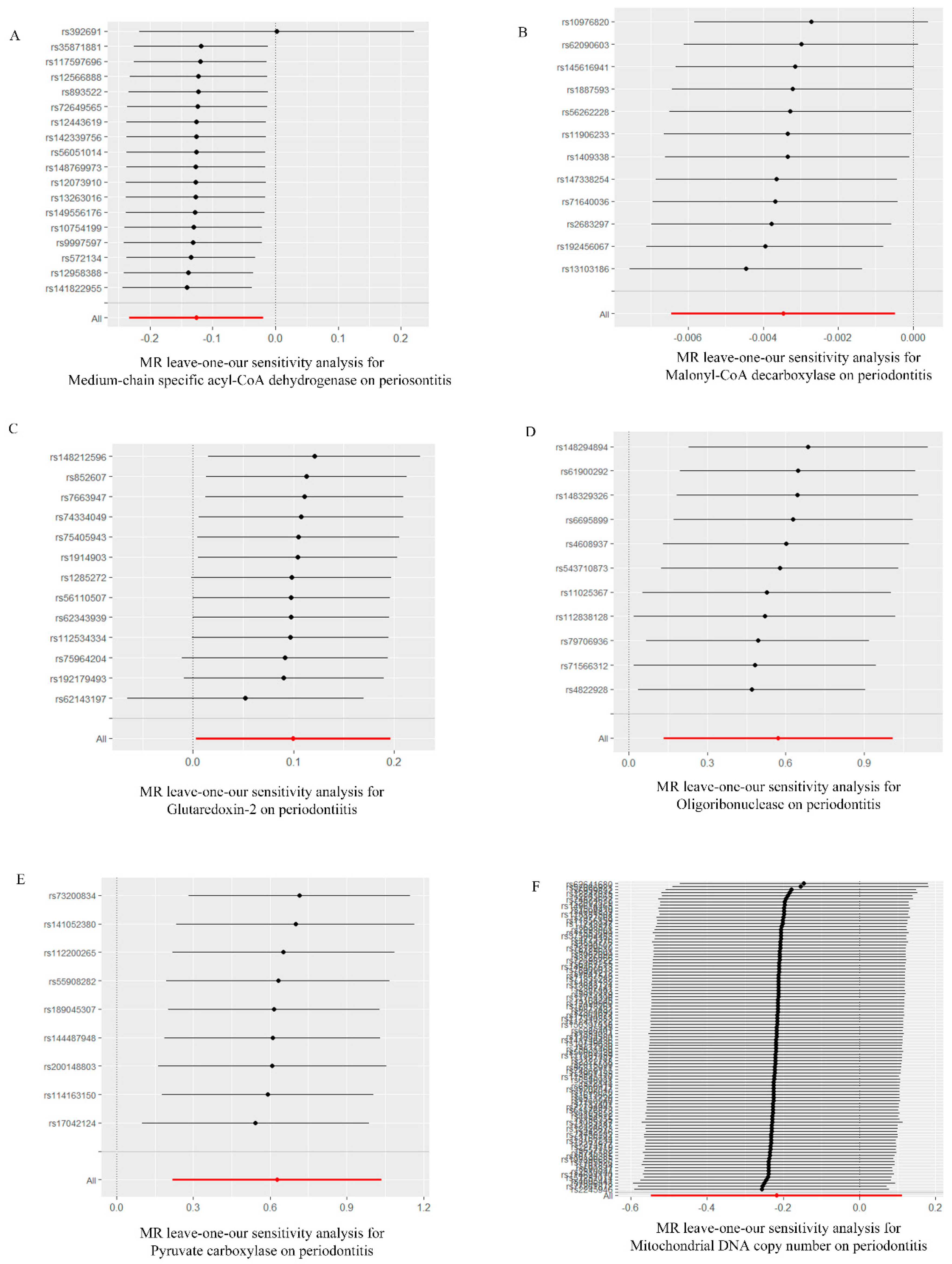
Based on the MR-Egger regression and IVW analysis results, there was no heterogeneity in any of the datasets (p > 0.05). Additionally, Egger regression analysis demonstrated that directional horizontal pleiotropy did not occur, as the results revealed p > 0.05 across all datasets (Table 2). In addition, the funnel plot showed a symmetrical distribution of SNPs, emphasizing the relative stability of the results (Figure 3). Subsequently, a leave-one-out method was applied to assess the effect of individual SNPs, and the results showed no significant effect on the effect sizes (Figure 4).

Table 2.
Heterogeneity analysis.
