Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Properties of In2O3-ZnO Heterojunction on SF6 Decomposition Gases
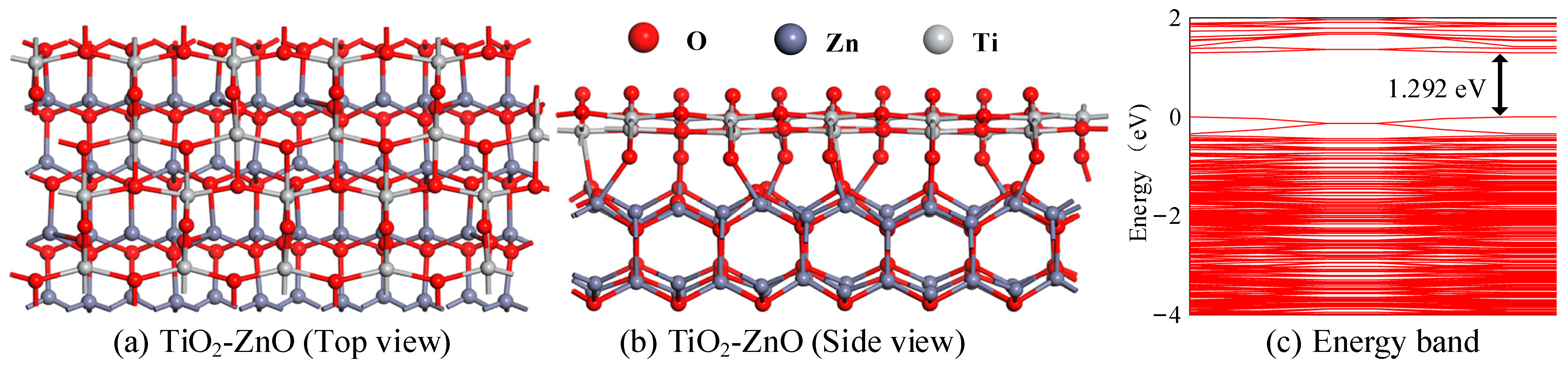
2.1.1. Construction and Energy Band Analysis of In2O3-ZnO Heterojunction Model
2.1.2. Adsorption Properties of In2O3-ZnO Heterojunction
- Gas adsorption structure analysis
- DOS, CDD, and molecular orbital analysis
2.2. Adsorption Properties of TiO2-ZnO Heterojunction on SF6 Decomposition Gases
2.2.1. Construction and Energy Band Analysis of TiO2-ZnO Heterojunction Model
2.2.2. Adsorption Properties of TiO2-ZnO Heterojunction
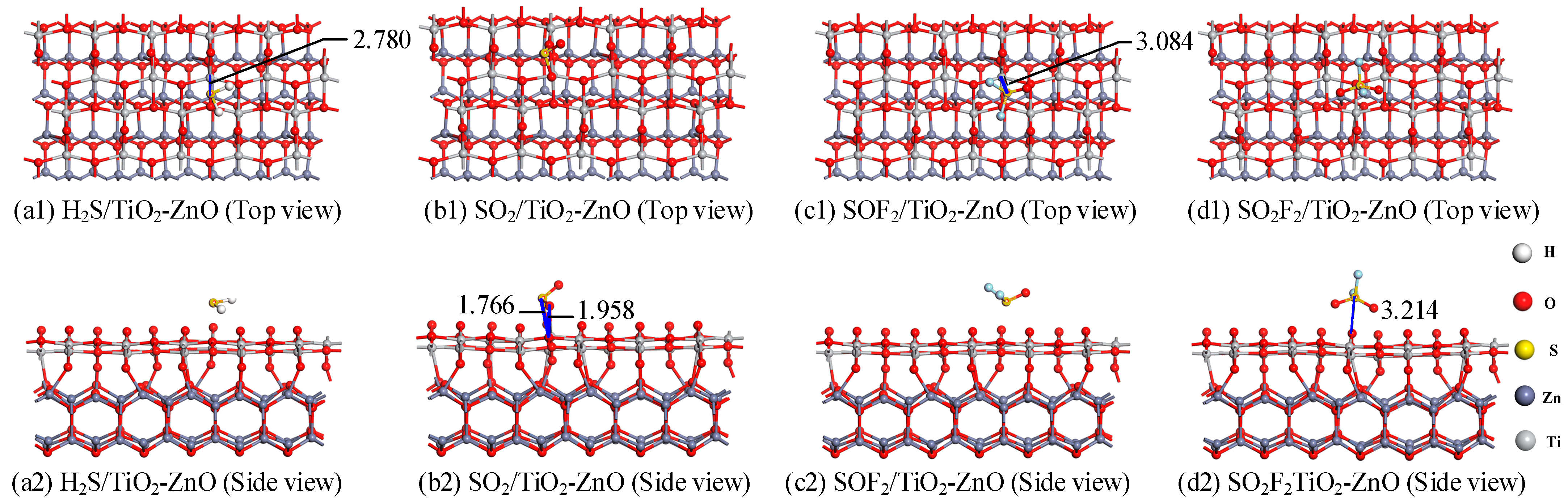
- Gas adsorption structure analysis
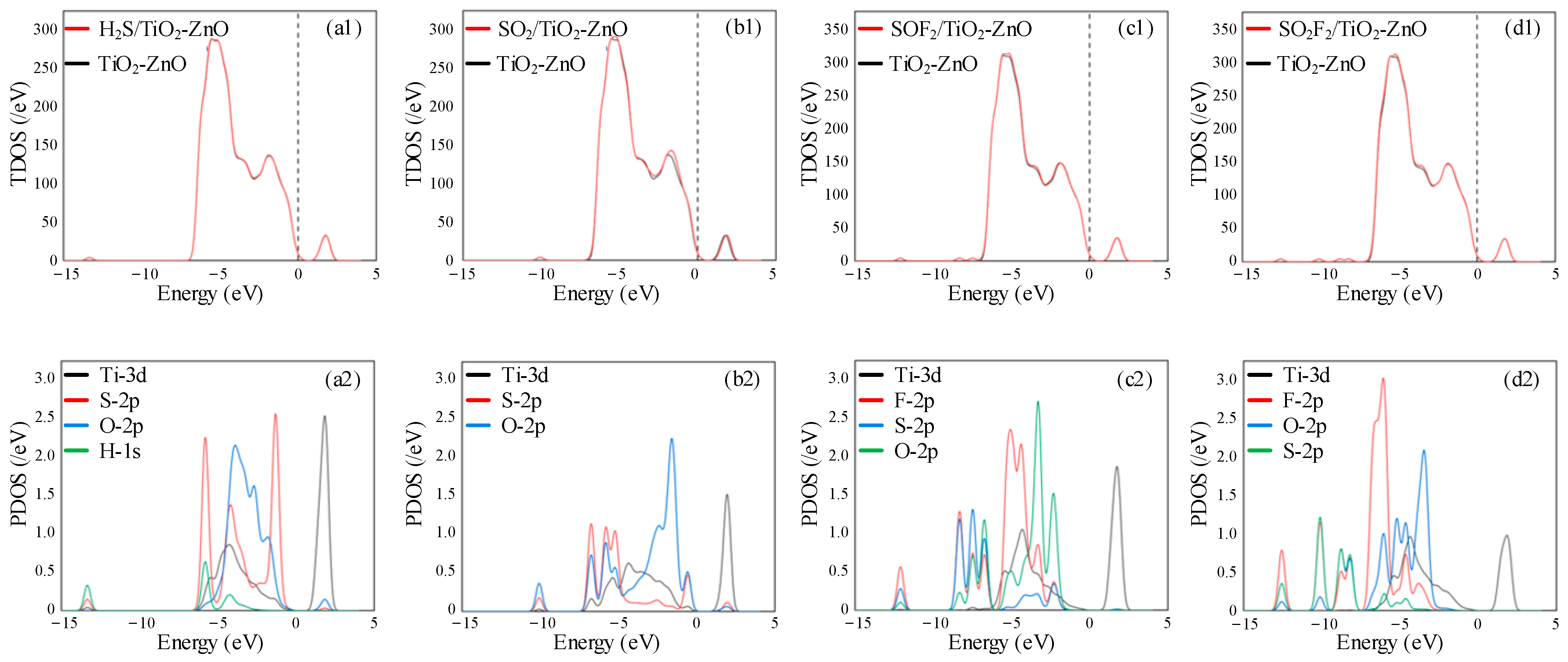
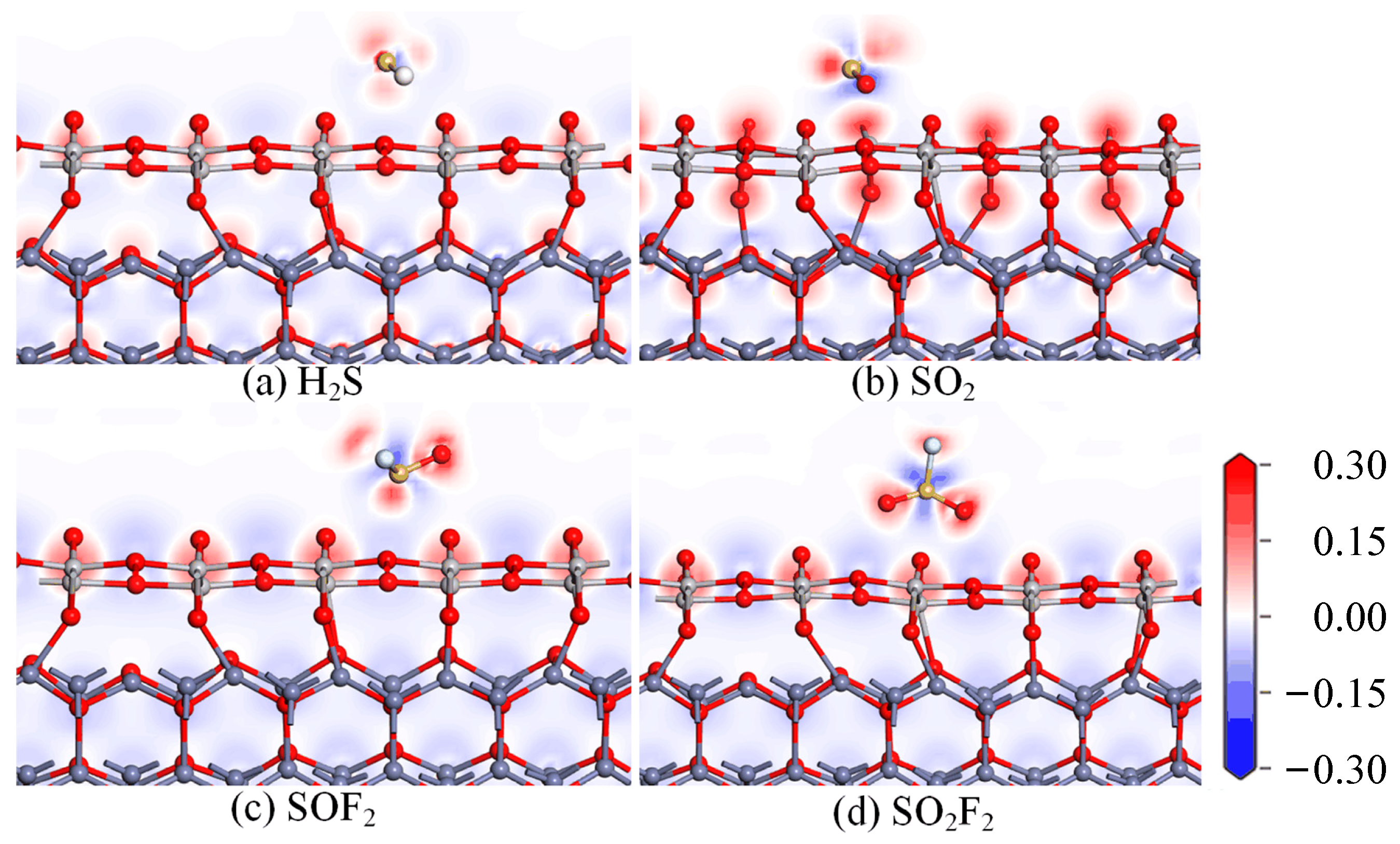
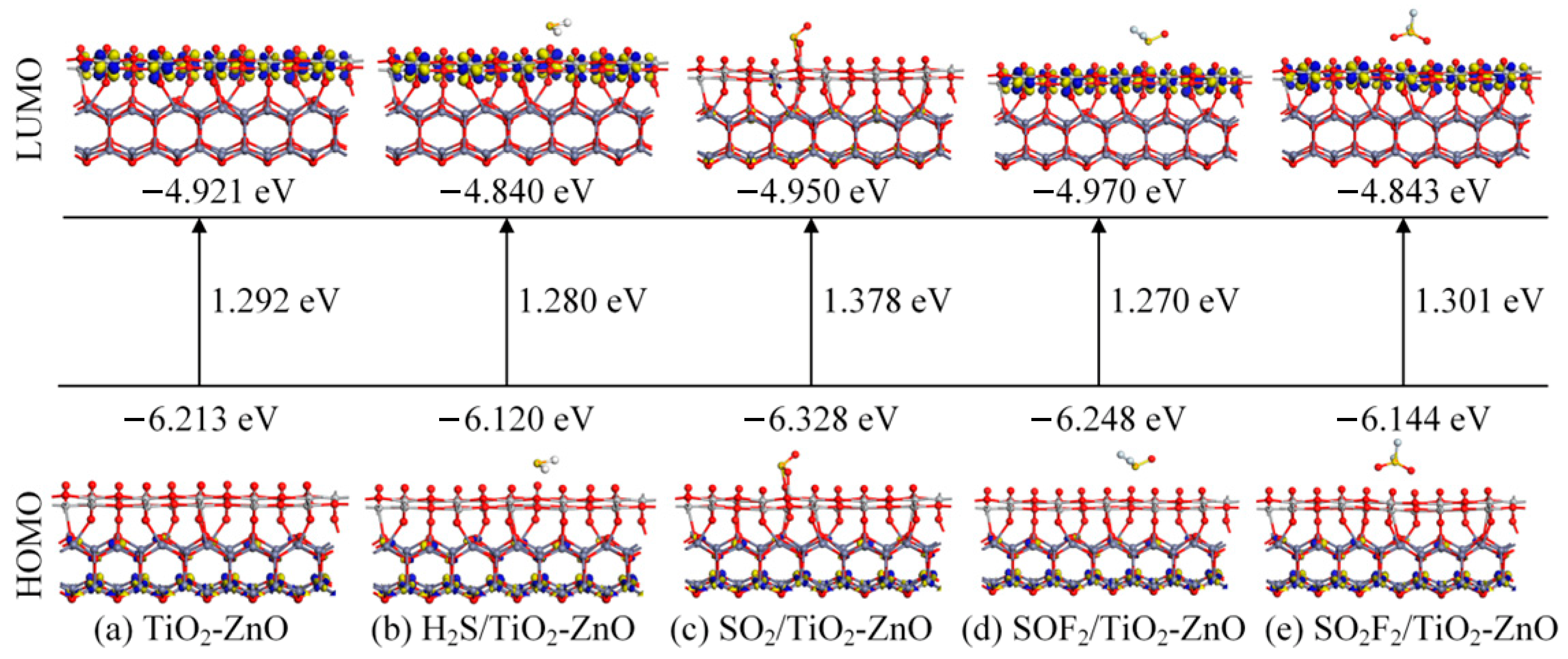
- DOS, CDD, and molecular orbital analysis
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SF6 | sulfur hexafluoride |
| GIS | gas insulated switchgear |
| TMDCs | two-dimensional metal sulfur compounds |
| VOCs | volatile organic compounds |
| DFT | density functional theory |
| DOS | density of states |
| TDOS | total density of states |
| PDOS | partial density of states |
