Transcriptome-Wide Identification of m6A Writers, Erasers and Readers and Their Expression Profiles under Various Biotic and Abiotic Stresses in Pinus massoniana Lamb.
Abstract
:1. Introduction
2. Results
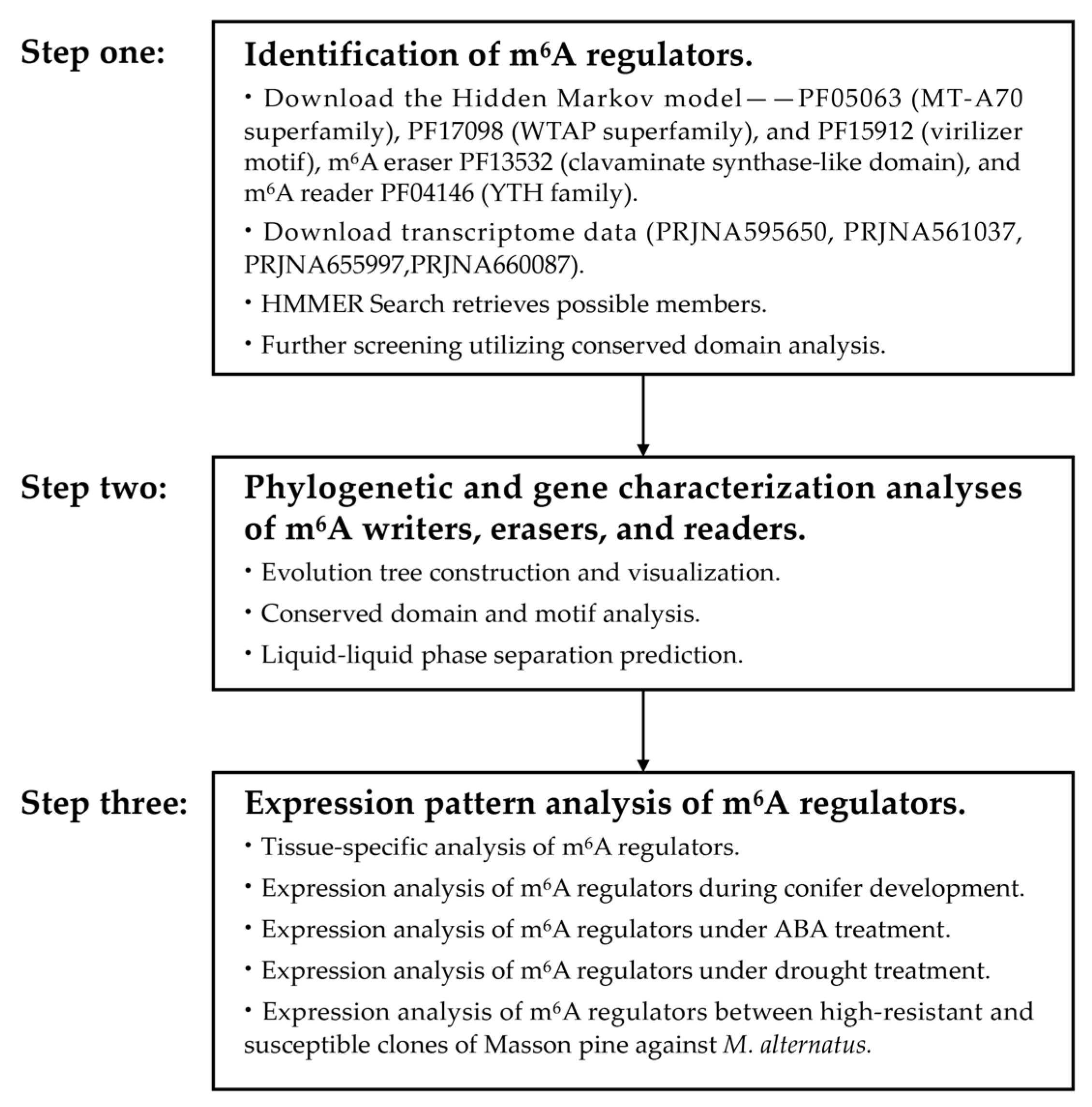
2.1. Identification and Characterization Analysis of m6A Pathway Genes in Masson Pine
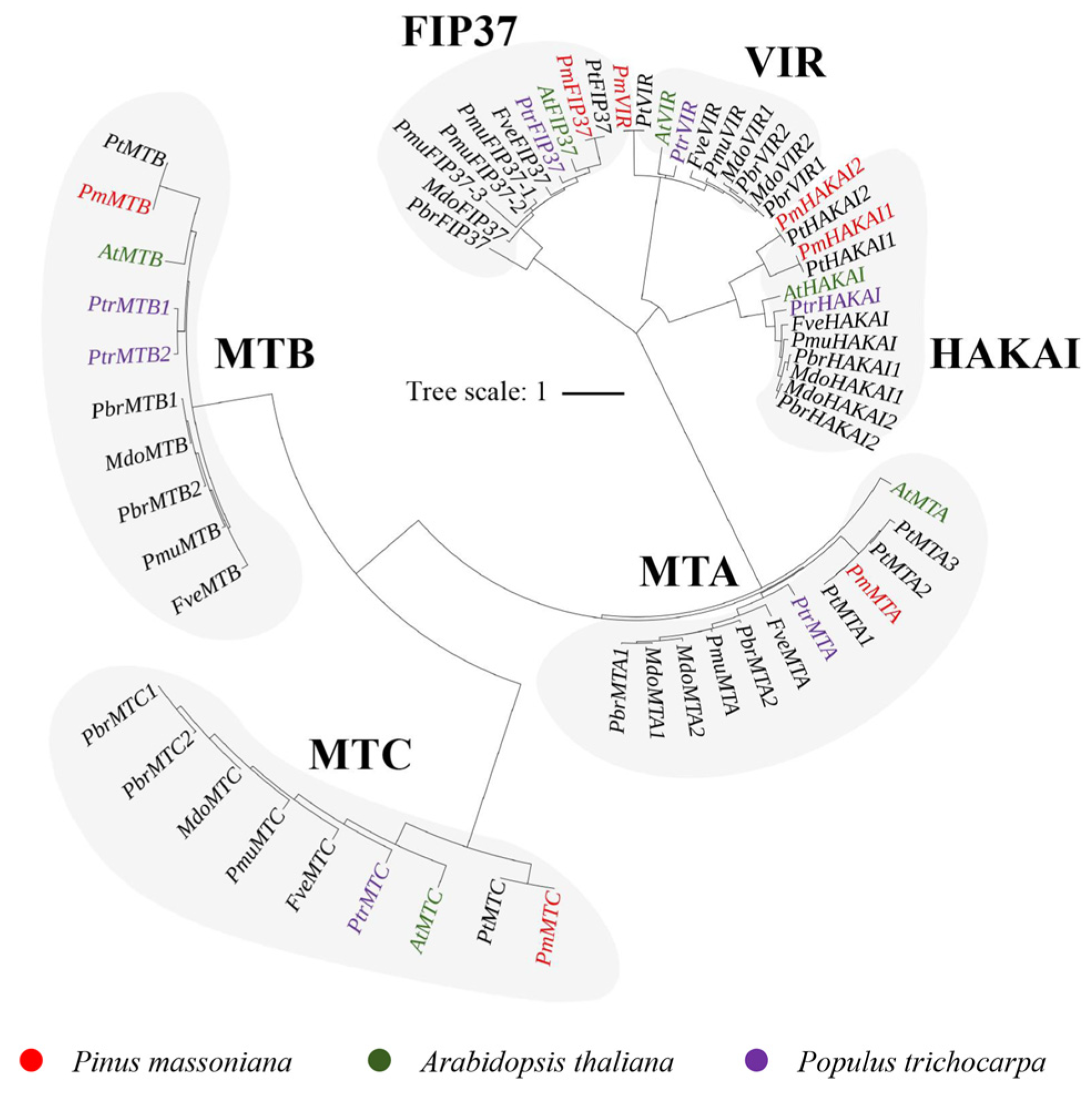
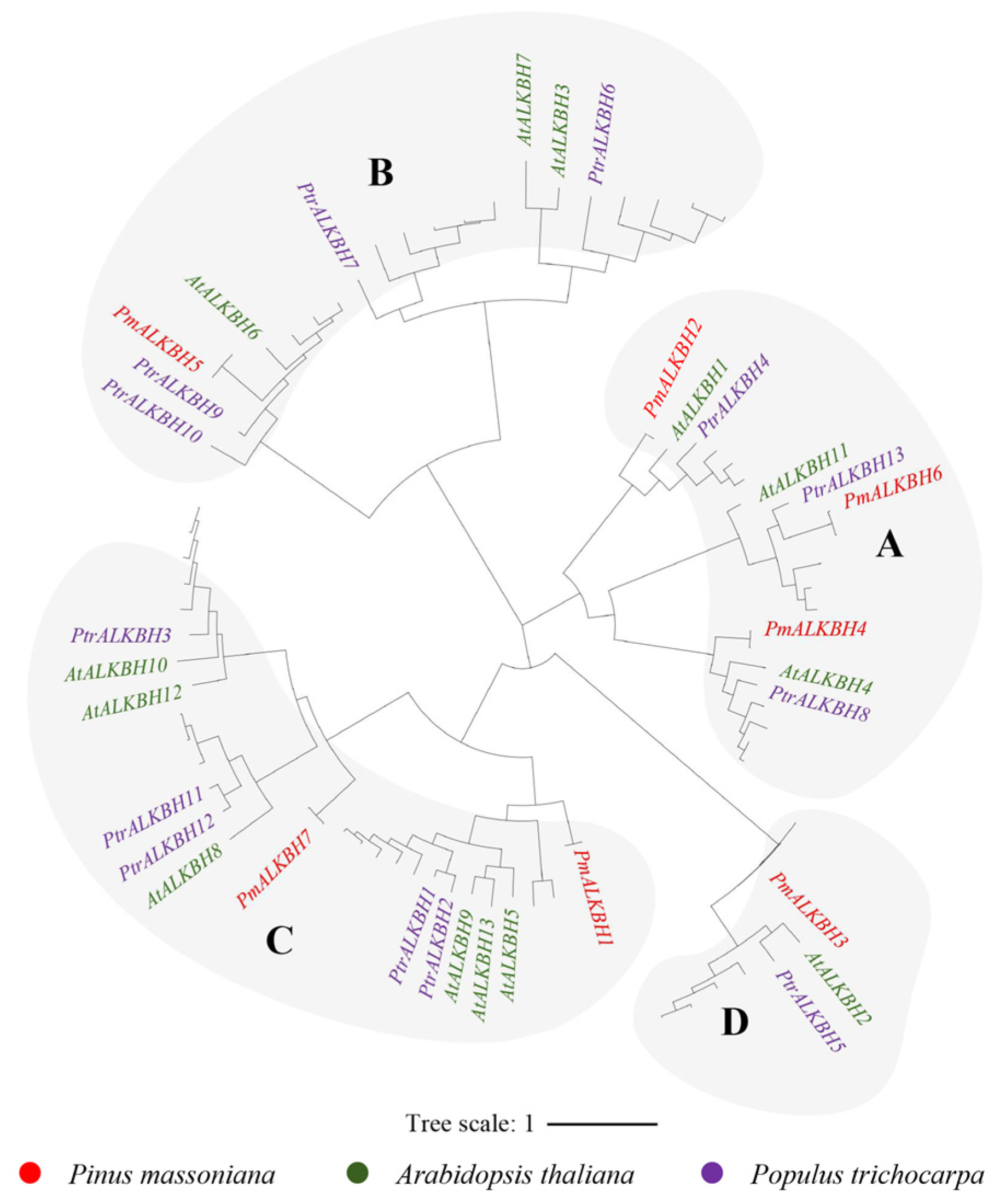
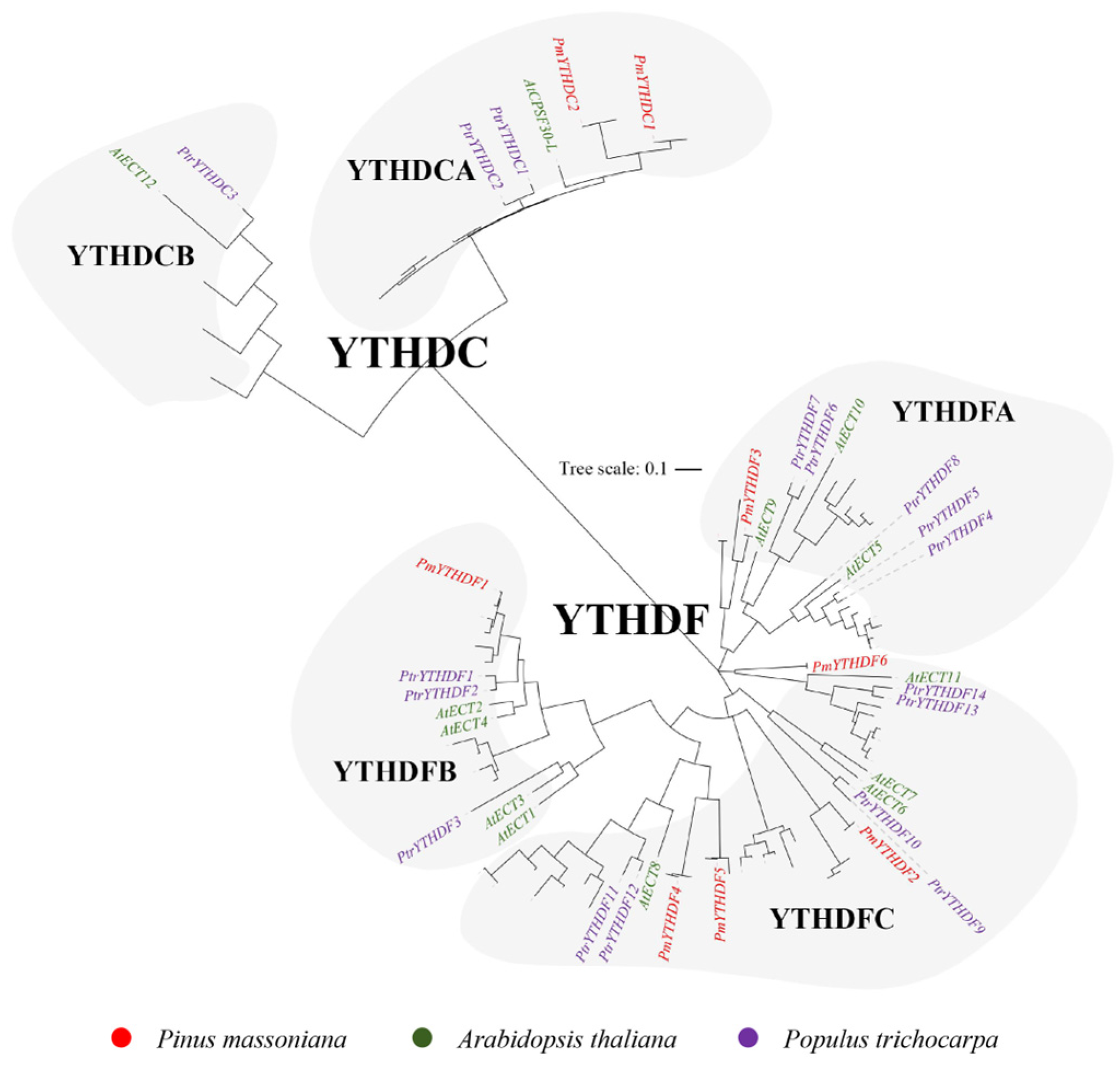
2.2. Phylogenetic and Gene Characterization Analyses of m6A Writers, Erasers, and Readers
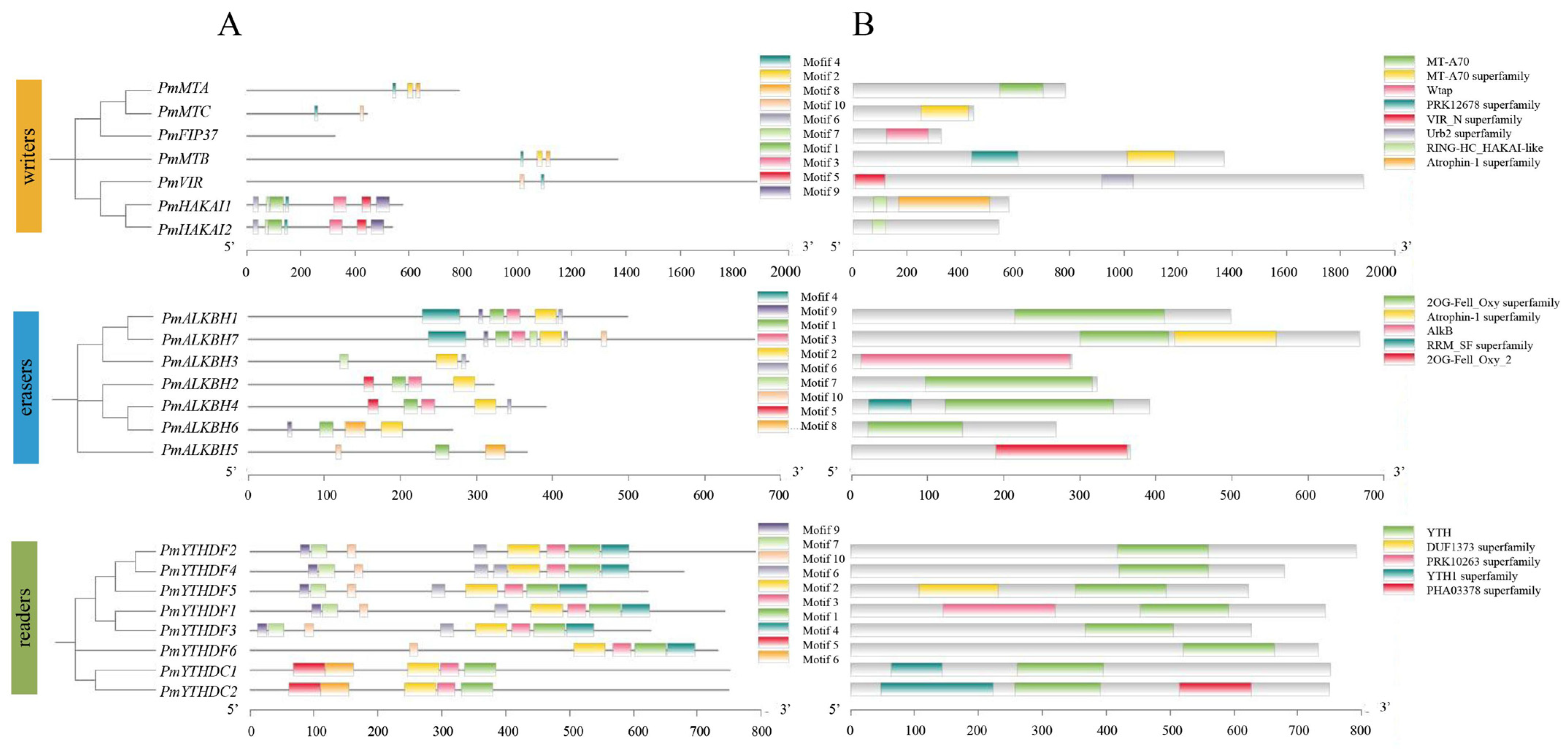
2.3. Gene Structure and Conserved Motif Analysis
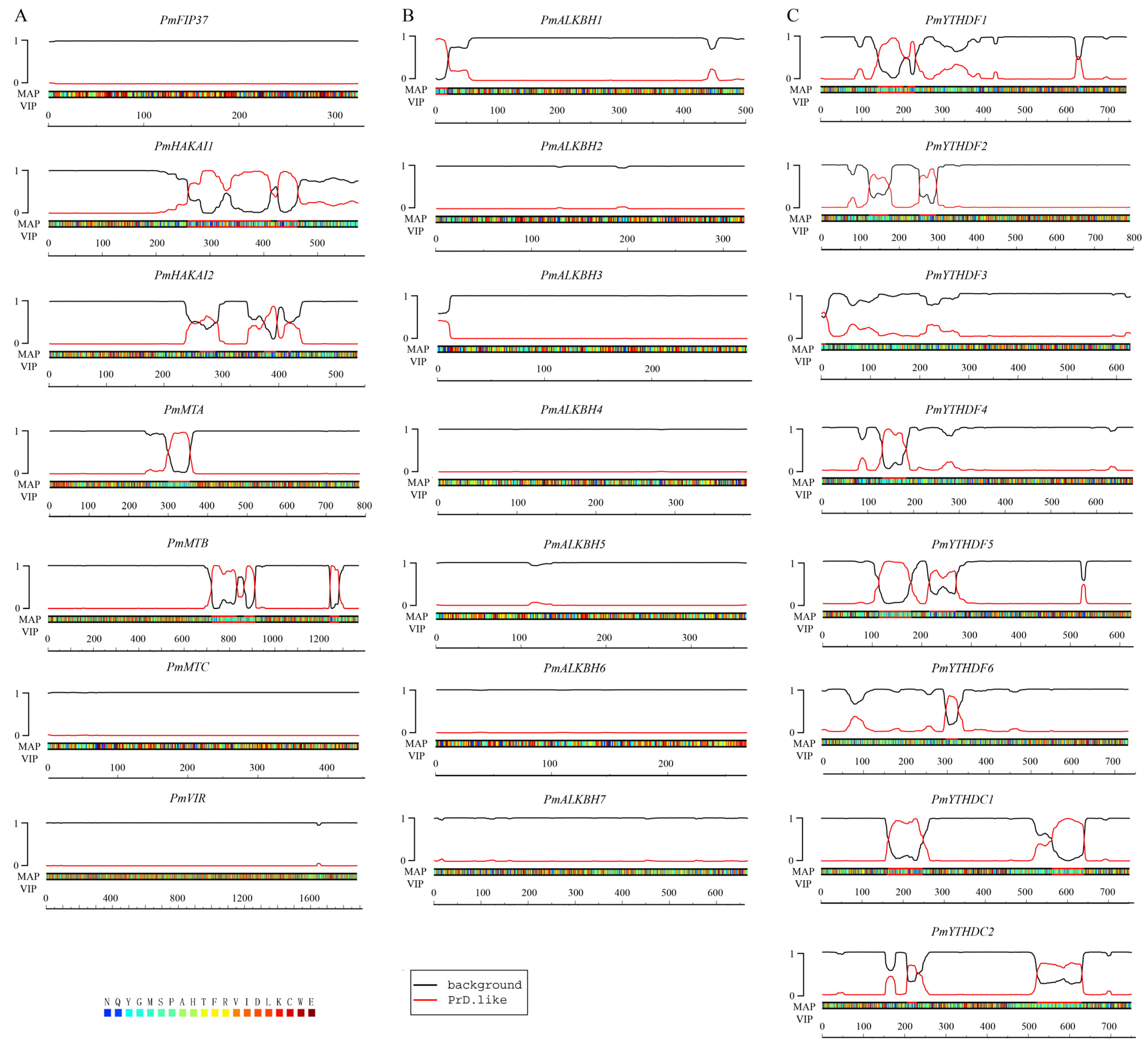
2.4. Certain Authors and Readers May Participate in the Process of Phase Separation
2.5. Expression Levels of m6A Regulators in Different Tissues
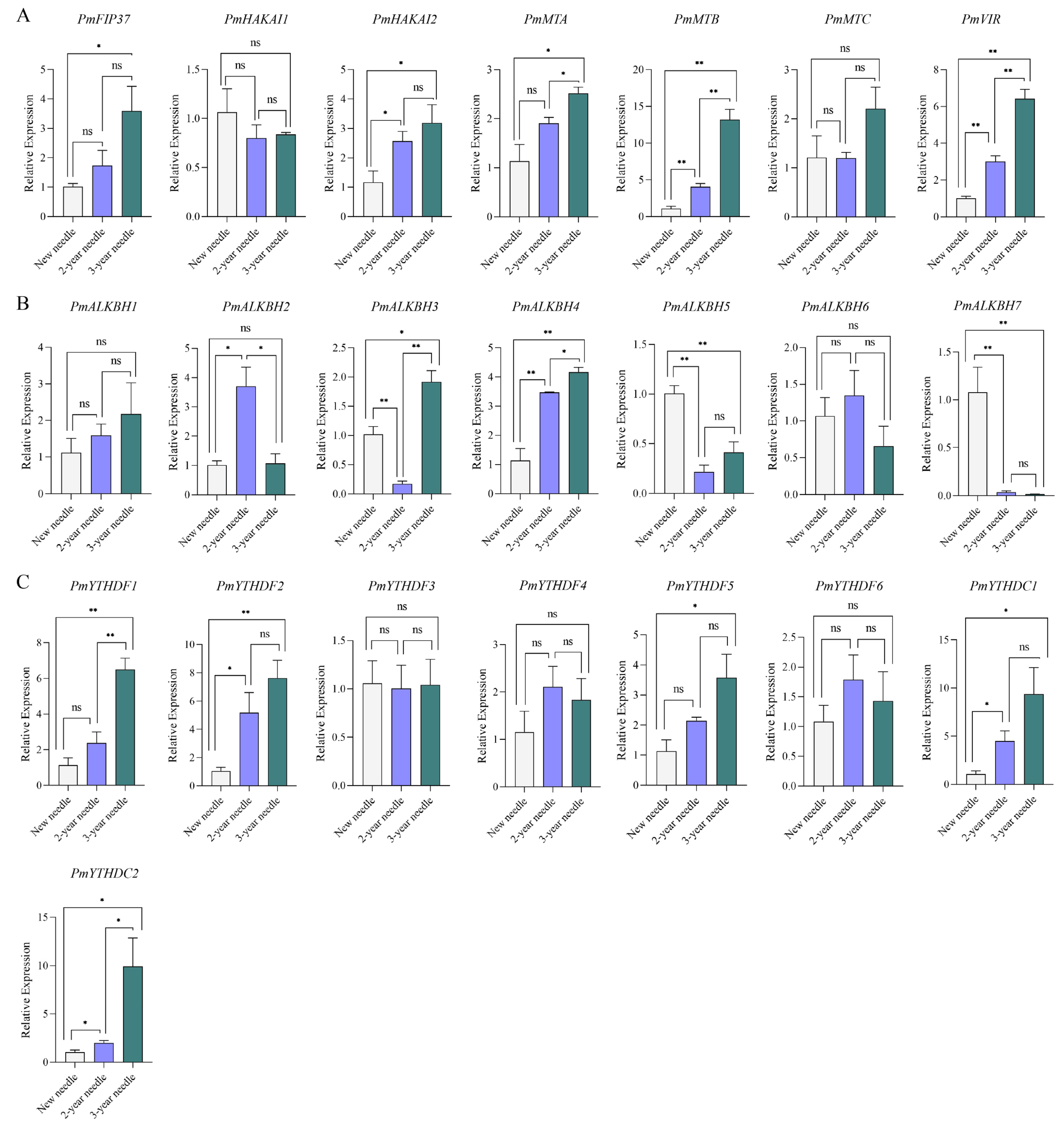
2.6. Expression Levels of m6A Regulators during Conifer Development
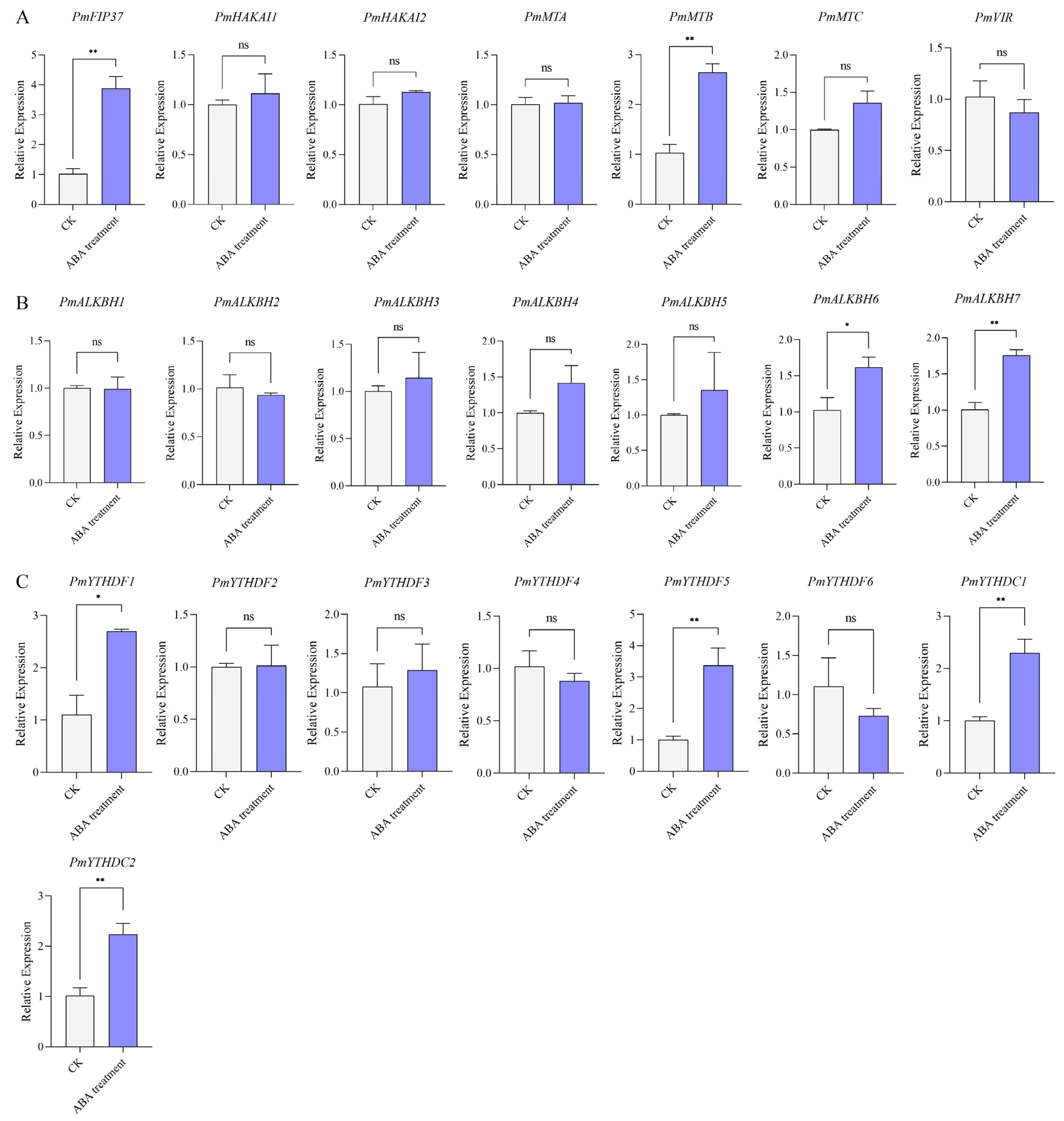
2.7. Expression Levels of m6A Regulators under ABA Treatment
2.8. Expression Levels of m6A Regulators under Different Stresses
3. Discussion
3.1. The m6A Regulators of Masson Pine May Have a Similar But Unique Molecular Mechanism to Them in Other Plants
3.2. The m6A Regulators May Participate in LLPS Process through PrLDs Domain
3.3. The m6A Regulators of Masson Pine Potentially Have a Crucial Impact on Both the Development of Reproductive Organs and the Senescence Process of Pine Needles
