Antimicrobial Evaluation of Two Polycyclic Polyprenylated Acylphloroglucinol Compounds: PPAP23 and PPAP53
Abstract
:1. Introduction
2. Results
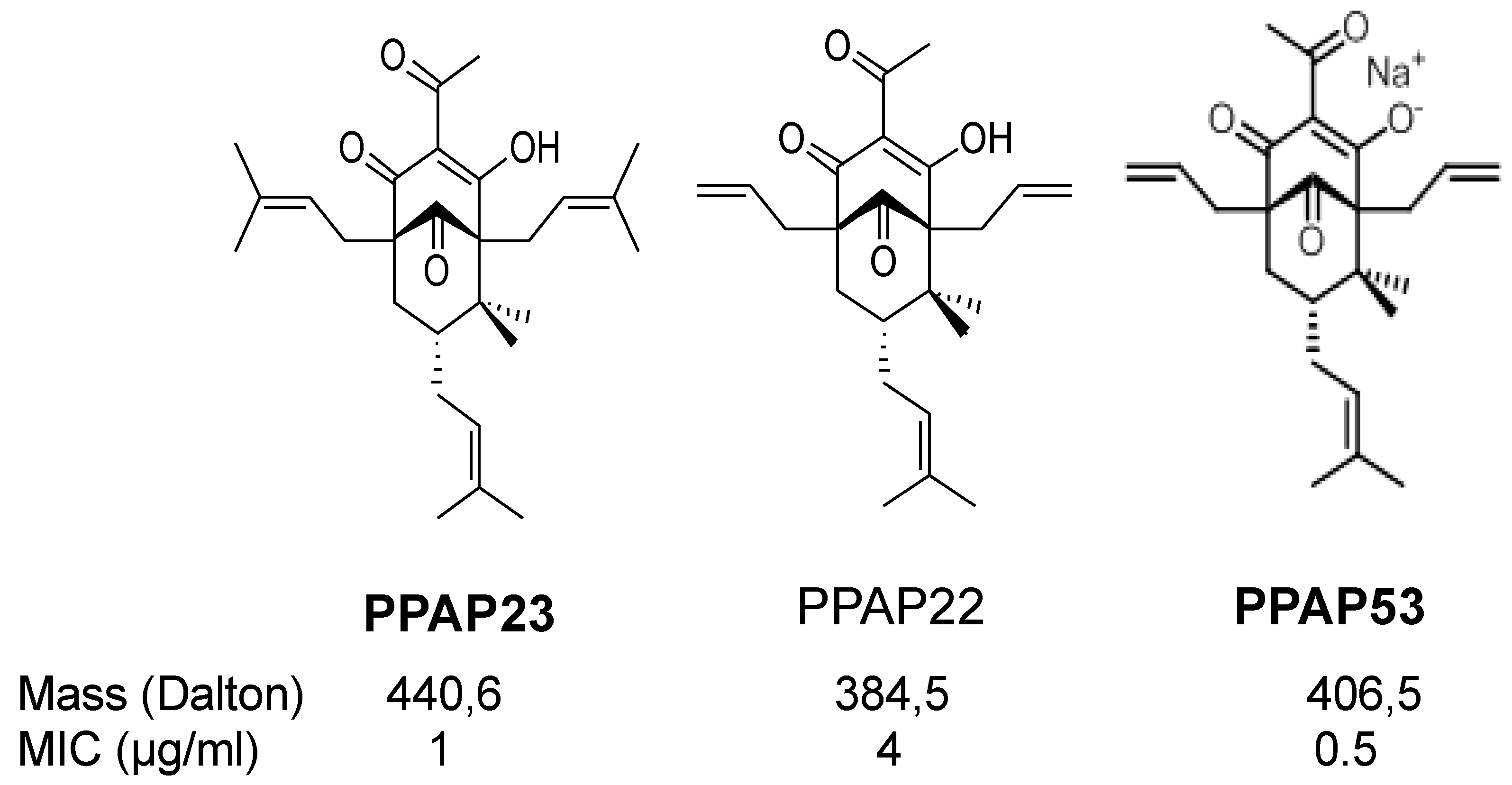
2.1. The PPAP Compounds 23 and 53
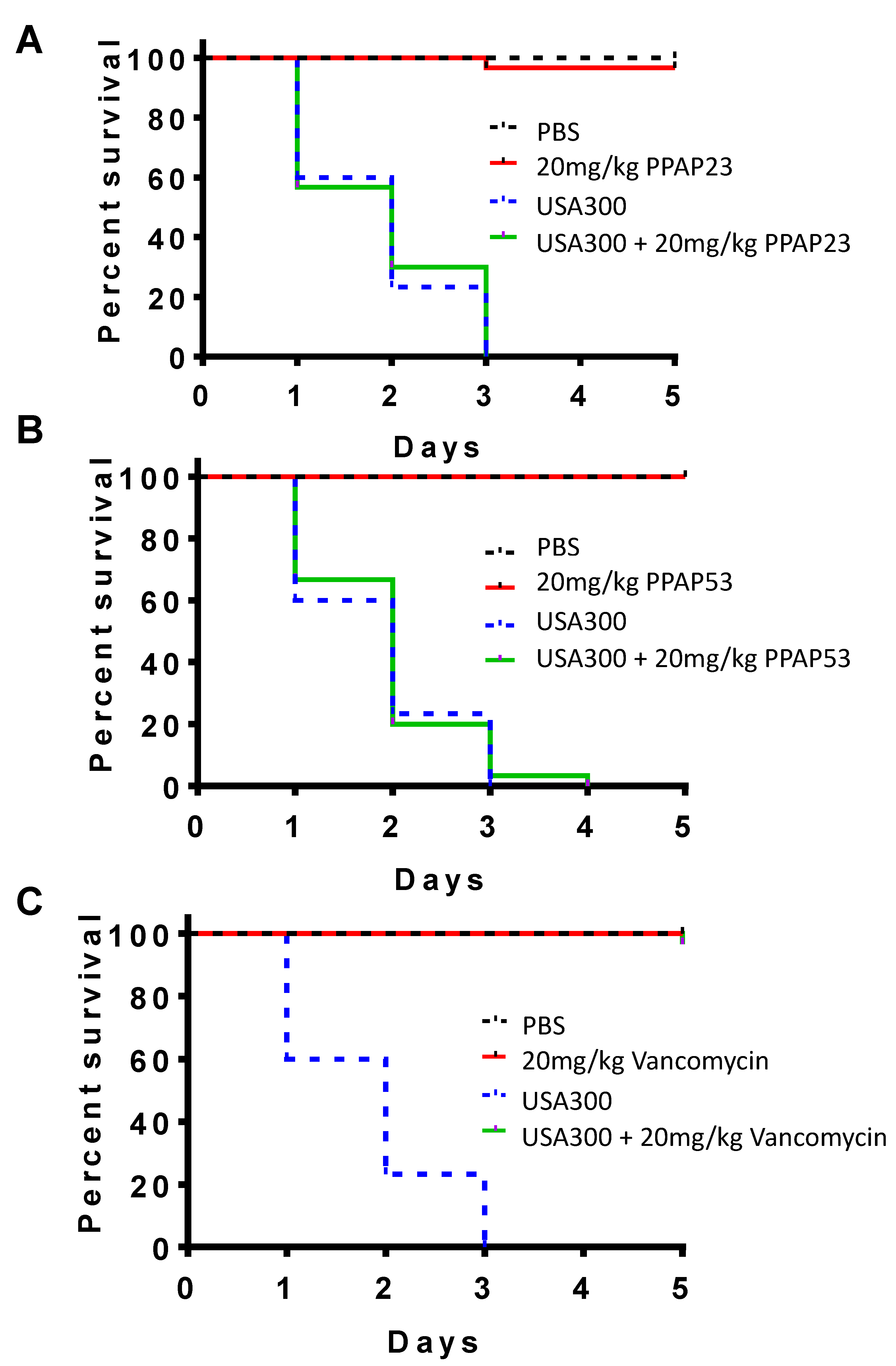
2.2. PPAP23 and PPAP53 Have No Toxic Effects on Larvae but Could Not Rescue Larvae in an Infection Model
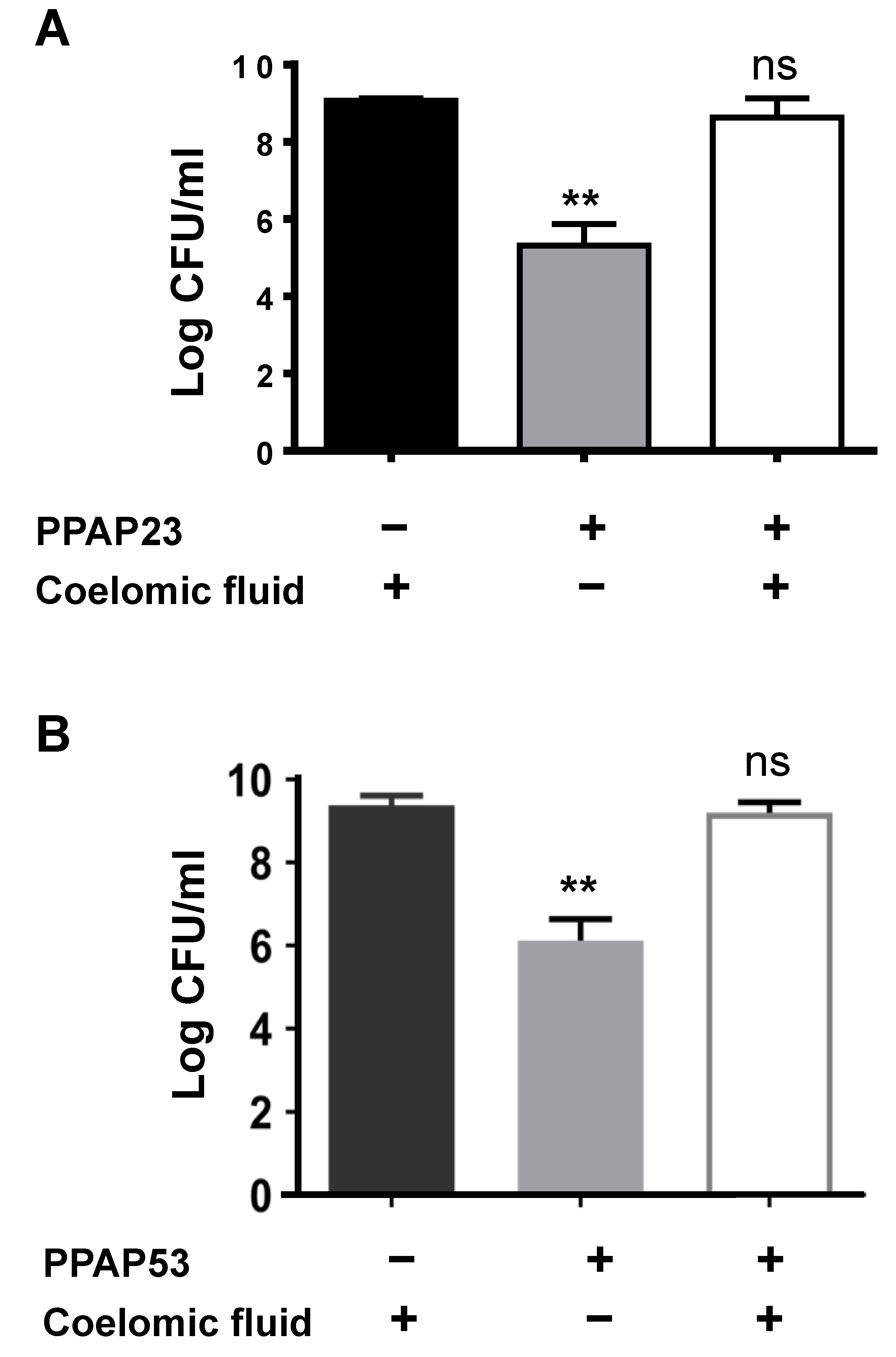
2.3. The Coelomic Fluid of the Larvae Antagonizes the Activity of PPAP23 and PPAP53
2.4. Bovine Serum and Albumin Abrogate the Bactericidal Activity of PPAP53
2.5. In Silico Docking Studies Show PPAP53 Binds to the Hemin Binding Pocket of HSA
2.6. Addition of Known Albumin Ligands Does Not Improve the In Vitro Antibacterial Activity of PPAP53
2.7. PPAP53 Reduces Growth of S. aureus USA300 in Subcutaneous Abscesses
2.8. PPAP23 Shows a Beneficial but Not Fully Protective Effect on S. aureus Septic Arthritis Mouse Model
2.9. PPAP23 and PPAP53 Show Activity against Gut, Gram+ Anaerobic Pathogens
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Conditions and Antibiotics
4.2. Synthesis of PPAP53
4.3. Bacterial Growth Kinetics
4.4. Antibiotic Susceptibility Testing
4.5. Galleria Mellonella Infection Model
4.6. Ex Vivo Killing Assay of Galleria Mellonella Larvae
4.7. In Silico Docking of PPAP with Human Serum Albumin (HSA)
4.8. Mouse Model for Hematogenous S. aureus Arthritis
4.9. Mouse Model of Subcutaneous Abscess Formation
4.10. IC25 Values of PPAP23 against Some of the Anaerobic Bacterial Strains
4.11. Quantification of Cytokines Using Enzyme-Linked Immunosorbent Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s Wort (Hypericum perforatum L.): A Review of Its Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.S.; Bhattacharya, S.K.; Wonnemann, M.; Singer, A.; Müller, W.E. Hyperforin as a Possible Antidepressant Component of Hypericum Extracts. Life Sci. 1998, 63, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ciochina, R.; Grossman, R.B. Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2006, 106, 3963–3986. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, N.S.; Dobrynin, V.N.; Kolosov, M.N.; Chernov, B.K.; Chervin, I.I. Structure of the Antibiotic Hyperforin. Dokl. Akad. Nauk. SSSR 1976, 226, 88–90. [Google Scholar] [PubMed]
