Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study
Abstract
1. Introduction
2. Results and Discussion
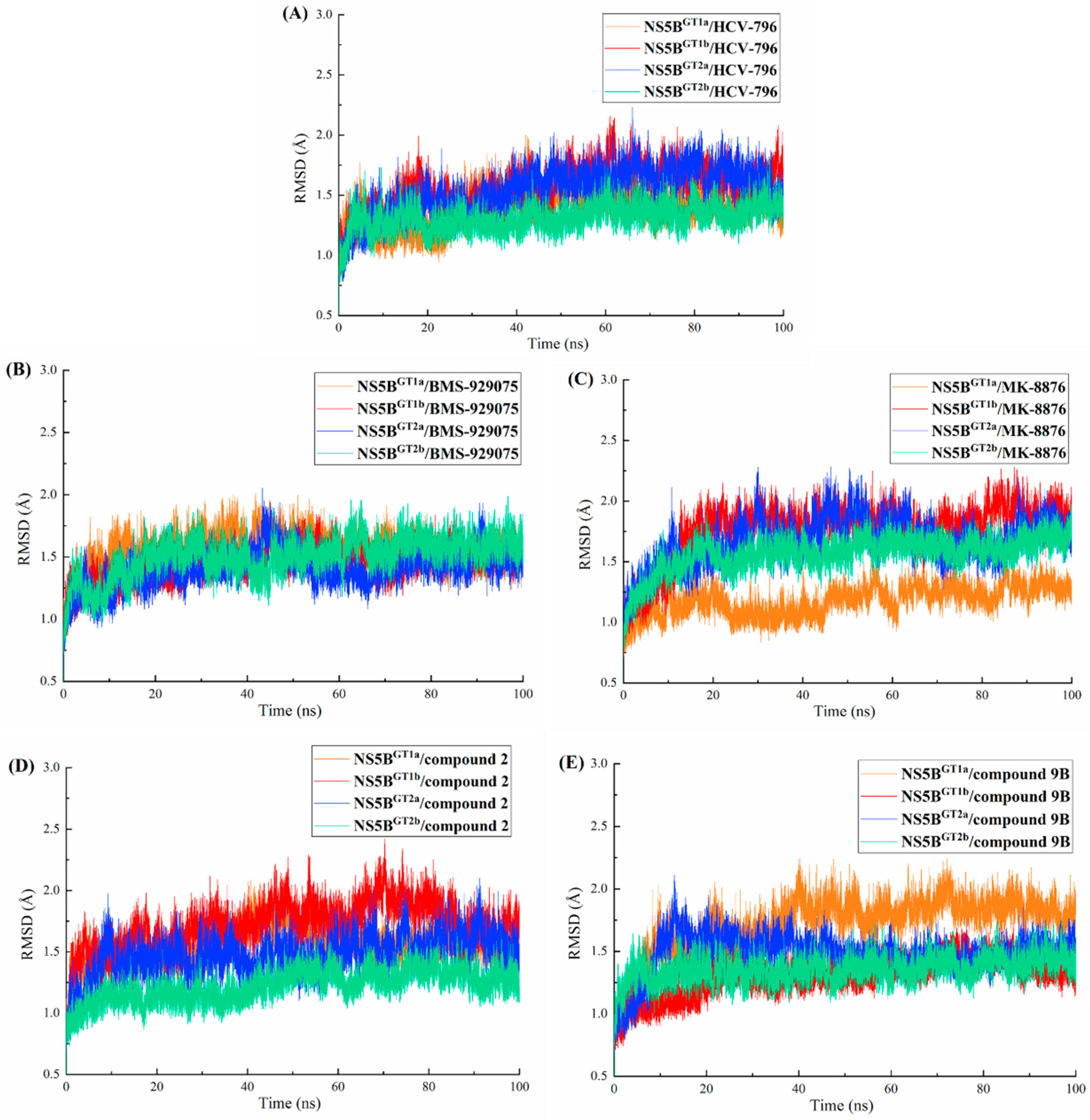