3.4. The m6A Regulators of Masson Pine Potentially Play a Pivotal Role in Drought and ABA Response and Confers Resistance Against the M. alternatus
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of m6A Regulators: Writers, Erasers, and Readers in Masson Pine
4.3. Sequence Analysis
4.4. Conserved Motifs and Functional Domains Analyses
4.5. LLPS Prediction
4.6. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR
4.7. RNA-Seq Data Analysis of m6A Regulators
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. Nucleic Acids Res. 2022, 50, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, X.; Yi, C. Epitranscriptome sequencing technologies: Decoding RNA modifications. Nat. Methods 2016, 14, 23–31. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; He, C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015, 29, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; SalmonDivon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernández, L.; Bressendorff, S.; Hansen, M.H.; Poulsen, C.; Erdmann, S.; Brodersen, P. An m6A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell 2018, 30, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.; Evans, M.; Pan, T.; He, C. Dynamic RNA modifications in gene expression regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Schwartz, S.; Agarwala, S.D.; Mumbach, M.R.; Jovanovic, M.; Mertins, P.; Shishkin, A.; Tabach, Y.; Mikkelsen, T.S.; Satija, R.; Ruvkun, G.; et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 2013, 155, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Li, C.; Hu, S.; Yu, J.; Song, S. Transcriptomewide N6-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014, 11, 1180–1188. [Google Scholar] [CrossRef]
- Parker, M.T.; Knop, K.; Sherwood, A.V.; Schurch, N.J.; Mackinnon, K.; Gould, P.D.; Hall, A.J.; Barton, G.J.; Simpson, G.G. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. Elife 2020, 9, 49658. [Google Scholar] [CrossRef]
- Zhong, S.; Li, H.; Bodi, Z.; Button, J.; Vespa, L.; Herzog, M.; Fray, R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 2008, 20, 1278–1288. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, X.; Zhong, M.; Li, X.; Qu, L.; Zhang, H.; Tang, D.; Liu, X.; He, C.; et al. The blue light receptor CRY1 interacts with FIP37 to promote N6-methyladenosine RNA modification and photomorphogenesis in Arabidopsis. New Phytol. 2022, 237, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, K.; Zhang, M.; Campilho, A.; Bodi, Z.; Kashif, M.; Saleh, M.; Eeckhout, D.; El-Showk, S.; Li, H.; Zhong, S.; et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017, 215, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.T.; Knop, K.; Zacharaki, V.; Sherwood, A.V.; Tomé, D.; Yu, X.; Martin, P.G.; Beynon, J.; Michaels, S.D.; Barton, G.J.; et