- Beerhues, L. Hyperforin. Phytochemistry 2006, 67, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, B.I.P.; Rosato, A.; Marilena, M.; Gibbons, S.; Bombardelli, E.; Verotta, L.; Franchini, C.; Corbo, F. Biological Evaluation of Hyperforin and Its Hydrogenated Analogue on Bacterial Growth and Biofilm Production. J. Nat. Prod. 2013, 76, 1819–1823. [Google Scholar] [CrossRef]
- Biber, N.; Möws, K.; Plietker, B. The Total Synthesis of Hyperpapuanone, Hyperibone L, epi-Clusianone and Oblongifolin A. Nat. Chem. 2011, 3, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Horeischi, F.; Biber, N.; Plietker, B. The Total Syntheses of Guttiferone A and 6-Epi-Guttiferone A. J. Am. Chem. Soc. 2014, 136, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Socolsky, C.; Plietker, B. Total Synthesis and Absolute Configuration Assignment of MRSA Active Garcinol and Isogarcinol. Chem. A Eur. J. 2015, 21, 3053–3061. [Google Scholar] [CrossRef]
- Verotta, L.; Appendino, G.; Belloro, E.; Bianchi, F.; Sterner, O.; Lovati, M.; Bombardelli, E. Synthesis and Biological Evaluation of Hyperforin Analogues. Part I. Modification of the Enolized Cyclohexanedione Moiety. J. Nat. Prod. 2002, 65, 433–438. [Google Scholar] [CrossRef]
- Peslalz, P.; Vorbach, A.; Bleisch, A.; Liberini, E.; Kraus, F.; Izzo, F.; Brötz-Oesterhelt, H.; Götz, F.; Plietker, B. Chemical Predictive Modelling and Natural Product-Based Divergent Synthesis—Design of Type B PPAPs with Nanomolar Activities against MRSA. Chem. A Eur. J. 2024, e202401955. [Google Scholar] [CrossRef]
- Guttroff, C.; Baykal, A.; Wang, H.; Popella, P.; Kraus, F.; Biber, N.; Krauss, S.; Götz, F.; Plietker, B. Polycyclic Polyprenylated Acylphloroglucinols: An Emerging Class of Non-Peptide-Based MRSA- and VRE-Active Antibiotics. Angew. Chem. Int. Ed. Engl. 2017, 56, 15852–15856. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kraus, F.; Popella, P.; Baykal, A.; Guttroff, C.; François, P.; Sass, P.; Plietker, B.; Götz, F. The Polycyclic Polyprenylated Acylphloroglucinol Antibiotic PPAP 23 Targets the Membrane and Iron Metabolism in Staphylococcus aureus. Front. Microbiol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Peslalz, P.; Grieshober, M.; Kraus, F.; Bleisch, A.; Izzo, F.; Lichtenstein, D.; Hammer, H.; Vorbach, A.; Momoi, K.; Zanger, U.M.; et al. Unnatural Endotype B PPAPs as Novel Compounds with Activity against Mycobacterium tuberculosis. J. Med. Chem. 2023, 66, 15073–15083. [Google Scholar] [CrossRef] [PubMed]
- Fredrick, W.S.; Ravichandran, S. Hemolymph Proteins in Marine Crustaceans. Asian Pac. J. Trop. Biomed. 2012, 2, 496–502. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Ghuman, J.; Komatsu, T.; Tsuchida, E.; Curry, S. Crystal Structural Analysis of Human Serum Albumin Complexed with Hemin and Fatty Acid. BMC Struct. Biol. 2003, 3, 6. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-Binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.; Schade, J.; Keinhörster, D.; Weller, N.; George, S.E.; Kull, L.; Bauer, J.; Grau, T.; Winstel, V.; Stoy, H.; et al. Wall Teichoic Acids Mediate Increased Virulence in Staphylococcus aureus. Nat. Microbiol. 2017, 2, 4. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Anticancer Properties and Mechanism of Action of Oblongifolin C, Guttiferone K and Related Polyprenylated Acylphloroglucinols. Nat. Prod. Bioprospect 2021, 11, 629. [Google Scholar] [CrossRef]
- Richard, J.A.; Pouwer, R.H.; Chen, D.Y.K. The Chemistry of the Polycyclic Polyprenylated Acylphloroglucinols. Angew. Chem. Int. Ed. 2012, 51, 4536–4561. [Google Scholar] [CrossRef]
- Yang, X.W.; Grossman, R.B.; Xu, G. Research Progress of Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef]
- Grossman, R.B. Table of PPAPs. Available online: https://organicchemistrydata.org/grossman/ppap/allPPAPs.html (accessed on 11 June 2024).
- Tsai, C.J.Y.; Loh, J.M.S.; Proft, T. Galleria mellonella Infection Models for the Study of Bacterial Diseases and for Antimicrobial Drug Testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Füller, J.; Kellner, T.; Gaid, M.; Beerhues, L.; Müller-Goymann, C.C. Stabilization of Hyperforin Dicyclohexylammonium Salt with Dissolved Albumin and Albumin Nanoparticles for Studying Hyperforin Effects on 2D Cultivation of Keratinocytes in vitro. Eur. J. Pharm. Biopharm. 