Exploring the Effects of an Alfalfa Leaf-Derived Adsorbent on Microbial Community, Ileal Morphology, Barrier Function, and Immunity in Turkey Poults during Chronic Aflatoxin B1 Exposure
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Source, Diets, and Experimental Design
4.2. Microbial Community
4.3. Bioinformatics and Statistical Analysis
4.4. Ileal Morphology
4.5. Barrier Function
4.6. Immunity
4.7. Statistical Analysis
4.8. Data Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaemmaghami, S.S.; Rouhanipour, H.; Sharifi, S.D. Aflatoxin levels in poultry feed: A comparison of mash and pellet forms. Poult. Sci. 2024, 103, 103254. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Al Hakeem, W.G.; Selvaraj, R.K.; Shanmugasundaram, R. Beyond protein synthesis: The emerging role of arginine in poultry nutrition and host-microbe interactions. Front. Physiol. 2024, 14, 1326809. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Herfel, T.M.; Dunkley, C.S.; Hester, P.Y.; Crenshaw, T.D.; Ricke, S.C. The effects of alfalfa-based molt diets on skeletal integrity of white leghorns. Poult. Sci. 2008, 87, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, A.Ö.; Şentürk, E.T.; Olgun, O. Use of alfalfa meal in layer diets–a review. J. World’s Poult. Sci. 2020, 76, 134–143. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Chen, D.; Zhao, W.; Zhang, X.; Yuan, W.; Si, H.; Deng, X.; Du, R.; Xu, C. Effects of dietary alfalfa meal supplementation on the growth performance, nutrient apparent digestibility, serum parameters, and intestinal microbiota of raccoon dogs (Nyctereutes procyonoides). Animals 2024, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Xu, M.; Jiang, Y.; Wang, W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2021, 96, 332–341. [Google Scholar] [CrossRef]
- Qin, P.; Hu, L.; Liu, Y.; Hu, X.; Zhang, X.; Rosado, A.S.; Wei, G.; Chen, C. Responses of soil microbial communities and nutrient dynamics under continuous alfalfa (Medicago sativa L.) cultivation. Appl. Soil Ecol. 2024, 197, 105356. [Google Scholar] [CrossRef]
