Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth.
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yields
2.2. GC and GC-MS Analyses of the EOs
2.3. Total Polyphenols and Flavonoids
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity Assay
2.4.2. β-Carotene Bleaching Test
2.4.3. Reducing Power Test
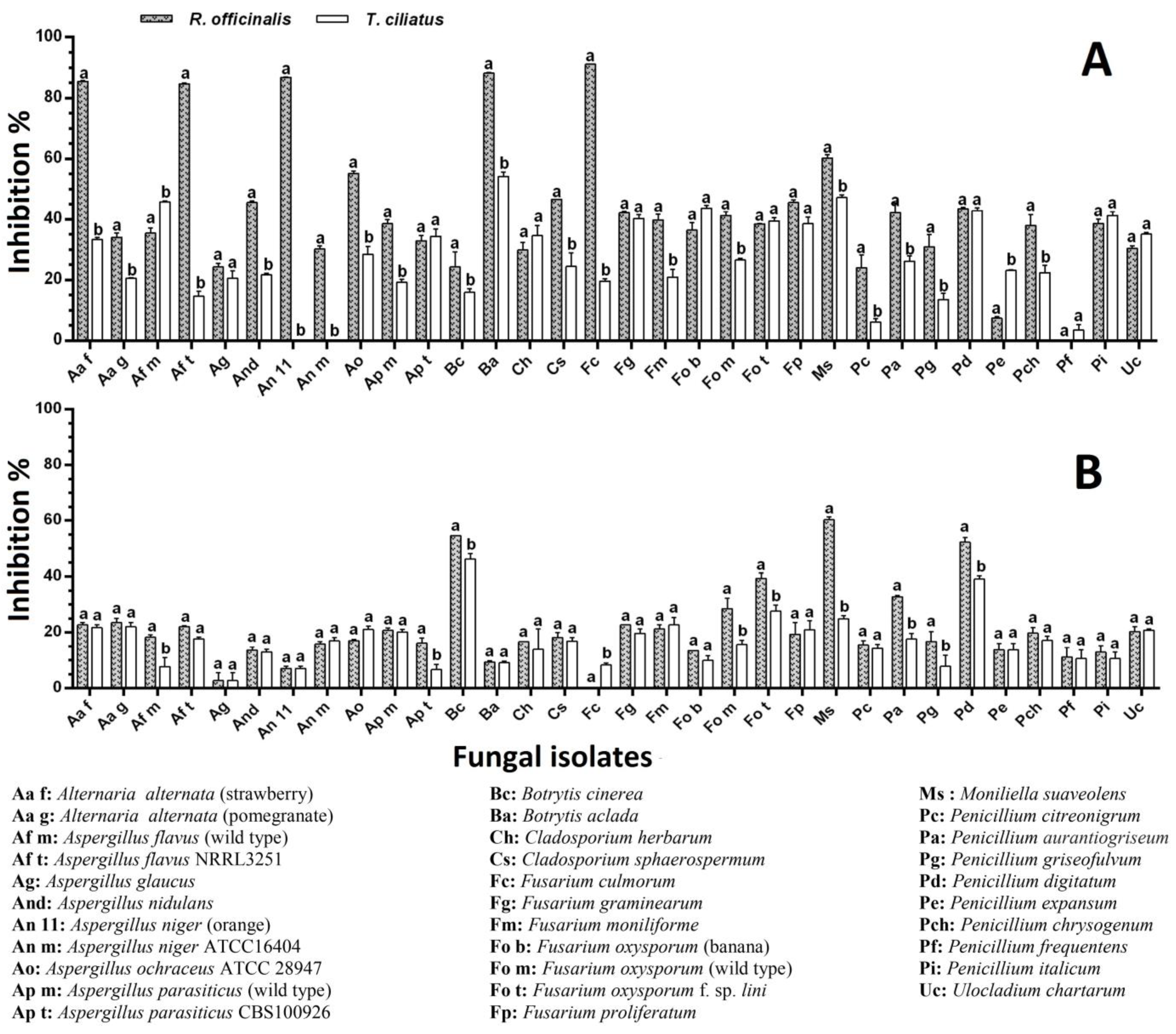
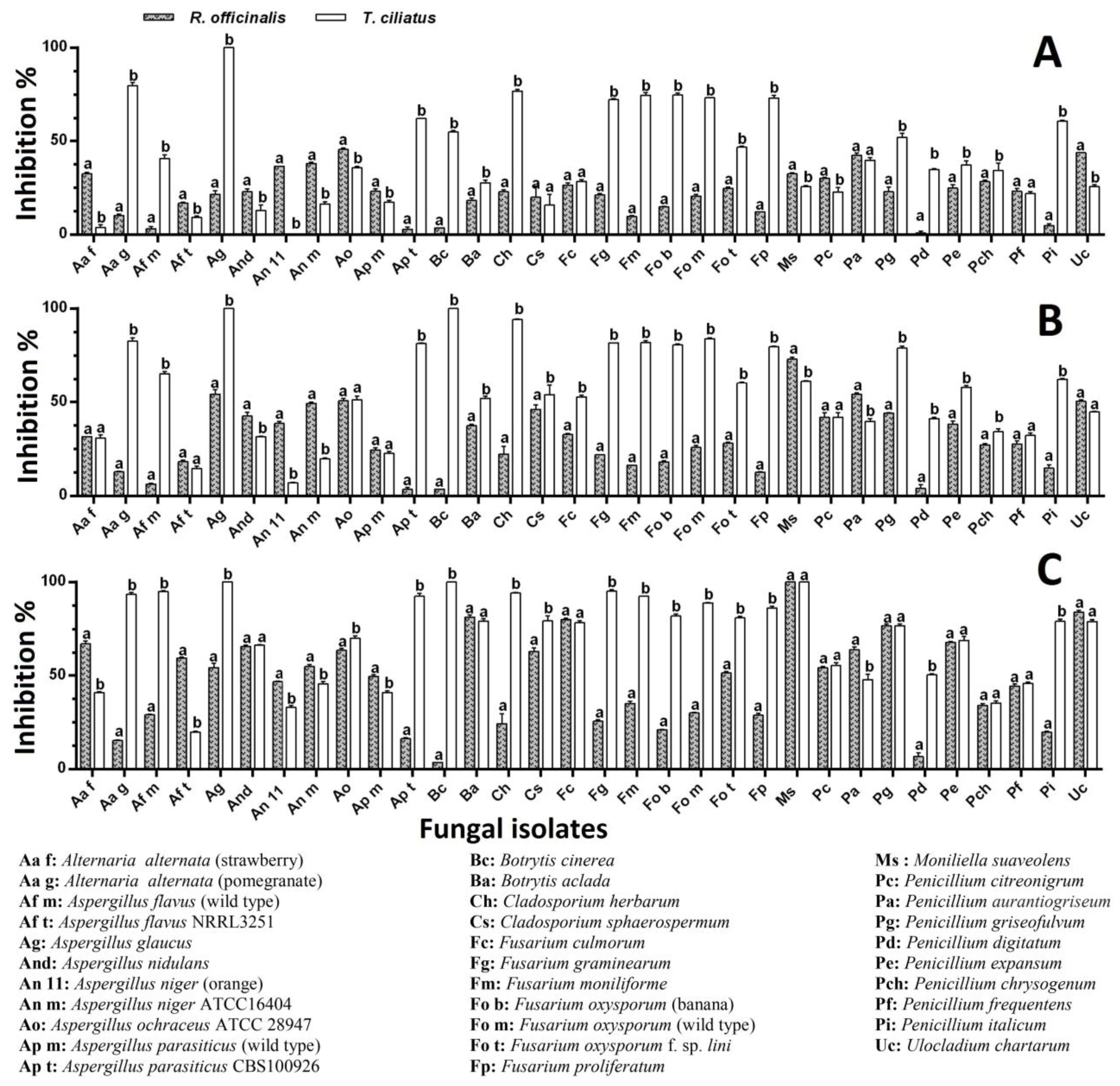
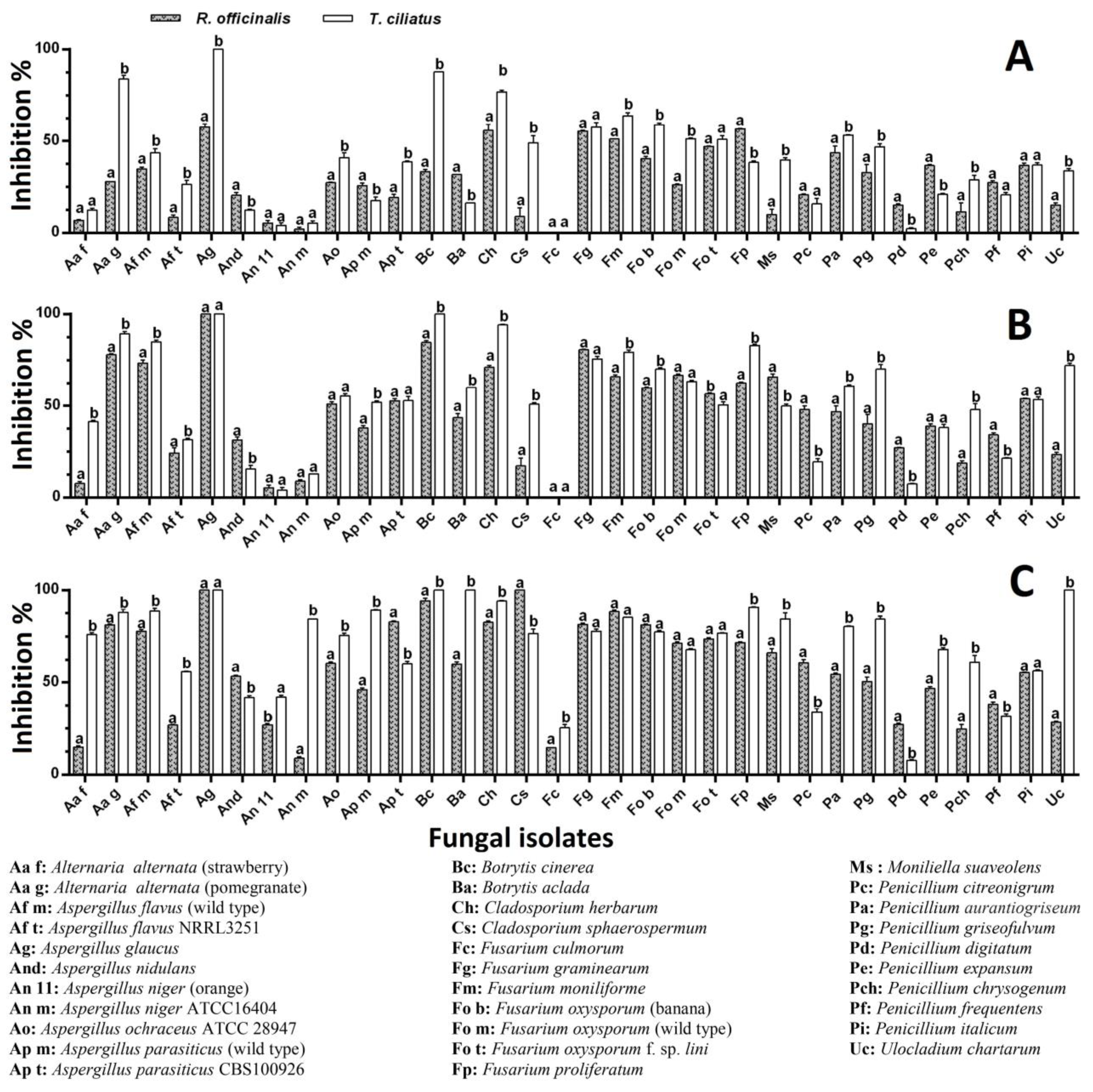
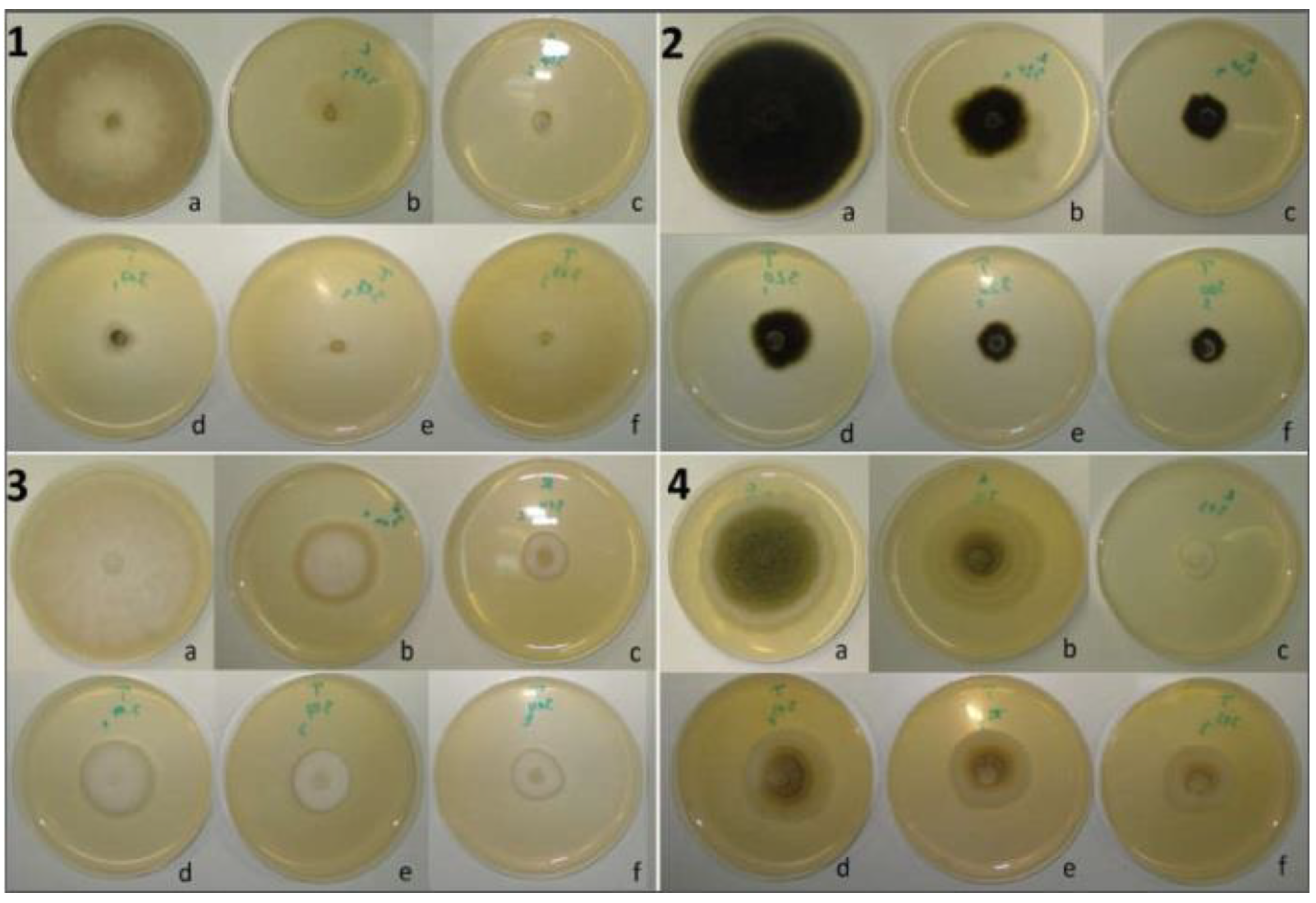
2.5. Antifungal Activity
3. Materials and Methods
3.1. Plant Material and Essential Oil Extraction
3.2. The Methanol Extract Preparation
3.3. GC- and GC-MS Analyses and Identification of the EOs Components
3.4. Determination of the Total Phenolic and Flavonoid Contents
3.5. Antioxidant Activity
3.5.1. DPPH Radical Scavenging Activity Assay
3.5.2. β-Carotene Bleaching Assay
3.5.3. Reducing Power Assay
3.6. Antifungal Activity
3.6.1. Fungal Strains
3.6.2. Antifungal Activity Assays
Effect of Plant Powder on the Mold Mycelial Growth
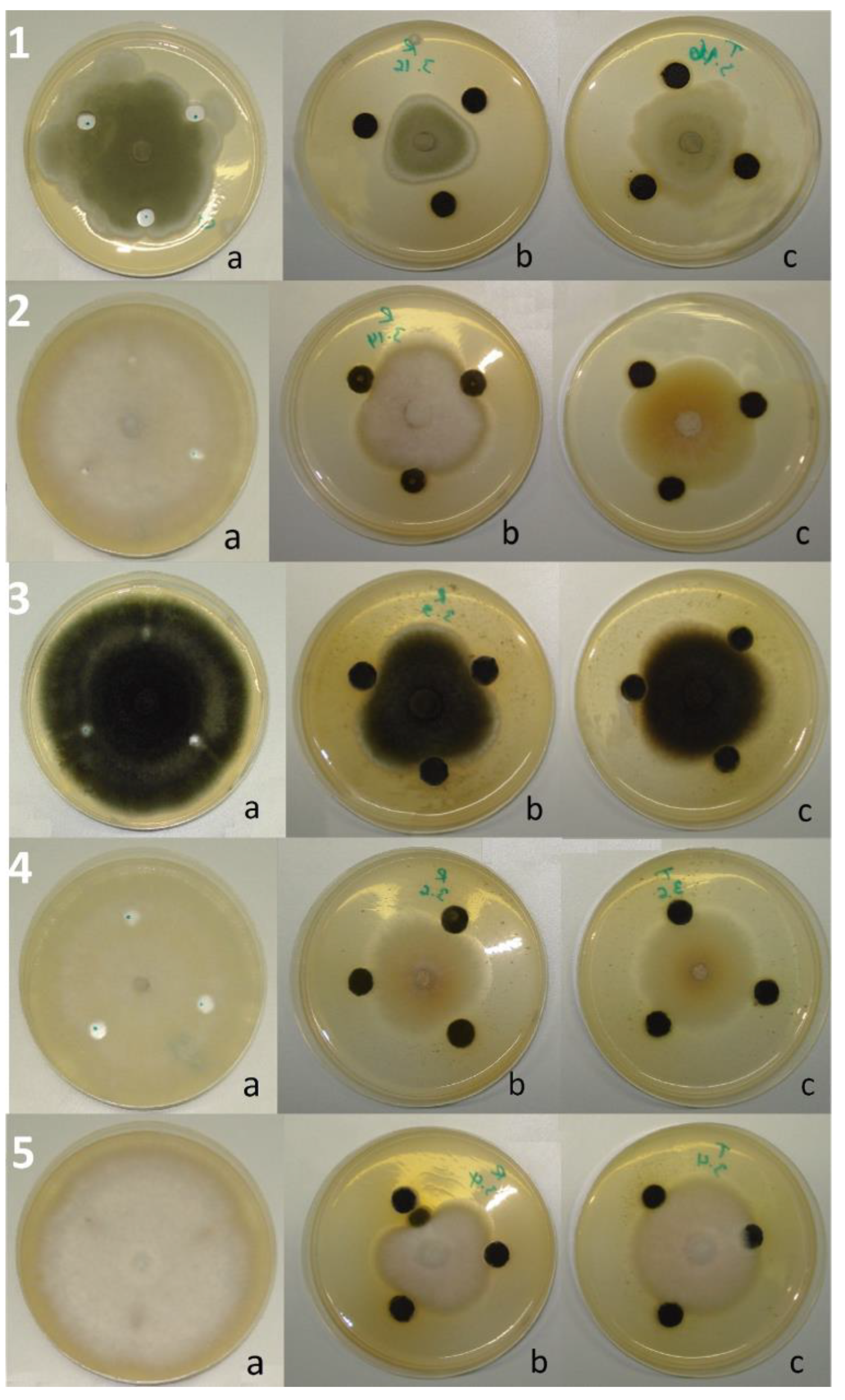
Agar-Well Diffusion Method
Fumigation Bioassay
Contact Bioassay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prieto, J.A.; Patiño, O.J.; Plazas, E.A.; Pabón, L.C.; Ávila, M.C.; Guzmán, J.D.; Delgado, W.A.; Cuca, L.E. Natural products from plants as potential source agents for controlling Fusarium. In Fungicides—Showcases of Integrated Plant Disease Management from Around the World; Nita, M., Ed.; InTech: London, UK, 2013; pp. 233–278. ISBN 978-953-51-1130-6. [Google Scholar] [CrossRef][Green Version]
- Deravel, J.; Krier, F.; Jacques, P. Les biopesticides, compléments et alternatives aux produits phytosanitaires chimiques (synthèse bibliographique). Biotechnol. Agron. Soc. Environ. 2014, 18, 220–232. [Google Scholar]
- Sharifi, S.; Moghaddam, F.A.; Abedi, A.; Maleki Dizaj, S.; Ahmadian, S.; Abdolahinia, E.D.; Khatibi, S.M.H.; Samiei, M. Phytochemicals impact on osteogenic differentiation of mesenchymal stem cells. BioFactors 2020, 46, 874–893. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, A. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Bulboacӑ, A.E. Mycotoxins. In Foodborne Diseases, 3rd ed.; Dodd, C., Aldsworth, T., Stein, R.A., Cliver, D., Riemann, H., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2017; pp. 407–446. [Google Scholar]
- Bouelet Ntsama, I.S.; Frazzoli, C.; Pouokam, G.B.; Colizzi, V. Occurrence and dietary risk assessment of mycotoxins in most consumed foods in cameroon: Exploring current data to understand futures challenges. Foods 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Scanavacca, J.; Bortolucci, W.C.; Jacomassi, E.; Baretta, I.P.; Faria, M.G.I.; Fernandez, C.M.M.; Tešević, V.; Glamoćlija, J.; Soković, M.; Colauto, N.B.; et al. Antimicrobial activity of Tetradenia riparia leaf essential oil. Bol. Latinoam Caribe Plant Med. Aromat. 2023, 22, 255–267. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Michalak, M.; Generalić Mekinić, I.; Kačániová, M.; Chaari, M.; Brini, F.; Ben Saad, R.; Mnif, W.; Garzoli, S.; Ben Hsouna, A. Mixture design of α-pinene, α-terpineol, and 1,8-cineole: A multiobjective response followed by chemometric approaches to optimize the antibacterial effect against various bacteria and antioxidant activity. Food Sci. Nutr. 2024, 12, 574–589. [Google Scholar] [CrossRef]