| CDD | charge difference density |
| LUMO | lowest unoccupied molecular orbital |
| HOMO | highest occupied molecular orbital |
| GGA | generalized gradient approximation |
| PBE | Perdew–Burke–Ernzerhof |
| DSSP | DFT semi-core pseudopotential |
References
- Wang, J.; Chen, W.; Wang, P.; Wan, F.; Zhang, Z.; Gao, S.; Wang, Y. Analysis of SF6 decomposed products by fibre-enhanced Raman spectroscopy for gas-insulated switchgear diagnosis. High Volt. 2024, 9, 206–216. [Google Scholar] [CrossRef]
- Khan, Q.; Refaat, S.S.; Abu-Rub, H.; Toliyat, H.A. Partial discharge detection and diagnosis in gas insulated switchgear: State of the art. IEEE Electr. Insul. Mag. 2019, 35, 16–33. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hu, X.; Tang, B.; Wang, S.; Cui, A.; Hou, K.; He, Y.; Zhu, L.; Li, W.; Chu, J. Effects of SF6 decomposition components and concentrations on the discharge faults and insulation defects in GIS equipment. Sci. Rep. 2020, 10, 15039. [Google Scholar] [CrossRef] [PubMed]
- Faizol, Z.; Zubir, F.; Saman, N.M.; Ahmad, M.H.; Rahim, M.K.A.; Ayop, O.; Jusoh, M.; Majid, H.A.; Yusoff, Z. Detection Method of Partial Discharge on Transformer and Gas-Insulated Switchgear: A Review. Appl. Sci. 2023, 13, 9605. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Zhou, Y.; Tang, N.; Zou, Z.; Li, X.; Huang, G.; Liu, M. Online detection technology for SF6 decomposition products in electrical equipment: A review. IET Sci. Meas. Technol. 2018, 12, 707–711. [Google Scholar] [CrossRef]
- Fang, H.; Chen, H.; Zhang, F.; Tan, R.; Han, C.; Ai, X.; Ma, X.; Cui, Y.; Wang, D. Trace detection of SF6 gas decomposition component H2S based on Pr6O11-In2O3. Sens. Actuators B Chem. 2024, 412, 135822. [Google Scholar] [CrossRef]
- Li, Z.; Jia, L.; Chen, J.; Cui, X.; Zeng, W.; Zhou, Q. Ag-modified hexagonal GaN monolayer as an innovative gas detector toward SF6 decomposed species: Insights from the first-principles computations. Appl. Surf. Sci. 2022, 589, 153000. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Yan, Y.; Qiu, H.; Guo, X.; Tang, J.; Zeng, F. Theoretical analysis of Ni atom-doped MXene for improving the catalytic degradation performance of SF6. Comput. Theor. Chem. 2023, 1229, 114325. [Google Scholar] [CrossRef]
