Cellular Effects of Enterobacteriaceae Polysaccharide Colanic Acid
Abstract
:1. Introduction
2. Results
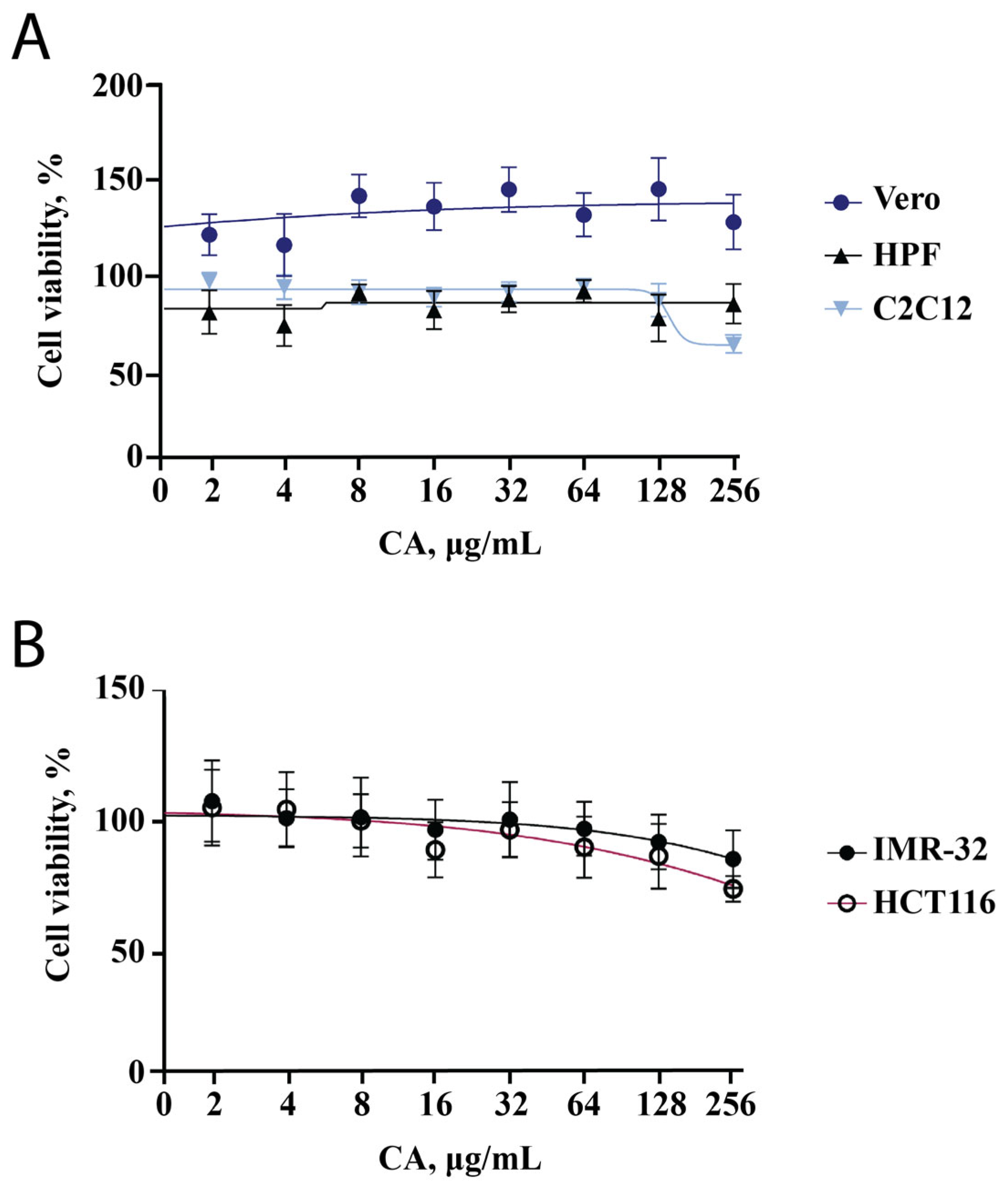
2.1. CA Cytotoxicity
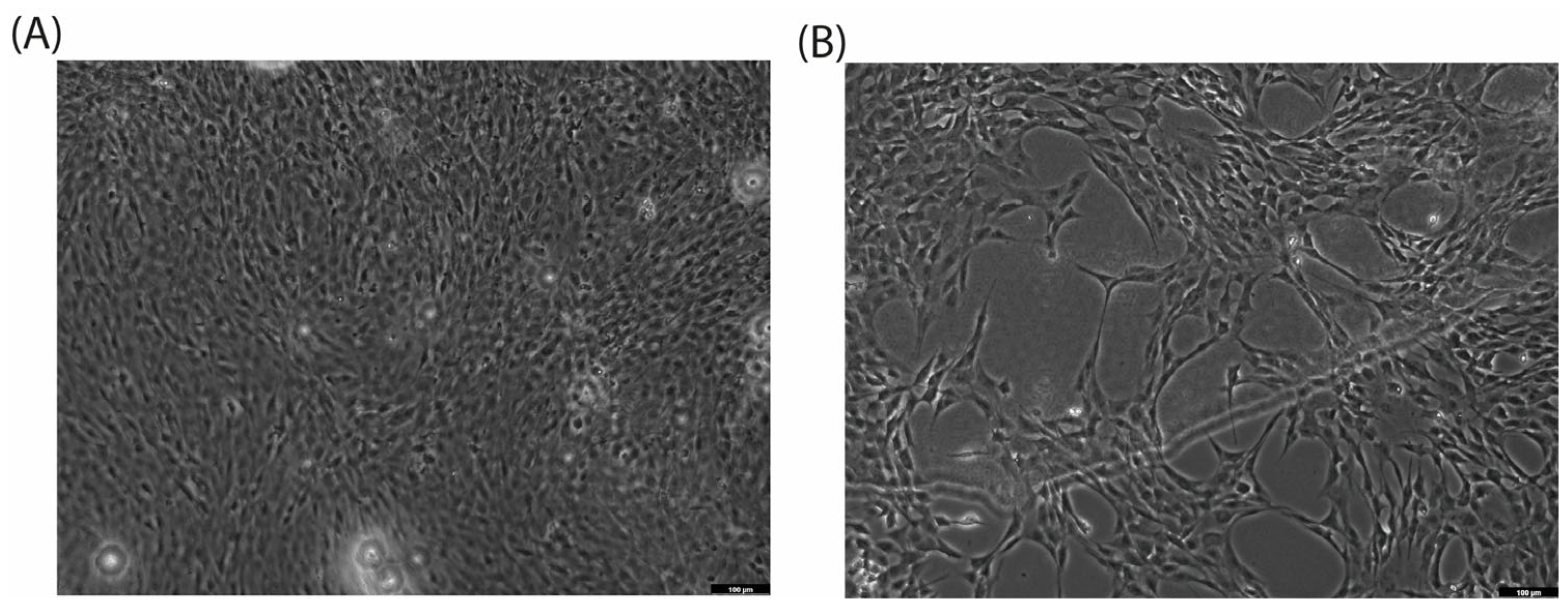
2.2. Cell Morphology Analysis
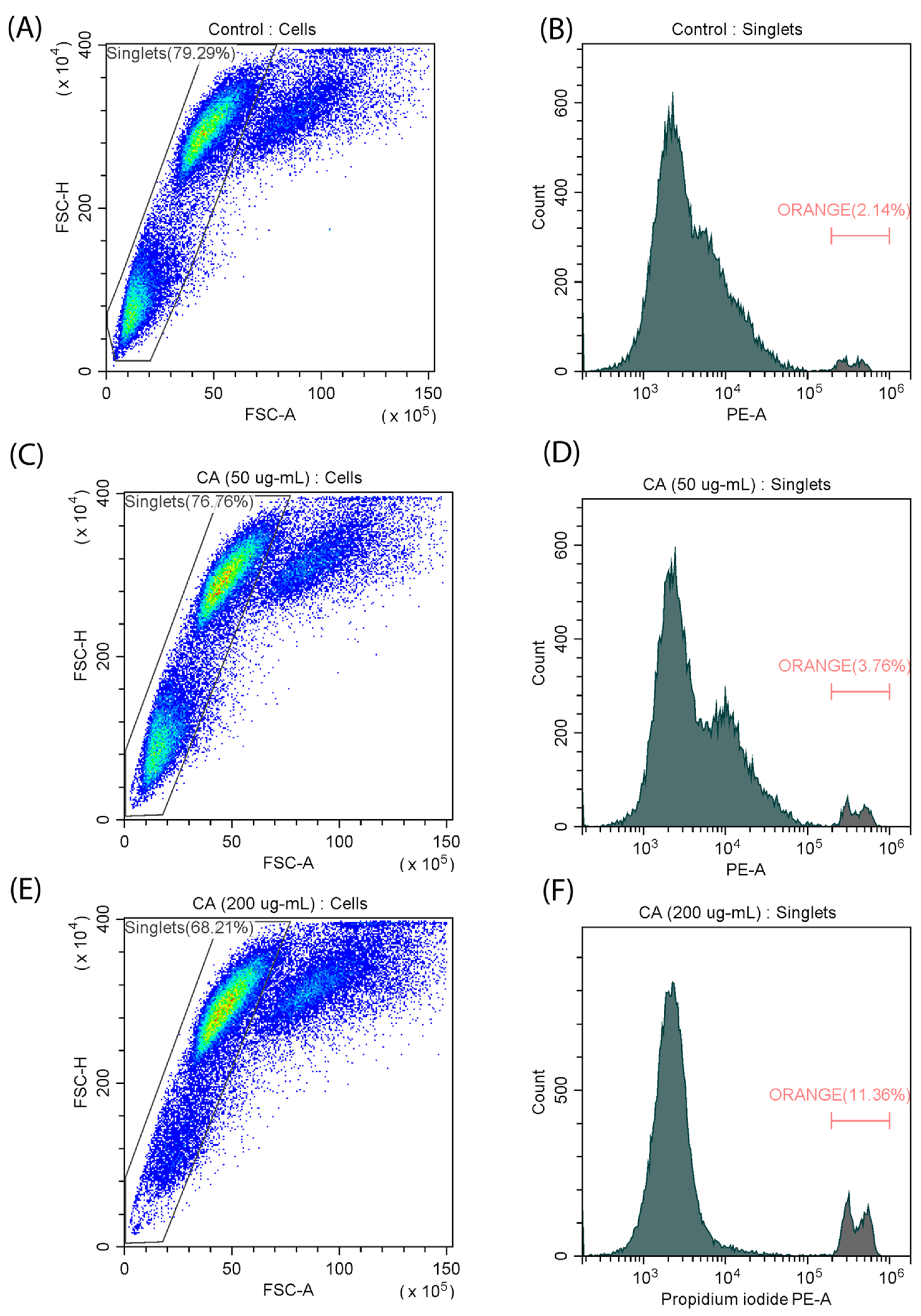
2.3. Cell Death Kinetics
2.4. ROS Detection
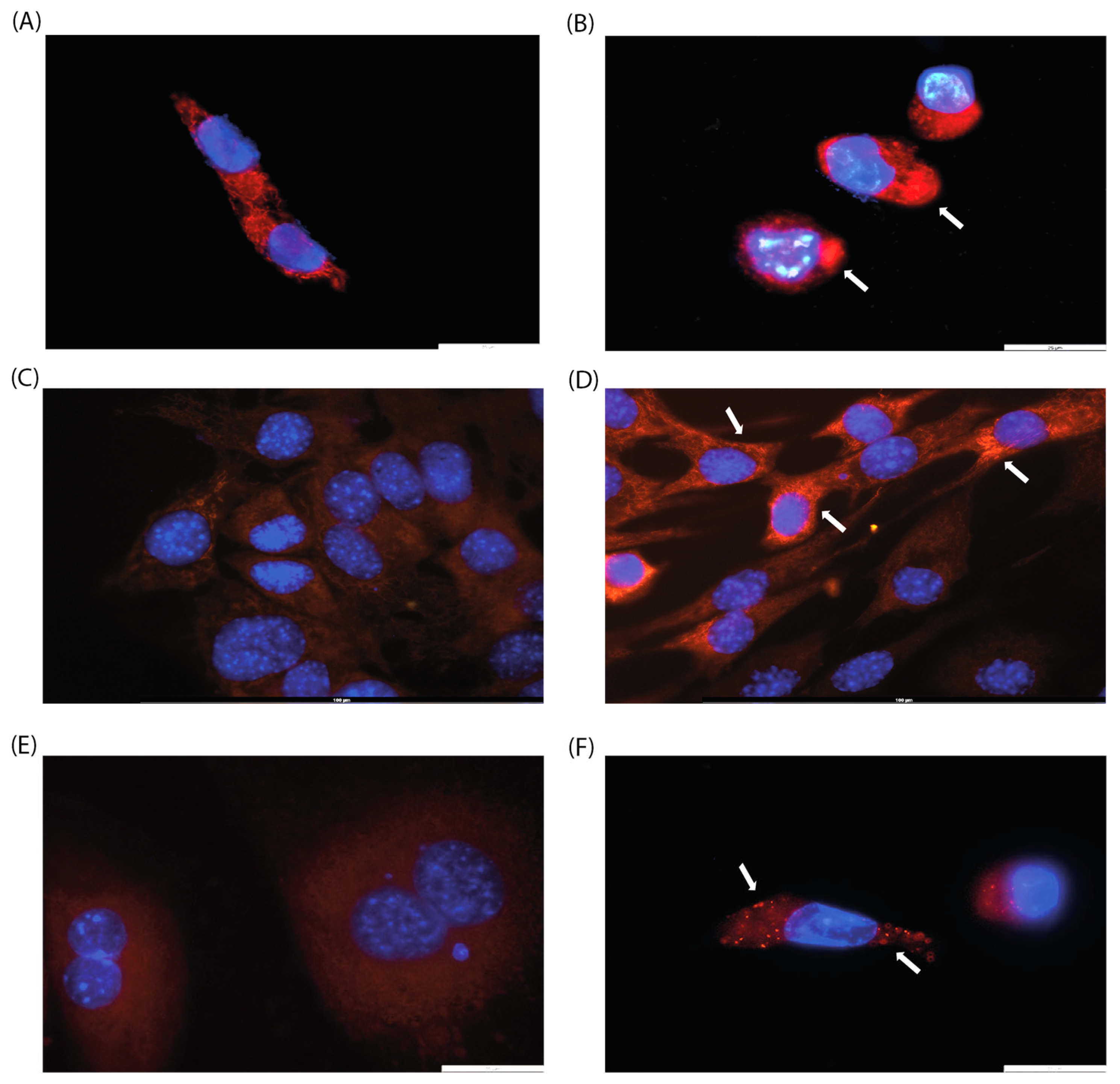
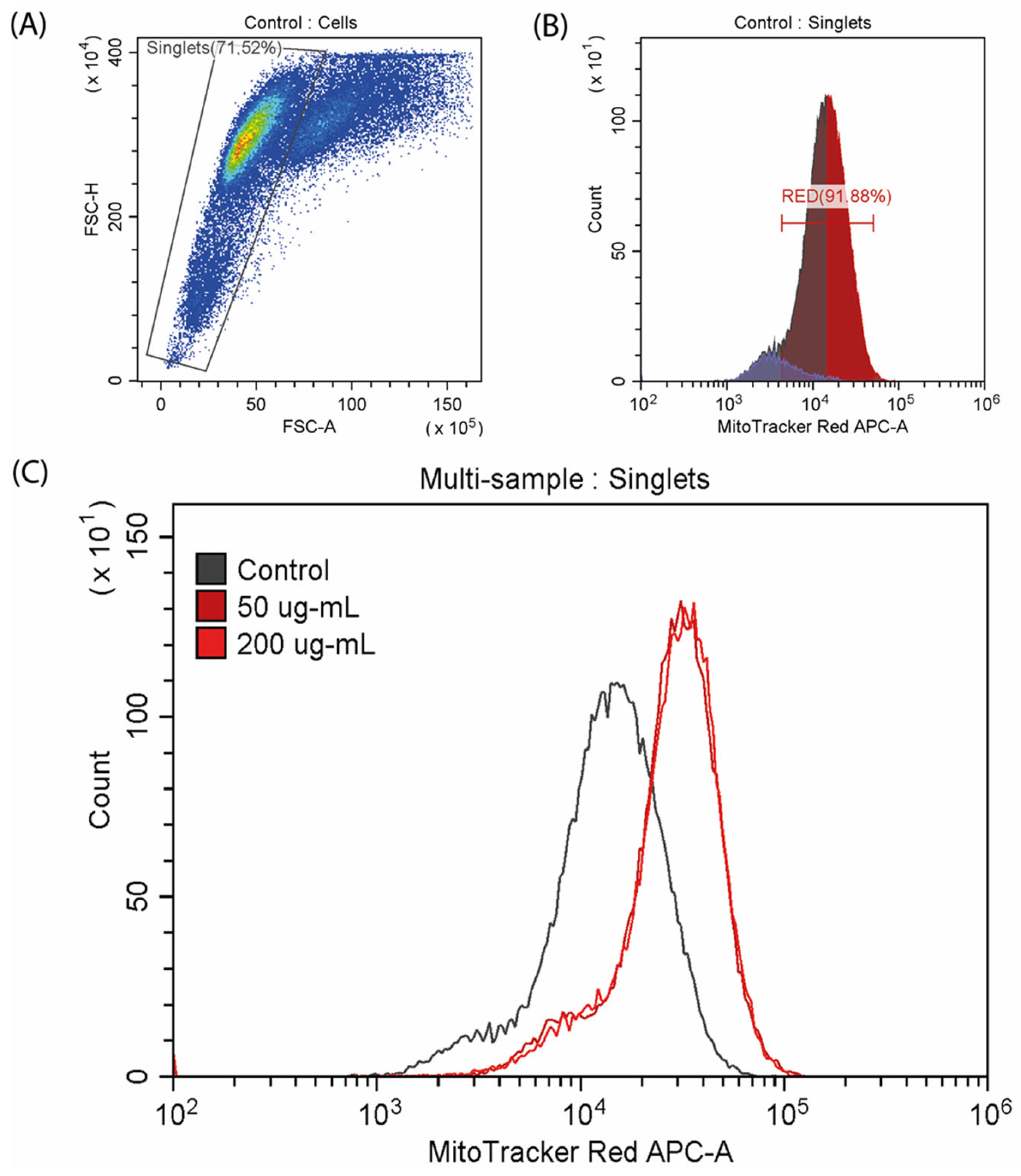
2.5. Analysis of Mitochondrial Membrane Potential
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garegg, P.J.; Lindberg, B.; Onn, T.; Sutherland, I.W. Comparative structural studies on the M-antigen from Salmonella typhimurium. Escherichia coli and Aerobacter cloacae. Acta Chem. Scand. 1971, 25, 2103–2108. [Google Scholar] [CrossRef]
- Whitfield, C.; Roberts, I.S. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 1999, 31, 1307–1319. [Google Scholar] [CrossRef]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef]
- Zamze, S.; Martinez-Pomares, L.; Jones, H.; Taylor, P.R.; Stillion, R.J.; Gordon, S.; Wong, S.Y. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J. Biol. Chem. 2002, 277, 41613–41623. [Google Scholar] [CrossRef]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 2006, 203, 2853. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 2010, 15, 25. [Google Scholar] [CrossRef]
- Hsieh, S.A.; Allen, P.M. Immunomodulatory Roles of Polysaccharide Capsules in the Intestine. Front. Immunol. 2020, 11, 525541. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of in Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as potential anti-tumor biomacromolecules—A review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Goebel, W.F.F. Colanic acid. Proc. Natl. Acad. Sci. USA 1963, 49, 464–471. [Google Scholar] [CrossRef]
- Sapelli, R.V.; Goebel, W.F. The capsular polysaccharide of a mucoid variant of E. coli K-12. Proc. Natl. Acad. Sci. USA 1964, 52, 265–271. [Google Scholar] [CrossRef]
- Grant, W.D.; Sutherland, I.W.; Wilkinson, J.F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J. Bacteriol. 1969, 100, 1187–1193. [Google Scholar] [CrossRef]
- Sutherland, I.W. Structural studies on colanic acid, the common exopolysaccharide found in the Enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem. J. 1969, 115, 935–945. [Google Scholar] [CrossRef]
- Pando, J.M.; Karlinsey, J.E.; Lara, J.C.; Libby, S.J.; Fang, F.C. The Rcs-regulated colanic acid capsule maintains membrane potential in Salmonella enterica serovar typhimurium. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Prigent-Combaret, C.; Prensier, G.; Le Thi, T.T.; Vidal, O.; Lejeune, P.; Dorel, C. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: Role of flagella, curli and colanic acid. Environ. Microbiol. 2000, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Sivaramakrishnan, P.; Lin, C.C.J.; Neve, I.A.; He, J.; Tay, L.W.R.; Sowa, J.N.; Sizovs, A.; Du, G.; Wang, J.; et al. Microbial Genetic Composition Tunes Host Longevity. Cell 2017, 169, 1249–1262. [Google Scholar] [CrossRef]
- Hartsough, L.A.; Park, M.; Kotlajich, M.V.; Lazar, J.T.; Han, B.; Lin, C.C.J.; Musteata, E.; Gambill, L.; Wang, M.C.; Tabor, J.J. Optogenetic control of gut bacterial metabolism to promote longevity. eLife 2020, 9, e56849. [Google Scholar] [CrossRef]
- Firozi, P.; Zhang, W.; Chen, L.; Quiocho, F.A.; Worley, K.C.; Templeton, N.S. Identification and removal of colanic acid from plasmid DNA preparations: Implications for gene therapy. Gene Ther. 2010, 17, 1484–1499. [Google Scholar] [CrossRef]
