Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-Ray Diffraction
2.2. Optical Analysis
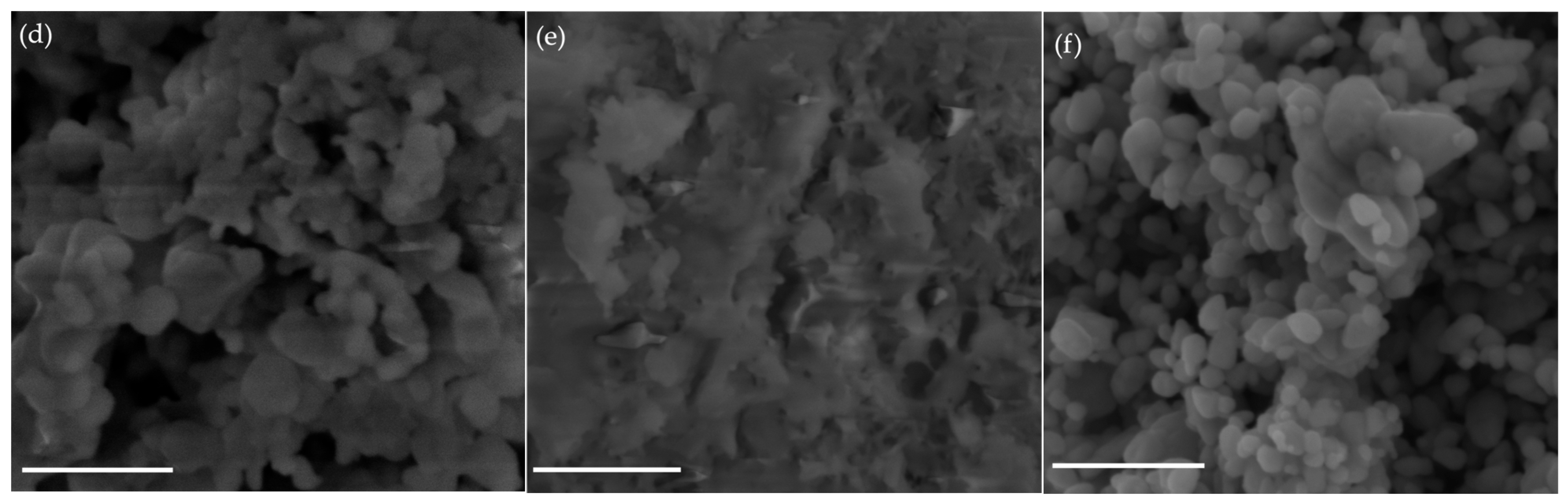
2.3. SEM Analysis
2.4. Photocatalytic Activity
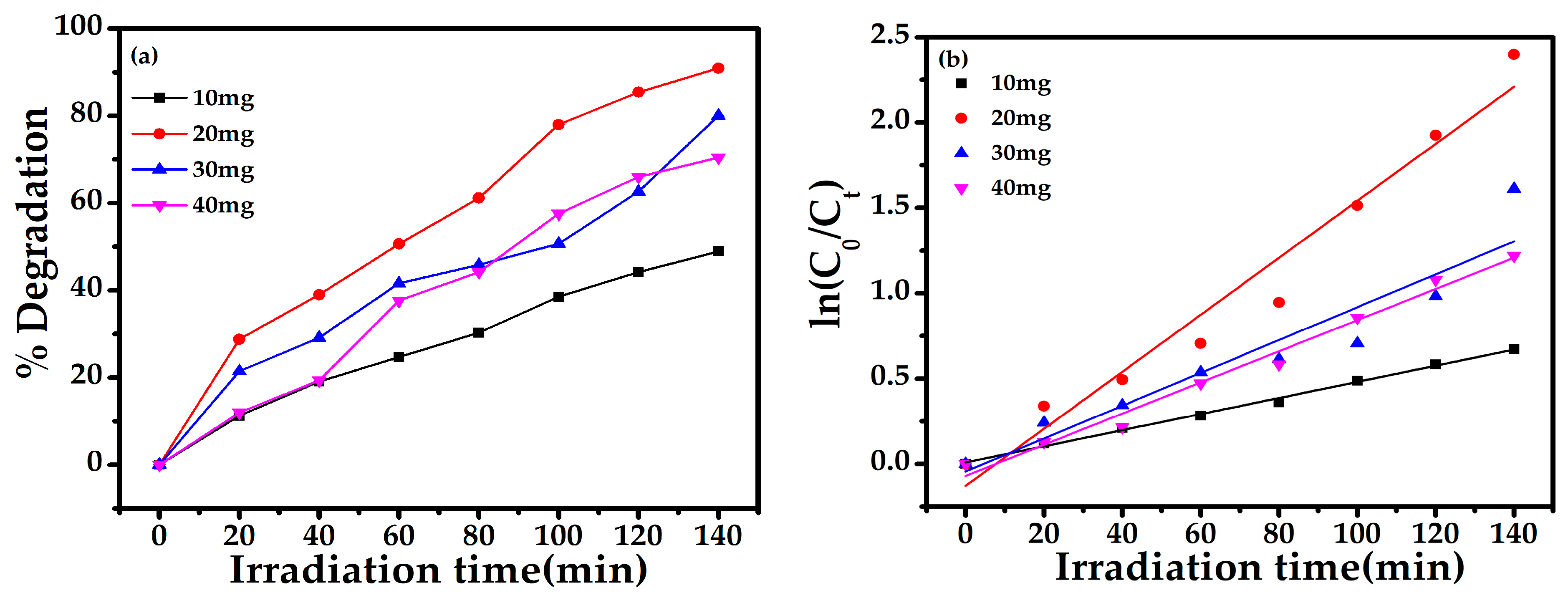
2.4.1. Effect of Photocatalyst Loading
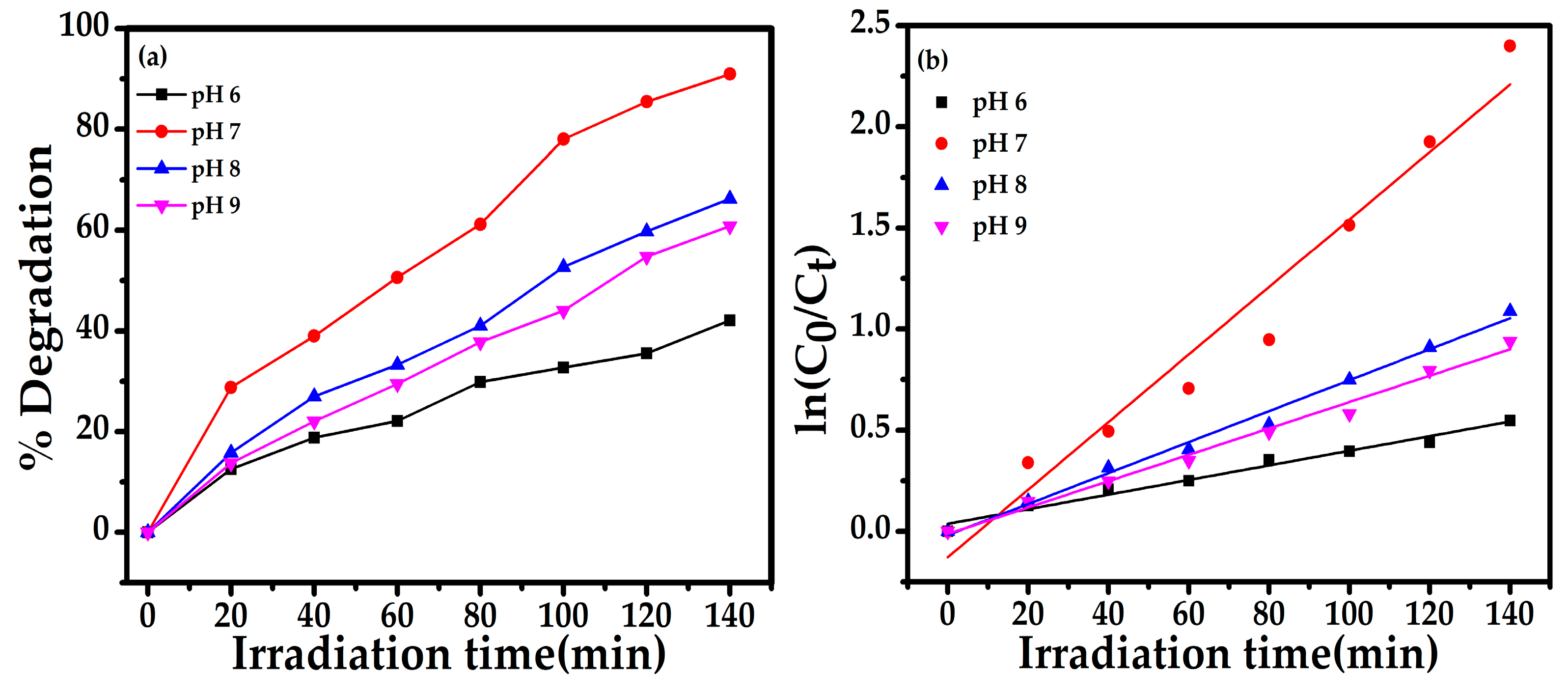
2.4.2. Effect of pH
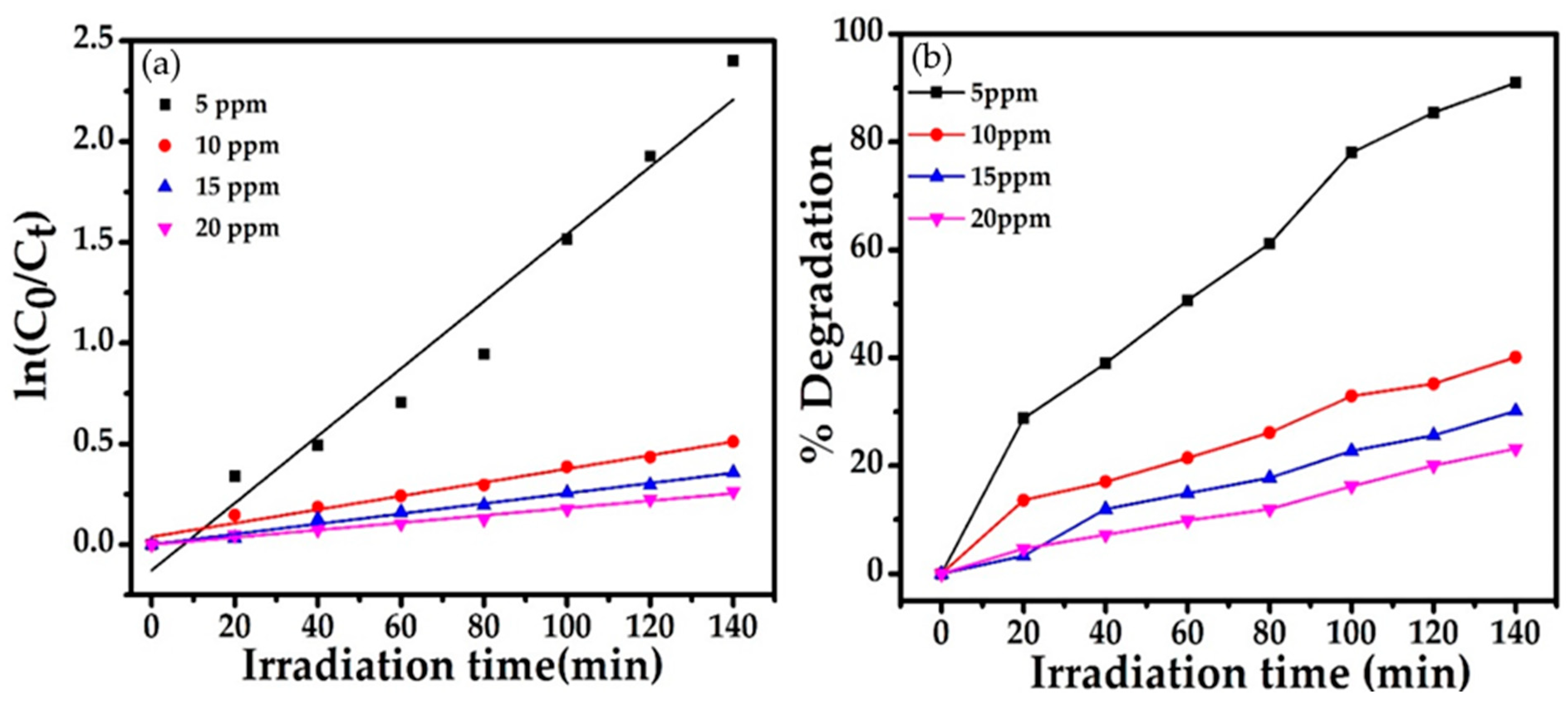
2.4.3. Effect of Bentazon Herbicide Concentration
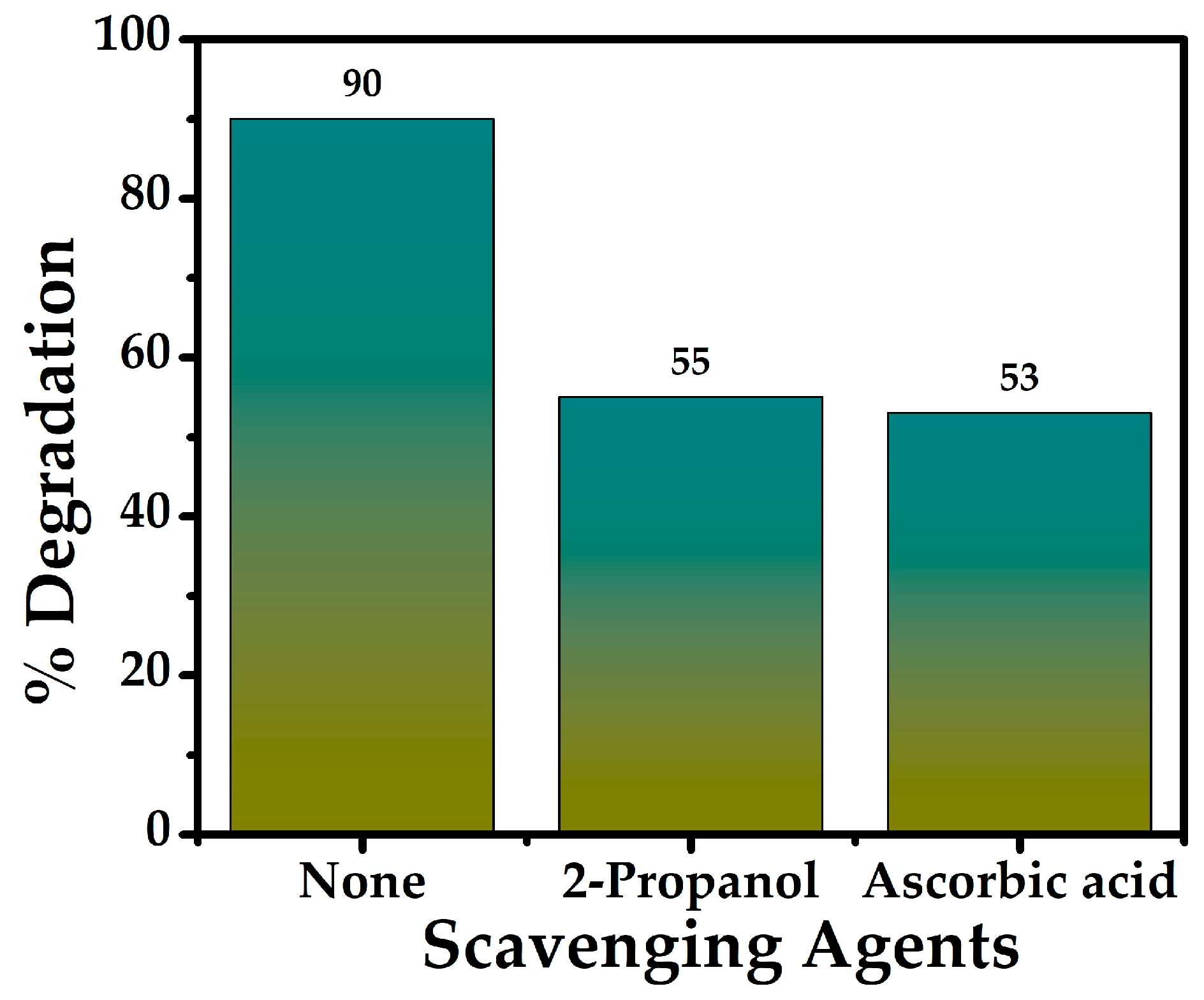
2.4.4. Scavenger Study
2.4.5. Photodegradation Mechanism
3. Materials and Methods
3.1. Chemicals
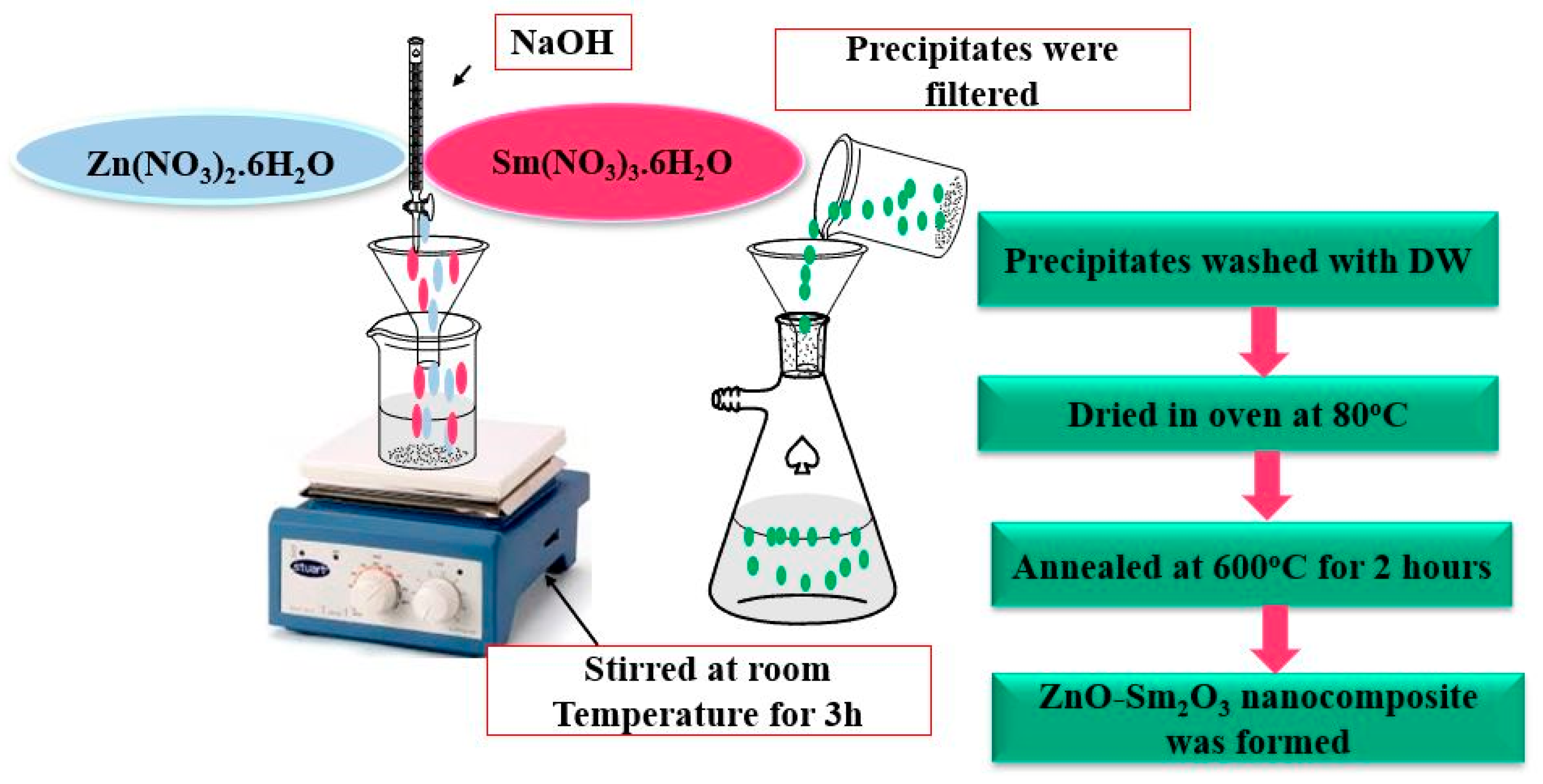
3.2. Synthesis of ZnO, Sm2O3 Nanoparticles and ZnO-Sm2O3 Nanocomposites
3.3. Photocatalytic Degradation Experiment
3.4. Instrumentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berberidou, C.; Kitsiou, V.; Kazala, E.; Lambropoulou, D.A.; Kouras, A.; Kosma, C.I.; Albanis, T.A.; Poulios, I. Study of the decomposition and detoxification of the herbicide bentazon by heterogeneous photocatalysis: Kinetics, intermediates and transformation pathways. Appl. Catal. B Environ. 2017, 200, 150–163. [Google Scholar] [CrossRef]
- Guelfi, D.R.V.; Brillas, E.; Gozzi, F.; Machulek, A.; de Oliveira, S.C.; Sirés, I. Influence of electrolysis conditions on the treatment of herbicide bentazon using artificial UVA radiation and sunlight. Identification of oxidation products. J. Environ. Manag. 2019, 231, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Jonidi-Jafari, A.; Farzadkia, M.; Esrafili, A.; Godini, K.; Shirzad-Siboni, M. Photocatalytic removal of bentazon by copper doped zinc oxide nanorods: Reaction pathways and toxicity studies. J. Environ. Manag. 2021, 294, 112962. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Burratti, L.; Zannotti, M.; Maranges, V.; Giovannetti, R.; Duranti, L.; De Matteis, F.; Francini, R.; Prosposito, P. Poly(ethylene glycol) Diacrylate Hydrogel with Silver Nanoclusters for Water Pb(II) Ions Filtering. Gels 2023, 9, 133. [Google Scholar] [CrossRef]
