Comparative Transcriptomics to Identify RNA Writers and Erasers in Microalgae
Abstract
1. Introduction
2. Results
2.1. New Functional Annotation of the Four Transcriptomes
2.2. Comparative Analysis and Network of Writers and Erasers
2.3. Genome Searching
2.4. Transcriptome Differential Expression Analysis
2.5. A. carterae Cultured in Various Stressful Culturing Conditions
2.6. Reference Gene Assessment for Reverse Transcription-Quantitative PCR (RT-qPCR) in A. carterae
2.7. RT-qPCR for ALKBH9B, ALKH10B, FIP37, and MTB in A. carterae
3. Discussion
4. Materials and Methods
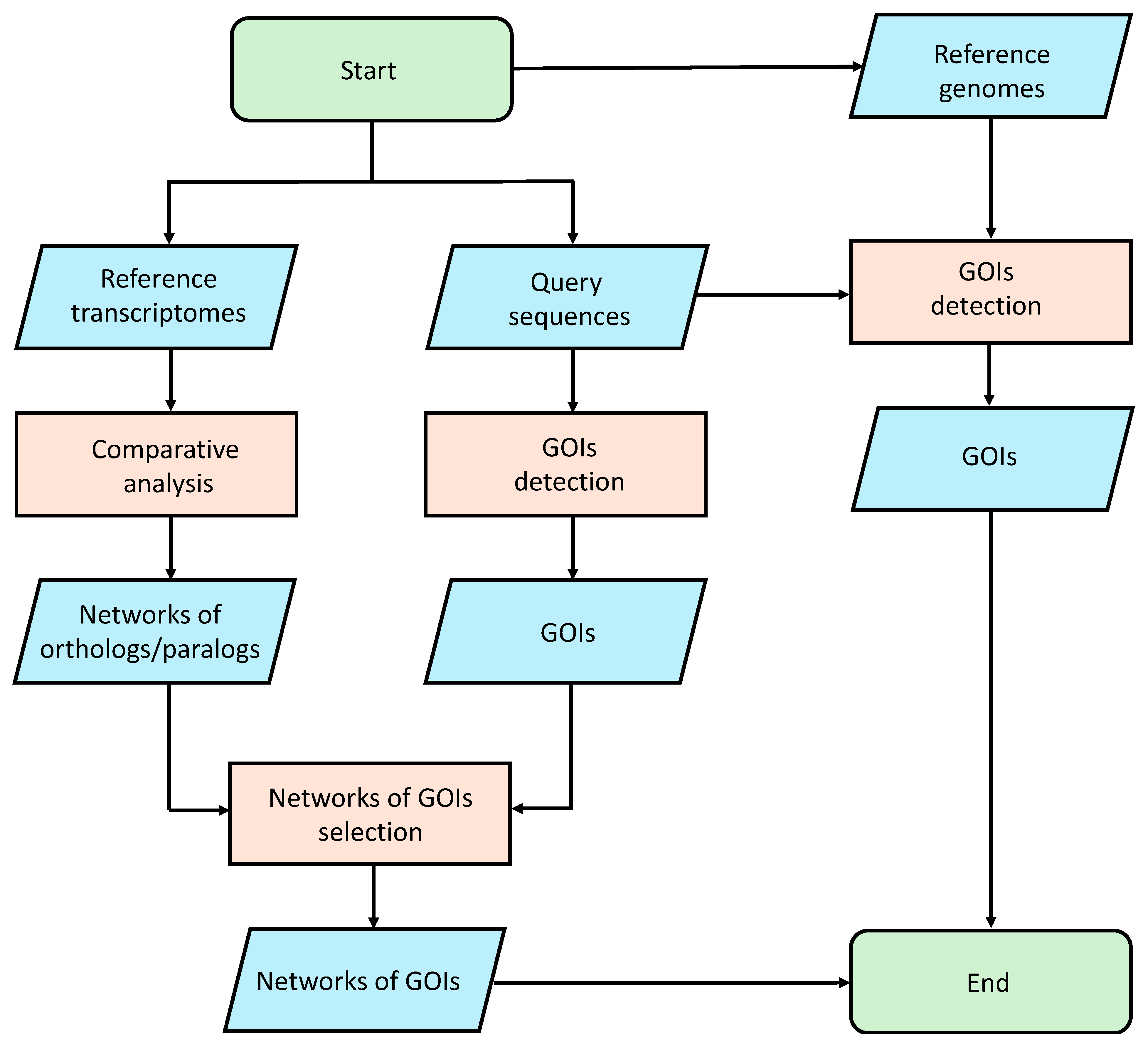
4.1. Experimental Design
4.2. Functional Annotation
4.3. Comparative Analysis
4.4. Query Sequences Selection and Screening
4.5. Genome Searching
4.6. Microalgal Culturing and Harvesting for Reverse Transcription-Quantitative PCR (RT-qPCR)
4.7. RNA Extraction, Retrotranscription, and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Hsu, P.J.; Chen, Y.-S.; Yang, Y.-G. Dynamic Transcriptomic m6A Decoration: Writers, Erasers, Readers and Functions in RNA Metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Shoaib, Y.; Usman, B.; Kang, H.; Jung, K.-H. Epitranscriptomics: An Additional Regulatory Layer in Plants’ Development and Stress Response. Plants 2022, 11, 1033. [Google Scholar] [CrossRef]
- Ke, S.; Alemu, E.A.; Mertens, C.; Gantman, E.C.; Fak, J.J.; Mele, A.; Haripal, B.; Zucker-Scharff, I.; Moore, M.J.; Park, C.Y.; et al. A Majority of m6A Residues Are in the Last Exons, Allowing the Potential for 3’ UTR Regulation. Genes Dev. 2015, 29, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Molinie, B.; Wang, J.; Lim, K.S.; Hillebrand, R.; Lu, Z.; Van Wittenberghe, N.; Howard, B.D.; Daneshvar, K.; Mullen, A.C.; Dedon, P.; et al. m6A-LAIC-Seq Reveals the Census and Complexity of the m6A Epitranscriptome. Nat. Methods 2016, 13, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N6-Methyladenosine by IGF2BP Proteins Enhances mRNA Stability and Translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Fustin, J.-M.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka, M.S.; Kakeya, H.; Manabe, I.; et al. RNA-Methylation-Dependent RNA Processing Controls the Speed of the Circadian Clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, K.I.; Parisien, M.; Dai, Q.; Diatchenko, L.; Pan, T. N6-Methyladenosine Alters RNA Structure to Regulate Binding of a Low-Complexity Protein. Nucleic Acids Res. 2017, 45, 6051–6063. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.-S.; Hao, Y.-J.; Sun, B.-F.; Sun, H.-Y.; Li, A.; Ping, X.-L.; Lai, W.-Y.; et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bartosovic, M.; Molares, H.C.; Gregorova, P.; Hrossova, D.; Kudla, G.; Vanacova, S. N6-Methyladenosine Demethylase FTO Targets Pre-mRNAs and Regulates Alternative Splicing and 3’-End Processing. Nucleic Acids Res. 2017, 45, 11356–11370. [Google Scholar] [CrossRef]
- Yoon, K.-J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.-S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m6A Methylation. Cell 2017, 171, 877–889.e17. [Google Scholar] [CrossRef]