- Nava-Ramírez, M.J.; Vázquez-Durán, A.; Figueroa-Cárdenas, J.d.D.; Hernández-Patlán, D.; Solís-Cruz, B.; Téllez-Isaías, G.; López-Coello, C.; Méndez-Albores, A. Removal of aflatoxin B1 using alfalfa leaves as an adsorbent material: A comparison between two in vitro experimental models. Toxins 2023, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Nava-Ramírez, M.J.; Maguey-González, J.A.; Gómez-Rosales, S.; Hernández-Ramírez, J.O.; Latorre, J.D.; Du, X.; López-Coello, C.; Hargis, B.M.; Téllez-Isaías, G.; Vázquez-Durán, A.; et al. Efficacy of powdered alfalfa leaves to ameliorate the toxic effects of aflatoxin B1 in turkey poults. Mycotoxin Res. 2024, 40, 269–277. [Google Scholar] [CrossRef]
- Alpsoy, L.; Yalvac, M.E. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam. Horm. 2024, 2011, 287–305. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shahidi, S.A.; Safari, R.; Motamedzadegan, A.; Ghorbani-HasanSaraei, A. Evaluation of stability and antibacterial properties of extracted chlorophyll from alfalfa (Medicago sativa L.). Food Chem. Toxicol. 2022, 163, 112980. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, M.S.; Hedayati, N.; Riahi-Zanjani, B.; Karimi, G. Immunosuppression following dietary aflatoxin B1 exposure: A review of the existing evidence. Toxin Rev. 2016, 35, 121–127. [Google Scholar] [CrossRef]
- Quezada, T.; Cuellar, H.; Jaramillo-Juarez, F.; Valdivia, A.G.; Reyes, J.L. Effects of aflatoxin B1 on the liver and kidney of broiler chickens during development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Antoniou, C.; Christodoulou, C.; Hapeshi, E.; Stavrou, I.; Michael, C.; Fatta-Kassomps, D.; Fotopoulos, V. Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Sci. Total Environ. 2016, 557, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ditta, Y.A.; Mahad, S.; Bacha, U. Aflatoxins: Their toxic effect on poultry and recent advances in their treatment. In Mycotoxins-Impact and Management Strategies; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Crispo, M.; Shivaprasad, H.L.; Cooper, G.L.; Bickford, A.A.; Stoute, S.T. Streptococcosis in commercial and noncommercial avian species in California: 95 cases (2000–2017). Avian Dis. 2018, 62, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Maguey-González, J.A.; Liu, J.; Zhang, G.; Latorre, J.D.; Hernández-Ramírez, J.O.; de Jesús Nava-Ramírez, M.; Senas-Cuesta, R.; Gómez-Rosales, S.; de Lourdes Ángeles, M.; Stein, A.; et al. Assessment of the impact of humic acids on intestinal microbiota, gut integrity, ileum morphometry, and cellular immunity of turkey poults fed an aflatoxin B1-contaminated diet. Toxins 2024, 16, 122. [Google Scholar] [CrossRef]