- IUCN (International Union for Conservation of Nature and Natural Resources) (Ed.) A Guide to Medicinal Plants in North Africa; IUCN Centre for Mediterranean Cooperation: Málaga, Spain, 2005; 256p, ISBN 2-8317-0893-1. [Google Scholar]
- Charles, D.J. Thyme. In Antioxidant Properties of Spices, Herbs and Other Sources; Charles, D.J., Ed.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 553–561. ISBN 978-1-4614-4309-4/978-1-4614-4310-0. [Google Scholar] [CrossRef]
- Baba Aissa, F. Encyclopédie des plantes utiles. In Flore d’Algérie et du Maghreb, Substances Végétales D’afrique, D’orient et D’occident; EDAS: Algiers, Algeria, 1999; 368p. [Google Scholar]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Syst. Ecol. 2010, 38, 659–670. [Google Scholar] [CrossRef]
- Goetz, P.; Ghédira, K. Phytothérapie Anti-Infectieuse; Springer: France, Paris, 2012; 382p, ISBN 978-2-8178-0057-8. [Google Scholar]
- Meccatti, V.M.; Oliveira, J.R.; Figueira, L.W.; Lagareiro Netto, A.A.; Zamarioli, L.S.; Marcucci, M.C.; Camargo, S.; Carvalho, C.; Deoliveira, L.D. Rosmarinus officinalis L.(rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. An. Acad. Bras. Ciênc. 2021, 93, e20190366. [Google Scholar] [CrossRef]
- Bekhechi, A.; Malti, C.E.W.; Babali, B.; Bouafia, M.; Bekhechi, C.; Casanova, J.; Mathieu, P.; Félix, T. Chemical variability and anti-inflammatory activity of Rosmarinus officinalis L. leaf essential oil from Algerian Sahara. Chem. Biodiv. 2024, 21, e202302077. [Google Scholar] [CrossRef]
- Ben Kaab, S.; Rebey, I.B.; Hanafi, M.; Berhal, C.; Fauconnier, M.L.; De Clerck, C.; Ksouri, R.; Jijakli1, H. Rosmarinus officinalis essential oil as an effective antifungal and herbicidal agent Span. J. Agric. Res. 2019, 17, e1006. [Google Scholar]
- Da Silva Bomfim, N.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Mikcha, J.M.G.; Filho, B.A.A.; Machinski, M., Jr. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A-Chem. 2020, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Boukhobza, Z.; Boulenouar, N.; Abdelkrim, C.; Kadri, Z. Essential oil of Rosmarinus officinalis L. from West Highlands of Algeria: Chemical characterization and in vitro antifungal activity against Fusarium oxysporum f. sp. albedinis. Nat. Vol. Essent. Oil. 2021, 8, 44–55. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kačániová, M. In vitro Antimicrobial activity of lavender, mint and rosemary essential oils and effect of their vapours on growth of Penicillium spp. in a bread model system. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- Beloued, A. Plantes Médicinales d’Algérie; O.P.U.: Ben Aknoun, Alger, 1998; 277p. [Google Scholar]