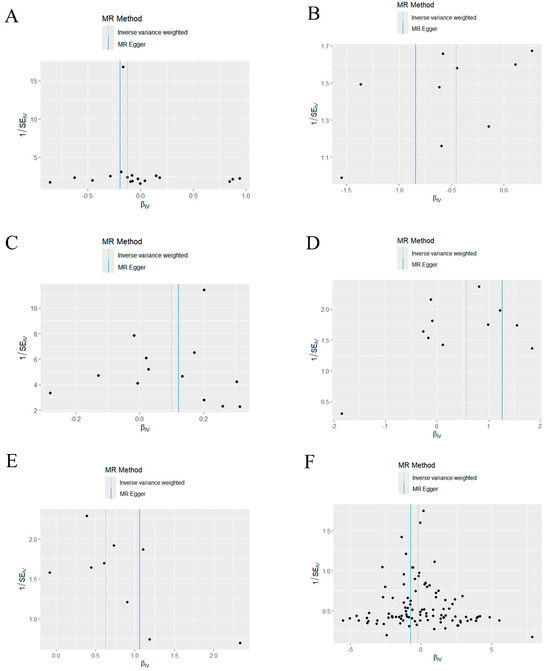
Figure 3.
Funnel plot of the causal relationship between mitochondria and periodontitis (IVW method). (A) Funnel plot of the causal relationship between medium−chain specific acyl−CoA dehydrogenase and periodontitis. (B) Funnel plot of the causal relationship between malonyl-CoA decarboxylase and periodontitis. (C) Funnel plot of the causal relationship between glutaredoxin− 2 and periodontitis. (D) Funnel plot of the causal relationship between oligoribonuclease and periodontitis. (E) Funnel plot of the causal relationship between pyruvate carboxylase and periodontitis. (F) Funnel plot of the causal relationship between mitochondrial DNA copy number and periodontitis. IVW, inverse variance weighting.
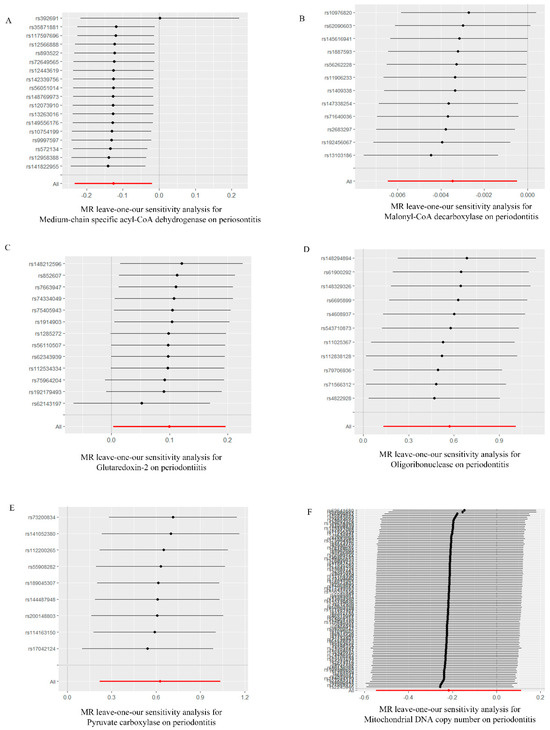
Figure 4.
“Leave−one−out” forest map of the causal relationship between mitochondria and periodontitis (IVW method). (A) “Leave−one−out” forest map of the causal relationship between medium−chain specific acyl−CoA dehydrogenase and periodontitis. (B) “Leave−one−out” forest map of the causal relationship between malonyl−CoA decarboxylase and periodontitis. (C) “Leave−one−out” forest map of the causal relationship between glutaredoxin−2 and periodontitis. (D) “Leave−one−out” forest map of the causal relationship between oligoribonuclease and periodontitis. (E) “Leave−one−out” forest map of the causal relationship between pyruvate carboxylase and periodontitis. (F) “Leave−one−out” forest map of the causal relationship between mitochondrial DNA copy number and periodontitis. IVW, inverse variance weighting.
3. Discussion
Compelling evidence has demonstrated the vital role of mitochondrial dysfunction in periodontitis; therefore, targeted mitochondrial therapy is potentially an effective strategy in periodontitis treatment [22,23,24]. In the present study, we demonstrated that there was a causal relationship between mitochondrial biological function and periodontitis, and discerned that MCAD, MLYCD, Grx2, ORN, and PC are significantly associated with periodontitis.