al. Widespread premature transcription termination of Arabidopsis thaliana NLR genes by the spen protein FPA. Elife 2021, 10, 65537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bodi, Z.; Mackinnon, K.; Zhong, S.; Archer, N.; Mongan, N.P.; Simpson, G.G.; Fray, R.G. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat. Commun. 2022, 13, 1127. [Google Scholar] [CrossRef]
- Bhat, S.S.; Bielewicz, D.; Gulanicz, T.; Bodi, Z.; Xiang, Y.; Anderson, S.J.; Szewc, L.; Bajczyk, M.; Dolata, J.; Grzelak, N.; et al. mRNA adenosine methylase (MTA) deposits m6A on pri-miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 21785–21795. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liang, Z.; Yu, H. N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Mech. Dev. 2017, 145, S171. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, Y.; Peng, R.; Dong, J.; Lan, Y.; Duan, S.; Chang, Z.; Ren, J.; Luo, G.; Liu, B.; et al. N6-methyladenosine RNA modification regulates photosynthesis during photodamage in plants. Nat. Commun. 2022, 13, 7441. [Google Scholar] [CrossRef]
- Hu, J.; Cai, J.; Park, S.J.; Lee, K.; Li, Y.; Chen, Y.; Yun, J.Y.; Xu, T.; Kang, H. N6-methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 2021, 106, 1759–1775. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Shi, Y.; Wang, W.L.; Song, S.H.; Lu, Z.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Hofmann, N.R. Epitranscriptomics and flowering: mRNA methylation/demethylation regulates flowering time. Plant Cell 2017, 29, 2949–2950. [Google Scholar] [CrossRef] [PubMed]