- Kholkhal, F.; Lazouni, H.A.; Bendahou, M.; Boublenza, I.; Chabane, S.D.; Chaouch, T. Étude phytochimique et évaluation de l’activité anti-oxydante de Thymus ciliatus ssp. coloratus. Afr. Sci. 2013, 9, 151–158. [Google Scholar]
- Ferhat, M.; Ghorab, H.; Laggoune, S.; Ghannadi, A.; Sajjadi, S.E.; Touzani, R.; Kabouche, A.; Kabouche, Z. Composition and antioxidant activity of the essential oil of thymus dreatensis from Algeria. Chem. Nat. Compd. 2014, 50, 747–749. [Google Scholar] [CrossRef]
- Amrouni, S.; Touati, M.; Hadef, Y.; Djahoudi, A. Effet de l’huile essentielle d’Origanum vulgare et de Thymus ciliatus sur Pseudomonas aeruginosa VIM-2 carbapénèmase. Phytothérapie 2014, 12, 309–313. [Google Scholar]
- Ameziane, N.; Boubaker, H.; Boudyach, H.; Msanda, F.; Jilal, A.; Ait Benaoumar, A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 2007, 27, 273–277. [Google Scholar] [CrossRef]
- Jamshidi, R.; Afzali, Z.; Afzali, D. Chemical composition of hydrodistillation essential oil of rosemary in different origins in Iran and comparison with other countries. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 78–81. [Google Scholar]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Kamli, T.E.; Errachidi, F.; Eloutassi, N.; Majid, H.; Chabir, R.; Bour, A. Comparaison quantitative et qualitative des huiles essentielles de Rosmarinus officinalis obtenues par différentes méthodes. Eur. Sci. J. 2017, 13, 1857–7881. [Google Scholar] [CrossRef]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosyst. 2018, 152, 1282–1292. [Google Scholar] [CrossRef]
- Abada, M.B.; Hamdi, S.H.; Masseoud, C.; Jroud, H.; Bousshih, E.; Jemâa, J.M.B. Variations in chemotypes patterns of Tunisian Rosmarinus officinalis essential oils and applications for controlling the date moth Ectomyelois ceratoniae (Pyralidae). S. Afr. J. Bot. 2020, 128, 18–27. [Google Scholar] [CrossRef]
- Amarti, F.; Satrani, B.; Ghanmi, M.; Farah, A.; Aafi, A.; Aarab, L.; El Ajjouri, M.; Chaouch, A. Composition chimique et activité antimicrobienne des huiles essentielles de Thymus algeriensis Boiss. & Reut. et Thymus ciliatus (Desf.) Benth. Du Maroc. Biotechnol. Agron. Soc. Environ. 2010, 14, 141–148. [Google Scholar]
- Ghorab, H.; Kabouche, A.; Kabouche, Z. Comparative compositions of essential oils of Thymus growing in various soils and climates of North Africa. J. Mater. Environ. Sci. 2014, 5, 298–303. [Google Scholar]
- Benomari, F.Z.; Djabou, N.; Moumani, M.; Hassani, F.; Muselli, A.; Costa, J. Chemical variability of essential oils of three subspecies of Thymus munbyanus Boiss. & Reut. from Western Algeria. J. Essent. Oil Res. 2020, 32, 474–484. [Google Scholar]
- Giordani, R.; Hadef, Y.; Kaloustian, J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia 2008, 79, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Hendel, N.; Napoli, E.; Sarri, M.; Saija, A.; Cristani, M.; Nostro, A.; Ginestra, G.; Ruberto, G. Essential oil from aerial parts of wild Algerian rosemary: Screening of chemical composition, antimicrobial and antioxidant activities. J. Essent. Oil Bear. Plants 2019, 22, 1–17. [Google Scholar] [CrossRef]