MCAD is a mitochondrial enzyme that is involved in the catalysis of fatty acid β-oxidation, a process that is important for the maintenance of energy homeostasis [25,26]. A deficiency in MCAD was demonstrated to affect fatty acid β-oxidation, resulting in lipid deposition in multiple organs. Previous studies have shown that empagliflozin can effectively reduce lipid deposition and ameliorate nonalcoholic steatohepatitis via activating AMPK/FOXA2 signaling to upregulate MCAD expression; thus, MCAD may be a potential therapeutic target for the treatment of nonalcoholic steatohepatitis [27]. Acetyl-CoA by sodium octanoate (8C) was demonstrated to markedly improve heart function in ischemia reperfusion rats and, more importantly, MCAD was verified to be a pivotal factor in the pathophysiology of reperfusion injury mediated by 8C [28]. Based on the obtained results, our findings indicated that MCAD may act as a protective element against periodontitis. MLYCD is a mitochondrial enzyme that catalyzes the breakdown of propionyl-CoA into acetyl-CoA and carbon dioxide, offering a metabolic pathway for propionyl-CoA in mitochondria [29]. Accumulating research has demonstrated that mutations in the MLYCD gene can result in a rare inherited metabolic disorder, which manifests as hypoglycemia, metabolic acidosis, and/or cardiomyopathy [30,31]. In addition, MLYCD was found to be downregulated in clear cell renal cell carcinoma (ccRCC), and low MLYCD expression was correlated with a poor prognosis in patients. However, overexpressing MLYCD was verified to effectively reduce tumor growth and reverse resistance to sunitinib; this was because MLYCD-mediated fatty acid oxidation can disrupt endoplasmic reticulum and mitochondrial homeostasis, indicating that MYLCD activation could be a promising strategy for treating ccRCC [32]. Additionally, MYLCD was demonstrated to be involved in the process by which lipid-induced mitochondrial stress contributes to skeletal muscle insulin resistance [33]. Our current study also indicated that MYLCD may be a critical mediator in the development of periodontitis, and may be used as a potential protective agent against periodontitis in the clinic.
Grx2 is an oxidoreductase located in mitochondria that plays a crucial role in the regulation of mitochondrial redox [34]. Previously, Grx2 was proved to protect lens epithelial cells from oxidative stress-related epithelial–mesenchymal transition via suppressing mitochondrial oxidative stress-related upregulation of integrin-linked kinase [35]. Of note, the lack of Grx2 in mitochondria led to changes in metabolic phenotype, as evidenced by increased body weight, the accumulation of fat in the liver, and augmented plasma lipid levels, which resulted in fatty liver diseases [36]. Based on our results, the expression of Grx2 is positively correlated with periodontitis, which may shed light on the pathological and physiological processes of periodontitis, and provide a potential therapeutic target for the treatment of periodontitis. Based on the current understanding, ORN is an enzyme that is involved in the terminal step of RNA digestion and deletion of ORN can affect pathways regulated by c-di-GMP signaling, for example, the response to oxidative stress [37]. ORN deletion can significantly weaken the motility of P. aeruginosa, reduce energy metabolism and adversely affect bacterial chemotaxis [38]. Our research corroborates this view, suggesting that ORN is positively correlated with periodontitis and may promote the occurrence and development of periodontitis by regulating mitochondrial function. PC is a mitochondrial enzyme that contributes to the ATP-dependent conversion of pyruvate to oxaloacetate. Previously, PC was identified to be present in some neurons in the human brain cortex, and further research verified that PC has important functions in the metabolomic cooperation between neurons and astrocytes, which are of importance in understanding the intercellular metabolism of neurons and astrocytes [39]. E.S. Selen et al. proved that PC is vital for mitochondrial pyruvate metabolism, because PC was involved in the high fat, ketogenic diet and fasting processes [40]. An adipocyte-specific lncRNA, ADIPINT, was demonstrated to regulate lipid metabolism by interacting with PC, and further studies revealed that reduced ADIPINT or PC expression can decrease the synthesis and content of lipids in adipocytes [41]. Thus, our results may unveil a novel gene that connects mitochondrial biological function with periodontitis. Additionally, the effect of mtDNA-CN on periodontitis was assessed and, based on the obtained results, there was no causal relationship between mtDNA-CN and periodontitis. Currently, a variety of studies have indicated that mitochondrial dysfunction serves as a pathological mechanism for periodontitis, and therapy based on regulating mitochondria may be an effective method for treating periodontitis [42,43,44]. Our study based on GWAS data indicates a causal association between mitochondrial biological function and periodontitis, and MCAD and MLYCD are causally linked to periodontitis, serving as protective factors, while, Grx2, ORN, and PC function as risk factors for periodontitis. These results may provide therapeutic targets for periodontitis treatment based on mitochondrial therapy.
