An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments
Abstract
1. Introduction
2. Biological Models and Underwater Adhesion Processes
2.1. Mussels
2.2. Sandcastle Worms
2.3. Barnacles
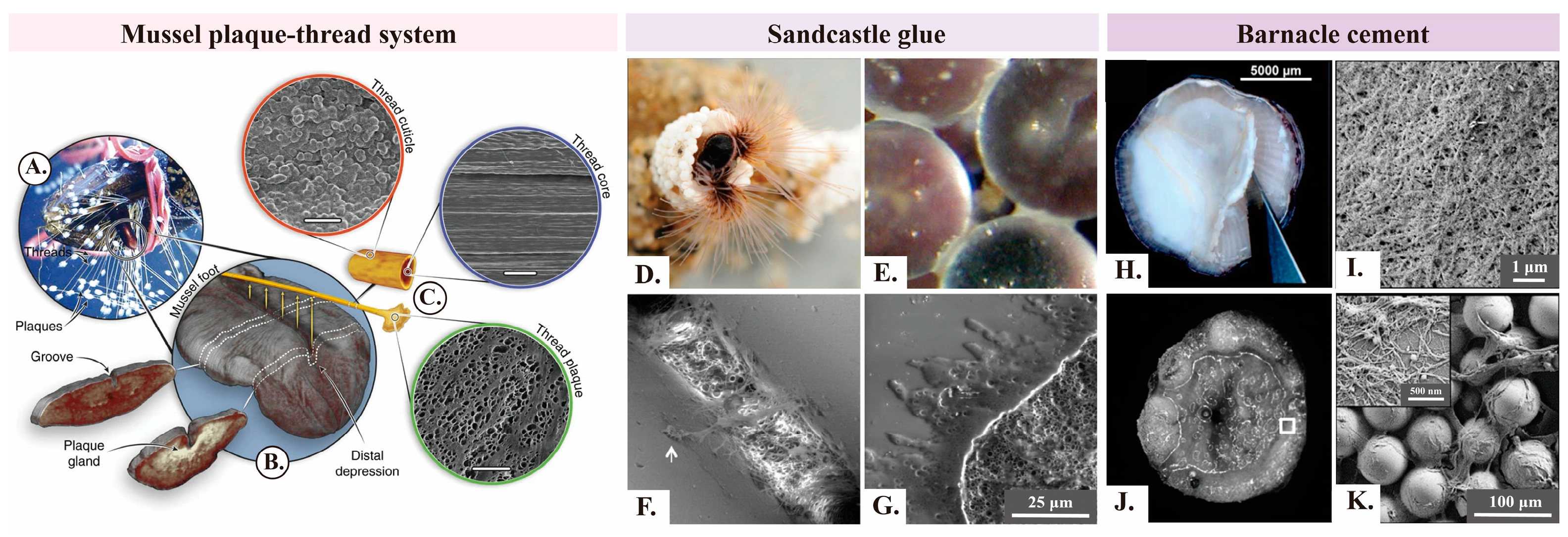
2.4. Summary of Adhesion Processes and Mechanisms
3. Underwater Adhesion Processes and Potential Mechanisms in Exemplary Biological Models
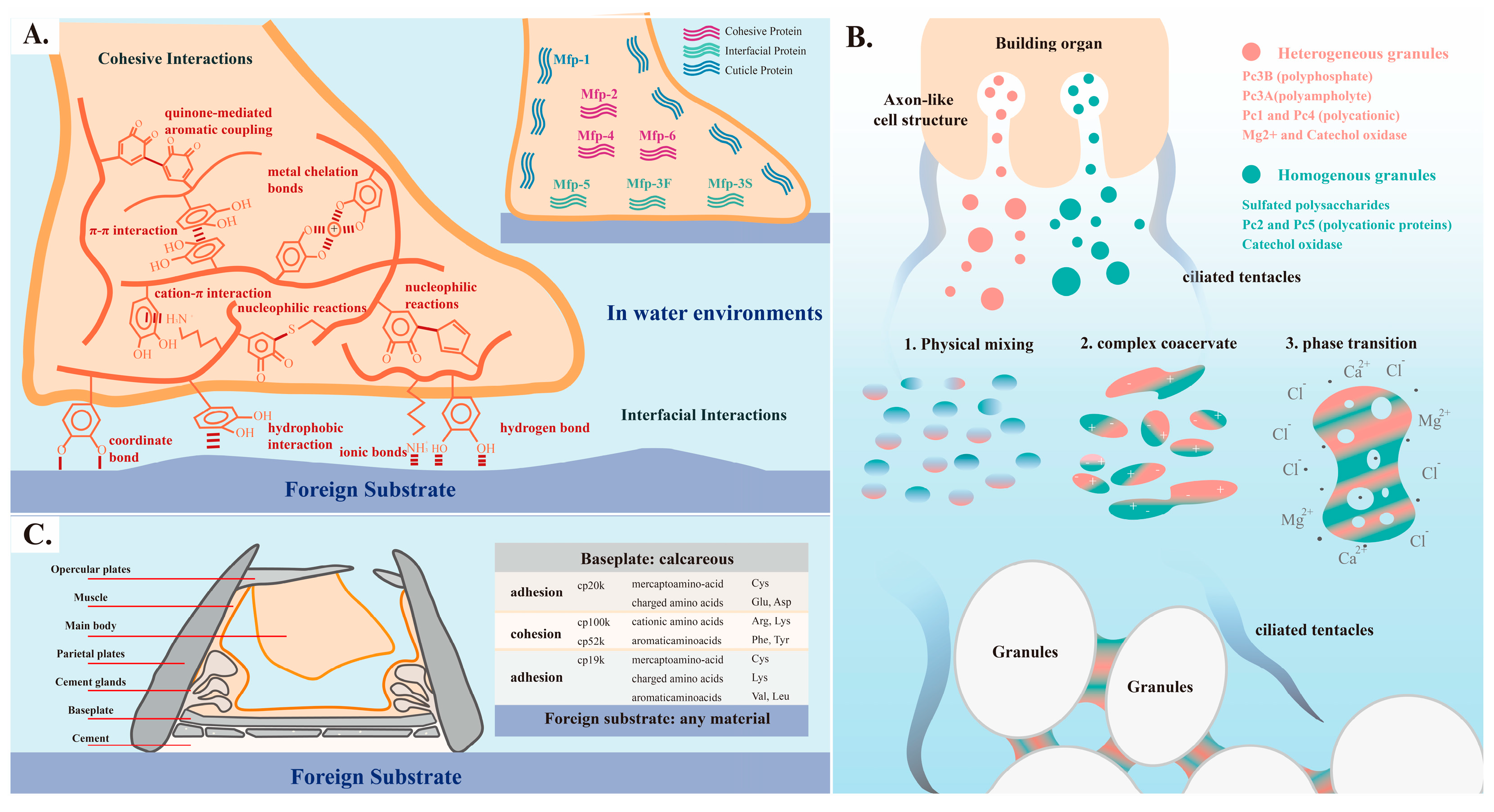
3.1. Mussel-Inspired Biomimetic Adhesives Based on DOPA
3.2. Sandcastle Worm-Inspired Biomimetic Adhesives Based on Coacervation and Phase Transition
3.3. Barnacle-Inspired Biomimetic Adhesives Based on Proteins’ Multiple Interactions and Self-Assembling
3.4. Development of Other Biomimetic Adhesives and Applications
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuen, H.Y.; Bei, H.P.; Zhao, X. Underwater and wet adhesion strategies for hydrogels in biomedical applications. Chem. Eng. J. 2022, 431, 133372. [Google Scholar] [CrossRef]
- Bu, Y.; Pandit, A. Cohesion mechanisms for bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- Zhu, W.; Chuah, Y.J.; Wang, D.A. Bioadhesives for internal medical applications: A review. Acta Biomater. 2018, 74, 1–16. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhao, X. Bioadhesive Technology Platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef]
- Yao, L.; Lin, C.; Duan, X.; Ming, X.; Chen, Z.; Zhu, H.; Zhu, S.; Zhang, Q. Autonomous underwater adhesion driven by water-induced interfacial rearrangement. Nat. Commun. 2023, 14, 6563. [Google Scholar] [CrossRef]
- Yi, H.; Lee, S.H.; Seong, M.; Kwak, M.K.; Jeong, H.E. Bioinspired Reversible Hydrogel Adhesive for Wet and Underwater Surfaces. J. Mater. Chem. B 2018, 6, 8064–8070. [Google Scholar] [CrossRef]
- Narayanan, A.; Dhinojwala, A.; Joy, A. Design principles for creating synthetic underwater adhesives. Chem. Soc. Rev. 2021, 50, 13321–13345. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef]
- Mao, X.; Yuk, H.; Zhao, X. Hydration and swelling of dry polymers for wet adhesion. J. Mech. Phys. Solids 2020, 137, 103863. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, L.; Zhang, M.; Chen, X.; Liu, M.; Fan, J.; Liu, J.; Zhou, F.; Wang, Z. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017, 8, 2218. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.J.; Li, S.; Lee, W.W.; Guo, H.; Cai, Y.; Wang, C.; Tee, B.C.K. Self-healing electronic skins for aquatic environments. Nat. Electron. 2019, 2, 75–82. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Tian, J.; Gao, A.; Tian, L.; Su, H.; Miao, S.; Tao, F.; Ren, H.; Yang, Q.; et al. Synthesis of robust underwater glues from common proteins via unfolding-aggregating strategy. Nat. Commun. 2023, 14, 5145. [Google Scholar] [CrossRef]
- Cai, C.; Chen, Z.; Chen, Y.; Li, H.; Yang, Z.; Liu, H. Mechanisms and applications of bioinspired underwater/wet adhesives. J. Polym. Sci. 2021, 59, 2911–2945. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Xu, Y.; Ma, C.; Cai, L.; Zhang, J.; Luo, J.; Li, J.; Li, J.; Shi, S.; et al. A bio-inspired multifunctional soy protein-based material: From strong underwater adhesion to 3D printing. Chem. Eng. J. 2022, 430, 133017. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Lv, K.; Zhu, Q.; Yin, H.; Feng, Y. Slow Curing of Epoxy Resin Underwater at High Temperatures. Ind. Eng. Chem. Res. 2022, 61, 16935–16945. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhang, H.; Chen, T.; Sun, G.; Han, Y.; Li, J. Double-Interpenetrating-Network Lignin-based Epoxy Resin Adhesives for Resistance to Extreme Environment. Biomacromolecules 2022, 23, 779–788. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Park, G.Y.; Felix, J.; Lee, C.H. Bioadhesives for musculoskeletal tissue regeneration. Acta Biomater. 2020, 117, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.A.; Goffredo, S.; Kim, Y.; Han, Y.; Guo, M.; Ryu, S.; Qin, Z. Why mussel byssal plaques are tiny yet strong in attachment. Matter 2022, 5, 710–724. [Google Scholar] [CrossRef]
- Kirkiz, I.; Cavas, L. First Barnacle (Amphibalanus amphitrite) Adhesion Strength Data on the Self-Polishing Coatings off the Aegean Sea. ACS Omega 2023, 8, 33675–33683. [Google Scholar] [CrossRef]
- Pan, G.; Li, B. A dynamic biointerface controls mussel adhesion. Science 2023, 382, 763–764. [Google Scholar] [CrossRef]
- Palanichamy, S.; Subramanian, G.; Eashwar, M. Corrosion behaviour and biofouling characteristics of structural steel in the coastal waters of the Gulf of Mannar (Bay of Bengal), India. Biofouling 2012, 28, 441–451. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Park, Y.; Jung, Y.M.; Cha, H.J. Coacervation of Interfacial Adhesive Proteins for Initial Mussel Adhesion to a Wet Surface. Small 2018, 14, e1803377. [Google Scholar] [CrossRef]