2018, 126, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Traeger, A.; Voelker, S.; Shkodra-Pula, B.; Kretzer, C.; Schubert, S.; Gottschaldt, M.; Schubert, U.S.; Werz, O. Improved Bioactivity of the Natural Product 5-Lipoxygenase Inhibitor Hyperforin by Encapsulation into Polymeric Nanoparticles. Mol. Pharm. 2020, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.N.; Blirup-Jensen, S.; Svendsen, P.J.; Wandall, J.H. Characterization and Quantification of Plasma Proteins Excreted in Faeces from Healthy Humans. Scand. J. Clin. Lab. Investig. 1995, 55, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Obradović, D.; Radan, M.; Đikić, T.; Nikolić, M.P.; Oljačić, S.; Nikolić, K. The Evaluation of Drug-Plasma Protein Binding Interaction on Immobilized Human Serum Albumin Stationary Phase, Aided by Different Computational Approaches. J. Pharm. Biomed. Anal. 2022, 211, 114593. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Bikadi, Z.; Malik, D.; Hari, P.; Pechan, I.; Berces, A.; Hazai, E. Evaluation of Drug-Human Serum Albumin Binding Interactions with Support Vector Machine Aided Online Automated Docking. Bioinformatics 2011, 27, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.J.; Lawson, N.; Hussaini, S.H.; Asquith, P. The Usefulness of Faecal Haemoglobin, Albumin and Alpha-1-Antitrypsin in the Detection of Gastrointestinal Bleeding. Ann. Clin. Biochem. 1990, 27 Pt 3, 208–212. [Google Scholar] [CrossRef]
- Su, Q.; Li, X.; Mo, W.; Yang, Z. Low Serum Bilirubin, Albumin, and Uric Acid Levels in Patients with Crohn’s Disease. Medicine 2019, 98, e15664. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile Colitis: Pathogenesis and Host Defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Eichner, M.; Augustin, C.; Fromm, A.; Piontek, A.; Walther, W.; Bücker, R.; Fromm, M.; Krause, G.; Schulzke, J.D.; Günzel, D.; et al. In Colon Epithelia, Clostridium perfringens Enterotoxin Causes Focal Leaks by Targeting Claudins Which Are Apically Accessible Due to Tight Junction Derangement. J. Infect. Dis. 2017, 217, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Legaria, M.C.; García, S.D.; Tudanca, V.; Barberis, C.; Cipolla, L.; Cornet, L.; Famiglietti, A.M.R.; Stecher, D.; Vay, C.A. Clostridium ramosum Rapidly Identified by MALDI-TOF MS. A Rare Gram-Variable Agent of Bacteraemia. Access Microbiol. 