- Bodi, Z.; Bottley, A.; Archer, N.; May, S.T.; Fray, R.G. Yeast m6A Methylated mRNAs Are Enriched on Translating Ribosomes during Meiosis, and under Rapamycin Treatment. PLoS ONE 2015, 10, e0132090. [Google Scholar] [CrossRef]
- Zhong, S.; Li, H.; Bodi, Z.; Button, J.; Vespa, L.; Herzog, M.; Fray, R.G. MTA Is an Arabidopsis Messenger RNA Adenosine Methylase and Interacts with a Homolog of a Sex-Specific Splicing Factor. Plant Cell 2008, 20, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Grozhik, A.V.; Vedanayagam, J.; Patil, D.P.; Pang, N.; Lim, K.-S.; Huang, Y.-C.; Joseph, B.; Lin, C.-J.; Despic, V.; et al. The m6A Pathway Facilitates Sex Determination in Drosophila. Nat. Commun. 2017, 8, 15737. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-Methyladenosine in Nuclear RNA Is a Major Substrate of the Obesity-Associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 Is a Mammalian RNA Demethylase That Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, S.; Qin, G. RNA Methylomes Reveal the m6A-Mediated Regulation of DNA Demethylase Gene SlDML2 in Tomato Fruit Ripening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef]
- Shen, L.; Liang, Z.; Gu, X.; Chen, Y.; Teo, Z.W.N.; Hou, X.; Cai, W.M.; Dedon, P.C.; Liu, L.; Yu, H. N6-Methyladenosine RNA Modification Regulates Shoot Stem Cell Fate in Arabidopsis. Dev. Cell 2016, 38, 186–200. [Google Scholar] [CrossRef]
- Dhingra, Y.; Gupta, S.; Gupta, V.; Agarwal, M.; Katiyar-Agarwal, S. The Emerging Role of Epitranscriptome in Shaping Stress Responses in Plants. Plant Cell Rep. 2023, 42, 1531–1555. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Y.; He, Q.; Qi, Z.; Zhang, G.; Xu, W.; Yi, T.; Wu, G.; Li, R. MTA, an RNA m6A Methyltransferase, Enhances Drought Tolerance by Regulating the Development of Trichomes and Roots in Poplar. Int. J. Mol. Sci. 2020, 21, 2462. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, C.-C.; Gao, Y.; Yang, Y.; Shi, B.; Yu, J.-L.; Lyu, C.; Sun, B.-F.; Wang, H.-L.; Xu, Y.; et al. OsNSUN2-Mediated 5-Methylcytosine mRNA Modification Enhances Rice Adaptation to High Temperature. Dev. Cell 2020, 53, 272–286.e7. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Cheng, Z.; Sun, J.; Gao, J.; Li, J.; Niu, X.; Amjid, M.W.; Yang, H.; Zhu, G.; et al. Transcriptome-Wide N6-Methyladenosine Profiling of Cotton Root Provides Insights for Salt Stress Tolerance. Environ. Exp. Bot. 2022, 194, 104729. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, S.; Yu, L.; Xiao, Y.; Zhang, S.; Wang, X.; Xu, Y.; Yu, H.; Li, Y.; Yang, J.; et al. RNA Demethylation Increases the Yield and Biomass of Rice and Potato Plants in Field Trials. Nat. Biotechnol. 2021, 39, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Ambrosino, L.; Jahnke, M.; Chiusano, M.; Barrote, I.; Procaccini, G.; Silva, J.; Dattolo, E. m6A RNA Methylation in Marine Plants: First Insights and Relevance for Biological Rhythms. Int. J. Mol. Sci. 2020, 21, 7508. [Google Scholar] [CrossRef] [PubMed]