- Barlow, D.E.; Dickinson, G.H.; Orihuela, B.; Kulp, J.L., III; Rittschof, D.; Wahl, K.J. Characterization of the Adhesive Plaque of the Barnacle Balanus amphitrite: Amyloid-Like Nanofibrils Are a Major Component. Langmuir 2010, 26, 6549–6556. [Google Scholar] [CrossRef]
- Deepankumar, K.; Guo, Q.; Mohanram, H.; Lim, J.; Mu, Y.; Pervushin, K.; Yu, J.; Miserez, A. Liquid-Liquid Phase Separation of the Green Mussel Adhesive Protein Pvfp-5 is Regulated by the Post-Translated Dopa Amino Acid. Adv. Mater. 2022, 34, e2103828. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, H.; Sun, C. The Research Progress on Mussel Adhesive Proteins. Adv. Mar. Sci. 2014, 32, 560–570. [Google Scholar]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-Mediated Cross-Linking in the Generation of a Marine-Mussel Adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef]
- Priemel, T.; Degtyar, E.; Dean, M.N.; Harrington, M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017, 8, 14539. [Google Scholar] [CrossRef]
- Renner-Rao, M.; Clark, M.; Harrington, M.J. Fiber Formation from Liquid Crystalline Collagen Vesicles Isolated from Mussels. Langmuir 2019, 35, 15992–16001. [Google Scholar] [CrossRef]
- Jehle, F.; Priemel, T.; Strauss, M.; Fratzl, P.; Bertinetti, L.; Harrington, M.J. Collagen Pentablock Copolymers form Smectic Liquid Crystals as Precursors for Mussel Byssus Fabrication. ACS Nano 2021, 15, 6829–6838. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Cheng, Z.; Wang, L.; Wang, W.; Liang, X.; Yang, J. Near-future levels of ocean temperature weaken the byssus production and performance of the mussel Mytilus coruscus. Sci. Total Environ. 2020, 733, 139347. [Google Scholar] [CrossRef]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. Lond. B 2002, 357, 121–132. [Google Scholar] [CrossRef]
- Krauss, S.; Metzger, T.H.; Fratzl, P.; Harrington, M.J. Self-Repair of a Biological Fiber Guided by an Ordered Elastic Framework. Biomacromolecules 2013, 14, 1520–1528. [Google Scholar] [CrossRef]
- Schmitt, C.N.Z.; Politi, Y.; Reinecke, A.; Harrington, M.J. Role of sacrificial protein-metal bond exchange in mussel byssal thread self-healing. Biomacromolecules 2015, 16, 2852–2861. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Fantner, G.E.; Hohlbauch, S.; Waite, J.H.; Zok, F.W. Protective coatings on extensible biofibres. Nat. Mater. 2007, 6, 669–672. [Google Scholar] [CrossRef]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef]
- Filippidi, E.; DeMartini, D.G.; De Molina, P.M.; Danner, E.W.; Kim, J.; Helgeson, M.E.; Waite, J.H.; Valentine, M.T. The microscopic network structure of mussel (Mytilus) adhesive plaques. J. R. Soc. Interface 2015, 12, 20150827. [Google Scholar] [CrossRef]
- Wilhelm, M.H.; Filippidi, E.; Waite, J.H.; Valentine, M.T. Influence of multi-cycle loading on the structure and mechanics of marine mussel plaques. Soft Matter 2017, 13, 7381–7388. [Google Scholar] [CrossRef]
- Burkett, J.R.; Wojtas, J.L.; Cloud, J.L.; Wilker, J. A Method for Measuring the Adhesion Strength of Marine Mussels. J. Adhes. 2009, 85, 601–615. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, M.; Wang, S.; Han, F.; Xu, P.; Teng, L.; Zhao, H.; Wang, P.; Yue, G.; Zhao, Y.; et al. Extensible and self-recoverable proteinaceous materials derived from scallop byssal thread. Nat. Commun. 2022, 13, 2731. [Google Scholar] [CrossRef]
- Qin, Z.; Buehler, M.J. Impact tolerance in mussel thread networks by heterogeneous material distribution. Nat. Commun. 2013, 4, 2187. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Xu, C.; Sebastin, M.; Lee, A.; Holwell, N.; Xu, C.; Miranda Nieves, D.; Mu, L.; Langer, R.S.; Lin, C.; et al. Bioinspired Nanoparticulate Medical Glues for Minimally Invasive Tissue Repair. Adv. Healthc. Mater. 2015, 4, 2587–2596. [Google Scholar] [CrossRef]
- Wang, C.S.; Stewart, R.J. Multipart copolyelectrolyte adhesive of the sandcastle worm, Phragmatopoma californica (Fewkes): Catechol oxidase catalyzed curing through peptidyl-DOPA. Biomacromolecules 2013, 14, 1607–1617. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S.; Song, I.T.; Jones, J.P. The role of coacervation and phase transitions in the sandcastle worm adhesive system. Adv. Colloid Interface Sci. 2017, 239, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Rosso, A.; Mastandrea, A.; Viola, A.; Deias, C.; Guido, A. Sabellaria alveolata sandcastle worm from the Mediterranean Sea: New insights on tube architecture and biocement. J. Morphol. 2019, 280, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Cleverley, R.; Webb, D.; Middlemiss, S.; Duke, P.; Clare, A.; Okano, K.; Harwood, C.; Aldred, N. In Vitro Oxidative Crosslinking of Recombinant Barnacle Cyprid Cement Gland Proteins. Mar. Biotechnol. 2021, 23, 928–942. [Google Scholar] [CrossRef]