- Braga, C.S.; Bessegato, G.G.; Maestre, K.; Espinoza-Quiñones, F.R.; Alves, H.J.; da Silva, L.C.; Eising, R.; Bariccatti, R.A. Photocatalytic Degradation of Bentazon Pesticide by a Fe2O3-TiO2 Composite Catalyst Irradiated by UVA, UVB, and Natural Light. J. Braz. Chem. Soc. 2023, 34, 1284–1292. [Google Scholar] [CrossRef]
- Yasmeen, S.; Burratti, L.; Duranti, L.; Sgreccia, E.; Prosposito, P. Photocatalytic Degradation of Organic Pollutants—Nile Blue, Methylene Blue, and Bentazon Herbicide—Using NiO-ZnO Nanocomposite. Nanomaterials 2024, 14, 470. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hasan, M.; Mahmood, K.; Hussain, A.; Ali, A.; Arshad, M.I.; Iqbal, F. Novel tri-phase heterostructured ZnO–Yb2O3–Pr2O3 nanocomposite; structural, optical, photocatalytic and antibacterial studies. Ceram. Int. 2020, 46, 11101–11114. [Google Scholar] [CrossRef]
- Rehani, D.; Saxena, M.; Solanki, P.R.; Sharma, S.N. Transition Metal and Rare-Earth Metal Doping in SnO2 Nanoparticles. J. Supercond. Nov. Magn. 2022, 35, 2573–2581. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, A.; Akram, M.; Iqbal, F. Novel direct dual-Z-scheme ZnO-Er2O3-Yb2O3 heterostructured nanocomposite with superior photocatalytic and antibacterial activity. Mater. Lett. 2020, 264, 127357. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Yasmeen, S.; Iqbal, F.; Munawar, T.; Nawaz, M.A.; Asghar, M.; Hussain, A. Synthesis, structural and optical analysis of surfactant assisted ZnO–NiO nanocomposites prepared by homogeneous precipitation method. Ceram. Int. 2019, 45, 17859–17873. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Peng, H.; Zheng, X. Core-shell Sm2O3@ZnO nano-heterostructure for the visible light driven photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 244–251. [Google Scholar] [CrossRef]