2020, 2, e000137. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides Distasonis: Intriguing Aerotolerant Gut Anaerobe with Emerging Antimicrobial Resistance and Pathogenic and Probiotic Roles in Human Health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and Characterization of Gut Microbiota Decarboxylases That Can Produce the Neurotransmitter Tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Rekdal, V.M.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and Inhibition of an Interspecies Gut Bacterial Pathway for Levodopa Metabolism. Science 2019, 364, 1055. [Google Scholar]
- Luqman, A.; Nega, M.; Nguyen, M.T.; Ebner, P.; Götz, F. SadA-Expressing Staphylococci in the Human Gut Show Increased Cell Adherence and Internalization. Cell Rep. 2018, 22, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Kharisma, V.D.; Ruiz, R.A.; Götz, F. In Silico and in Vitro Study of Trace Amines (TA) and Dopamine (DOP) Interaction with Human Alpha 1-Adrenergic Receptor and the Bacterial Adrenergic Receptor QseC. Cell Physiol. Biochem. 2020, 54, 888–898. [Google Scholar]
- Chen, H.; Nwe, P.-K.; Yang, Y.; Ring, A.M.; Crawford, J.M.; Palm Correspondence, N.W. A Forward Chemical Genetic Screen Reveals Gut Microbiota Metabolites That Modulate Host Physiology. Cell 2019, 177, 1217–1231. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Luck, B.; Horvath, T.D.; Engevik, K.A.; Ruan, W.; Haidacher, S.J.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; Haag, A.M.; Versalovic, J.; et al. Neurotransmitter Profiles Are Altered in the Gut and Brain of Mice Mono-Associated with Bifidobacterium dentium. Biomolecules 2021, 11, 1091. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Sorokina, E.V.; Zarubina, A.P.; Parkhomenko, I.M. Testing Neurotransmitters for Toxicity with a Luminescent Biosensor: Implications for Microbial Endocrinology. J. Pharm. Nutr. Sci. 2017, 7, 88–94. [Google Scholar] [CrossRef]