- Liang, C.; Strickland, J.; Ye, Z.; Wu, W.; Hu, B.; Rittschof, D. Biochemistry of Barnacle Adhesion: An Updated Review. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Lee, Y.J.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5, 4414. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Sarrafian, T.L.; Mao, X.; Varela, C.E.; Roche, E.T.; Griffiths, L.G.; Nabzdyk, C.S.; Zhao, X. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nat. Biomed. Eng. 2021, 5, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5, 1700762. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.-L.; Byern, J.; Flammang, P.; Klepal, W.; Power, A.M. Unusual adhesive production system in the barnacle Lepas anatifera: An ultrastructural and histochemical investigation. J. Morphol. 2012, 273, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, C.; Zhang, X.; Li, J.; Huang, J.; Zeng, L.; Ye, Z.; Hu, B.; Wu, W. Amyloid fibril aggregation: An insight into the underwater adhesion of barnacle cement. Biochem. Biophys. Res. Commun. 2017, 493, 654–659. [Google Scholar] [CrossRef] [PubMed]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6, 36219. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.; Riek, R. Functional Amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Priemel, T.; Palia, R.; Babych, M.; Thibodeaux, C.J.; Bourgault, S.; Harrington, M.J. Compartmentalized processing of catechols during mussel byssus fabrication determines the destiny of DOPA. Proc. Natl. Acad. Sci. USA 2020, 117, 7613–7621. [Google Scholar] [CrossRef]
- Li, Y.; Liang, C.; Gao, L.; Li, S.; Zhang, Y.; Zhang, J.; Cao, Y. Hidden complexity of synergistic roles of Dopa and lysine for strong wet adhesion. Mater. Chem. Front. 2017, 1, 2664–2668. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, J.; Arisawa, T.; Hayashi, K.; Richardson, J.J.; Shibuta, Y.; Ejima, H. Ultrastrong underwater adhesion on diverse substrates using non-canonical phenolic groups. Nat. Commun. 2022, 13, 1892. [Google Scholar] [CrossRef]
- Lee, D.; Bae, H.; Ahn, J.; Kang, T.; Seo, D.-G.; Hwang, D.S. Catechol-thiol-based dental adhesive inspired by underwater mussel adhesion. Acta Biomater. 2020, 103, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2021, 31, 2009334. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Siebert, H.M.; Wilker, J.J. Integrating mussel chemistry into a bio-based polymer to create degradable adhesives. Macromolecules 2017, 50, 561–568. [Google Scholar] [CrossRef]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Interfacial pH during mussel adhesive plaque formation. Biofouling 2015, 31, 221–227. [Google Scholar] [CrossRef]
- Waite, J.H. Mussel adhesion-essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S.; Shao, H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011, 167, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lipik, V.; Miserez, A. Complex coacervates of oppositely charged co-polypeptides inspired by the sandcastle worm glue. J. Mater. Chem. B 2016, 4, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Dompé, M.; Cedano-Serrano, F.J.; Heckert, O.; van den Heuvel, N.; van der Gucht, J.; Tran, Y.; Hourdet, D.; Creton, C.; Kamperman, M. Thermoresponsive Complex Coacervate-Based Underwater Adhesive. Adv. Mater. 2019, 31, 1808179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lee, D.W.; Ahn, B.K.; Seo, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 2016, 15, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Valois, E.; Mirshafian, R.; Waite, J.H. Phase-dependent Redox Insulation in Mussel Adhesion. Sci. Adv. 2020, 6, eaaz6486. [Google Scholar] [CrossRef] [PubMed]
- So, C.; Liu, J.; Fears, K.; Leary, D.; Golden, J.; Wahl, K. Self-Assembly of Protein Nanofibrils Orchestrates Calcite Step Movement through Selective Nonchiral Interactions. ACS Nano 2015, 9, 5782–5791. [Google Scholar] [CrossRef] [PubMed]
- So, C.R.; Yates, E.A.; Estrella, L.A.; Fears, K.P.; Schenck, A.M.; Yip, C.M.; Wahl, K.J. Molecular Recognition of Structures Is Key in the Polymerization of Patterned Barnacle Adhesive Sequences. ACS Nano 2019, 13, 5172–5183. [Google Scholar] [CrossRef]