MR research that leverages GWAS data can, to a certain degree, control confounding factors within observational studies and be used to provide accurate and stable estimates. In addition, potential biases were mitigated effectively by employing LD analysis, association analysis, and weak IVs eliminations [45,46]. Additionally, a symmetrical distribution of SNPs in the funnel plot was observed within our studies. Nevertheless, the present study also has several limitations. First, the present study focused mainly on the European population rather than the Asian and African populations, which may lead to racial biases. Second, the number of IVs for SNPs within this study was relatively modest; thus, it is necessary to utilize larger sample datasets to further validate the results in subsequent analyses. Third, although the causal relationship between mitochondria and periodontitis was explored via MR, it is unclear whether mitochondrial dysfunction influences periodontitis onset or its progress, as the underlying mechanisms were not elucidated, and more in-depth research is needed.
4. Materials and Methods
4.1. Study Design
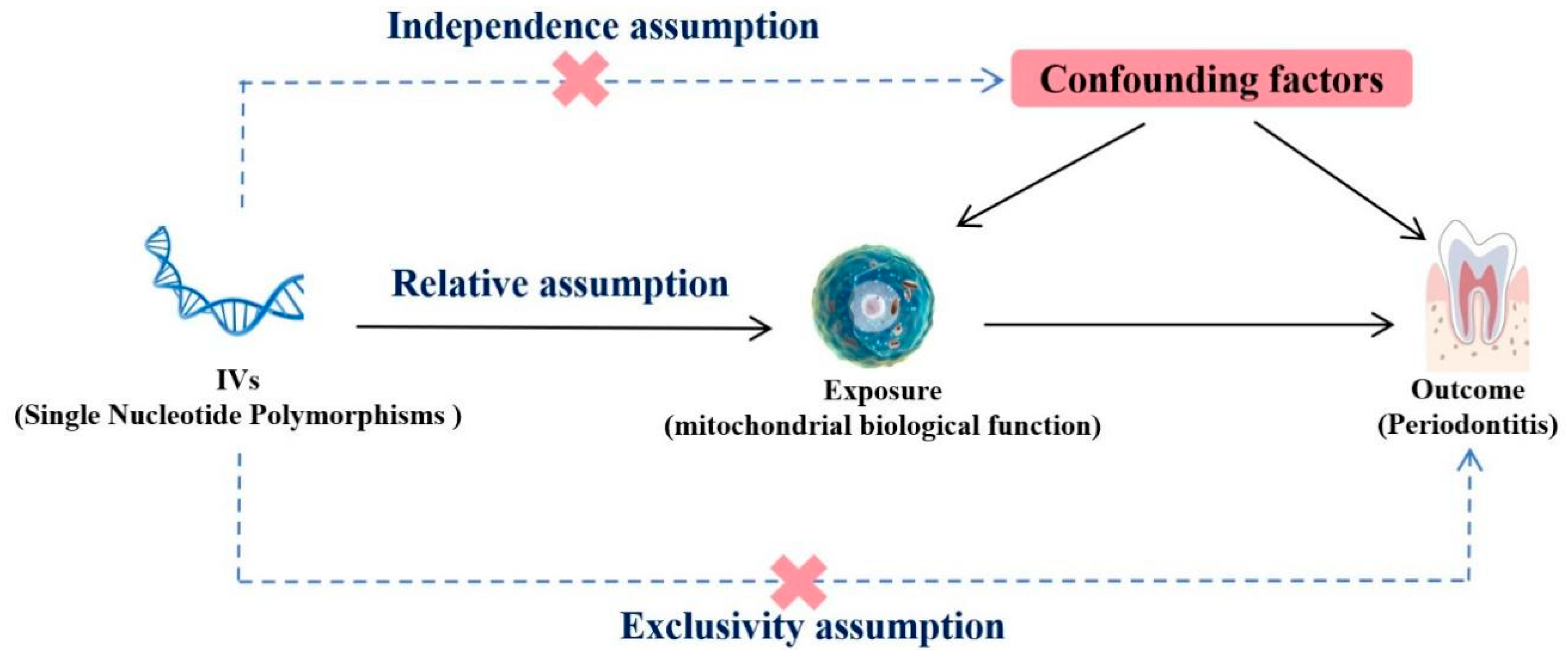
The present MR analysis was predicated on three fundamental assumptions, as shown in Figure 5. The initial assumption was that genetic IVs exhibit a robust association with mitochondria (the exposure). The second assumption was that IVs and periodontitis (the outcome variables) remain unaffiliated with any other confounding factors. The third assumption was that IVs solely impact periodontitis by regulating mitochondrial activity, without any additional pathways.