- Bacova, R.; Klejdus, B.; Ryant, P.; Cernei, N.; Adam, V.; Huska, D. The Effects of 5-azacytidine and Cadmium on Global 5-methylcytosine Content and Secondary Metabolites in the Freshwater Microalgae Chlamydomonas reinhardtii and Scenedesmus quadricauda. J. Phycol. 2019, 55, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chaumier, T.; Manirakiza, E.; Veluchamy, A.; Tirichine, L. PhaeoEpiView: An Epigenome Browser of the Newly Assembled Genome of the Model Diatom Phaeodactylum tricornutum. Sci. Rep. 2023, 13, 8320. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; De Luca, D.; Amoroso, M.; Benfatto, S.; Maestri, S.; Racioppi, C.; Esposito, F.; Ianora, A. New Molecular Insights on the Response of the Green Alga Tetraselmis suecica to Nitrogen Starvation. Sci. Rep. 2019, 9, 3336. [Google Scholar] [CrossRef] [PubMed]
- Elagoz, A.M.; Ambrosino, L.; Lauritano, C. De Novo Transcriptome of the Diatom Cylindrotheca closterium Identifies Genes Involved in the Metabolism of Anti-Inflammatory Compounds. Sci. Rep. 2020, 10, 4138. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; De Luca, D.; Ferrarini, A.; Avanzato, C.; Minio, A.; Esposito, F.; Ianora, A. De Novo Transcriptome of the Cosmopolitan Dinoflagellate Amphidinium carterae to Identify Enzymes with Biotechnological Potential. Sci. Rep. 2017, 7, 11701. [Google Scholar] [CrossRef]
- Vingiani, G.M.; Štālberga, D.; De Luca, P.; Ianora, A.; De Luca, D.; Lauritano, C. De Novo Transcriptome of the Non-Saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates. Mar. Drugs 2020, 18, 386. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Leflaive, J.; Ten-Hage, L. Chemical Interactions in Diatoms: Role of Polyunsaturated Aldehydes and Precursors. New Phytol. 2009, 184, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.; Stonik, I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Jung, G.; Jin, E. Transcriptome Analysis of Acclimatory Responses to Thermal Stress in Antarctic Algae. Biochem. Biophys. Res. Commun. 2008, 367, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the Neurotoxin Domoic Acid in a Bloom-Forming Diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Cervin, G.; Pavia, H. Effects of Nitrate and Phosphate on Grazer-Induced Toxin Production in Alexandrium minutum. Limnol. Oceanogr. 2008, 53, 523–530. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Dobson, A.D.W.; Adams, C.; O’Gara, F. Emerging Concepts Promising New Horizons for Marine Biodiscovery and Synthetic Biology. Mar. Drugs 2015, 13, 2924–2954. [Google Scholar] [CrossRef]
- El-Hawary, S.; Sayed, A.; Mohammed, R.; Hassan, H.; Zaki, M.; Rateb, M.; Mohammed, T.; Amin, E.; Abdelmohsen, U. Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Roy, N.; De Paepe, A. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m6A Demethylase Activity Modulates Viral Infection of a Plant Virus and the m6A Abundance in Its Genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, P.; De Rango, F.; Paparazzo, E.; Mandalà, M.; Bellizzi, D.; Passarino, G. Impact of Nutrition on Age-Related Epigenetic RNA Modifications in Rats. Nutrients 2022, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z. The Epitranscriptomic Mechanism of Metal Toxicity and Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 11830. [Google Scholar] [CrossRef]