- Mohanram, H.; Georges, T.; Pervushin, K.; Azaïs, T.; Miserez, A. Self-Assembly of a Barnacle Cement Protein (MrCP20) into Adhesive Nanofibrils with Concomitant Regulation of CaCO3 Polymorphism. Chem. Mater. 2021, 33, 9715–9724. [Google Scholar] [CrossRef]
- Kumar, A.; Mohanram, H.; Li, J.; Ferrand, H.L.; Verma, C.S.; Miserez, A. Disorder-Order Interplay of a Barnacle Cement Protein Triggered by Interactions with Calcium and Carbonate Ions: A Molecular Dynamics Study. Chem. Mater. 2020, 32, 8845–8859. [Google Scholar] [CrossRef]
- Tilbury, M.A.; Mccarthy, S.; Domagalska, M.; Ederth, T.; Power, A.M.; Wall, J.G. The expression and characterization of recombinant cp19k barnacle cement protein from Pollicipes pollicipes. Philos. Trans. R. Soc. B 2019, 374, 20190205. [Google Scholar] [CrossRef]
- Nakano, M.; Shen, J.-R.; Kamino, K. Self-Assembling Peptide Inspired by a Barnacle Underwater Adhesive Protein. Biomacromolecules 2007, 8, 1830–1835. [Google Scholar] [CrossRef]
- Li, B.; Song, J.; Mao, T.; Zeng, L.; Ye, Z.; Hu, B. An essential role of disulfide bonds for the hierarchical self-assembly and underwater affinity of CP20-derived peptides. Front. Bioeng. Biotechnol. 2022, 10, 998194. [Google Scholar] [CrossRef] [PubMed]
- Estrella, L.A.; Yates, E.A.; Fears, K.P.; Schultzhaus, J.N.; Ryou, H.; Leary, D.H.; So, C.R. Engineered Escherichia coli Biofilms Produce Adhesive Nanomaterials Shaped by a Patterned 43 kDa Barnacle Cement Protein. Biomacromolecules 2021, 22, 365–373. [Google Scholar] [CrossRef]
- Ciapetti, G.; Stea, S.; Cenni, E.; Sudanese, A.; Marraro, D.; Toni, A.; Pizzoferrato, A. Toxicity of cyanoacrylates in vitro using extract dilution assay on cell cultures. Biomaterials 1994, 15, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.C.M.; Herrmann, J.B.M.; Cameron, J.L.M.; Brandes, G.B.S.; Pulaski, C.E.M.; Leonard, F.P.D. Histotoxicity of Cyanoacrylate Tissue Adhesive in the Rat. Ann. Surg. 1965, 162, 113–122. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules 2022, 27, 8982. [Google Scholar] [CrossRef]
- Park, K.H.; Seong, K.-Y.; Yang, S.Y.; Seo, S. Advances in medical adhesives inspired by aquatic organisms’ adhesion. Biomater. Res. 2017, 21, 16. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheong, H.; Hwang, B.H.; Cha, H.J. Mussel-Inspired Protein Nanoparticles Containing Iron(III)-DOPA Complexes for pH-Responsive Drug Delivery. Angew. Chem. Int. Ed. 2015, 127, 7426–7430. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021, 12, 1689. [Google Scholar] [CrossRef]
- Jones, J.P.; Sima, M.; O’Hara, R.G.; Stewart, R.J. Water-Borne Endovascular Embolics Inspired by the Undersea Adhesive of Marine Sandcastle Worms. Adv. Healthc. Mater. 2016, 5, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, B.-H.; Jun, S.H.; Cha, H.J. Sandcastle Worm-Inspired Blood-Resistant Bone Graft Binder Using a Sticky Mussel Protein for Augmented In Vivo Bone Regeneration. Adv. Healthc. Mater. 2016, 5, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bao, G.; Li, J. Multifaceted Design and Emerging Applications of Tissue Adhesives. Adv. Mater. 2021, 33, 2007663. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wan, C.; Wang, T.; Li, Q.; Chen, G.; Wang, J.; Liu, Z.; Yang, H.; Liu, X.; Chen, X. Water-Resistant Conformal Hybrid Electrodes for Aquatic Endurable Electrocardiographic Monitoring. Adv. Mater. 2020, 32, 2001496. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, L.; Lu, L.; Du, R.; Ma, W.; Cai, Y.; An, X.; Wu, H.; Luo, Q.; Xu, Q.; et al. A Highly-Adhesive and Self-Healing Elastomer for Bio-Interfacial Electrode. Adv. Funct. Mater. 2021, 31, 2006432. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Xue, Y.; Duan, Q.; Liang, X.; Lin, T.; Wu, Z.; Tan, Y.; Zhao, Q.; Zheng, W.; et al. Fatigue-Resistant Conducting Polymer Hydrogels as Strain Sensor for Underwater Robotics. Adv. Healthc. Mater. 2023, 33, 1–9. [Google Scholar] [CrossRef]
- Yang, B.; Ayyadurai, N.; Yun, H.; Choi, Y.S.; Hwang, B.H.; Huang, J.; Lu, Q.; Zeng, H.; Cha, H.J. In Vivo residue-specific dopa-incorporated engineered mussel bioglue with enhanced adhesion and water resistance. Angew. Chem. Int. Ed. 2014, 53, 13360–13364. [Google Scholar] [CrossRef] [PubMed]
- Wasim, M.; Sabir, A.; Shafiq, M.; Khan, R.U. Mussel inspired surface functionalization of polyamide membranes for the removal and adsorption of crystal violet dye. Dyes Pigments 2022, 206, 110606. [Google Scholar] [CrossRef]