- Huang, S.; Lin, L.; Wang, S.; Ding, W.; Zhang, C.; Shaukat, A.; Xu, B.; Yue, K.; Zhang, C.; Liu, F. Total flavonoids of Rhizoma drynariae mitigates aflatoxin B1-induced liver toxicity in chickens via microbiota-gut-liver axis interaction mechanisms. Antioxidants 2023, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease. Front. Nutr. 2021, 8, 798038. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Donalson, L.M.; Mitchell, A.D.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Effects of alfalfa and fructooligosaccharide on molting parameters and bone qualities using dual energy X-ray absorptiometry and conventional bone assays. Poult. Sci. 2006, 85, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, X.S.; Li, F.H.; Usman, S.; Zhang, Y.X.; Ding, Z.T. Antioxidant, flavonoid, α-tocopherol, β-carotene, fatty acids, and fermentation profiles of alfalfa silage inoculated with novel Lactiplantibacillus plantarum and Pediococcus acidilactici strains with high-antioxidant activity. Anim. Feed. Sci. Technol. 2022, 288, 115301. [Google Scholar] [CrossRef]
- Jin, J.; Beekmann, K.; Ringø, E.; Rietjens, I.M.; Xing, F. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 2021, 126, 107998. [Google Scholar] [CrossRef]
- Sitkin, S.; Pokrotnieks, J. Clinical potential of anti-inflammatory effects of Faecalibacterium prausnitzii and butyrate in inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, e40–e41. [Google Scholar] [CrossRef] [PubMed]
- Nyangale, E.P.; Farmer, S.; Cash, H.A.; Keller, D.; Chernoff, D.; Gibson, G.R. Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 2015, 145, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Shi, J.; Li, H.; An, N.; Li, S.; Ciu, K.; Guo, H.; Li, Z. Coprococcus eutactus, a potent probiotic, alleviates colitis via acetate-mediated IgA response and microbiota restoration. J. Agric. Food Chem. 2023, 71, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Palma-Hidalgo, J.M.; Jiménez, E.; Yáñez-Ruiz, D.R. Enhancing rumen microbial diversity and its impact on energy and protein metabolism in forage-fed goats. Front. Vet. Sci. 2023, 10, 1272835. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Ali Akbari, S.K.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125. [Google Scholar] [CrossRef]