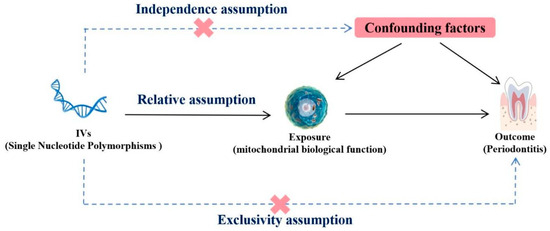
Figure 5.
Mendelian randomization design of the present study.
4.2. Genetic Instrument Selection
We employed GWAS data pertaining to mitochondrial biological function as an exposure variable. The selection of IVs associated to mitochondrial function within this study was based on three genome-wide analyses. A total of 10,708 cases and 15,566,792 controls (average age 48.6 years old) were reported by M. Pietzner et al. [17]. A total of 3301 cases and 10,534,735 controls (aged ≥18 years old) were reported by B.B. Sun et al. [18], and 383,476 samples (aged 40–69 years) were reported by M. Chong et al. [21]. All of the above studies collected data based on the European population, and mixed populations or non-European populations were excluded (Table 3). For the present study, summary data can be accessed at https://gwas.mrcieu.ac.uk (accessed on 1 May 2024). Single nucleotide polymorphisms (SNPs) associated with mitochondrial function that met the significance threshold of p < 5 × 10−6 were selected as potential IVs. In addition, a clumping procedure was performed to set the linkage disequilibrium (LD) coefficient R2 to less than 0.001 in a 10,000 kb window, to determine the independent SNPs. Additionally, F statistical analysis was utilized to assess the strength of SNPs, with F < 10 indicating a possible weak tool. Ambiguous SNPs were deleted to ensure effectiveness and stability.

Table 3.
Mitochondria-related GWAS data.
4.3. Outcome Data Source
The GWAS data for periodontitis were selected from the FinnGen database (https://www.finngen.fi/en, accessed on 1 May 2024). The latest whole-genome sequencing results of periodontitis were used in the MR analysis, which included 195,395 controls and 3413 individuals who were diagnosed with periodontitis (acute periodontitis: 367, chronic periodontitis: 3046; the original data are classified according to the 1999 classification of periodontal diseases and conditions).
4.4. MR Analysis
The causal relationship between mitochondrial function and periodontitis was determined via the following five methods: inverse variance weighting (IVW), MR-Egger, weighted median, weighted mode, and simple mode. Typically, the IVW method was primarily utilized because of its high statistical efficiency and common application, with a significance threshold of p < 0.05 representing a positive result, which is ideal for large samples [47]. The other four robust methods were utilized as complementary methods. MR-Egger can provide a reliable and unbiased estimate, even if all of the SNPs in the selection are invalid [48]. Weighted median can produce reliable estimates of the causal effects, even if as much as 50% of the data come from variations of interest that are invalid IVs [49]. The weighted mode approach maintains validity, even if other instrumental variables do not qualify for the causal inference of the MR technique [50]. Although the simple mode is not as powerful as IVW, this mode-based analysis approach provides robustness to pleiotropy [51]. The results of MR analysis were visualized by the TwoSampleMR package, featuring forest plots, scatter plots, and sensitivity analysis plots.
4.5. Sensitivity Analyses
Sensitivity analyses were performed to assess the robustness of our findings. Specifically, MR-Egger and IVW tests were conducted to assess heterogeneity, with a p < 0.05 significance level indicating the presence of heterogeneity. In addition, the MR-Egger intercept test was performed to assess the pleiotropy of our findings, and a p-value < 0.05 denoted the presence of pleiotropy. For the sensitivity analysis, the “leave-one-out” method was employed to observe the influence of individual SNPs on the overall estimates.
4.6. Statistical Analysis
R software (version 4.3.1, the University of Auckland, Auckland, New Zealand), and the TwoSampleMR software package (version 0.5.11) were used to evaluate causality in MR analysis. A threshold of statistical significance of p < 0.05 was adopted.
5. Conclusions
The present MR study based on GWAS data indicates a causal association between mitochondrial biological function and periodontitis. Notably, MCAD and MLYCD are causally linked to periodontitis, serving as protective factors. Grx2, ORN, and PC function as risk factors for periodontitis. Future studies into the underlying biological mechanisms between periodontitis and these factors may facilitate the development of strategies aimed at treating periodontitis through mitochondrial regulation.