- Li, X.; Hu, E.; Wang, F.; Lund, P.D.; Wang, J. N−N Heterostructure Sm2O3/ZnO Electrolyte with Enhanced Proton Conduction for Fuel Cell Application. ACS Appl. Energy Mater. 2024, 7, 4629–4638. [Google Scholar] [CrossRef]
- Subhan, M.A.; Fahim AM, M.; Saha, P.C.; Rahman, M.M.; Begum, K.; Azad, A.K. Structural study, photoluminescence and photocatalytic properties of La2O3⋅Fe3O4⋅ZnO, AgO⋅NiO⋅ZnO and La2O3⋅AgO⋅ZnO nanocomposites. Nano-Struct. Nano-Objects 2017, 10, 30–41. [Google Scholar] [CrossRef]
- Subhan, M.A.; Uddin, N.; Sarker, P.; Azad, A.K.; Begum, K. Photoluminescence, photocatalytic and antibacterial activities of CeO2·CuO·ZnO nanocomposite fabricated by co-precipitation method. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 149, 839–850. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Thangamani, C.; Thiyagarajulu, N.; Thangaraj, K.; Paramasivam, D.; Thangavel, S.; Kannan, K.; Parvathiraja, C.; Visalakshi, V.B.; Velmurugan, P.; et al. Facile synthesis of ZnO-Y2O3 nanocomposite for photocatalytic and biological applications. Catal. Commun. 2023, 184, 106786. [Google Scholar] [CrossRef]
- AlAbdulaal, T.; AlShadidi, M.; Hussien, M.; Ganesh, V.; Bouzidi, A.-F.; Rafique, S.; Algarni, H.; Zahran, H.; Abdel-wahab, M.; Yahia, I. Multifunctional and smart Er2O3–ZnO nanocomposites for electronic ceramic varistors and visible light degradation of wastewater treatment. Environ. Sci. Pollut. Res. 2022, 29, 19109–19131. [Google Scholar] [CrossRef]
- Wang, H.; Hu, E.; Wang, F.; Zhu, B. Energy Band Alignment in ZnO-Sm2O3 Heterostructure for Low Temperature Ceramic Fuel Cells. J. Electrochem. Soc. 2023, 170, 104503. [Google Scholar] [CrossRef]
- Munawar, T.; Mukhtar, F.; Nadeem, M.S.; Mahmood, K.; Hussain, A.; Ali, A.; Arshad, M.I.; Nabi, M.A.U.; Iqbal, F. Structural, optical, electrical, and morphological studies of rGO anchored direct dual-Z-scheme ZnO-Sm2O3–Y2O3 heterostructured nanocomposite: An efficient photocatalyst under sunlight. Solid State Sci. 2020, 106, 106307. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.U.; Mahmood, K.; Batool, S.; Hasan, M.; Rehman, K.U.; Iqbal, F. Enhancement in carrier separation of ZnO-Ho2O3-Sm2O3 hetrostuctured nanocomposite with rGO and PANI supported direct dual Z-scheme for antimicrobial inactivation and sunlight driven photocatalysis. Adv. Powder Technol. 2021, 32, 3770–3787. [Google Scholar] [CrossRef]
- Arulkumar, E.; Shree, S.S.; Thanikaikarasan, S. Photocatalytic degradation efficiency of Rhodamine-B for CuO/CdO nanosheets attained through simple co-precipitation method. Results Chem. 2023, 6, 101169. [Google Scholar] [CrossRef]
- Singh, R.P.P.; Hudiara, I.S.; Panday, S.; Rana, S.B. Effect of Ni Doping on Structural, Optical, and Magnetic Properties of Fe-Doped ZnO Nanoparticles. J. Supercond. Nov. Magn. 2015, 28, 3685–3691. [Google Scholar] [CrossRef]
- Kite, S.V.; Kadam, A.N.; Sathe, D.J.; Patil, S.; Mali, S.S.; Hong, C.K.; Lee, S.W.; Garadkar, K.M. Nanostructured TiO2Sensitized with MoS2Nanoflowers for Enhanced Photodegradation Efficiency Toward Methyl Orange. ACS Omega 2021, 6, 17071–17085. [Google Scholar] [CrossRef] [PubMed]
- Caglar, A.; Kivrak, H.; Aktas, N. The effect of titanium dioxide-supported CdSe photocatalysts enhanced for photocatalytic glucose electrooxidation under UV illumination. Int. J. Hydrogen Energy 2022, 47, 21130–21145. [Google Scholar] [CrossRef]
- Bensaid, S.; Piumetti, M.; Novara, C.; Giorgis, F.; Chiodoni, A.; Russo, N.; Fino, D. Catalytic Oxidation of CO and Soot over Ce-Zr-Pr Mixed Oxides Synthesized in a Multi-Inlet Vortex Reactor: Effect of Structural Defects on the Catalytic Activity. Nanoscale Res. Lett. 2016, 11, 494. [Google Scholar] [CrossRef]
- Zheng, X.; Kang, F.; Huang, C.; Lv, S.; Zhang, J.; Peng, H. Enhanced photocatalytic capacity of ZnS–ZnO–Sm2O3 composites for the removal of dyes and antibiotics in visible light region. J. Ind. Eng. Chem. 2020, 88, 186–195. [Google Scholar] [CrossRef]