- Meng, H.; Liu, Z.; Wang, X.; Feng, L. A fluorinated greenhouse gas sensor based on N-doped tin oxide materials. Environ. Sci.-Nano 2024, 11, 459–469. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO Nanosheets Abundant in Oxygen Vacancies Derived from Metal-Organic Frameworks for ppb-Level Gas Sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, M.R. Hierarchical zinc oxide nanorings with superior sensing properties. Mater. Sci. Eng. B 2018, 236–237, 132–138. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Li, A.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Effects of rare earth elements doping on gas sensing properties of ZnO nanowires. Ceram. Int. 2021, 47, 24218–24226. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.-Y.; Gao, P.-X. Low-Concentration NOx Gas Analysis Using Single Bimodular ZnO Nanorod Sensor. ACS Sens. 2021, 6, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Razavi, R.; Nejad, F.G.; Ahmadi, S.A.; Beitollahi, H. Synthesis of ZnO@TiO2 nanoparticles and its application to construct an electrochemical sensor for determination of hydrazine. Electrochem. Commun. 2024, 159, 107639. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Haidry, A.A.; Xie, L.; Wang, Z.; Zavabeti, A.; Li, Z.; Plecenik, T.; Gregor, M.; Roch, T.; Plecenik, A. Remarkable Improvement in Hydrogen Sensing Characteristics with Pt/TiO2 Interface Control. ACS Sens. 2019, 4, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Tshabalala, Z.P.; Swart, H.C.; Motaung, D.E. Fabrication of TiO2 nanofibers based sensors for enhanced CH4 performance induced by notable surface area and acid treatment. Vacuum 2021, 187, 110102. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B-Chem. 2022, 357. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, S.; Din, S.T.U.; Shahid, A.; Amu-Darko, J.N.O.; Wang, M.; Tianyan, Y.; Liu, G.; Qiao, G. A review on In2O3 nanostructures for gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112538. [Google Scholar] [CrossRef]
- Sui, N.; Zhang, P.; Zhou, T.; Zhang, T. Selective ppb-level ozone gas sensor based on hierarchical branch-like In2O3 nanostructure. Sens. Actuators B Chem. 2021, 336, 129612. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Du, L.; Zhang, M. Effect of resonant tunneling modulation on ZnO/In2O3 heterojunction nanocomposite in efficient detection of NO2 gas at room temperature. Sens. Actuators B Chem. 2021, 329, 129230. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal Oxide Based Heterojunctions for Gas Sensors: A Review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.-Y.; Lai, R.-P.; Dong, X.-H.; Wu, X.; Luan, Q.-T.; Wang, J.; Lin, H.-F.; Ding, W.-H.; Wu, G.-L.; Xie, W.-F. Recent advances in ethanol gas sensors based on metal oxide semiconductor heterojunctions. Rare Met. 2022, 41, 1818–1842. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, C.; Zhang, H.; Zhang, Y. Preparation and study of ammonia gas sensor based on ZnO/CuO heterojunction with high performance at room temperature. Mater. Sci. Semicond. Process. 2022, 146, 106700. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Alharbi, F.; Al-Hossainy, A.F.; Abdel-Aziz, M.H. Preparation of hybrid conducting polymers blend nanocomposite for energy conversion using experimental data and TD-DFT/DMOl3 computations. J. Mater. Res. Technol. 2023, 23, 2852–2867. [Google Scholar] [CrossRef]
- Haas, P.; Tran, F.; Blaha, P. Calculation of the lattice constant of solids with semilocal functionals. Phys. Rev. B 2009, 79, 085104. [Google Scholar] [CrossRef]
- Bučko, T.; Lebègue, S.; Hafner, J.; Ángyán, J.G. Tkatchenko-Scheffler van der Waals correction method with and without self-consistent screening applied to solids. Phys. Rev. B 2013, 87, 064110. [Google Scholar] [CrossRef]
- Muvva, C.; Murugan, N.A.; Subramanian, V. Assessment of Amyloid Forming Tendency of Peptide Sequences from Amyloid Beta and Tau Proteins Using Force-Field, Semi-Empirical, and Density Functional Theory Calculations. Int. J. Mol. Sci. 2021, 22, 3244. [Google Scholar] [CrossRef]
- Gui, Y.; Lin, Y.; Ji, C.; Luo, P.; Chen, X. Density Functional Theory Calculations for the Adsorption Property of Hazardous Industrial Gasses on Transition-Metal-Modified MoS2 Nanosheets. ACS Appl. Nano Mater. 2022, 5, 11111–11118. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components on Pt3-TiO2(1 0 1) surface: A DFT study. Appl. Surf. Sci. 2018, 459, 242–248. [Google Scholar] [CrossRef]

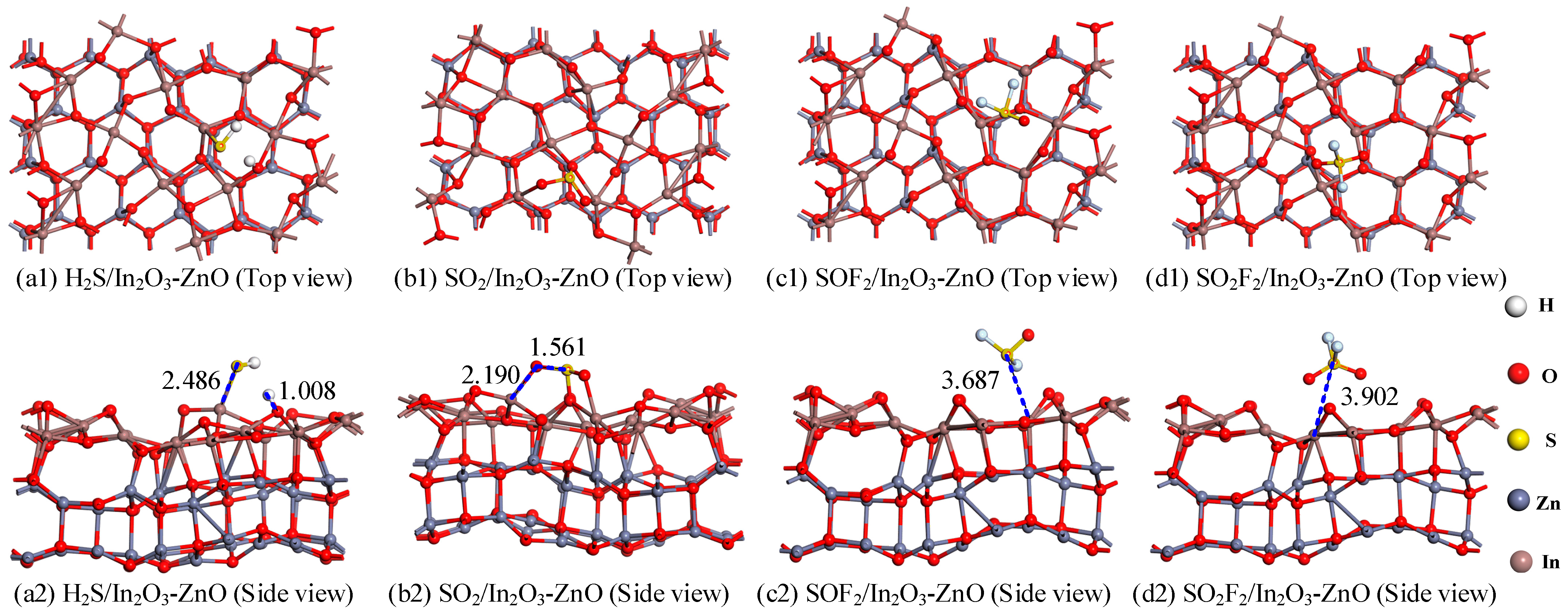

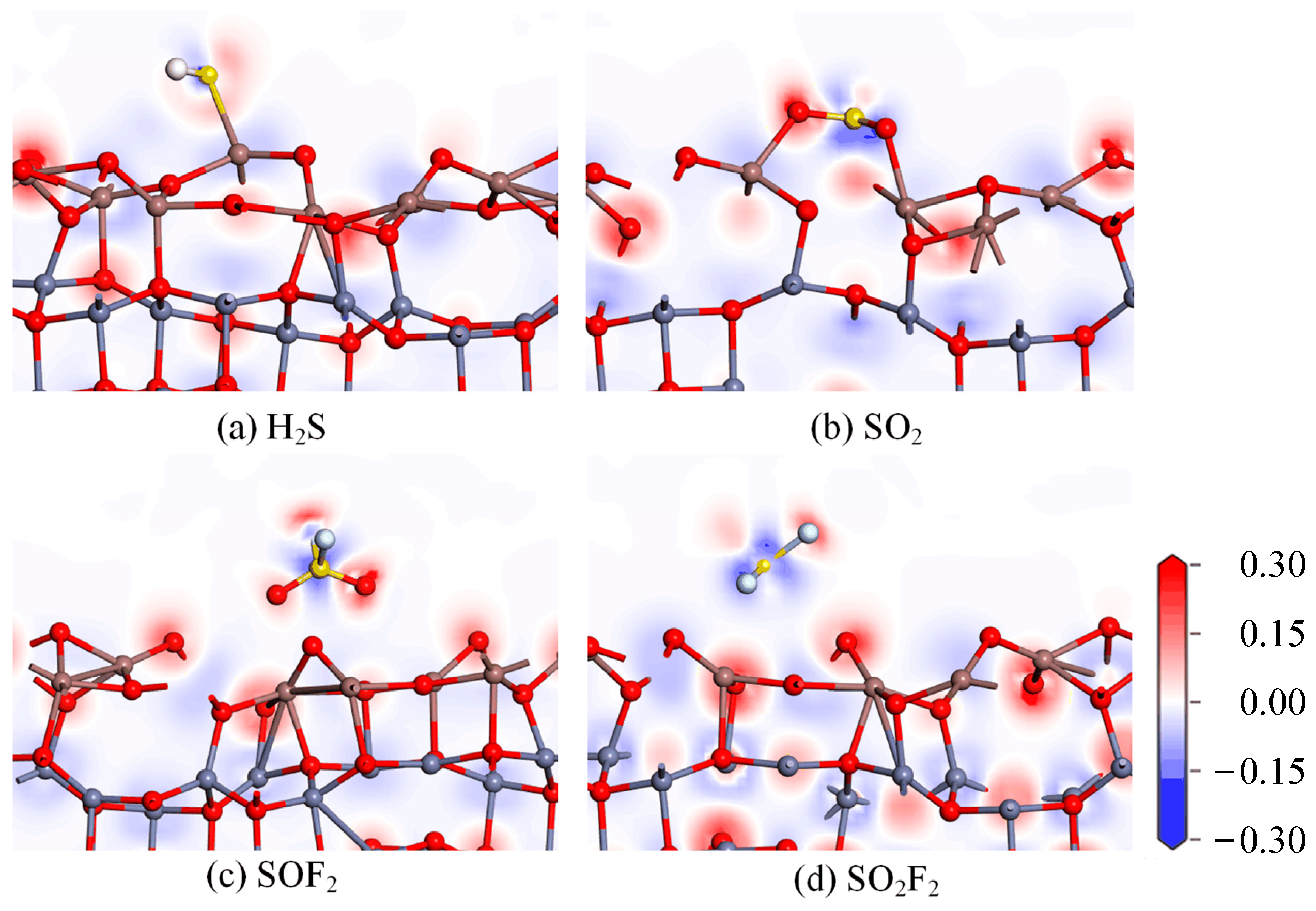
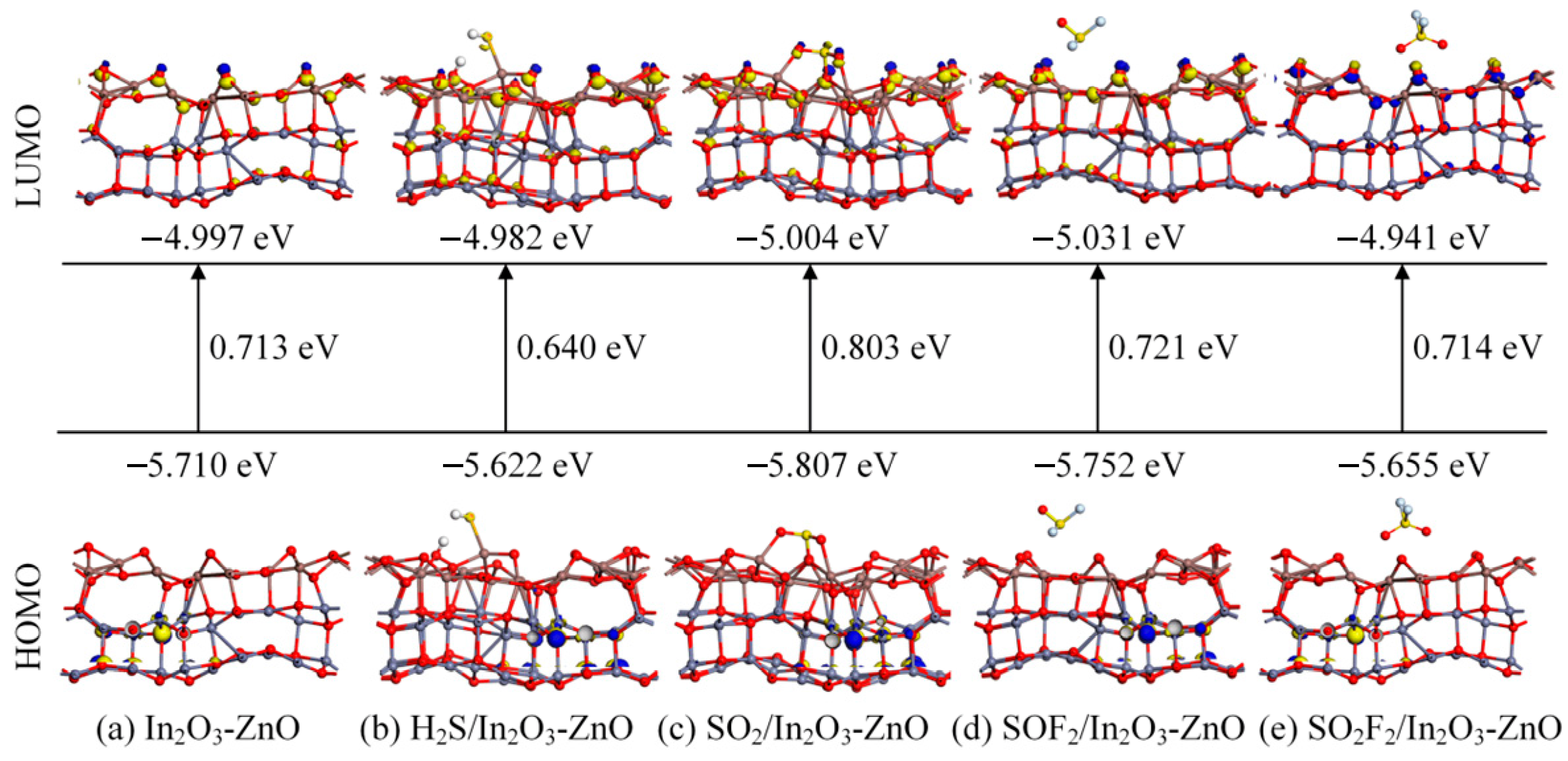
| System | Distance (Å) | Eads (eV) | Qt (e) |
|---|---|---|---|
| H2S/In2O3-ZnO | In-S:2.486 H-O:1.008 | −1.924 | 0.255 |
| SO2/In2O3-ZnO | In-O:2.190 S-O:1.561 | −1.992 | −0.361 |