Author Contributions
Conceptualization, H.Z. and D.-D.P.; methodology, Y.-X.Q., X.-X.Z. and R.-Y.C.; data analysis and interpretation, Y.-X.Q. and R.-Y.C.; writing—original draft preparation, H.Z. and Y.-X.Q.; writing—review and editing, A.L. and D.-D.P.; funding acquisition, H.Z., A.L. and D.-D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82201066, 82170927, 82071078), and the Outstanding Youth Science Foundation Project of Shaanxi Provincial Department of Science and Technology (No. 2022JC-57).
Institutional Review Board Statement
Not applicable. All data used in this study were derived from publicly available GWAS summary datasets, which had received approval from the relevant ethics committees. No human data were collected in this study; therefore, further ethical approval was not necessary.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available in the GWAS Catalog (https://gwas.mrcieu.ac.uk/ (accessed on 13 June 2024)).
Acknowledgments
The authors wish to acknowledge the participants and investigators of the FinnGen study: EBI study and Sun et al., and express their appreciation for the GWAS summary statistics provided by FinnGen, EBI and Sun et al.
Conflicts of Interest
The authors have no competing interests to declare.
References
- Cui, Y.; Hong, S.; Xia, Y.; Li, X.; He, X.; Hu, X.; Li, Y.; Wang, X.; Lin, K.; Mao, L. Melatonin Engineering M2 Macrophage-Derived Exosomes Mediate Endoplasmic Reticulum Stress and Immune Reprogramming for Periodontitis Therapy. Adv. Sci. 2023, 10, e2302029. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Barry, M.J.; Nicholson, W.K.; Silverstein, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; Li, L.; Ogedegbe, G.; et al. Screening and Preventive Interventions for Oral Health in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2023, 330, 1773–1779. [Google Scholar]
- Puletic, M.; Velikic, G.; Maric, D.M.; Supic, G.; Maric, D.L.; Radovic, N.; Avramov, S.; Vojvodic, D. Clinical Efficacy of Extracellular Vesicle Therapy in Periodontitis: Reduced Inflammation and Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 5753. [Google Scholar] [CrossRef]
- Sun, X.; Wu, T.; Yang, Z.; Chen, S.; Zhao, Z.; Hu, C.; Wu, S.; Wu, J.; Mao, Y.; Liu, J.; et al. Regulatory role of PDK1 via integrated gene analysis of mitochondria-immune response in periodontitis. Gene 2024, 918, 148476. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Cao, Z.; Chen, Y.; Si, C.; Sun, X.; Huang, S. The role of mitochondrial dysfunction in periodontitis: From mechanisms to therapeutic strategy. J. Periodontal Res. 2023, 58, 853–863. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Murphy, M.P. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Du, Y.; Yao, H.; Zhao, B.; Wang, Z.; Chen, R.; Ji, Y.; Du, M. Isobavachin attenuates osteoclastogenesis and periodontitis-induced bone loss by inhibiting cellular iron accumulation and mitochondrial biogenesis. Biochem. Pharmacol. 2024, 224, 116202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ren, X.; Mao, J.; Zeng, J.; Jiang, W.; Zhou, R.; Han, Y.; Wang, H.; Mao, Y.; Sun, X.; et al. 3-methyl-1H-indol-1-yl dimethylcarbamodithioate attenuates periodontitis through targeting MAPK signaling pathway-regulated mitochondrial function. J. Periodontal Res. 2024, 59, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, L.; Zhong, W.; Wang, H.; Lan, Y.; Chen, Q.; Yu, S.; Yang, F.; Yan, P.; Peng, H.; et al. Integrated analyses revealed the potential role and immune link of mitochondrial dysfunction between periodontitis and type 2 diabetes mellitus. Int. Immunopharmacol. 2024, 130, 111796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, S.Q.; Chen, Y.X.; Pan, X.; Chen, Y.Z.; Zhuang, Y.G. Causal Relationship between Mitochondrial-Associated Proteins and Sepsis in ICU Patients: A Mendelian Randomization Study. ACS Omega 2024, 9, 8457–8463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Shi, J.; Yang, R.; Zhou, C.; Liu, Z. Evidence based on Mendelian randomization: Causal relationship between mitochondrial biological function and lung cancer and its subtypes. Neoplasia 2023, 46, 100950. [Google Scholar] [CrossRef]