- Ishaque, M.Z.; Zaman, Y.; Arif, A.; Siddique, A.B.; Shahzad, M.; Ali, D.; Aslam, M.; Zaman, H.; Faizan, M. Fabrication of ternary metal oxide (ZnO:NiO:CuO) nanocomposite heterojunctions for enhanced photocatalytic and antibacterial applications. RSC Adv. 2023, 13, 30838–30854. [Google Scholar] [CrossRef]
- Eskalen, H.; Kavgaci, M. Degradation of methylene blue and rhodamine B by hollow ZnO microspheres formed of radially oriented nanorods. Ömer Halisdemir Üniversitesi Mühendislik Bilim. Derg. 2023, 12, 998–1006. [Google Scholar] [CrossRef]
- Altındal, Ş.; Barkhordari, A.; Özçelik, S.; Pirgholi-Givi, G.; Mashayekhi, H.R.; Azizian-Kalandaragh, Y. A comparison of electrical characteristics of Au/n-Si (MS) structures with PVC and (PVC: Sm2O3) polymer interlayer. Phys. Scr. 2021, 96, 125838. [Google Scholar] [CrossRef]
- Ullah, S.; Shahid, W.; Shahid, S.; Khan, M.I.; Ansar, N.; Khizar, M.; Ali, S.; Choi, J.R.; Almutairi, B.S.; Alreshidi, M.A. Advancing photocatalysis: Innovative approaches using novel V2O5/ZnO nanocomposites for efficient photocatalytic degradation of tubantin red. J. Saudi Chem. Soc. 2023, 27, 101766. [Google Scholar] [CrossRef]
- Warshagha, M.Z.A.; Muneer, M. Facile synthesis of CdO-ZnO heterojunction photocatalyst for rapid removal of organic contaminants from water using visible light. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100728. [Google Scholar] [CrossRef]
- Chand, P.; Gaur, A.; Kumar, A. Structural and optical properties of ZnO nanoparticles synthesized at different pH values. J. Alloys Compd. 2012, 539, 174–178. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Wang, B.; Li, S.; Jiang, B.; Yang, L.; Fu, W.; Fu, H.; Sun, J. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J. The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance. Nanomaterials 2022, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 249, 122983. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Wu, X.; Siddique, M.S.; Su, Z.; Liu, M.; Yu, W. Reducing ROS generation and accelerating the photocatalytic degradation rate of PPCPs at neutral pH by doping Fe-N-C to g-C3N4. Appl. Catal. B Environ. 2022, 301, 120790. [Google Scholar] [CrossRef]