- Tsvetikova, S.A.; Zabavkina, A.A.; Nikonorova, V.G.; Tsymbal, S.A.; Dukhinova, M.S.; Chrishtop, V.V.; Koshel, E.I. Stimulatory Effect on Mice by Bacterial Exopolysaccharide, Colanic Acid. Russ. J. Bioorg. Chem. 2024, 50, 594–603. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V.; Chernikov, O.V. Structure and in vitro bioactivity against cancer cells of the capsular polysaccharide from the marine bacterium Psychrobacter marincola. Mar. Drugs 2020, 18, 268. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, A.S.; Romanenko, L.A.; Kokoulin, M.S. Cell-cycle arrest and mitochondria-dependent apoptosis induction in T-47D cells by the capsular polysaccharide from the marine bacterium Kangiella japonica KMM 3897. Carbohydr. Polym. 2023, 320, 121237. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.K.; Anderson, P.A.; Nassar, R.; Bunting, J.B.; Saba, Z.; Oakeley, A.E.; Malouf, N.N. C2C12 cells: Biophysical, biochemical, and immunocytochemical properties. Am. J. Physiol. Cell Physiol. 1994, 266, C1795–C1802. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C.; Dremina, E.; Galeva, N.; Sharov, V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis 2014, 19, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Malinska, D.; Kudin, A.P.; Bejtka, M.; Kunz, W.S. Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion 2012, 12, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, S.; Sengupta, S.; Biswas, S.; Sinha, T.K.; Sen, R.; Basak, R.K.; Adhikari, B.; Bhattacharyya, A. Bacterial fucose-rich polysaccharide stabilizes MAPK-mediated Nrf2/Keap1 signaling by directly scavenging reactive oxygen species during hydrogen peroxide-induced apoptosis of human lung fibroblast cells. PLoS ONE 2014, 9, e113663. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.C.; Khan, I. Bacterial polysaccharides—A big source for prebiotics and therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Sengupta, S.; Biswas, S.; Sen, R.; Sinha, T.K.; Basak, R.K.; Adhikari, B.; Bhattacharyya, A. Low fucose containing bacterial polysaccharide facilitate mitochondria-dependent ROS-induced apoptosis of human lung epithelial carcinoma via controlled regulation of MAPKs-mediated Nrf2/Keap1 homeostasis signaling. Mol. Carcinog. 2015, 54, 1636–1655. [Google Scholar] [CrossRef]