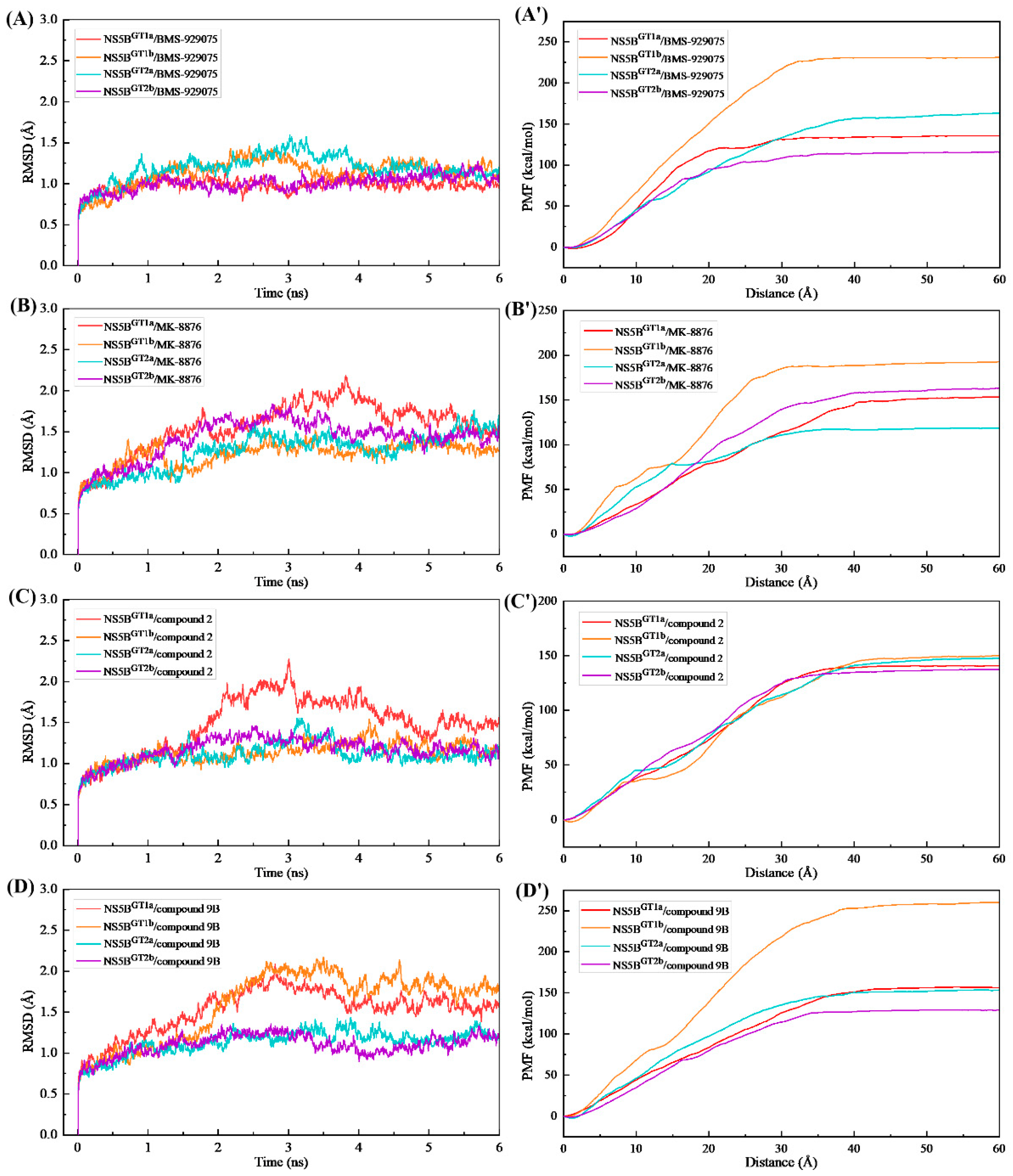
2.1. Stability of the NS5B/Inhibitor Complexes during the MD Simulations