- Rais, A.; Chand, P.; Malik, M.; Prasad, T. Human microbiota: Role in cancer progression and therapy. In Microbial Crosstalk with Immune System; Academic Press: New Delhi, India, 2002; pp. 145–175. [Google Scholar]
- Hernández-Ramírez, J.O.; Nava-Ramírez, M.J.; Merino-Guzmán, R.; Téllez-Isaías, G.; Vázquez-Durán, A.; Méndez-Albores, A. The effect of moderate-dose aflatoxin B 1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. 2020, 36, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.O.; Ajiboye, T.O.; Iliasu, G.A.; Abdussalam, F.A.; Balogun, A.; Ojewuyi, O.B.; Yakubu, M.T. Phenolic extract of Dialium guineense pulp enhances reactive oxygen species detoxification in aflatoxin B1 hepatocarcinogenesis. J. Med. Food 2014, 17, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Olayem, B.S.; Olaitan, O.B.; Akinola, A.B. Immunomodulatory plant based foods, it’s chemical, biochemical and pharmacological approaches. In Medicinal Plants-Chemical, Biochemical, and Pharmacological Approaches; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Zhang, Y.; Usman, S.; Li, Q.; Li, F.; Zhang, X.; Nussio, L.G.; Guo, X. Effects of antioxidant-rich Lactiplantibacillus plantarum inoculated alfalfa silage on rumen fermentation, antioxidant and immunity status, and mammary gland gene expression in dairy goats. J. Anim. Sci. Biotechnol. 2024, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sang, R.; Feng, G.; Feng, Y.; Zhang, R.; Yan, X. Microbiological and metabolic pathways analysing the mechanisms of alfalfa polysaccharide and sulfated alfalfa polysaccharide in alleviating obesity. Int. J. Biol. Macromol. 2024, 263, 130334. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, e00191–e00216. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.F.; Merino-Guzman, R.; Latorre, J.D.; Mahaffey, B.D.; Yang, Y.; Teague, K.D.; Graham, L.E.; Wolfenden, A.D.; Hernandez-Velasco, X.; Bielke, L.R.; et al. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, Package Version 2.5-5. 2019. Available online: https://github.com/vegandevs/vegan (accessed on 6 May 2024).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/Share: 9.4 User’s Guide, 2nd ed.; SAS Documentation: Cary, NC, USA, 2002; 288p. [Google Scholar]


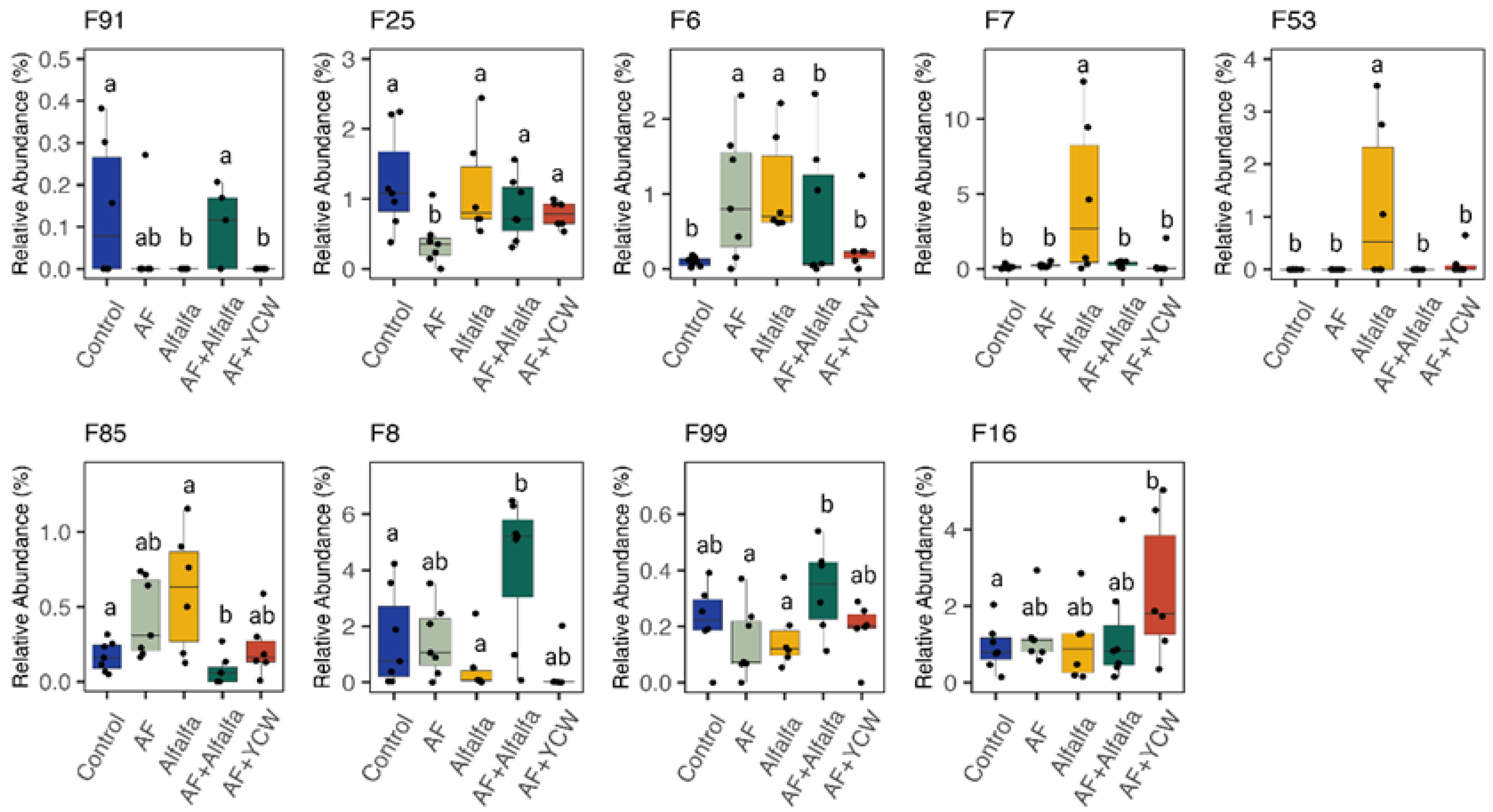