- Mubeen, K.; Safeen, K.; Irshad, A.; Safeen, A.; Ghani, T.; Shah, W.H.; Khan, R.; Ahmad, K.S.; Casin, R.; Rashwan, M.A.; et al. ZnO/CuSe composite-mediated bandgap modulation for enhanced photocatalytic performance against methyl blue dye. Sci. Rep. 2023, 13, 19580. [Google Scholar] [CrossRef]
- Varadi, A.; Leostean, C.; Stefan, M.; Popa, A.; Toloman, D.; Pruneanu, S.; Tripon, S.; Macavei, S. Fe3O4-ZnO:V Nanocomposites with Modulable Properties as Magnetic Recoverable Photocatalysts. Inorganics 2024, 12, 119. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Batoo, K.M.; Khursheed, H.; Nadeem, M.S.; Hussain, S.; Ponraj, J.; Koc, M.; Iqbal, F. Oxygen vacancies generation in CeO2 via Y/Nd co-doping with accelerated charge separation by decorating on rGO sheets for sunlight-driven photodegradation of hazardous dyes. Ceram. Int. 2024, 50, 11486–11499. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of pH on the photocatalytic degradation of remazol brilliant blue dye using zirconia catalyst. Mater. Today Proc. 2020, 31, 260–262. [Google Scholar] [CrossRef]
- Cametti, C.; Fratoddi, I.; Venditti, I.; Russo, M.V. Dielectric Relaxations of Ionic Thiol-Coated Noble Metal Nanoparticles in Aqueous Solutions: Electrical Characterization of the Interface. Langmuir 2011, 27, 7084–7090. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Fatima, S.; Batoo, K.M.; Nadeem, M.S.; Mukhtar, F.; Hussain, S.; Khan, S.A.; Koc, M.; Iqbal, F. Enhanced charge carriers separation/transportation via S-scheme ZnCeS–ZnWO heterostructure nanocomposite for photodegradation of synthetic dyes under sunlight. Mater. Chem. Phys. 2024, 314, 128938. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Synthesis and characterization of novel Sm2O3/S-doped g-C3N4 nanocomposites with enhanced photocatalytic activities under visible light irradiation. Appl. Surf. Sci. 2018, 427, 375–387. [Google Scholar] [CrossRef]