- Hannour, K.; Boughdad, A.; Maataoui, A.; Bouchelta, A. Chemical composition of Rosmarinus officinalis (Lamiaceae) essential oils and evaluation of their toxicity against Bruchus rufimanus (Coleoptera: Chrysomelidae: Bruchinae) in Morocco. Int. J. Trop. Insect Sci. 2018, 38, 192–204. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef]
- Souadia, A. Chemical composition and antioxidant activity of Thymus ciliatus (Desf.) Benth. essential oils of Algeria. Nat. Prod. Commun. 2022, 17, 1934578X221080337. [Google Scholar] [CrossRef]
- Jamali, C.A.; El Bouzidi, L.; Bekkouche, K.; Lahcen, H.; Markouk, M.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical composition and antioxidant and anticandidal activities of essential oils from different wild Moroccan Thymus species. Chem. Biodivers. 2012, 9, 1188–1197. [Google Scholar] [CrossRef]
- Nickavar, B.; Mojab, F.; Dolat-Abadi, R. Analysis of the essential oils of two Thymus species from Iran. Food Chem. 2005, 90, 609–611. [Google Scholar] [CrossRef]
- Jaafari, A.; Ait Mouse, H.; El Mostapha, R.; Ait M’barek, L.; Tilaoui1, M.; Benbakhta, C.; Boulli, A.; Abbad, A.; Zyad, A. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Braz. J. Pharmacogn. 2007, 17, 477–491. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. In vitro antimicrobial, cytotoxic and radical scavenging activities and chemical constituents of the andemic Thymus laevigatus (Vahl). Rec. Nat. Prod. 2010, 4, 49–63. [Google Scholar]
- Kasrati, A.; Jamali, C.A.; Fadli, M.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Antioxidative activity and synergistic effect of Thymus saturejoides Coss. essential oils with cefixime against selected food-borne bacteria. Ind. Crop. Prod. 2014, 61, 338–344. [Google Scholar] [CrossRef]
- Tefiani, C.; Riazi, A.; Youcefi, F.; Aazza, S.; Gago, C.; Faleiro, M.L.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C.; Megías, C.; et al. Ammoides pusilla (Apiaceae) and Thymus munbyanus (Lamiaceae) from Algeria essential oils: Chemical composition, antimicrobial, antioxidant and antiproliferative activities. J. Essent. Oil Res. 2015, 27, 131–139. [Google Scholar] [CrossRef]
- Kabouche, A.; Ghannadi, A.; Kabouche, Z. Thymus ciliatus—The highest thymol containing essential oil of the genus. Nat. Prod. Commun. 2009, 4, 1251–1252. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221–232. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Girgin, G.; Orhan, H.; Wichers, H.J.; Bedir, E.; Vardar-Sukan, F. Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus fficinalis extracts with focus on location and harvesting times. Eur. Food Res. Technol. 2007, 224, 443–451. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmianskii, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Santos, R.D.; Shetty, K.; Cecchini, A.L.; Miglioranza, L.H.S. Phenolic compounds and total antioxidant activity determination in rosemary and oregano extracts and its use in cheese spread. Semin. Ciênc. Agrár. 2012, 33, 655–666. [Google Scholar] [CrossRef]