- Mielecki, D.; Zugaj, D.Ł.; Muszewska, A.; Piwowarski, J.; Chojnacka, A.; Mielecki, M.; Nieminuszczy, J.; Grynberg, M.; Grzesiuk, E. Novel AlkB dioxygenases-alternative models for in silico and in vivo studies. PLoS ONE 2012, 7, 30588. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.C.; Wei, L.H.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.; Chen, P.R.; He, C.; Jia, G. ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, S.; Qin, G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Hong, Y.; Huang, L.; Li, X.; Zhang, Y.; Ouyang, Z.; Song, F. Genome-wide identification, biochemical characterization, and expression analyses of the YTH domain-containing RNA-binding protein family in Arabidopsis and Rice. Plant Mol. Biol. Rep. 2014, 32, 1169–1186. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosinedependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Scutenaire, J.; Deragon, J.; Jean, V.; Benhamed, M.; Raynaud, C.; Favory, J.; Merret, R.; Bousquet-Antonelli, C. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 2018, 30, 986–1005. [Google Scholar] [CrossRef]
- Wei, L.H.; Song, P.Z.; Wang, Y.; Lu, Z.K.; Tang, Q.; Yu, Q.; Xiao, Y.; Zhang, X.; Duan, H.C.; Jia, G.F. The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell 2018, 30, 968–985. [Google Scholar] [CrossRef]