- Lin, S. Genomic Understanding of Dinoflagellates. Res. Microbiol. 2011, 162, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, F.M.; Zippay, M.L.; Pezzolesi, L.; Rein, K.S.; Johnson, J.G.; Morey, J.S.; Wang, Z.; Pistocchi, R. Subcellular Localization of Dinoflagellate Polyketide Synthases and Fatty Acid Synthase Activity. J. Phycol. 2013, 49, 1118–1127. [Google Scholar] [CrossRef]
- Kohli, G.S.; John, U.; Figueroa, R.I.; Rhodes, L.L.; Harwood, D.T.; Groth, M.; Bolch, C.J.S.; Murray, S.A. Polyketide Synthesis Genes Associated with Toxin Production in Two Species of Gambierdiscus (Dinophyceae). BMC Genomics 2015, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, L.; Ruggieri, V.; Bostan, H.; Miralto, M.; Vitulo, N.; Zouine, M.; Barone, A.; Bouzayen, M.; Frusciante, L.; Pezzotti, M.; et al. Multilevel Comparative Bioinformatics to Investigate Evolutionary Relationships and Specificities in Gene Annotations: An Example for Tomato and Grapevine. BMC Bioinform. 2018, 19, 435. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, A.; Meslet-Cladière, L.; Morin-Sardin, S.; Coton, E.; Jany, J.-L.; Barbier, G.; Corre, E. Comparative Analysis of Five Mucor Species Transcriptomes. Genomics 2019, 111, 1306–1314. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, J.; Zhu, J.; Hu, Z.; Diao, Y. Comparative Transcriptome Analysis and Identification of Candidate Adaptive Evolution Genes of Miscanthus lutarioriparius and Miscanthus sacchariflorus. Physiol. Mol. Biol. Plants 2021, 27, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiang, W.; Li, T.; Long, L. Transcriptome Analysis for Phosphorus Starvation-Induced Lipid Accumulation in Scenedesmus sp. Sci. Rep. 2018, 8, 16420. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Wang, X.; Peng, Q.; Han, Y.; Liu, X.; Liu, K.; Dou, S.; Li, L.; Liu, G.; et al. Starch Accumulation Dynamics and Transcriptome Analysis of Chlorella sorokiniana during Transition of Sulfur Nutritional Status. Front. Mar. Sci. 2022, 9, 986400. [Google Scholar] [CrossRef]
- López García de Lomana, A.; Schäuble, S.; Valenzuela, J.; Imam, S.; Carter, W.; Bilgin, D.D.; Yohn, C.B.; Turkarslan, S.; Reiss, D.J.; Orellana, M.V.; et al. Transcriptional Program for Nitrogen Starvation-Induced Lipid Accumulation in Chlamydomonas reinhardtii. Biotechnol. Biofuels 2015, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Sirikhachornkit, A. De Novo Transcriptome Analysis and Gene Expression Profiling of an Oleaginous Microalga Scenedesmus acutus TISTR8540 during Nitrogen Deprivation-Induced Lipid Accumulation. Sci. Rep. 2018, 8, 3668. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.; De Maayer, P.; Cowan, D. The Genome of the Antarctic Polyextremophile Nesterenkonia sp. AN1 Reveals Adaptive Strategies for Survival under Multiple Stress Conditions. FEMS Microbiol. Ecol. 2016, 92, fiw032. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, M.; Fabris, M.; Broos, S.; Vyverman, W.; Goossens, A. Profiling of the Early Nitrogen Stress Response in the Diatom Phaeodactylum tricornutum Reveals a Novel Family of RING-Domain Transcription Factors. Plant Physiol. 