- Bányai, E.S.; Tulok, M.H.; Hegedûs, A.; Renner, C.; Varga, I.S. Antioxidant effect of various rosemary (Rosmarinus officinalis L.) clones. Acta Biol. Szeged. 2003, 47, 111–113. [Google Scholar]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Douar-Latreche, S. Effect of heat treatment on the chemical composition and the antioxidant activity of essential oil of Thymus pallescens de Noé from Algeria. J. Essent. Oil Res. 2013, 25, 308–314. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crop. Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013, 5, 729–739. [Google Scholar]
- Okoh, O.O.; Sadimenko, A.P.; Afolayan, A.J. Antioxidant activities of Rosmarinus officinalis L. essential oil obtained by hydrodistillation and solvent free microwave extraction. Afr. J. Biotechnol. 2011, 10, 4207–4211. [Google Scholar]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Grujicic, S.S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Sana, A.M.; Van Baren, C.M.; Elechosa, A.M.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, H.; Kabouche, A.; Semra, Z.; Ghannadi, A.; Sajjadi, E.B.; Touzani, R.; Kabouche, Z. Biological activities and compositions of the essential oil of Thymus ciliatus from Algeria. Pharm. Lett. 2013, 5, 28–32. [Google Scholar]
- Nickavar, B.; Esbati, N. Evaluation of the antioxidant capacity and phenolic content of three Thymus Species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef]
- El Bouzidi, L.; Jamali, C.A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical composition, antioxidant and antimicrobial activities of essential oils obtained from wild and cultivated Moroccan Thymus species. Ind. Crop. Prod. 2013, 43, 450–456. [Google Scholar] [CrossRef]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-Butylhydroquinone. J. Agric. Food Chem. 2004, 52, 5183–5186. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant activity of rosemary and thyme by-products and synergism with added antioxidant in a liposome system. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Yigit, F.; Ozcan, M.; Akgul, A. Inhibitory effect of some spice essential oils on Penicillium digitatum causing postharvest rot in citrus. Grasas Aceites 2000, 51, 237–240. [Google Scholar] [CrossRef]
- Park, J.; Rho, S.J.; Kim, Y.R. Enhancing antioxidant and antimicrobial activity of carnosic acid in rosemary (Rosmarinus officinalis L.) extract by complexation with cyclic glucans. Food Chem. 2019, 299, 125119. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.P.; Hamedi, B.; Abdizadeh, R.; Malekpoor, F. Antifungal activity of the essential oil of Iranian medicinal plants. J. Med. Plants Res. 2011, 5, 5089–5093. [Google Scholar]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Bouchra, C.; Achouri, M.; Idrissi, H.L.M.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef] [PubMed]