- Arribas-Hernández, L.; Simonini, S.; Hansen, M.H.; Paredes, E.B.; Bressendorff, S.; Dong, Y.; Østergaard, L.; Brodersen, P. Recurrent requirement for the m6A-ECT2/ECT3/ECT4 axis in the control of cell proliferation during plant organogenesis. Development 2020, 147, 189134. [Google Scholar] [CrossRef]
- Song, P.; Yang, J.; Wang, C.; Lu, Q.; Shi, L.; Tayier, S.; Jia, G. Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol. Plant 2021, 14, 571–587. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Q.; Song, P.; Tian, E.; Yang, J.; Jia, G. The m6A reader ECT8 is an abiotic stress sensor that accelerates mRNA decay in Arabidopsis. Plant Cell 2024, koae149. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Liao, J.; Yu, Y.; Zhou, Y.; Feng, Y.; Yang, Y.; Lei, M.; Bai, M.; Wu, H.; et al. The subunit of RNA N6-methyladenosine methyltransferase OsFIP regulates early degeneration of microspores in rice. PLoS Genet. 2019, 15, e1008120. [Google Scholar] [CrossRef]
- Song, P.; Wei, L.; Chen, Z.; Cai, Z.; Lu, Q.; Wang, C.; Tian, E.; Jia, G. m6A readers ECT2/ECT3/ECT4 enhance mRNA stability through direct recruitment of the poly(A) binding proteins in Arabidopsis. Genome Biol. 2023, 24, 103. [Google Scholar] [CrossRef]
- Han, R.; Shoaib, Y.; Cai, J.; Kang, H. ALKBH10B-mediated m6A demethylation is crucial for drought tolerance by affecting mRNA stability in Arabidopsis. Environ. Exp. Bot. 2023, 209, 105306. [Google Scholar] [CrossRef]