- Sangeetha, R.; Kumar, R.; Doble, M. Barnacle cement: An etchant for stainless steel 316L? Colloids Surf. B Biointerfaces 2010, 79, 524–530. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Li, R.; Shi, S.; Gong, Y.-K. Mussel adhesion and cell membrane antifouling mimetic strategies for durable fouling-resistant coating. Prog. Org. Coat. 2023, 182, 107636. [Google Scholar] [CrossRef]
- Pandey, N.; Hakamivala, A.; Xu, C.; Hariharan, P.; Radionov, B.; Huang, Z.; Liao, J.; Tang, L.; Zimmern, P.; Nguyen, K.T.; et al. Biodegradable Nanoparticles Enhanced Adhesiveness of Mussel-Like Hydrogels at Tissue Interface. Adv. Healthc. Mater. 2018, 7, e1701069. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cui, Y.; Han, M.; Jia, P.; Zhao, Y.; Zhang, M.; Sun, Y.; Nian, R. Mussel-inspired chemistry in producing mechanically robust and bioactive hydrogels as skin dressings. Mater. Today Chem. 2023, 27, 101272. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Shang, X.; Hu, W.; Gao, G.; Duan, L. Bio-inspired adhesive and self-healing hydrogels as flexible strain sensors for monitoring human activities. Mater. Sci. Eng. C 2020, 106, 110168. [Google Scholar] [CrossRef]
- Mao, X.; Liu, L.; Cheng, L.; Cheng, R.; Zhang, L.; Deng, L.; Sun, X.; Zhang, Y.; Sarmento, B.; Cui, W. Adhesive nanoparticles with inflammation regulation for promoting skin flap regeneration. J. Control. Release 2019, 297, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.; Ma, X.Y.D.; Li, Z.; He, C.; Lu, X. Facile anchoring mussel adhesive mimic tentacles on biodegradable polymer cargo carriers via self-assembly for microplastic-free cosmetics. J. Colloid Interface Sci. 2022, 612, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Chen, J.; Wang, Z.; Jin, X.; Dai, C. Mussel-inspired hydrogel particles with selective adhesion characteristics for applications in reservoir fracture control. J. Mol. Liq. 2022, 361, 119598. [Google Scholar] [CrossRef]
- Xie, T.; Ding, J.; Han, X.; Jia, H.; Yang, Y.; Liang, S.; Wang, W.; Liu, W.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horiz. 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Hu, S.; Yang, Z.; Zhai, Q.; Li, D.; Zhu, X.; He, Q.; Li, L.; Cannon, R.D.; Wang, H.; Tang, H.; et al. An All-in-One “4A Hydrogel”: Through First-Aid Hemostatic, Antibacterial, Antioxidant, and Angiogenic to Promoting Infected Wound Healing. Small 2023, 19, e2207437. [Google Scholar] [CrossRef]
- George, M.N.; Liu, X.; Miller, A.L.; Zuiker, E.; Xu, H.; Lu, L. Injectable pH-responsive adhesive hydrogels for bone tissue engineering inspired by the underwater attachment strategy of marine mussels. Biomater. Adv. 2022, 133, 112606. [Google Scholar] [CrossRef]
- Gan, D.; Wang, Z.; Xie, C.; Wang, X.; Xing, W.; Ge, X.; Yuan, H.; Wang, K.; Tan, H.; Lu, X. Mussel-Inspired Tough Hydrogel with In Situ Nanohydroxyapatite Mineralization for Osteochondral Defect Repair. Adv. Healthc. Mater. 2019, 8, 1901103. [Google Scholar] [CrossRef]
- Xia, G.; Lin, M.; Jiayu, Y.; Jinkang, H.; Bowen, L.; Mu, Y.; Wan, X. Solvent-Free Mussel-Inspired Adhesive with Rapid Underwater Curing Capability. Adv. Mater. Interfaces. 2021, 8, 2101544. [Google Scholar] [CrossRef]
- Cheng, Q.; Peng, Y.-Y.; Asha, A.B.; Zhang, L.; Li, J.; Shi, Z.; Cui, Z.; Narain, R. Construction of antibacterial adhesion surfaces based on bioinspired borneol-containing glycopolymers. Biomater. Sci. 2022, 10, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.; Park, J.; Choi, W.; Hong, J.; Lee, D.W.; Kim, B.-S. Mussel-Inspired Multiloop Polyethers for Antifouling Surfaces. Biomacromolecules 2021, 22, 5173–5184. [Google Scholar] [CrossRef]
- Cai, Y.; Xin, L.; Li, H.; Sun, P.; Liu, C.; Fang, L. Mussel-inspired controllable drug release hydrogel for transdermal drug delivery: Hydrogen bond and ion-dipole interactions. J. Control. Release 2024, 365, 161–175. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, C.; Gong, K.; Li, H.; Song, H.; Zhang, Y.; Ding, D.; Liu, J.; Guo, J.; Fang, L. Mussel-inspired quaternary composite hydrogels with high strength and high tissue adhesion for transdermal drug delivery: Synergistic hydrogen bonding and drug release mechanism. Chem. Eng. J. 2023, 465, 142942. [Google Scholar] [CrossRef]
- Winslow, B.D.; Shao, H.; Stewart, R.J.; Tresco, P.A. Biocompatibility of adhesive complex coacervates modeled after the sandcastle glue of Phragmatopoma californica for craniofacial reconstruction. Biomaterials 2010, 31, 9373–9381. [Google Scholar] [CrossRef]