| Taxon | Control | AF | Alfalfa | Alfalfa + AF | AF + YCW | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Phyla | |||||||
| Firmicutes | 79.42 | 74.04 | 81.02 | 82.45 | 72.78 | 1.92 | 0.13 |
| Proteobacteria | 14.10 | 20.76 | 16.52 | 12.93 | 20.82 | 1.64 | 0.17 |
| Tenericutes | 3.85 | 2.84 | 1.11 | 2.94 | 4.57 | 0.58 | 0.45 |
| Cyanobacteria | 0.78 | 1.45 | 0.08 | 0.26 | 0.02 | 0.26 | 0.62 |
| Actinobacteria | 0.45 | 0.08 | 0.08 | 0.42 | 0.15 | 0.08 | 0.39 |
| Families | |||||||
| Oscillospiraceae | 32.72 | 32.53 | 40.05 | 39.90 | 30.04 | 2.06 | 0.06 |
| Lachnospiraceae | 32.28 | 25.69 | 28.66 | 28.64 | 28.21 | 1.05 | 0.15 |
| Enterobacteriaceae | 14.10 | 20.75 | 16.52 | 12.89 | 20.82 | 1.64 | 0.17 |
| Clostridiales_unidentified | 4.85 | 5.97 | 4.11 | 3.29 | 4.05 | 0.45 | 0.24 |
| Erysipelotrichaceae | 3.81 | 1.70 | 4.74 | 2.54 | 4.04 | 0.54 | 0.09 |
| Bacillaceae | 1.55 | 1.47 | 0.52 | 4.20 | 2.12 | 0.61 | 0.06 |
| Mollicutes_unidentified | 3.04 | 2.56 | 0.81 | 2.19 | 4.01 | 0.52 | 0.30 |
| Lactobacillaceae | 1.67 | 1.84 | 0.36 | 1.51 | 1.74 | 0.27 | 0.10 |
| Christensenellaceae | 0.76 | 0.81 | 0.80 | 0.93 | 1.23 | 0.08 | 0.68 |
| Streptococcaceae | 0.09 b | 0.97 a | 1.09 a | 0.71 a | 0.45 a | 0.18 | 0.04 |
| Vampirovibrio_unidentified | 0.78 | 1.45 | 0.08 | 0.26 | 0.02 | 0.26 | 0.62 |
| Enterococcaceae | 0.67 | 0.14 | 0.10 | 0.20 | 0.23 | 0.10 | 0.18 |
| Clostridia_unidentified | 0.14 | 0.85 | 0.03 | 0 | 0 | 0.16 | 0.58 |
| Peptostreptococcaceae | 0.41 | 0.29 | 0.09 | 0.13 | 0.09 | 0.06 | 0.12 |
| Clostridiaceae 1 | 0.25 | 0.29 | 0.19 | 0.12 | 0.19 | 0.02 | 0.69 |
| Taxon | Control | AF | Alfalfa | Alfalfa + AF | AF + YCW | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Genera | |||||||
| Escherichia/Shigella | 13.16 | 20.71 | 16.37 | 12.76 | 20.67 | 1.73 | 0.17 |
| Oscillospiraceae_unidentified | 10.21 | 11.14 | 10.97 | 14.11 | 11.05 | 0.67 | 0.68 |
| Mediterraneibacter | 10.54 | 7.50 | 8.76 | 9.49 | 8.96 | 0.49 | 0.62 |
| Lachnospiraceae_unidentified | 6.27 | 6.07 | 6.29 | 7.30 | 6.83 | 0.22 | 0.69 |
| Subdoligranulum | 8.70 | 4.93 | 7.29 | 6.65 | 3.03 | 0.98 | 0.59 |
| Pseudoflavonifractor | 5.04 | 5.91 | 4.36 | 7.05 | 5.76 | 0.45 | 0.32 |
| Enterocloster | 4.64 | 3.54 | 5.24 | 5.05 | 3.99 | 0.32 | 0.48 |
| Clostridiales_unidentified | 4.85 | 5.97 | 4.11 | 3.29 | 4.05 | 0.45 | 0.24 |