2016, 170, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Ravaglioli, C.; Lauritano, C.; Buia, M.C.; Balestri, E.; Capocchi, A.; Fontanini, D.; Pardi, G.; Tamburello, L.; Procaccini, G.; Bulleri, F. Nutrient Loading Fosters Seagrass Productivity Under Ocean Acidification. Sci. Rep. 2017, 7, 13732. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, E.; Lauritano, C.; Mangoni, O.; Bolinesi, F.; Saggiomo, M. Chlamydomonas Responses to Salinity Stress and Possible Biotechnological Exploitation. J. Mar. Sci. Eng. 2021, 9, 1242. [Google Scholar] [CrossRef]
- Verde, C.; Giordano, D.; di Prisco, G. The Adaptation of Polar Fishes to Climatic Changes: Structure, Function and Phylogeny of Haemoglobin. IUBMB Life 2008, 60, 29–40. [Google Scholar] [CrossRef]
- Sato, S.; Kurihara, T.; Kawamoto, J.; Hosokawa, M.; Sato, S.B.; Esaki, N. Cold Adaptation of Eicosapentaenoic Acid-Less Mutant of Shewanella livingstonensis Ac10 Involving Uptake and Remodeling of Synthetic Phospholipids Containing Various Polyunsaturated Fatty Acids. Extremophiles 2008, 12, 753–761. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, J.; Tang, X.X.; Zhou, B. Description of a Zostera marina Catalase Gene Involved in Responses to Temperature Stress. PeerJ 2018, 6, e4532. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wang, M.; Sun, S. Effects of Elevated Temperature and Food Supply on the Termination of Over-Summering and Subsequent Development of the Calanoid Copepod Calanus sinicus: Morphology, Physiology and Gene Expression. PLoS ONE 2016, 11, e0161838. [Google Scholar] [CrossRef]
- Le Franc, L.; Bernay, B.; Petton, B.; Since, M.; Favrel, P.; Rivière, G. A Functional m6A-RNA Methylation Pathway in the Oyster Crassostrea gigas Assumes Epitranscriptomic Regulation of Lophotrochozoan Development. FEBS J. 2021, 288, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M. Understanding Epitranscriptomic Changes Associated with Heat Stress in Acropora hemprichii Using Direct RNA Sequencing. Ph.D. Thesis, KAUST University Library, Thuwal, Saudi Arabia, 2024. [Google Scholar]
- Qadri, M.; Nalli, Y.; Jain, S.K.; Chaubey, A.; Ali, A.; Strobel, G.A.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. An Insight into the Secondary Metabolism of Muscodor yucatanensis: Small-Molecule Epigenetic Modifiers Induce Expression of Secondary Metabolism-Related Genes and Production of New Metabolites in the Endophyte. Microb. Ecol. 2017, 73, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The Green Microalga Tetraselmis suecica Reduces Oxidative Stress and Induces Repairing Mechanisms in Human Cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the Culture of Oceanic Ultraphytoplankton1,2. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Leinonen, R.; Sugawara, H.; Shumway, M.; International Nucleotide Sequence Database Collaboration. The Sequence Read Archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings of the 1st Conference on Culture of Marine Invertebrate Animals Greenport, New York, NY, USA, October 1975; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.; Gould, M.; Stewart, R.; Kendziorski, C. EBSeq: An Empirical Bayes Hierarchical Model for Inference in RNA-Seq Experiments. Bioinforma. Oxf. Engl. 2013, 29, 1035–1043. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Overbeek, R.; Fonstein, M.; D’Souza, M.; Pusch, G.D.; Maltsev, N. The Use of Gene Clusters to Infer Functional Coupling. Proc. Natl. Acad. Sci. USA 1999, 96, 2896–2901. [Google Scholar] [CrossRef]