- Shao, H.; Stewart, R.J. Biomimetic Underwater Adhesives with Environmentally Triggered Setting Mechanisms. Adv. Mater. 2010, 22, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Chen, Q.; Jiang, W.; Wang, Y.; Xie, J.; Ma, K.; Shi, C.; Zhang, H.; Chen, M.; et al. A sandcastle worm-inspired strategy to functionalize wet hydrogels. Nat. Commun. 2021, 12, 6331. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhou, W.; Shi, S.Q.; Xie, X.; Gao, Q.; Zeng, L.; Li, J. Sandcastle worm-inspired phytic acid and magnesium oxychloride cement copolymerization for performance enhancement. J. Hazard. Mater. 2021, 404, 123992. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, S.; Mensah, A.; Wang, Q.; Wei, Q. Hydrogel transformed from sandcastle-worm-inspired powder for adhering wet adipose surfaces. J. Colloid Interface Sci. 2023, 646, 472–483. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ku, Y.H.; Wang, K.C.; Chiang, H.C.; Hsu, Y.P.; Cheng, M.T.; Wang, C.S.; Wee, Y. Bioinspired Sandcastle Worm-Derived Peptide-Based Hybrid Hydrogel for Promoting the Formation of Liver Spheroids. Gels 2022, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, Q.; Ke, X.; Wang, H.; Li, M.; Xie, J.; Luo, J.; Li, J. Bioinspired by Sandcastle Worm Glue: An Underwater Reversible Adhesive Modulated by pH Environments Based on Urushiol. Ind. Eng. Chem. Res. 2023, 62, 19690–19701. [Google Scholar] [CrossRef]
- You, X.; Xiao, K.; Yu, Q.; Wu, H.; Yuan, J.; Zhang, R.; Ma, Y.; Li, Y.; Huang, T.; Jiang, Z. Fouling-resistant robust membranes via electrostatic complexation for water purification. Chem. Eng. J. 2021, 416, 129139. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Z.; Wan, X.; Wang, Z.; Zhang, Y.; Meng, J.; Jiang, L.; Wang, S. Colonial sandcastle-inspired low-carbon building materials. Matter 2023, 6, 3864–3876. [Google Scholar] [CrossRef]
- Lin, Y.X.; Fu, T.; Guo, D.M.; Tang, Y.L.; He, J.H.; Liu, C.; Wu, S.G.; Liu, B.W.; Chen, L.; Wang, Y.Z. A Sandcastle-Worm-Inspired Strategy toward Antimicrobial Fouling and Fireproof Composite. ACS Mater. Lett. 2024, 6, 627–639. [Google Scholar] [CrossRef]
- Brennan, M.J.; Kilbride, B.F.; Wilker, J.J.; Liu, J.C. A bioinspired elastin-based protein for a cytocompatible underwater adhesive. Biomaterials 2017, 124, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Jia, M.; Mu, Y.; Jiang, W.; Wan, X. Humid bonding with a water-soluble adhesive inspired by mussels and sandcastle worms. Macromol. Chem. Phys. 2015, 216, 450–459. [Google Scholar] [CrossRef]
- Han, K.; Park, T.Y.; Yong, K.; Cha, H.J. Combinational Biomimicking of Lotus Leaf, Mussel, and Sandcastle Worm for Robust Superhydrophobic Surfaces with Biomedical Multifunctionality: Antithrombotic, Antibiofouling, and Tissue Closure Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 9777–9785. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Liu, J.; Zhou, C.; Zhu, Y.; Yuan, Y.; Fu, D.; Lv, Q.; Song, Y.; Zou, M.; et al. Bio-Inspired Self-Hydrophobized Sericin Adhesive with Tough Underwater Adhesion Enables Wound Healing and Fluid Leakage Sealing. Adv. Funct. Mater. 2022, 32, 2201108. [Google Scholar] [CrossRef]
- Clancy, S.K.; Sodano, A.; Cunningham, D.J.; Huang, S.S.; Zalicki, P.J.; Shin, S.; Ahn, B.K. Marine Bioinspired Underwater Contact Adhesion. Biomacromolecules 2016, 17, 1869–1874. [Google Scholar] [CrossRef]
- Presti, M.L.; Rizzo, G.; Farinola, G.M.; Omenetto, F.G. Bioinspired Biomaterial Composite for All-Water-Based High-Performance Adhesives. Adv. Sci. 2021, 8, e2004786. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Li, X.; Yang, E.; Tian, Y.; Jiang, L. Barnacle inspired high-strength hydrogel for adhesive. Front. Bioeng. Biotechol. 2023, 11, 1183799. [Google Scholar] [CrossRef] [PubMed]
- Daisuke Fujii, K.T.; Takagi, A.; Kamino, K.; Hirano, Y. Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. Int. J. Mol. Sci. 2021, 22, 1240. [Google Scholar] [CrossRef] [PubMed]
- Mondarte, E.A.Q.; Wang, J.; Yu, J. Adaptive Adhesions of Barnacle-Inspired Adhesive Peptides. ACS Biomater. Sci. Eng. 2023, 9, 5679–5686. [Google Scholar] [CrossRef]
- He, S.; Guo, B.; Sun, X.; Shi, M.; Zhang, H.; Yao, F.; Sun, H.; Li, J. Bio-Inspired Instant Underwater Adhesive Hydrogel Sensors. ACS Appl. Mater. Interfaces 2022, 14, 45869–45879. [Google Scholar] [CrossRef]
| Materials | Biological Model | Application Fields | Effect | Ref. | |
|---|---|---|---|---|---|
| 1 | Mixing of N-hydroxysuccinimide modified poly (lactic-co-glycolic acid) nanoparticles (PLGA-NHS) and alginate-dopamine polymer (Alg-Dopa) | Mussel | Biodegradable tissue adhesive | Lap shear strength of 33 ± 3 kPa for porcine skin-muscle interface; degradable; cytocompatible; minimal inflammatory responses. | [101] |