| Blautia | 4.24 | 2.67 | 2.36 | 1.56 | 2.37 | 0.44 | 0.21 |
| Bacillus | 1.55 | 1.47 | 0.52 | 4.20 | 2.12 | 0.61 | 0.06 |
| Faecalibacterium | 0.18 | 0.26 | 7.70 | 0.37 | 0.66 | 1.46 | 0.07 |
| Coprobacillaceae_unidentified | 1.72 ab | 0.44 b | 3.06 a | 0.69 b | 2.24 a | 0.48 | 0.02 |
| Mollicutes_unidentified | 3.04 | 2.56 | 0.81 | 2.19 | 4.01 | 0.52 | 0.30 |
| Anaerostipes | 2.51 | 0.75 | 1.35 | 1.43 | 1.33 | 0.28 | 0.11 |
| Eisenbergiella | 1.50 | 1.55 | 1.23 | 0.78 | 1.44 | 0.14 | 0.46 |
| ASVs | |||||||
| Escherichia/Shigella_F1 | 12.8 | 20.24 | 15.86 | 12.54 | 20.23 | 1.69 | 0.14 |
| Subdoligranulum_variabile_F4 | 5.11 | 1.88 | 3.80 | 3.36 | 1.93 | 0.60 | 0.81 |
| Mediterraneibacter_F2 | 2.65 | 2.02 | 1.37 | 2.54 | 3.79 | 0.39 | 0.25 |
| Mediterraneibacter_F3 | 2.14 | 3.31 | 3.41 | 1.02 | 2.03 | 0.44 | 0.35 |
| Enterocloster_F5 | 2.65 | 1.25 | 2.56 | 2.08 | 2.06 | 0.24 | 0.44 |
| Bacillus_F8 | 1.55 | 1.47 | 0.52 | 4.20 | 2.12 | 0.61 | 0.06 |
| Mollicutes_unidentified_F9 | 2.77 | 1.19 | 0.64 | 0.80 | 3.30 | 0.54 | 0.50 |
| Blautia_obeum_F18 | 2.93 | 1.67 | 1.34 | 1.07 | 1.31 | 0.33 | 0.82 |
| Coprobacillaceae_unidentified_F13 | 1.72 ab | 0.44 b | 3.06 a | 0.69 b | 2.24 a | 0.48 | 0.02 |
| Enterocloster_F11 | 1.24 | 1.36 | 1.51 | 1.68 | 1.04 | 0.10 | 0.79 |
| Pseudoflavonifractor_capillosus_F16 | 0.93 | 1.22 | 1.03 | 1.30 | 2.43 | 0.27 | 0.45 |
| Pseudoflavonifractor_F14 | 1.10 | 1.57 | 1.17 | 1.48 | 1.29 | 0.08 | 0.57 |
| Oscillospiraceae_unidentified_F17 | 0.80 | 0.92 | 1.06 | 1.39 | 1.96 | 0.20 | 0.98 |
| Oscillospiraceae_unidentified_F23 | 2.08 | 0.33 | 0.78 | 1.09 | 1.73 | 0.31 | 0.30 |
| Pseudoflavonifractor_F20 | 1.35 | 1.14 | 0.55 | 2.06 | 0.44 | 0.29 | 0.21 |
| Butyricicoccus_pullicaecorum_F24 | 1.29 | 0.13 | 1.72 | 1.98 | 0.47 | 0.35 | 0.68 |
| Anaerostipes_butyraticus_F19 | 1.77 | 0.56 | 1.20 | 0.71 | 1.10 | 0.21 | 0.18 |
| Faecalibacterium_F7 | 0.13 b | 0.24 b | 4.60 a | 0.34 b | 0.36 b | 0.86 | 0.02 |
| Pseudoflavonifractor_F21 | 0.82 | 1.07 | 0.73 | 1.25 | 1.23 | 0.10 | 0.60 |
| Oscillibacter_F15 | 0.92 | 1.25 | 0.58 | 1.30 | 0.91 | 0.13 | 0.73 |
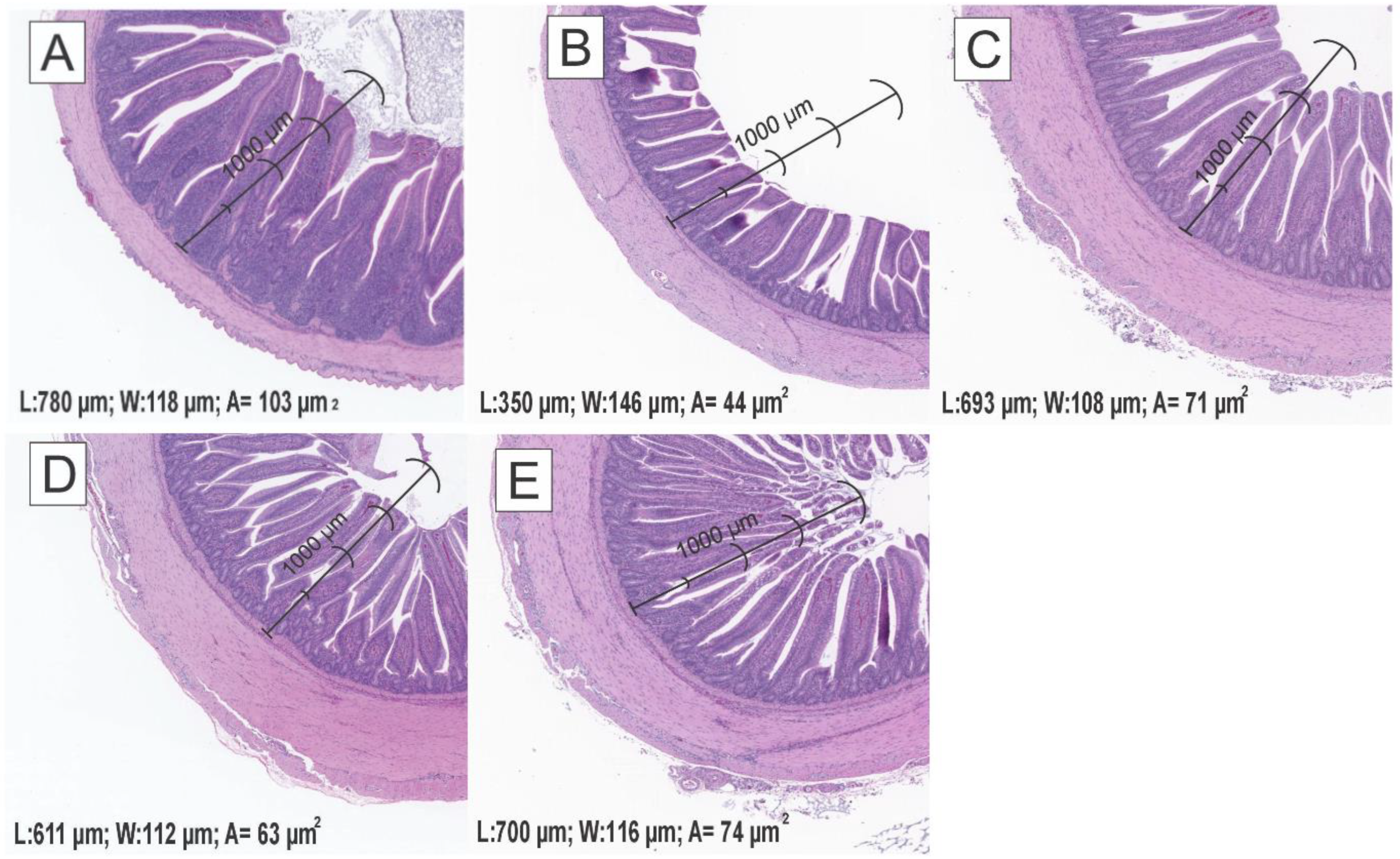
| Control | AF | Alfalfa | Alfalfa + AF | AF + YCW | SEM * | p-Value | |
|---|---|---|---|---|---|---|---|
| Villus height (μm) | 780.0 c | 350.6 a | 693.3 bc | 611.9 b | 700.0 bc | 271.8 | <0.0001 |