- Richards, B.J.; Slavin, M.; Oliveira, A.N.; Sanfrancesco, V.C.; Hood, D.A. Mitochondrial protein import and UPRmt in skeletal muscle remodeling and adaptation. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 28–36. [Google Scholar]
- Lorenzon, P.; Bandi, E.; de Guarrini, F.; Pietrangelo, T.; Schäfer, R.; Zweyer, M.; Wernig, A.; Ruzzier, F. Ageing affects the differentiation potential of human myoblasts. Exp. Gerontol. 2004, 39, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Pääsuke, R.; Eimre, M.; Piirsoo, A.; Peet, N.; Laada, L.; Kadaja, L.; Roosimaa, M.; Pääsuke, M.; Märtson, A.; Seppet, E.; et al. Proliferation of human primary myoblasts is associated with altered energy metabolism in dependence on ageing in vivo and in vitro. Oxidative Med. Cell Longev. 2016, 2016, 8296150. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, S.; Ji, M.; Chen, Q.; Shi, J.; Sun, J. Activation of colanic acid biosynthesis linked to heterologous expression of the polyhydroxybutyrate pathway in Escherichia coli. Int. J. Biol. Macromol. 2019, 128, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.; Szczepanowska, J.; Koopman, W.J.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetikova, S.A.; Zabavkina, A.A.; Ivankova, O.; Koshel, E.I. Cellular Effects of Enterobacteriaceae Polysaccharide Colanic Acid. Int. J. Mol. Sci. 2024, 25, 8017. https://doi.org/10.3390/ijms25158017
Tsvetikova SA, Zabavkina AA, Ivankova O, Koshel EI. Cellular Effects of Enterobacteriaceae Polysaccharide Colanic Acid. International Journal of Molecular Sciences. 2024; 25(15):8017. https://doi.org/10.3390/ijms25158017
Chicago/Turabian StyleTsvetikova, Sofia A., Alina A. Zabavkina, Olesia Ivankova, and Elena I. Koshel. 2024. "Cellular Effects of Enterobacteriaceae Polysaccharide Colanic Acid" International Journal of Molecular Sciences 25, no. 15: 8017. https://doi.org/10.3390/ijms25158017
APA StyleTsvetikova, S. A., Zabavkina, A. A., Ivankova, O., & Koshel, E. I. (2024). Cellular Effects of Enterobacteriaceae Polysaccharide Colanic Acid. International Journal of Molecular Sciences, 25(15), 8017. https://doi.org/10.3390/ijms25158017