- Kocić-Tamackov, S.D.; Dimić, G.R. Antifugal activity of essential oils in the control of foodborne fungi growth and mycotoxine biosynthesis in food. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 4, pp. 838–849. [Google Scholar]
- Wang, Y.; Liu, H.; Zhan, F. Effects of natural borneol on germ tube formation and preformed biofilm activity in Candida albicans. Nat. Prod. Commun. 2022, 17, 1934578X221129128. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Nakassugi, L.P.; Oliveira, J.F.P.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Filho, B.A.A.; Machinski, M., Jr. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Mukhia, S.; Kapoor, S.; Bhatt, V.; Kumar, R.; Kumar, R. Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya. Sci. Rep. 2022, 12, 3305. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agrifood commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-based untargeted lipidomics reveal mechanism of antifungal activity of carvacrol against Aspergillus flavus. Foods 2022, 11, 93. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Ebani, V.V.; Pieracci, Y.; Cagnoli, G.; Bertelloni, F.; Munafò, C.; Nardoni, S.; Pistelli, L.; Mancianti, F. In vitro antimicrobial activity of Thymus vulgaris, Origanum vulgare, Satureja montana and their mixture against clinical isolates responsible for canine otitis externa. Vet. Sci. 2023, 10, 30. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatographic/Quadrupole Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Eskici, M.; Tepe, B.; Can, Ş.; Mete, E. Essential oil composition and antioxidant activity of Thymus longicaulis C. Presl subsp. longicaulis var. longicaulis. Food Chem. Toxicol. 2010, 48, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) Sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea seeds. Food Control 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Sonboli, A. Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against oxidative cardiac cell injury. Food Chem. Toxicol. 2010, 48, 846–853. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009; 524p. [Google Scholar]
- Botton, B.; Breton, A.; Fevre, M.; Gauthier, S.; Guy, P.H.; Larpent, J.P.; Reymond, P.; Sanglier, J.J.; Vayssier, Y.; Veau, P. Moisissures Utiles et Nuisibles, Importance Industrielle, 2nd ed.; Masson: Paris, France, 1990; 512p, revue et complétée. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi; Morphologies of Cultured Fungi and Key to Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; 486p. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; 388p. [Google Scholar]
- Campbell, C.K.; Johnson, E.M.; Warnock, D.W. Identification of Pathogenic Fungi, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; 350p. [Google Scholar]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett. Appl. Microbiol. 2012, 55, 155–161. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Marandi, R.J.; Hassani, A.; Ghosta, Y.; Abdollahi, A.; Pirzad, A.; Sefidkon, F. Control of Penicillium expansum and Botrytis cinerea on pear with Thymus kotschyanus, Ocimum basilicum and Rosmarinus officinalis essential oils. J. Med. Plant. Res. 2011, 5, 626–634. [Google Scholar]