| 2 | Poly (ethylene glycol) diacrylate/alginate double network hydrogels and 3,4-dihydroxy-L-phenylalanine as a crosslinker | Mussel | Skin dressings | High mechanical strength and self-healing properties with a highly transparent appearance. | [102] |
| 3 | Poly (acrylamide-co-dopamine) with lithium chloride | Mussel | Flexible strain sensors | Self-healing, stretchable, adhesive, and conductive. | [103] |
| 4 | Multipotent flap-protective adhesive mangiferin (MF)-loaded liposomes (A-MF-Lip) | Mussel | Local drug delivery for promoting the generation of skin flaps | Liposomes exhibit great adhesion properties, and adherent MF-loaded liposomes possess multipotent flap-protective therapeutic effects such as pro-neovascularization, cytoprotection, anti-apoptosis, and anti-inflammatory. | [104] |
| 5 | Chitosan-graft-L-lysine-L-DOPA | Mussel | Fragrance delivery systems in personal care products | CLD can facilitate the deposition of biodegradable fragrance carriers on diverse surfaces, including hair, cotton, and skin. | [105] |
| 6 | Catechol-modified polyacrylamide | Mussel | Reservoir fracture control | Excellent reservoir adaptability (96 °C; 4.7 × 104 mg/L); capable of withstanding water flushing and maintaining stable adhesion to the fracture wall to guarantee the long-term control effect. | [106] |
| 7 | Mixing HB-PBAE, poly (1-vinylimidazole) (PVI), and gelatin solution, followed by adding Fe3+ | Mussel | Wound-healing dressings | Capable of accelerating the wound-healing process and rapidly reducing adhesion; the strength is significantly enhanced upon the spraying of the Zn2+ solution. | [107] |
| 8 | PVA-DOPA-Cu2+ (PDPC) hydrogel | Mussel | Wound healing | Tissue adhesive, antioxidative, photothermal, antibacterial, and hemostatic | [108] |
| 9 | Catechol functional groups (DOPA) are crosslinked with the synthetic oligomer oligo [poly (ethylene glycol) fumarate] (OPF) | Mussel | Bone tissue engineering | Capable of enhancing the pre-osteoblast cell attachment and proliferation; DOPA-mediated interfacial adhesive interactions prevent the displacement of scaffolds. | [109] |
| 10 | GelMA-PDA hydrogel with TGF-β3 as a cartilage repair layer; GelMA-PDA/HA hydrogel with BMP-2 as a subchondral bone repair layer | Mussel | Bone tissue engineering | The hydrogel exhibits a bone area ratio of 65% in a rabbit’s knee joint with full-thickness cartilage defect. | [110] |
| 11 | PUP-PPG-DBHP | Mussel | Underwater engineering field | The adhesive can be applied underwater directly, reaching a bonding strength of approximately 1.2 MPa within around 30 s on glass substrates. | [111] |
| 12 | Poly (LAEMA-co-GMA-co-BA) | Mussel | Coating materials | The coated surfaces exhibit flatness, smoothness, great antibacterial adhesion properties, and low cytotoxicity. | [112] |
| 13 | Poly (TEG-co-CAG)n | Mussel | Antifouling | Polymer-coated surfaces exhibit reduced protein adsorption and a decreased cell count when compared to the control group. | [113] |
| 14 | PAHDP | Mussel | Drug delivery | The PAHDP hydrogel, with excellent adhesion properties and safety profiles, can deliver over 10 types of drugs, especially small-molecule drugs. | [114,115] |
| 15 | Dense coacervates formed by aminated collagen and phosphodopa copolymer at 25 °C | Sandcastle worm | Craniofacial reconstruction | The adhesive can maintain 3D bone alignment in freely moving rats over a 12-week indwelling period, and it is degradable. | [116,117] |
| 16 | Amine-terminated DbaYKY tripeptide links to functionalized molecules | Sandcastle worm | Synthesis of functional hydrogels | The modified hydrogel possesses biological functions such as cell adhesion, antibacterial, and wound repair. | [118] |
| 17 | Phytic acid (PA) as the crosslinker for magnesium oxychloride cement (MOC) | Sandcastle worm | The research of magnesium oxychloride cement (MOC) | The integration of phytic acid improves the water resistance, workability, and applicability of MOC, and it is environmentally friendly. | [119] |
| 18 | Oppositely-charged polyelectrolytes (PEI and PAA) and catechol-functionalized cellulose nanofibers (TA-CNF) | Sandcastle worm | Medical adhesion | Capable of absorbing fluids and transforming into a hydrogel (<3 s) with great ductility (~14 times its original form), self-healing ability, and an efficient drug-loading capacity. | [120] |
| 19 | Poly (glycerol sebacate)-acrylate nanoparticles | Sandcastle worm | Tissue adhesion | Capable of quickly assembling viscous glue. | [44] |