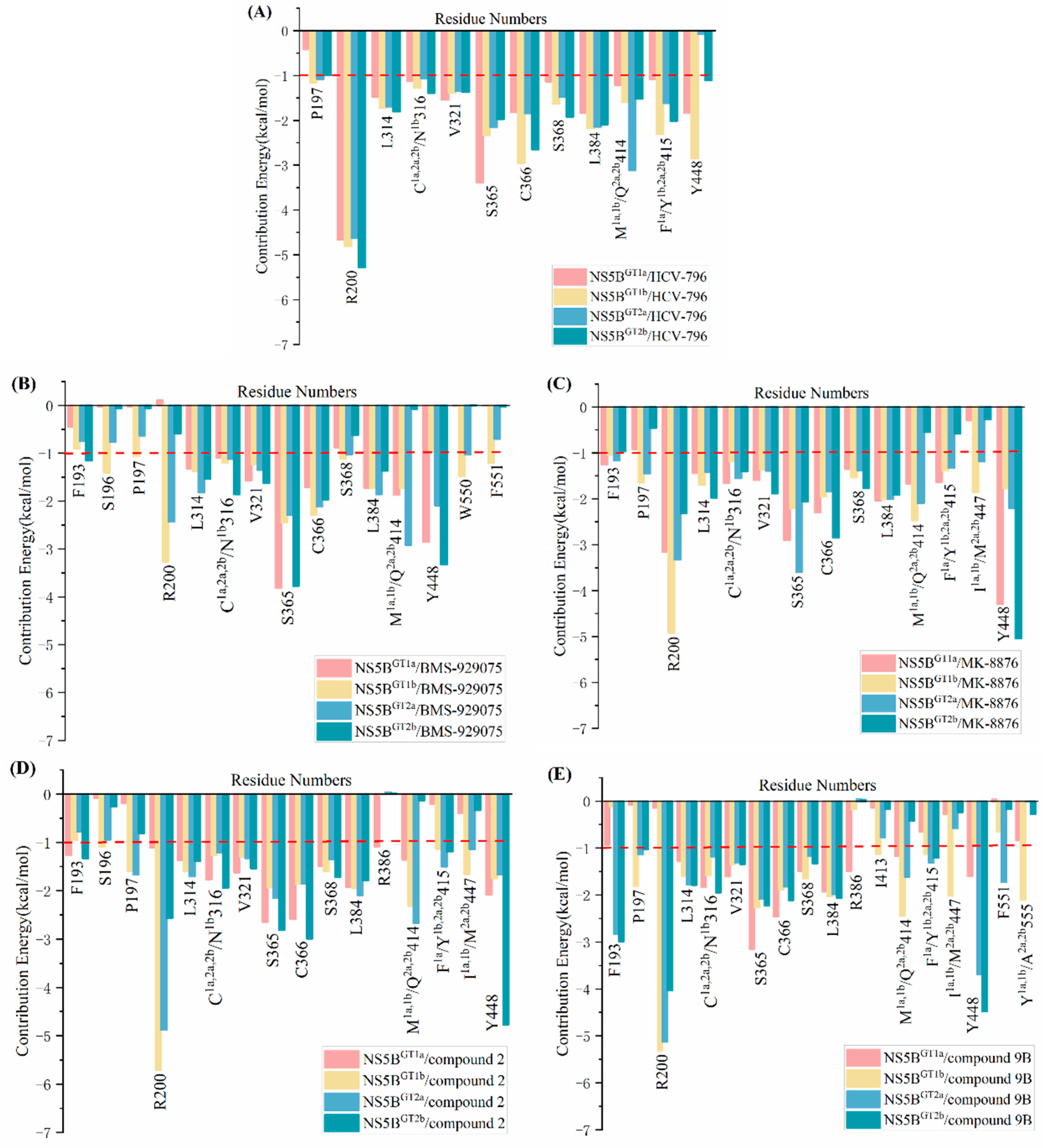
2.2. Binding Free Energies Predicted by MM/GBSA Method
2.3. The Binding Modes of the Benzofuran Core