- Aghaei, M.; Sajjadi, S.; Keihan, A.H. Sono-coprecipitation synthesis of ZnO/CuO nanophotocatalyst for removal of parathion from wastewater. Environ. Sci. Pollut. Res. 2020, 27, 11541–11553. [Google Scholar] [CrossRef] [PubMed]
- Mungondori, H.H.; Tichagwa, L.; Katwire, D.M.; Aoyi, O. Preparation of photo-catalytic copolymer grafted asymmetric membranes (N-TiO2-PMAA-g-PVDF/PAN) and their application on the degradation of bentazon in water. Iran. Polym. J. 2016, 25, 135–144. [Google Scholar] [CrossRef]
- Yasmeen, S.; Munawar, T.; Asghar, M.; Khan, M.A.; Hussain, A.; Iqbal, F. Synthesis and photocatalytic study of Zn0.90Co0.10O and Zn0.90Co0.05M0.05O (M = Ca, Ba, Cr, Pb) nanocrystals: Structural, optical and electrical investigations. J. Mater. Res. Technol. 2020, 9, 4076–4096. [Google Scholar] [CrossRef]
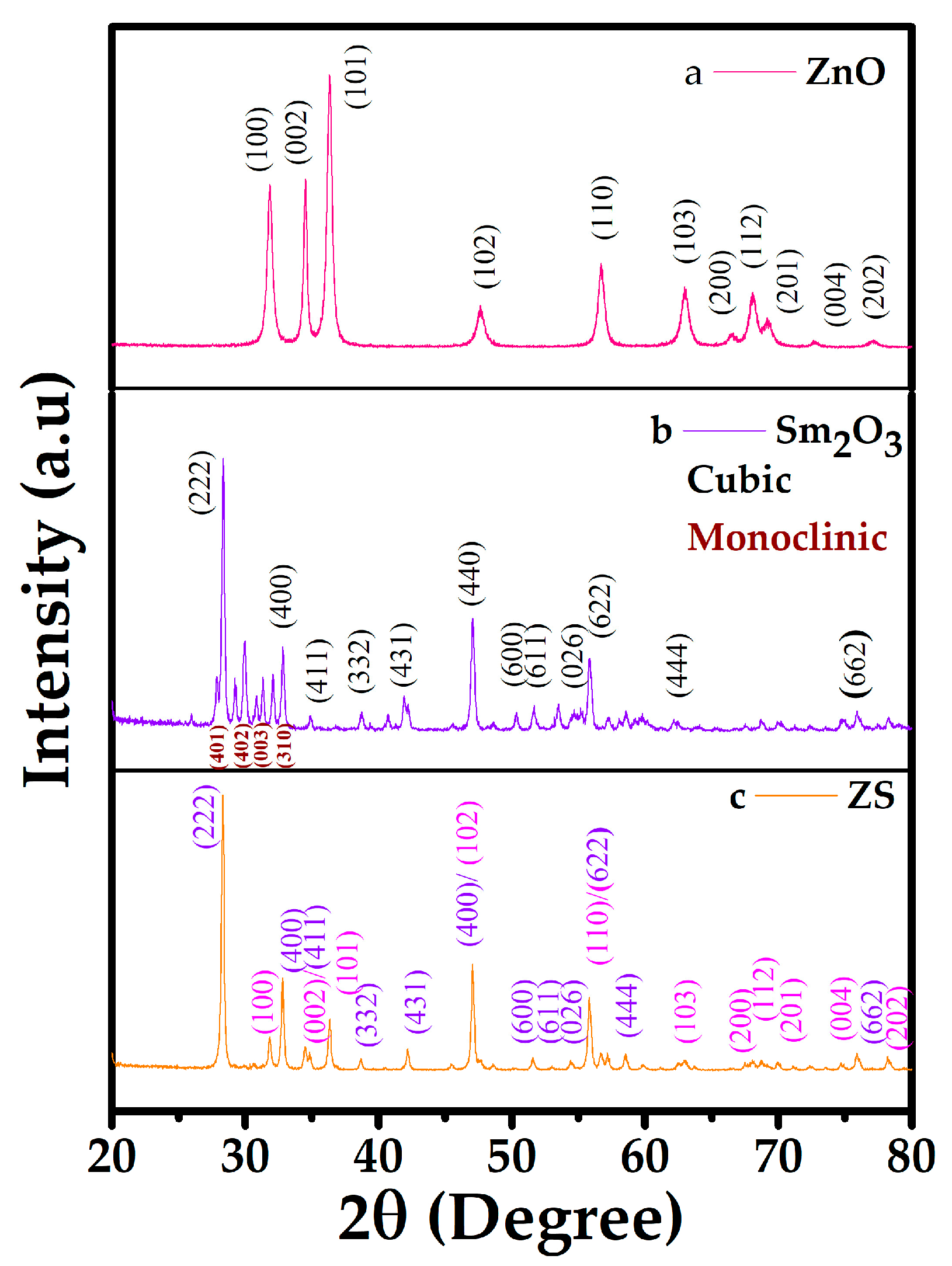
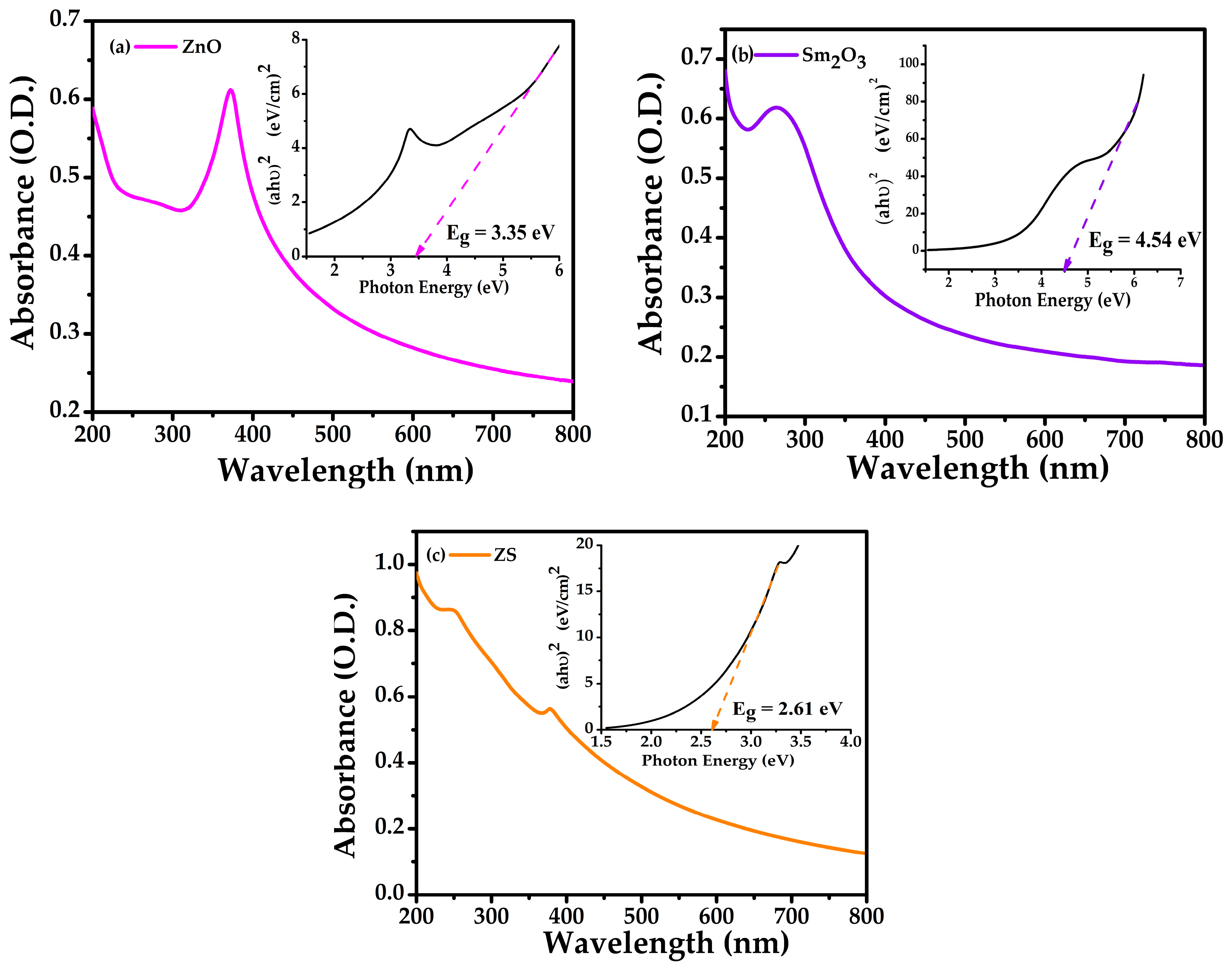
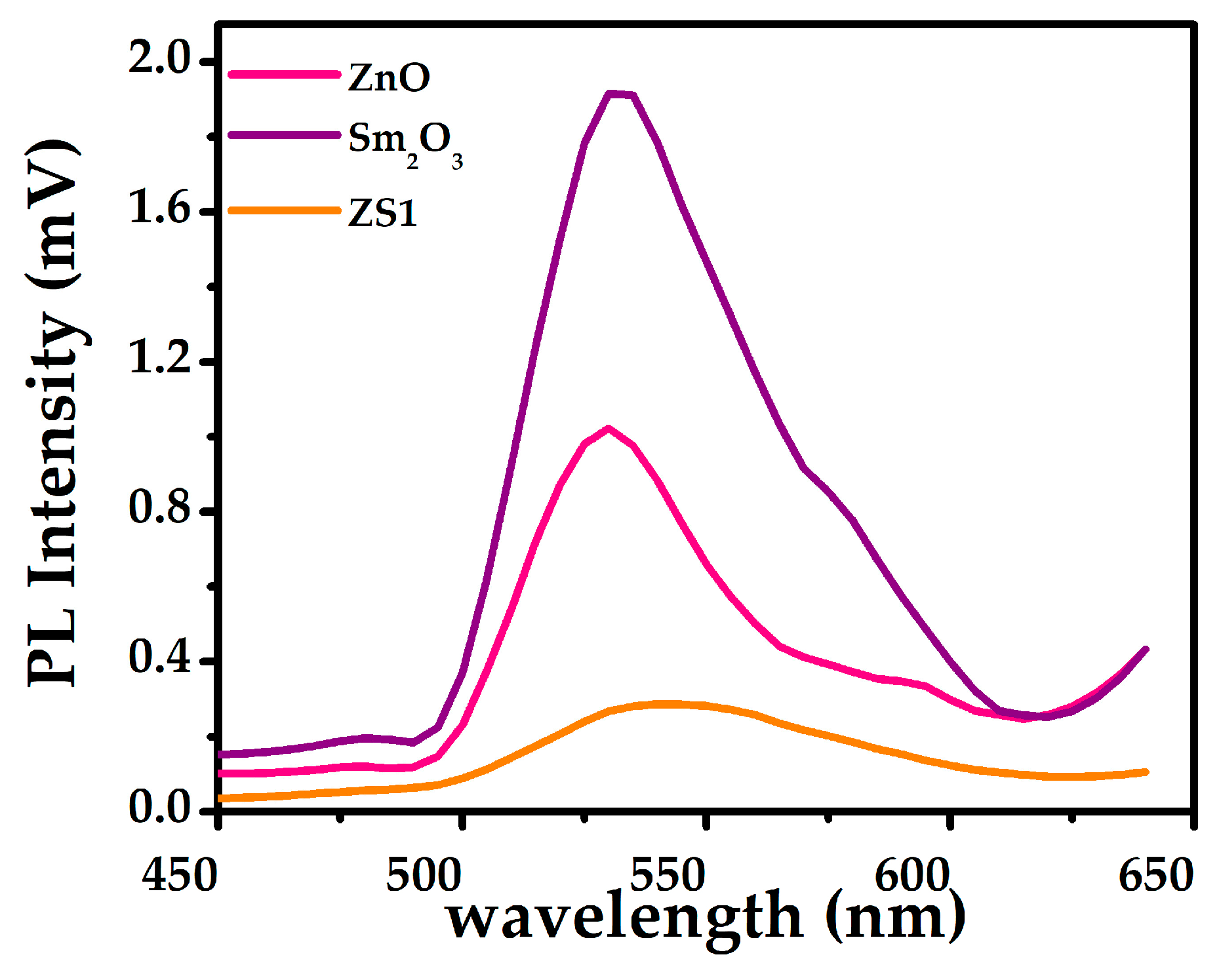
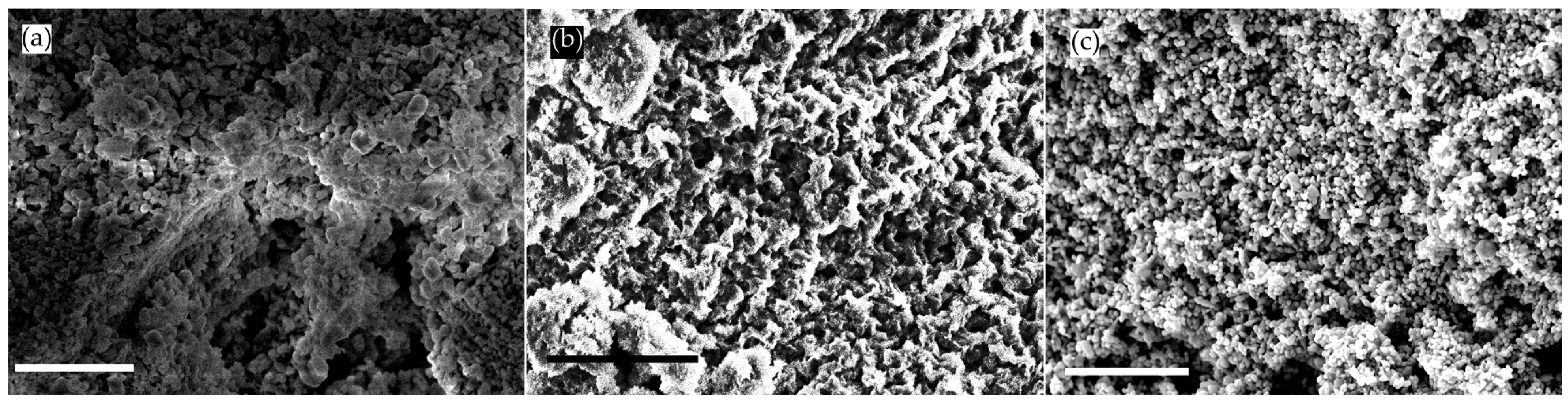


| Samples | a (Å) = b (Å) | c () | c/a | Volume () | d-Spacing | Average Crystallite Size (nm) | Dislocation Density 10−3 (nm−2) |
|---|---|---|---|---|---|---|---|
| Nanoparticles | |||||||
| ZnO Sm2O3 | 3.244 10.897 | 5.197 - | 1.601 1 | 47.368 1294.031 | 1.829 2.071 | 21.767 45.361 | 2.210 0.485 |
| ZS nanocomposite | |||||||
| ZnO Sm2O3 | 3.246 10.916 | 5.198 - | 1.601 1 | 47.441 1299.167 | 1.924 2.281 | 33.838 45.866 | 1.5944 0.62301 |
| Catalyst Loading | Bentazon Concentration | pH | % Degradation | K (min−1) | R2 | y = a + bx |
|---|---|---|---|---|---|---|
| 10 mg | 5 ppm | 7 | 49 | 0.00472 | 0.9958 | 0.0094 + 0.0047x |
| 20 mg | -- | -- | 90 | 0.01669 | 0.9572 | −0.1273 + 0.0166x |
| 30 mg | -- | -- | 80 | 0.00961 | 0.8790 | −0.0429 + 0.0096x |