| 20 | PC4/Cultrex hybrid hydrogel | Sandcastle worm | Hydrogels for the formation of liver spheroids | Capable of enhancing HepG2 cells to form spheroids and hepatic differentiation. | [121] |
| 21 | 3-(acrylamidophenyl) boronic acid (AAPBA) and N-2-hydroxyethyl acrylamide (HEAA) | Sandcastle worm | Responsive reversible wet adhesion | Capable of acquiring pH-responsive reversible adhesion. | [122] |
| 22 | Multidentate organophosphate, quaternized cellulose, and perfluorinated sulfonic acid are assembled onto polyethersulfone (PES) substrate | Sandcastle worm | Membrane-based water treatment | The water permeance is 93.3 L m−2 h−1 bar−1 with a rejection rate to organic dyes ranging from 90.0 to 99.9%. | [123] |
| 23 | Quaternized chitosan and alginate are mixed with various solid materials (nLCBMs/±) | Sandcastle worm | Building material | Excellent mechanical performance (compressive elastic modulus of nearly 400 MPa), recyclability, anti-weathering property, and scalability. | [124] |
| 24 | Tyramine-ammonium polyphosphate (TA-APP) serves as an adhesive along with vinyl ester resin to bond with carbon fibers | Sandcastle worm | Functional material | The material possesses broad-spectrum antibacterial and anti-algae capabilities, in addition to a superior flame-retardant effect. | [125] |
| 25 | DOPA-rich ELP | Sandcastle worm and mussel | Biomedical glue | It exhibits adhesion strengths of ∼240 MPa in wet environments and >2 MPa in dry environments and is capable of coacervating in physiological conditions. | [126] |
| 26 | Grafting catechol and bis-phosphoric acid groups to the polyoxetane backbone | Sandcastle worm and mussel | Underwater bonding | A bonding strength of 0.35 MPa is achieved under humid conditions. | [127] |
| 27 | IMglue-SiO2(TiO2/SiO2)2 SH coating | Sandcastle worm, mussel, and lotus leaf | Tissue closure | Antibiofouling, durable, biocompatible, and antithrombotic. | [128] |
| 28 | Reduced sericin-tannic acid (rSer-TA) | Barnacle and mussel | Wound healing in vivo and the sealing of fluid leakage in vivo | A bonding strength of >0.1 MPa for tissues and >0.5 MPa for solid plates. | [129] |
| 29 | Aromatic, ionic moieties, and nonpolar functionalized copolymer films | Barnacle and mussel | Potential applications in biomedicine or engineering | The wet contact adhesion is ~15.0 N/cm2 in deionized water and ~9.0 N/cm2 in seawater at a pH of approximately 7. | [130] |
| 30 | A composite composed of a silk fibroin (SF) solution and polydopamine (PDA) | Barnacle and mussel | Underwater adhesion | The synthesis of polymers is simple, characterized by a completely biological composition. A high adhesion strength (>2 MPa) can be achieved using a relatively low mass (1–2 mg). | [131] |
| 31 | PEI and PMAA | Barnacle | Hydrogel for adhesive | High mechanical strength (2.66 ± 0.18 MPa) and adhesion strength (1.99 ± 0.11 MPa under water and 2.70 ± 0.21 MPa under silicon oil). | [132] |
| 32 | Coating RGD-containing peptides on a polystyrene plate | Barnacle | Tissue engineering scaffolds | Capable of facilitating cell adhesion and spreading. | [133] |
| 33 | Poly (LAEMA-co-GMA-co-BA) | Mussel | Coating materials | The coated surfaces exhibit flatness, smoothness, great antibacterial adhesion properties, and low cytotoxicity. | [112] |
| 34 | Mrcp19k-inspired low-complexity STGA-rich adhesive peptides (Mr-AP1 and Mr-AP1C) | Barnacle | Underwater adhesion | The adhesive peptides generate adhesive patches under conditions of low pH and low ionic strength. | [134] |
| 35 | Prepared MXene/PHMP hydrogel using PEA, MEA, and HEAA in the presence of conductive MXene nanosheets | Barnacle | Underwater sensing | It exhibits rapid and reversible adhesion with minimal swelling, which facilitates the manufacturing of stable and sensitive underwater sensors. | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Song, J.; Zeng, L.; Hu, B. An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 7994. https://doi.org/10.3390/ijms25147994
Liu J, Song J, Zeng L, Hu B. An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments. International Journal of Molecular Sciences. 2024; 25(14):7994. https://doi.org/10.3390/ijms25147994
Chicago/Turabian StyleLiu, Jiani, Junyi Song, Ling Zeng, and Biru Hu. 2024. "An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments" International Journal of Molecular Sciences 25, no. 14: 7994. https://doi.org/10.3390/ijms25147994
APA StyleLiu, J., Song, J., Zeng, L., & Hu, B. (2024). An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments. International Journal of Molecular Sciences, 25(14), 7994. https://doi.org/10.3390/ijms25147994