| Class/Compound | R. officinalis | T. ciliatus | |||
|---|---|---|---|---|---|
| N° a | RI Exp, b | RI Lit, c | Monoterpene Hydrocarbons | 43.97 | 53.11 |
| 1 | 922 | 926 | tricyclene | 0.11 | 0.05 |
| 2 | 927 | 930 | α-thujene | 2.01 | |
| 3 | 936 | 939 | α-pinene | 17.49 | 22.18 |
| 4 | 951 | 954 | camphene | 18.14 | 0.60 |
| 5 | 955 | 952 | fenchene | 0.08 | |
| 6 | 972 | 975 | sabinene | 0.40 | |
| 7 | 976 | 979 | β-pinene | 0.51 | 7.73 |
| 9 | 987 | 990 | myrcene | 0.42 | 13.13 |
| 10 | 1001 | 1002 | α-phellandrene | 0.11 | 0.13 |
| 11 | 1015 | 1017 | α-terpinene | 0.31 | 0.28 |
| 12 | 1023 | 1024 | p-cymene | 2.45 | 2.11 |
| 13 | 1029 | 1029 | limonene | 3.98 | 3.32 |
| 14 | 1036 | 1037 | cis-ocimene | t | |
| 15 | 1046 | 1050 | trans-ocimene | 0.35 | |
| 16 | 1059 | 1060 | γ-terpinene | 0.22 | 0.57 |
| 17 | 1088 | 1089 | terpinolene | 0.23 | 0.17 |
| Oxygenated Monoterpenes | 50.09 | 7.16 | |||
| 18 | 1032 | 1031 | 1,8-cineole | 4.90 | 2.58 |
| 19 | 1081 | 1086 | trans-linalool oxide | 0.21 | |
| 20 | 1099 | 1096 | linalool | 2.29 | |
| 21 | 1122 | 1121 | exo-Fenchol | 0.12 | |
| 22 | 1152 | 1146 | camphor | 41.22 | 0.76 |
| 23 | 1166 | 1164 | pinocarvone | t | |
| 24 | 1169 | 1169 | borneol | 2.55 | 0.12 |
| 25 | 1179 | 1177 | terpinen-4-ol | 0.41 | |
| 26 | 1192 | 1188 | α-terpineol | 1.18 | 0.71 |
| 27 | 1197 | 1195 | myrtenol | 0.08 | |
| 28 | 1290 | 1289 | bornyl acetate | t | |
| 29 | 1298 | 1290 | thymol | t | |
| 30 | 1304 | 1299 | carvacrol | 0.12 | |
| Sesquiterpenes | 2.97 | 39.33 | |||
| 31 | 1375 | 1375 | ylangene | 0.18 | |
| 32 | 1379 | 1376 | α-copaene | 0.21 | 0.88 |
| 33 | 1388 | 1388 | β-bourbonene | 1.15 | |
| 34 | 1388 | 1393 | β-cubebene | 0.61 | |
| 35 | 1424 | 1419 | β-caryophyllene | 0.63 | 10.21 |
| 36 | 1433 | 1434 | α-trans-Bergamotene | ||
| 37 | 1434 | 1432 | β-copaene | 0.82 | |
| 38 | 1440 | 1441 | aromadendrene | 0.09 | |
| 39 | 1445 | 1450 | cis-muurola-3,5-diene | t | |
| 40 | 1451 | 1453 | trans-muurola-3,5-diene | 0.26 | |
| 41 | 1455 | 1454 | α-humulene | 0.91 | |
| 42 | 1458 | 1456 | β-trans-farnesene | t | 0.51 |
| 43 | 1482 | 1479 | γ-muurolene | 1.14 | |
| 44 | 1485 | 1484 | α-amorphene | 0.32 | |
| 45 | 1486 | 1484 | germacrene D | 0.12 | 9.90 |
| 46 | 1486 | 1486 | β-selinene | 0.14 | |
| 47 | 1490 | 1490 | trans-muurola-4(14),5-diene | 0.52 | |
| 48 | 1495 | 1495 | bicyclogermacrene | 0.42 | |
| 49 | 1495 | 1495 | γ-Amorphene | 0.11 | |
| 50 | 1498 | 1496 | valencene | t | |
| 51 | 1500 | 1500 | α-muurolene | 0.27 | |
| 52 | 1505 | 1505 | β-bisabolene | 0.10 | 0.18 |
| 53 | 1512 | 1513 | γ-cadinene | 0.11 | |
| 54 | 1513 | 1512 | δ-amorphene | 1.51 | |
| 55 | 1520 | 1523 | δ-cadinene | 0.32 | 2.16 |
| 56 | 1535 | 1534 | trans-cadina-1(2),4-diene | 0.05 | |
| 57 | 1538 | 1538 | α-cadinene | t | 0.06 |
| 58 | 1548 | 1545 | α-calacorene | t | t |