- Cui, C.; Ma, Z.; Wan, H.; Gao, J.; Zhou, B. GhALKBH10 negatively regulates salt tolerance in cotton. Plant Physiol. Biochem. 2022, 192, 87–100. [Google Scholar] [CrossRef]
- Wu, X.; Su, T.; Zhang, S.; Zhang, Y.; Wong, C.; Ma, J.; Shao, Y.; Hua, C.; Shen, L.; Yu, H. N6-methyladenosine-mediated feedback regulation of abscisic acid perception via phase-separated ECT8 condensates in Arabidopsis. Nat. Plants 2024, 10, 469–482. [Google Scholar] [CrossRef]
- Tang, J.; Yang, J.; Duan, H.; Jia, G. ALKBH10B, an mRNA m6A demethylase, modulates ABA response during seed germination in Arabidopsis. Front. Plant Sci. 2021, 12, 712713. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yang, J.; Lu, Q.; Tang, Q.; Chen, S.; Jia, G. The RNA N6-methyladenosine demethylase ALKBH9B modulates ABA responses in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 2361–2373. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, Y.; Zhang, X.; Guo, L.; Zhu, L.; Sun, D.; Sun, K.; Xu, X.; Yang, X.; Xie, W.; et al. RNA m6A methylation suppresses insect juvenile hormone degradation to minimize fitness costs in response to a pathogenic attack. Adv. Sci. 2024, 11, 2307650. [Google Scholar] [CrossRef]
- Han, C.; Dong, H.; Qiao, Q.; Dai, Y.; Huang, X.; Zhang, S. Comparative genomic analysis of N6-methyladenosine regulators in nine rosaceae species and functional characterization in response to drought stress in pear. Hortic. Plant J. 2023, 9, 693–704. [Google Scholar] [CrossRef]