- Rosenfeld, J.A.; DeSalle, R. E Value Cutoff and Eukaryotic Genome Content Phylogenetics. Mol. Phylogenet. Evol. 2012, 63, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, L.; Chiusano, M.L. Transcriptologs: A Transcriptome-Based Approach to Predict Orthology Relationships. Bioinform. Biol. Insights 2017, 11, 1177932217690136. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Watanabe, C.K. GMAP: A Genomic Mapping and Alignment Program for mRNA and EST Sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Baroni, É.G.; Yap, K.Y.; Webley, P.A.; Scales, P.J.; Martin, G.J.O. The Effect of Nitrogen Depletion on the Cell Size, Shape, Density and Gravitational Settling of Nannochloropsis salina, Chlorella sp. (Marine) and Haematococcus pluvialis. Algal Res. 2019, 39, 101454. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-Induced Differential Oxidative Stress and Antioxidant Responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villarreal, M. Bloom Dynamics of Dinophysis acuta in an Upwelling System: In Situ Growth versus Transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative Expression Software Tool (REST©) for Group-Wise Comparison and Statistical Analysis of Relative Expression Results in Real-Time PCR. Nucleic Acids Res. 2002, 30, 10. [Google Scholar] [CrossRef] [PubMed]
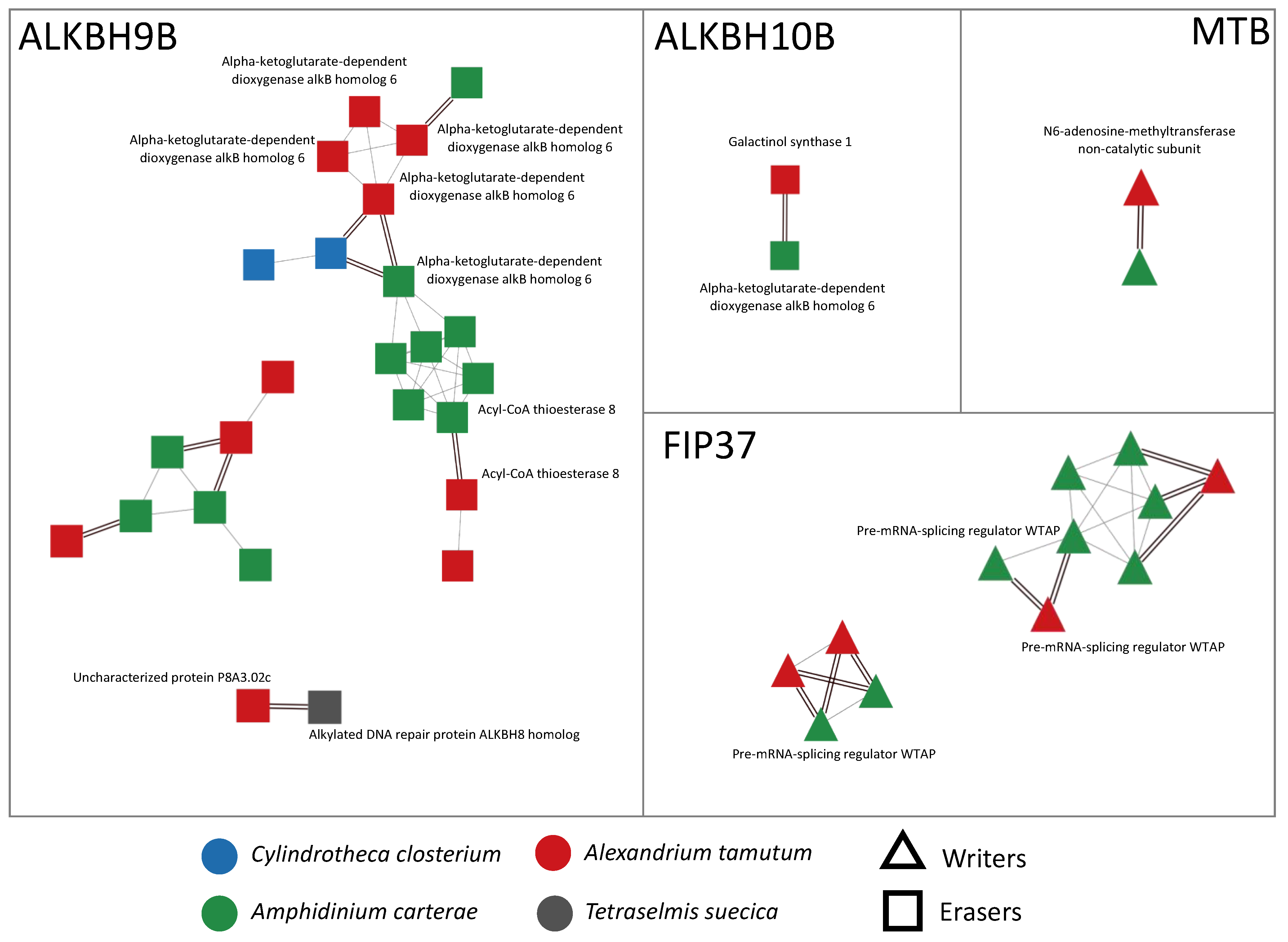
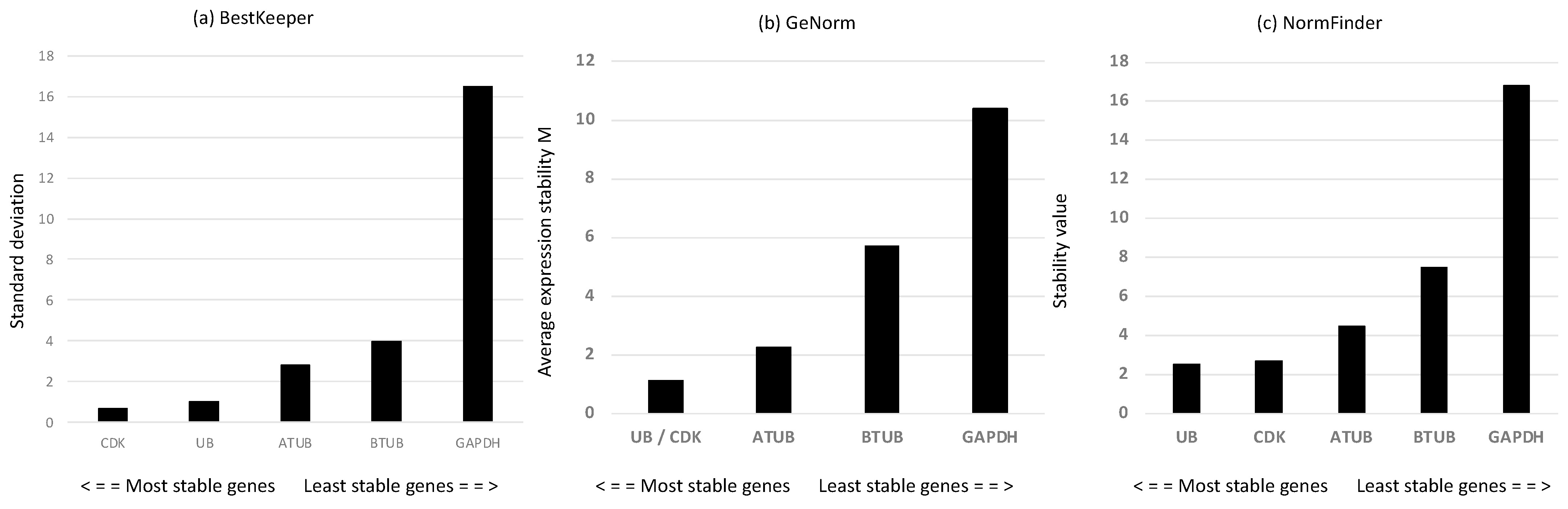
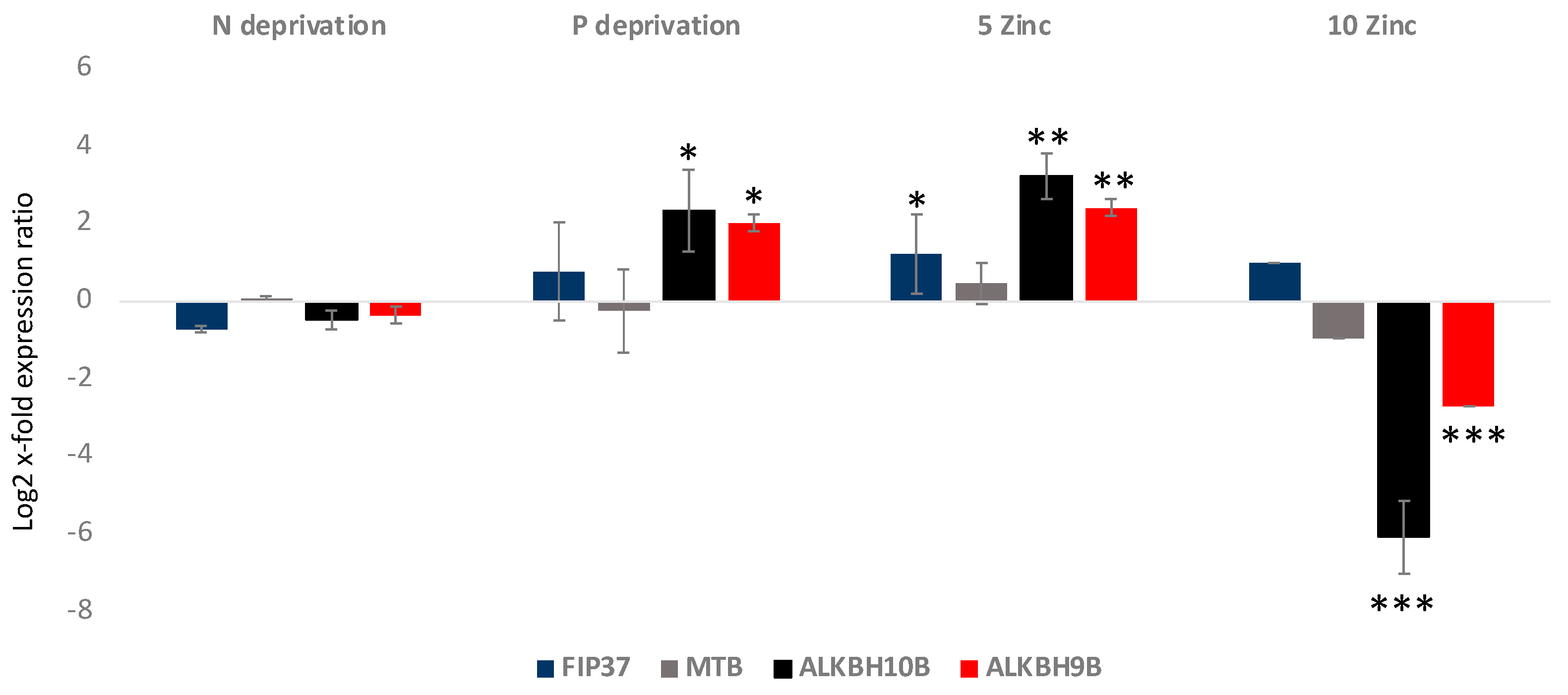
| GENE ID | Species |
|---|---|
| Eraser ALKBH9B | |
| TR13281-c0_g1_i1 | Alexandrium tamutum |
| TR95800-c0_g1_i1 | Alexandrium tamutum |
| TR95800-c0_g2_i1 | Alexandrium tamutum |
| tri_n_comp20163_c0_seq35 | Amphidinium carterae |
| tri_n_comp20163_c0_seq44 | Amphidinium carterae |
| tri_n_comp20163_c0_seq46 | Amphidinium carterae |
| vo_c3_Locus_29131_Transcript_3/4_Confidence_0.500_Length_4920 | Amphidinium carterae |
| TR10254-c0_g1_i1 | Alexandrium tamutum |
| SampleTS2_Locus_13766_Transcript_2/4_Confidence_0.400_Length_902 | Tetraselmis suecica |
| TR111002-c0_g1_i1 | Alexandrium tamutum |
| TR111002-c0_g1_i2 | Alexandrium tamutum |
| TR5514-c0_g2_i1 | Alexandrium tamutum |
| TR74917-c0_g1_i1 | Alexandrium tamutum |
| TR74917-c0_g1_i2 | Alexandrium tamutum |
| TR98782-c0_g1_i1 | Alexandrium tamutum |
| tri_c_comp46027_c0_seq1 | Amphidinium carterae |
| tri_c_comp48811_c0_seq1 | Amphidinium carterae |
| vo_c1_Locus_8663_Transcript_1/7_Confidence_0.300_Length_2289 | Amphidinium carterae |
| vo_c1_Locus_8663_Transcript_2/7_Confidence_0.600_Length_1953 | Amphidinium carterae |
| vo_c1_Locus_8663_Transcript_7/7_Confidence_0.000_Length_2852 | Amphidinium carterae |
| vo_c2_Locus_8417_Transcript_1/6_Confidence_0.333_Length_2144 | Amphidinium carterae |