- Miller, R.J.; Crosby, H.A.; Schilcher, K.; Wang, Y.; Ortines, R.V.; Mazhar, M.; Dikeman, D.A.; Pinsker, B.L.; Brown, I.D.; Joyce, D.P.; et al. Development of a Staphylococcus aureus Reporter Strain with Click Beetle Red Luciferase for Enhanced in vivo Imaging of Experimental Bacteremia and Mixed Infections. Sci. Rep. 2019, 9, 16663. [Google Scholar] [CrossRef]
- Na, M.; Wang, W.; Fei, Y.; Josefsson, E.; Ali, A.; Jin, T. Both Anti-TNF and CTLA4 Ig Treatments Attenuate the Disease Severity of Staphylococcal Dermatitis in Mice. PLoS ONE 2017, 12, e0173492. [Google Scholar] [CrossRef]
- Peslalz, P.; Kraus, F.; Izzo, F.; Bleisch, A.; El Hamdaoui, Y.; Schulz, I.; Kany, A.M.; Hirsch, A.K.H.; Friedland, K.; Plietker, B. Selective Activation of a TRPC6 Ion Channel Over TRPC3 by Metalated Type-B Polycyclic Polyprenylated Acylphloroglucinols. J. Med. Chem. 2023, 66, 15061–15072. [Google Scholar] [CrossRef]
- Gemmell, C.G.; Edwards, D.I.; Fraise, A.P.; Gould, F.K.; Ridgway, G.L.; Warren, R.E. Guidelines for the Prophylaxis and Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in the UK. J. Antimicrob. Chemother. 2006, 57, 589–608. [Google Scholar] [CrossRef]
- Rennie, R.P.; Turnbull, L.; Brosnikoff, C.; Cloke, J. First Comprehensive Evaluation of the M.I.C. Evaluator Device Compared to Etest and CLSI Reference Dilution Methods for Antimicrobial Susceptibility Testing of Clinical Strains of Anaerobes and Other Fastidious Bacterial Species. J. Clin. Microbiol. 2012, 50, 1153–1157. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Popella, P.; Krauss, S.; Ebner, P.; Nega, M.; Deibert, J.; Götz, F. VraH Is the Third Component of the Staphylococcus aureus VraDEH System Involved in Gallidermin and Daptomycin Resistance and Pathogenicity. Antimicrob. Agents Chemother. 2016, 60, 2391. [Google Scholar] [CrossRef] [PubMed]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to Optimize the Drop Plate Method for Enumerating Bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the General Anesthetics Propofol and Halothane to Human Serum Albumin. High Resolution Crystal Structures. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Welin, A.; Schwarze, J.C.; Svensson, M.N.D.; Na, M.; Jarneborn, A.; Magnusson, M.; Mohammad, M.; Kwiecinski, J.; Josefsson, E.; et al. CTLA4 Immunoglobulin but Not Anti-Tumor Necrosis Factor Therapy Promotes Staphylococcal septic Arthritis in Mice. J. Infect. Dis. 2015, 212, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; de la Cuesta-Zuluaga, J.; Kuhn, M.; Baghai Arassi, M.; Treis, T.; Blasche, S.; Zimmermann, M.; Bork, P.; Patil, K.R.; Typas, A.; et al. High-Throughput Anaerobic Screening for Identifying Compounds Acting against Gut Bacteria in Monocultures or Communities. Nat. Protoc. 2024, 19, 668–699. [Google Scholar] [CrossRef]
- Sutherland, R.; Boon, R.J.; Griffin, K.E.; Masters, P.J.; Slocombe, B.; White, A.R. Antibacterial Activity of Mupirocin (Pseudomonic Acid), a New Antibiotic for Topical Use. Antimicrob. Agents Chemother. 1985, 27, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Deplano, A.; Hallin, M.; Bustos Sierra, N.; Michel, C.; Prevost, B.; Martiny, D.; Yin, N. Persistence of the Staphylococcus aureus Epidemic European Fusidic Acid-Resistant Impetigo Clone (EEFIC) in Belgium. J. Antimicrob. Chemother. 2023, 78, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Galindo, E.; Hebert, A.A. A Comparative Review of Current Topical Antibiotics for Impetigo. Expert. Opin. Drug Saf. 2021, 20, 677–683. [Google Scholar]
- Ron, G.G.; Arranz, M.V. New Therapeutic Applications of Ozenoxacin in Superficial Skin Infections. Dermatol. Rep. 2021, 14, 9289. [Google Scholar]
- Schachner, L.A.; Andriessen, A.; Benjamin, L.T.; Claro, C.; Eichenfield, L.F.; Esposito, S.M.; Keller, L.; Kircik, L.H.; Kwong, P.C.; Mccuaig, C.; et al. Do Antimicrobial Resistance Patterns Matter? An Algorithm for the Treatment of Patients with Impetigo. J. Drugs Dermatol. 2021, 20, 134–142. [Google Scholar] [CrossRef]




| Medium | MIC (µg/mL) | ||
|---|---|---|---|
| PPAP23 | PPAP53 | Vancomycin | |
| MHB | 1 | 0.5 | 1 |
| MHB + 25% FBS | 32 | 32 | 2 |
| MHB + 0.5% BSA | 4 | 8 | 1 |
| MHB + 1% BSA | 8 | 8 | 1 |
| MHB + 2.5% BSA | 16 | 16 | 1 |
| MHB + 5% BSA | 32 | 32 | 2 |
| MHB + 1% IgG | 1 | 0.5 | 1 |
| MHB + 2.5% IgG | 1 | 0.5 | 1 |
| MHB + 1% Fg | 1 | 0.5 | 1 |
| MHB + 2.5% Fg | 1 | 0.5 | 1 |
| Strains | PPAP23 IC25 a (µM) | PPAP53 IC25 (µM) | |
|---|---|---|---|
| Pathogenic G (+) gut anaerobes | Clostridium difficile | <2 | >80 |