| Villus width (μm) | 118.9 a | 146.3 b | 108.8 a | 112.7 a | 116.9 a | 36.9 | 0.02 |
| Total area (μm2) | 103.2 c | 44.7 a | 71.7 b | 63.6 b | 74.1 b | 33.3 | <0.001 |
| FITC-d (ng/mL) | 263.3 b | 858.2 a | 214.8 b | 213.5 b | 230.7 b | 654.7 | 0.007 |
| CBH (mm) | 0.37 b | 0.50 b | 0.53 b | 0.86 a | 0.96 a | 0.23 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Ramírez, M.d.J.; Liu, J.; Hernández-Ramírez, J.O.; Hernandez-Velasco, X.; Latorre, J.D.; Vázquez-Durán, A.; Zhang, G.; Senas-Cuesta, R.; Gómez-Rosales, S.; Stein, A.; et al. Exploring the Effects of an Alfalfa Leaf-Derived Adsorbent on Microbial Community, Ileal Morphology, Barrier Function, and Immunity in Turkey Poults during Chronic Aflatoxin B1 Exposure. Int. J. Mol. Sci. 2024, 25, 7977. https://doi.org/10.3390/ijms25147977
Nava-Ramírez MdJ, Liu J, Hernández-Ramírez JO, Hernandez-Velasco X, Latorre JD, Vázquez-Durán A, Zhang G, Senas-Cuesta R, Gómez-Rosales S, Stein A, et al. Exploring the Effects of an Alfalfa Leaf-Derived Adsorbent on Microbial Community, Ileal Morphology, Barrier Function, and Immunity in Turkey Poults during Chronic Aflatoxin B1 Exposure. International Journal of Molecular Sciences. 2024; 25(14):7977. https://doi.org/10.3390/ijms25147977
Chicago/Turabian StyleNava-Ramírez, María de Jesús, Jing Liu, Juan Omar Hernández-Ramírez, Xochitl Hernandez-Velasco, Juan D. Latorre, Alma Vázquez-Durán, Guolong Zhang, Roberto Senas-Cuesta, Sergio Gómez-Rosales, Andressa Stein, and et al. 2024. "Exploring the Effects of an Alfalfa Leaf-Derived Adsorbent on Microbial Community, Ileal Morphology, Barrier Function, and Immunity in Turkey Poults during Chronic Aflatoxin B1 Exposure" International Journal of Molecular Sciences 25, no. 14: 7977. https://doi.org/10.3390/ijms25147977
APA StyleNava-Ramírez, M. d. J., Liu, J., Hernández-Ramírez, J. O., Hernandez-Velasco, X., Latorre, J. D., Vázquez-Durán, A., Zhang, G., Senas-Cuesta, R., Gómez-Rosales, S., Stein, A., Hargis, B. M., Téllez-Isaías, G., Méndez-Albores, A., & Maguey-González, J. A. (2024). Exploring the Effects of an Alfalfa Leaf-Derived Adsorbent on Microbial Community, Ileal Morphology, Barrier Function, and Immunity in Turkey Poults during Chronic Aflatoxin B1 Exposure. International Journal of Molecular Sciences, 25(14), 7977. https://doi.org/10.3390/ijms25147977