| 40 mg | -- | -- | 70 | 0.00912 | 0.9829 | −0.0693 + 0.0091x |
| 20 mg | 5 ppm | 6 | 42 | 0.00361 | 0.9779 | 0.0379 + 0.0036x |
| -- | -- | 7 | 90 | 0.01669 | 0.9585 | −0.1273 + 0.0166x |
| -- | -- | 8 | 66 | 0.00765 | 0.9704 | −0.0174 + 0.0076x |
| -- | -- | 9 | 60 | 0.00651 | 0.9875 | −0.0117 + 0.0065x |
| 20 mg | 5 ppm | 7 | 90 | 0.01669 | 0.9585 | −0.1273 + 0.0166x |
| -- | 10 ppm | -- | 40 | 0.00341 | 0.9780 | 0.0392 + 0.0033x |
| -- | 15 ppm | -- | 30 | 0.00253 | 0.9871 | 0.0017 + 0.0025x |
| -- | 20 ppm | -- | 21 | 0.00182 | 0.9872 | −0.5076 + 0.0018x |
| Photocatalyst | Source | Irradiation Time (min) | Degradation Efficiency (%) | Refs. |
|---|---|---|---|---|
| ZnO | UV light | 60 | 32% | [45] |
| CuO | - | 60 | 10% | - |
| ZnO | - | 140 | 70% | Present work |
| Sm2O3 | - | 140 | 40% | Present work |
| N–TiO2–PMAA-g-PVDF/PAN | - | 180 | 90% | [46] |
| Fe2O3-TiO2 | - | 120 | 51% | [1] |
| NiO-ZnO | - | 100 | 70% | [7] |
| ZnO-Sm2O3 | - | 140 | 90% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmeen, S.; Burratti, L.; Duranti, L.; Agresti, A.; Prosposito, P. Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light. Int. J. Mol. Sci. 2024, 25, 13319. https://doi.org/10.3390/ijms252413319
Yasmeen S, Burratti L, Duranti L, Agresti A, Prosposito P. Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light. International Journal of Molecular Sciences. 2024; 25(24):13319. https://doi.org/10.3390/ijms252413319
Chicago/Turabian StyleYasmeen, Sadaf, Luca Burratti, Leonardo Duranti, Antonio Agresti, and Paolo Prosposito. 2024. "Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light" International Journal of Molecular Sciences 25, no. 24: 13319. https://doi.org/10.3390/ijms252413319
APA StyleYasmeen, S., Burratti, L., Duranti, L., Agresti, A., & Prosposito, P. (2024). Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light. International Journal of Molecular Sciences, 25(24), 13319. https://doi.org/10.3390/ijms252413319