| 59 | 1560 | 1549 | elemol | 1.52 | |
| 60 | 1588 | 1578 | spathulenol | t | |
| 61 | 1597 | 1583 | caryophyllene oxide | 0.70 | |
| 62 | 1627 | 1631 | eremoligenol | 0.21 | |
| 63 | 1649 | 1632 | γ-eudesmol | 0.51 | |
| 64 | 1654 | 1654 | α-cadinol | 2.1 | |
| 65 | 1661 | 1656 | α-muurolol | 0.04 | |
| 66 | 1664 | 1660 | β-eudesmol | 1.11 | |
| 67 | 1676 | 1663 | α-eudesmol | 1.17 | |
| 68 | 1691 | 1685 | α-bisabolol | 1.15 | |
| Others | 0.05 | ||||
| 8 | 985 | 984 | 3-octanone | 0.05 | |
| Monoterpene hydrocarbons | 43.97 | 53.11 | |||
| Oxygenated monoterpenes | 50.09 | 7.16 | |||
| Sesquiterpenes | 2.97 | 39.33 | |||
| Others | 0.05 | ||||
| Total | 97.03 | 99.65 |
| Extract/Standard | Polyphenols (µg GAE/mg) | Flavonoids (µg QE/mg) |
|---|---|---|
| T. ciliatus ME (MET) | 81.97 ± 1.19 a * | 48.01 ± 0.99 a * |
| R. officinalis ME (MER) | 127.1 ± 2.40 b | 38.61 ± 0.75 a |
| T. ciliatus EO (EOT) | 13.24 ± 0.09 c | 0.02 ± 0.01 b |
| R. officinalis EO (EOR) | 7.81 ± 0.41 d | 0.01 ± 0.00 b |
| Extract/Standard | DPPH IC50 (µg/mL) | β-Carotene/Linoleic Acid IC50 (µg/mL) | RP EC50 (µg/mL) |
|---|---|---|---|
| MET | 17.03 ± 0.16 a * | 165.70 ± 3.82 a * | 53.86 ± 1.68 a * |
| MER | 13.43 ± 0.14 b | 39.01 ± 2.16 b | 15.03 ± 1.43 b |
| EOT # | 3.82 ± 0.10 c | 0.96 ± 0.14 c | 0.37 ± 0.01 c |
| EOR # | 3.37 ± 0.05 c | 0.78 ± 0.01 c | 0.69 ± 0.01 d |
| BHT | 21.91 ± 0.21 e | 0.58 ± 0.03 d | 5.37 ± 0.25 e |
| Contact Bioassay (Incorporation) (µL/L) | Fumigation Bioassay (µL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fungal Isolates | R. officinalis | T. ciliatus | R. officinalis | T. ciliatus | ||||||||
| 500 | 1000 | 1500 | 500 | 1000 | 1500 | 5 | 10 | 15 | 5 | 10 | 15 | |
| A. glaucus | F | F | F | F | F | F | F | F | ||||
| B. cinerea | F | F | F | F | ||||||||
| B. aclada | F | |||||||||||
| Cl. herbarum | F | |||||||||||
| Cl. sphaerospermum | F | |||||||||||
| M. suaveolens | F | F | ||||||||||
| U. chartarum | F | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendel, N.; Sarri, D.; Sarri, M.; Napoli, E.; Palumbo Piccionello, A.; Ruberto, G. Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. Int. J. Mol. Sci. 2024, 25, 7989. https://doi.org/10.3390/ijms25147989
Hendel N, Sarri D, Sarri M, Napoli E, Palumbo Piccionello A, Ruberto G. Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. International Journal of Molecular Sciences. 2024; 25(14):7989. https://doi.org/10.3390/ijms25147989
Chicago/Turabian StyleHendel, Noui, Djamel Sarri, Madani Sarri, Edoardo Napoli, Antonio Palumbo Piccionello, and Giuseppe Ruberto. 2024. "Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth." International Journal of Molecular Sciences 25, no. 14: 7989. https://doi.org/10.3390/ijms25147989
APA StyleHendel, N., Sarri, D., Sarri, M., Napoli, E., Palumbo Piccionello, A., & Ruberto, G. (2024). Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. International Journal of Molecular Sciences, 25(14), 7989. https://doi.org/10.3390/ijms25147989