- Pietzner, M.; Wheeler, E.; Carrasco-Zanini, J.; Raffler, J.; Kerrison, N.D.; Oerton, E.; Auyeung, V.P.W.; Luan, J.; Finan, C.; Casas, J.P.; et al. Genetic architecture of host proteins involved in SARS-CoV-2 infection. Nat. Commun. 2020, 11, 6397. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Chong, M.R.; Narula, S.; Morton, R.; Judge, C.; Akhabir, L.; Cawte, N.; Pathan, N.; Lali, R.; Mohammadi-Shemirani, P.; Shoamanesh, A.; et al. Mitochondrial DNA Copy Number as a Marker and Mediator of Stroke Prognosis: Observational and Mendelian Randomization Analyses. Neurology 2022, 98, e470–e482. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- Chong, M.; Mohammadi-Shemirani, P.; Perrot, N.; Nelson, W.; Morton, R.; Narula, S.; Lali, R.; Khan, I.; Khan, M.; Judge, C.; et al. GWAS and ExWAS of blood mitochondrial DNA copy number identifies 71 loci and highlights a potential causal role in dementia. eLife 2022, 11, e70382. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ping, Y.; Li, X.; Mao, Y.; Chen, Y.; Shi, L.; Hong, X.; Chen, L.; Chen, S.; Cao, Z.; et al. Activation of PGC-1α-dependent mitochondrial biogenesis supports therapeutic effects of silibinin against type I diabetic periodontitis. J. Clin. Periodontol. 2023, 50, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qin, H.; Zhou, M.; Zhang, T.; Zhang, Y.; Ding, H.; Xu, L.; Song, J. Knockdown of SIRT3 perturbs protective effects of irisin against bone loss in diabetes and periodontitis. Free Radic. Biol. Med. 2023, 200, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, J.; Jiang, L.; Li, H.; Lei, L. PINK1-mediated mitophagy reduced inflammatory responses to Porphyromonas gingivalis in macrophages. Oral Dis. 2023, 29, 3665–3676. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.L.; Kiriazis, H.; Wadley, G.D.; Masterman, E.I.; Sergienko, N.M.; Raaijmakers, A.J.A.; Trewin, A.J.; Harmawan, C.A.; Yildiz, G.S.; Liu, Y.; et al. A gene therapy targeting medium-chain acyl-CoA dehydrogenase (MCAD) did not protect against diabetes-induced cardiac pathology. J. Mol. Med. 2024, 102, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Herrema, H.; Derks, T.G.; van Dijk, T.H.; Bloks, V.W.; Gerding, A.; Havinga, R.; Tietge, U.J.; Müller, M.; Smit, G.P.; Kuipers, F.; et al. Disturbed hepatic carbohydrate management during high metabolic demand in medium-chain acyl-CoA dehydrogenase (MCAD)-deficient mice. Hepatology 2008, 47, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Q.L.; Xin, Q.; Sun, B.; Zhang, S.; Fang, Q.H.; Shi, Y.X.; Niu, W.Y.; Lin, J.N.; Li, C.J. MCAD activation by empagliflozin promotes fatty acid oxidation and reduces lipid deposition in NASH. J. Mol. Endocrinol. 2022, 69, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Lei, I.; Tian, S.; Gao, W.; Liu, L.; Guo, Y.; Tang, P.; Chen, E.; Wang, Z. Acetyl-CoA production by specific metabolites promotes cardiac repair after myocardial infarction via histone acetylation. eLife 2021, 10, e60311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Liu, J.; Chen, Q.; Cai, C.; Miao, X.; Wu, T.; Cheng, X. Identification of mitochondrial-related signature and molecular subtype for the prognosis of osteosarcoma. Aging 2023, 15, 12794–12816. [Google Scholar] [CrossRef]
- Prada, C.E.; Jefferies, J.L.; Grenier, M.A.; Huth, C.M.; Page, K.I.; Spicer, R.L.; Towbin, J.A.; Leslie, N.D. Malonyl coenzyme A decarboxylase deficiency: Early dietary restriction and time course of cardiomyopathy. Pediatrics 2012, 130, e456–e460. [Google Scholar] [CrossRef]