| vo_n1_Locus_3421_Transcript_10/10_Confidence_0.100_Length_2245 | Amphidinium carterae |
| vo_n1_Locus_3421_Transcript_6/10_Confidence_0.200_Length_2273 | Amphidinium carterae |
| TR12427-c0_g1_i1 | Cylindrotheca closterium |
| TR12745-c0_g1_i1 | Cylindrotheca closterium |
| Eraser ALKBH10B | |
| TR65690-c0_g1_i1 | Alexandrium tamutum |
| vo_c2_Locus_19652_Transcript_2/2_Confidence_0.250_Length_1986 | Amphidinium carterae |
| Writer MTB | |
| TR59452-c0_g1_i1 | Alexandrium tamutum |
| tri_c_comp49311_c0_seq1 | Amphidinium carterae |
| Writer FIP37 | |
| TR51739-c0_g1_i1 | Alexandrium tamutum |
| TR75118-c0_g1_i1 | Alexandrium tamutum |
| tri_n_comp42309_c0_seq1 | Amphidinium carterae |
| vo_c1_Locus_4428_Transcript_3/4_Confidence_0.879_Length_868 | Amphidinium carterae |
| vo_c3_Locus_26772_Transcript_3/6_Confidence_0.529_Length_887 | Amphidinium carterae |
| vo_n1_Locus_16_Transcript_73/225_Confidence_0.003_Length_1180 | Amphidinium carterae |
| vo_n2_Locus_4713_Transcript_1/2_Confidence_0.333_Length_891 | Amphidinium carterae |
| vo_n3_Locus_21951_Transcript_1/2_Confidence_0.985_Length_839 | Amphidinium carterae |
| TR126122-c0_g12_i1 | Alexandrium tamutum |
| TR126122-c0_g5_i1 | Alexandrium tamutum |
| vo_c3_Locus_6426_Transcript_2/2_Confidence_0.250_Length_1098 | Amphidinium carterae |
| vo_n2_Locus_6399_Transcript_2/2_Confidence_0.500_Length_1101 | Amphidinium carterae |
| (1) Amphidinium carterae | ||
| query_ID | query_length | identity% |
| AT1G14710.1_ALKBH10B | 2429 bp | 44.2 |
| AT1G14710.2_ALKBH10B | 2513 bp | 45.8 |
| AT1G48980.1_ALKBH9B | 1830 bp | 48.2 |
| AT1G48980.2_ALKBH9B | 1818 bp | 46.6 |
| AT1G48980.3_ALKBH9B | 1832 bp | 48.2 |
| AT1G48980.4_ALKBH9B | 1745 bp | 48.2 |
| AT2G17970.1_ALKBH9B | 1797 bp | 45.1 |
| AT2G17970.2_ALKBH9B | 1981 bp | 45.7 |
| AT2G17970.3_ALKBH9B | 2119 bp | 45.4 |
| AT2G48080.1_ALKBH10B | 1519 bp | 46.7 |
| AT3G05680.1_VIRILIZER | 6833 bp | 100 |
| AT3G05680.2_VIRILIZER | 6904 bp | 100 |
| AT3G54170.1_FIP37 | 1262 bp | 45.7 |
| AT4G02940.1_ALKBH10B | 2112 bp | 44.4 |
| AT4G09980.1_MTB | 3393 bp | 43.4 |
| AT4G09980.2_MTB | 3342 bp | 46.5 |
| AT4G10760.1_MTA | 2227 bp | 46.9 |
| AT4G36090.1_ALKBH9B | 1830 bp | 44.8 |
| AT4G36090.2_ALKBH9B | 2025 bp | 100 |
| AT4G36090.3_ALKBH9B | 1707 bp | 45.7 |
| AT5G01160.1_HAKAI | 1404 bp | 46 |
| NP_001042682.1_MTB XM_015763885.2 | 4254 bp | 40.5 |
| NP_001047707.1_MTA XM_015769953.1 | 2476 bp | 45.4 |
| NP_001048963.2_MTB XM_015776811.2 | 2832 bp | 42.9 |
| NP_001049502.1_ALKBH10B XM_015775307.2 | 2532 bp | 100 |
| NP_001056738.1_ALKBH9B XM_015787389.2 | 2221 bp | 46.3 |
| NP_001057630.2_FIP37 XM_015788828.2 | 1428 bp | 42.6 |
| NP_001064055.1_ALKBH10B XM_015759337.2 | 2380 bp | 42.5 |
| NP_001064723.2_MTB XM_015759054.2 | 3607 bp | 100 |
| NP_001064945.2_HAKAI XM_015758095.2 | 1685 bp | 43.3 |
| (2) Cylindrotheca fusiformis | ||
| query_ID | query_length | identity% |
| AT1G14710.1_ALKBH10B | 2429 bp | 49.8 |
| AT1G14710.2_ALKBH10B | 2513 bp | 46.3 |
| AT1G48980.1_ALKBH9B | 1830 bp | 42 |
| AT1G48980.2_ALKBH9B | 1818 bp | 42.4 |