| Clostridium perfringens | <2 | 5 | |
| Ruminococcus gnavus | <2 | 5 | |
| Clostridium ramosum | <2 | 10 | |
| Commensal gut anaerobes (+/−) | Streptococcus salivarius | <2 | 5 |
| Dorea formicigenerans | 4 | 10 | |
| Streptococcus parasanguinis | 4 | 10 | |
| Roseburia intestinalis | 4 | 10 | |
| Coprococcus comes | 4 | 10 | |
| Collinsella aerofaciens | 4 | 10 | |
| Eubacterium rectale | 4 | 5 | |
| Clostridium bolteae | 22 | 10 | |
| Parabacteroides merdae | 22 | 5 | |
| Clostridium saccharolyticum | 22 | 10 | |
| Fusobacterium nucleatum subsp. Nucleatum | >45 | 40 | |
| Bacteroides vulgatus | 22 | 5 | |
| Bacteroides uniformis | >45 | 10 | |
| Bacteroides thetaiotaomicron | >45 | 20 | |
| Bacteroides fragilis NT | 22 | 10 | |
| Pathogenic G (–) gut anaerobes | Yersinia pseudotuberculosis | >22 | 2.5 |
| Yersinia enterocolitica WA-314 | >45 | >80 | |
| Vibrio cholerae | >45 | 80 | |
| Shigella sonnei 53G | >45 | >80 | |
| Shigella flexneri | >45 | >80 | |
| Salmonella enterica Typhimurium LT2 | >45 | >80 | |
| Salmonella enterica Typhimurium | >45 | >80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammanath, A.V.; Matsuo, M.; Wang, H.; Kraus, F.; Bleisch, A.; Peslalz, P.; Mohammad, M.; Deshmukh, M.; Grießhammer, A.; Purkayastha, M.; et al. Antimicrobial Evaluation of Two Polycyclic Polyprenylated Acylphloroglucinol Compounds: PPAP23 and PPAP53. Int. J. Mol. Sci. 2024, 25, 8023. https://doi.org/10.3390/ijms25158023
Ammanath AV, Matsuo M, Wang H, Kraus F, Bleisch A, Peslalz P, Mohammad M, Deshmukh M, Grießhammer A, Purkayastha M, et al. Antimicrobial Evaluation of Two Polycyclic Polyprenylated Acylphloroglucinol Compounds: PPAP23 and PPAP53. International Journal of Molecular Sciences. 2024; 25(15):8023. https://doi.org/10.3390/ijms25158023
Chicago/Turabian StyleAmmanath, Aparna Viswanathan, Miki Matsuo, Huanhuan Wang, Frank Kraus, Anton Bleisch, Philipp Peslalz, Majd Mohammad, Meghshree Deshmukh, Anne Grießhammer, Moushumi Purkayastha, and et al. 2024. "Antimicrobial Evaluation of Two Polycyclic Polyprenylated Acylphloroglucinol Compounds: PPAP23 and PPAP53" International Journal of Molecular Sciences 25, no. 15: 8023. https://doi.org/10.3390/ijms25158023