- Zhu, P.H.; Ma, Y.Y.; Zhu, L.Z.; Chen, Y.; Ji, K.S. Selection of suitable reference genes in Pinus massoniana Lamb. under different abiotic stresses for qPCR normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Song, Y.; Cheng, X.; Wang, D.; Li, Q.; Zhang, J.; Chen, Q.; Yu, Q.; Ji, K. Transcriptome-Wide Identification of m6A Writers, Erasers and Readers and Their Expression Profiles under Various Biotic and Abiotic Stresses in Pinus massoniana Lamb. Int. J. Mol. Sci. 2024, 25, 7987. https://doi.org/10.3390/ijms25147987
Yao S, Song Y, Cheng X, Wang D, Li Q, Zhang J, Chen Q, Yu Q, Ji K. Transcriptome-Wide Identification of m6A Writers, Erasers and Readers and Their Expression Profiles under Various Biotic and Abiotic Stresses in Pinus massoniana Lamb. International Journal of Molecular Sciences. 2024; 25(14):7987. https://doi.org/10.3390/ijms25147987
Chicago/Turabian StyleYao, Sheng, Yidan Song, Xiang Cheng, Dengbao Wang, Qianzi Li, Jingjing Zhang, Qingyang Chen, Qiong Yu, and Kongshu Ji. 2024. "Transcriptome-Wide Identification of m6A Writers, Erasers and Readers and Their Expression Profiles under Various Biotic and Abiotic Stresses in Pinus massoniana Lamb." International Journal of Molecular Sciences 25, no. 14: 7987. https://doi.org/10.3390/ijms25147987