| AT1G48980.3_ALKBH9B | 1832 bp | 42 |
| AT1G48980.4_ALKBH9B | 1745 bp | 44.9 |
| AT2G17970.1_ALKBH9B | 1797 bp | 49.7 |
| AT2G17970.2_ALKBH9B | 1981 bp | 45.1 |
| AT2G17970.3_ALKBH9B | 2119 bp | 47.6 |
| AT2G48080.1_ALKBH10B | 1519 bp | 42.1 |
| AT3G05680.1_VIRILIZER | 6833 bp | 100 |
| AT3G05680.2_VIRILIZER | 6904 bp | 100 |
| AT3G54170.1_FIP37 | 1262 bp | 44.6 |
| AT4G02940.1_ALKBH10B | 2112 bp | 42 |
| AT4G09980.1_MTB | 3393 bp | 100 |
| AT4G09980.2_MTB | 3342 bp | 100 |
| AT4G10760.1_MTA | 2227 bp | 84.6 |
| AT4G36090.1_ALKBH9B | 1830 bp | 46.7 |
| AT4G36090.2_ALKBH9B | 2025 bp | 46.9 |
| AT4G36090.3_ALKBH9B | 1707 bp | 46.6 |
| AT5G01160.1_HAKAI | 1404 bp | 43.1 |
| NP_001047707.1_MTA XM_015769953.1 | 2476 bp | 45.6 |
| NP_001049502.1_ALKBH10B XM_015775307.2 | 2532 bp | 40.6 |
| NP_001056738.1_ALKBH9B XM_015787389.2 | 2221 bp | 53 |
| NP_001057630.2_FIP37 XM_015788828.2 | 1428 bp | 40.5 |
| NP_001064055.1_ALKBH10B XM_015759337.2 | 2380 bp | 47 |
| NP_001064723.2_MTB XM_015759054.2 | 3607 bp | 40.4 |
| NP_001064945.2_HAKAI XM_015758095.2 | 1685 bp | 42.5 |
| (3) Tetraselmis striata | ||
| query_ID | query_length | identity% |
| AT1G14710.1_ALKBH10B | 2429 bp | 40.5 |
| AT1G14710.2_ALKBH10B | 2513 bp | 40.5 |
| AT1G48980.1_ALKBH9B | 1830 bp | 43.7 |
| AT1G48980.3_ALKBH9B | 1832 bp | 43.7 |
| AT1G48980.4_ALKBH9B | 1745 bp | 43.7 |
| AT2G48080.1_ALKBH10B | 1519 bp | 44 |
| AT3G05680.1_VIRILIZER | 6833 bp | 100 |
| AT3G05680.2_VIRILIZER | 6904 bp | 100 |
| AT3G54170.1_FIP37 | 1262 bp | 42 |
| AT4G10760.1_MTA | 2227 bp | 42.4 |
| AT4G36090.1_ALKBH9B | 1830 bp | 46.7 |
| AT4G36090.2_ALKBH9B | 2025 bp | 46.1 |
| AT4G36090.3_ALKBH9B | 1707 bp | 46.1 |
| AT5G01160.1_HAKAI | 1404 bp | 42.6 |
| NP_001042682.1_MTB XM_015763885.2 | 4254 bp | 100 |
| NP_001047707.1_MTA XM_015769953.1 | 2476 bp | 48.8 |
| NP_001048963.2_MTB XM_015776811.2 | 2832 bp | 40.9 |
| NP_001056738.1_ALKBH9B XM_015787389.2 | 2221 bp | 42.9 |
| NP_001057630.2_FIP37 XM_015788828.2 | 1428 bp | 45 |
| NP_001064055.1_ALKBH10B XM_015759337.2 | 2380 bp | 45.9 |
| NP_001064945.2_HAKAI XM_015758095.2 | 1685 bp | 48.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosino, L.; Riccardi, A.; Welling, M.S.; Lauritano, C. Comparative Transcriptomics to Identify RNA Writers and Erasers in Microalgae. Int. J. Mol. Sci. 2024, 25, 8005. https://doi.org/10.3390/ijms25158005
Ambrosino L, Riccardi A, Welling MS, Lauritano C. Comparative Transcriptomics to Identify RNA Writers and Erasers in Microalgae. International Journal of Molecular Sciences. 2024; 25(15):8005. https://doi.org/10.3390/ijms25158005
Chicago/Turabian StyleAmbrosino, Luca, Alessia Riccardi, Melina S. Welling, and Chiara Lauritano. 2024. "Comparative Transcriptomics to Identify RNA Writers and Erasers in Microalgae" International Journal of Molecular Sciences 25, no. 15: 8005. https://doi.org/10.3390/ijms25158005
APA StyleAmbrosino, L., Riccardi, A., Welling, M. S., & Lauritano, C. (2024). Comparative Transcriptomics to Identify RNA Writers and Erasers in Microalgae. International Journal of Molecular Sciences, 25(15), 8005. https://doi.org/10.3390/ijms25158005