- de Wit, M.C.; de Coo, I.F.; Verbeek, E.; Schot, R.; Schoonderwoerd, G.C.; Duran, M.; de Klerk, J.B.; Huijmans, J.G.; Lequin, M.H.; Verheijen, F.W.; et al. Brain abnormalities in a case of malonyl-CoA decarboxylase deficiency. Mol. Genet. Metab. 2006, 87, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Luo, Y.; Liu, Y.; Zeng, Y.; Tong, J.; Li, M.; Hou, Y.; Du, K.; Qi, Y.; Pan, W.; et al. Fatty Acid Oxidation Mediated by Malonyl-CoA Decarboxylase Represses Renal Cell Carcinoma Progression. Cancer Res. 2023, 83, 3920–3939. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Brzozowa-Zasada, M.; Piecuch, A.; Bajdak-Rusinek, K.; Gołąbek, K.; Michalski, M.; Janelt, K.; Matysiak, N. Glutaredoxin 2 Protein (Grx2) as an Independent Prognostic Factor Associated with the Survival of Colon Adenocarcinoma Patients. Int. J. Mol. Sci. 2024, 25, 1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Li, C.; Li, J.; Zhang, S.; Liang, C.; Deng, Q.; Guo, Z.; Guo, C.; Yan, H. Glutaredoxin 2 protects lens epithelial cells from epithelial-mesenchymal transition by suppressing mitochondrial oxidative stress-related upregulation of integrin-linked kinase. Exp Eye Res. 2023, 234, 109609. [Google Scholar] [CrossRef] [PubMed]
- Scalcon, V.; Folda, A.; Lupo, M.G.; Tonolo, F.; Pei, N.; Battisti, I.; Ferri, N.; Arrigoni, G.; Bindoli, A.; Holmgren, A.; et al. Mitochondrial depletion of glutaredoxin 2 induces metabolic dysfunction-associated fatty liver disease in mice. Redox Biol. 2022, 51, 102277. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, P.; Ahmad, I.; Sharma, R.; Taneja, B. Structural investigation and gene deletion studies of mycobacterial oligoribonuclease reveal modulation of c-di-GMP-mediated phenotypes. Int. J. Biol. Macromol. 2022, 223, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, L.; Wang, M.; Bajinka, O.; Wu, G.; Qin, L.; Tan, Y. Oligoribonuclease mediates high adaptability of P. aeruginosa through metabolic conversion. BMC Microbiol. 2024, 24, 25. [Google Scholar] [CrossRef]
- Gondáš, E.; Kráľová Trančíková, A.; Šofranko, J.; Majerová, P.; Lučanský, V.; Dohál, M.; Kováč, A.; Murín, R. The presence of pyruvate carboxylase in the human brain and its role in the survival of cultured human astrocytes. Physiol. Res. 2023, 72, 403–414. [Google Scholar] [CrossRef]
- Selen, E.S.; Rodriguez, S.; Cavagnini, K.S.; Kim, H.B.; Na, C.H.; Wolfgang, M.J. Requirement of hepatic pyruvate carboxylase during fasting, high fat, and ketogenic diet. J. Biol. Chem. 2022, 298, 102648. [Google Scholar] [CrossRef]
- Kerr, A.G.; Wang, Z.; Wang, N.; Kwok, K.H.M.; Jalkanen, J.; Ludzki, A.; Lecoutre, S.; Langin, D.; Bergo, M.O.; Dahlman, I.; et al. The long noncoding RNA ADIPINT regulates human adipocyte metabolism via pyruvate carboxylase. Nat. Commun. 2022, 13, 2958. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xiao, J.; Ma, L.; Wang, C.; Wang, X.; Huang, X.; Cao, Z. Mitochondrial Dysfunction in Periodontitis and Associated Systemic Diseases: Implications for Pathomechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1024. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Ding, T.; Feng, S.; Liu, H.; Liu, M.; Ge, S. Capsaicin attenuates Porphyromonas gingivalis-suppressed osteogenesis of periodontal ligament stem cells via regulating mitochondrial function and activating PI3K/AKT/mTOR pathway. J. Periodontal Res. 2024, 59, 798–811. [Google Scholar] [CrossRef]
- Cao, N.; Liu, X.; Hou, Y.; Deng, Y.; Xin, Y.; Xin, X.; Xiang, X.; Liu, X.; Yu, W. 18-α-glycyrrhetinic acid alleviates oxidative damage in periodontal tissue by modulating the interaction of Cx43 and JNK/NF-κB pathways. Front. Pharmacol. 2023, 14, 1221053. [Google Scholar] [CrossRef]
- Ke, T.M.; Lophatananon, A.; Muir, K.R. Strengthening the Evidence for a Causal Link between Type 2 Diabetes Mellitus and Pancreatic Cancer: Insights from Two-Sample and Multivariable Mendelian Randomization. Int. J. Mol. Sci. 2024, 25, 4615. [Google Scholar] [CrossRef]
- Rodriguez-Martin, I.; Villanueva-Martin, G.; Guillen-Del-Castillo, A.; Ortego-Centeno, N.; Callejas, J.L.; Simeón-Aznar, C.P.; Martin, J.; Acosta-Herrera, M. Contribution of Telomere Length to Systemic Sclerosis Onset: A Mendelian Randomization Study. Int. J. Mol. Sci. 2023, 24, 15589. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Lázaro García, C. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017, 49, 1767–1778. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).