| SOF2/In2O3-ZnO | In-S:3.687 | −0.465 | 0.016 |
| SO2F2/In2O3-ZnO | In-S:3.902 | −0.501 | 0.011 |
| System | Distance (Å) | Eads (eV) | Qt (e) |
|---|---|---|---|
| H2S/TiO2-ZnO | Ti-S:2.780 | −0.788 | 0.249 |
| SO2/TiO2-ZnO | S-O:1.766 Ti-O:1.958 | −0.608 | −0.142 |
| SOF2/TiO2-ZnO | Ti-S:3.084 | −0.402 | 0.083 |
| SO2F2/TiO2-ZnO | S-O:3.214 | −0.549 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Ma, D.; Gui, Y. Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study. Int. J. Mol. Sci. 2024, 25, 8009. https://doi.org/10.3390/ijms25158009
Zeng T, Ma D, Gui Y. Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study. International Journal of Molecular Sciences. 2024; 25(15):8009. https://doi.org/10.3390/ijms25158009
Chicago/Turabian StyleZeng, Tingting, Donglin Ma, and Yingang Gui. 2024. "Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study" International Journal of Molecular Sciences 25, no. 15: 8009. https://doi.org/10.3390/ijms25158009
APA StyleZeng, T., Ma, D., & Gui, Y. (2024). Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study. International Journal of Molecular Sciences, 25(15), 8009. https://doi.org/10.3390/ijms25158009