Inhibitors with NS5B Polymerases
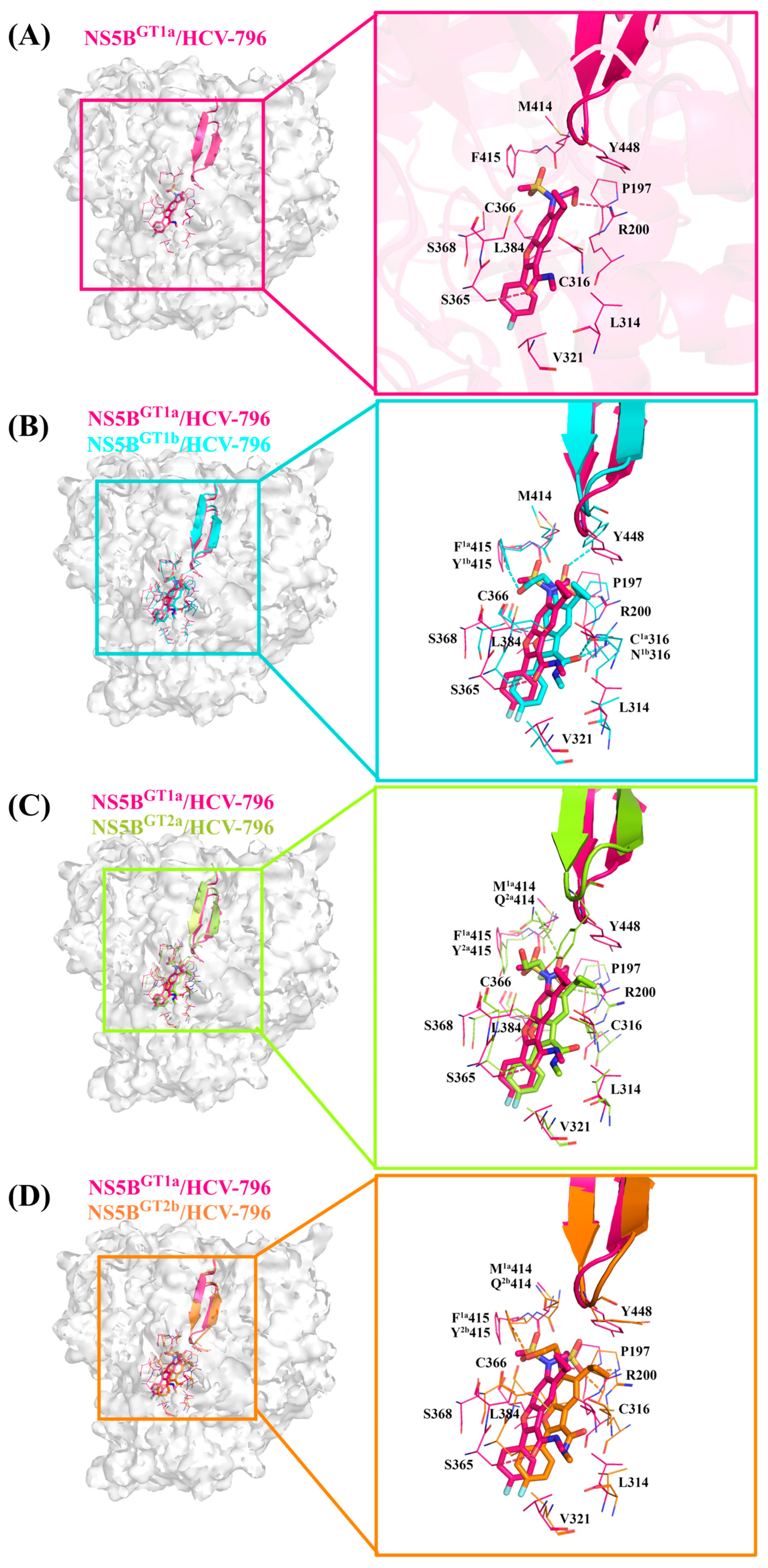
2.3.1. The Binding Modes of HCV-796 Binding to NS5B Polymerases
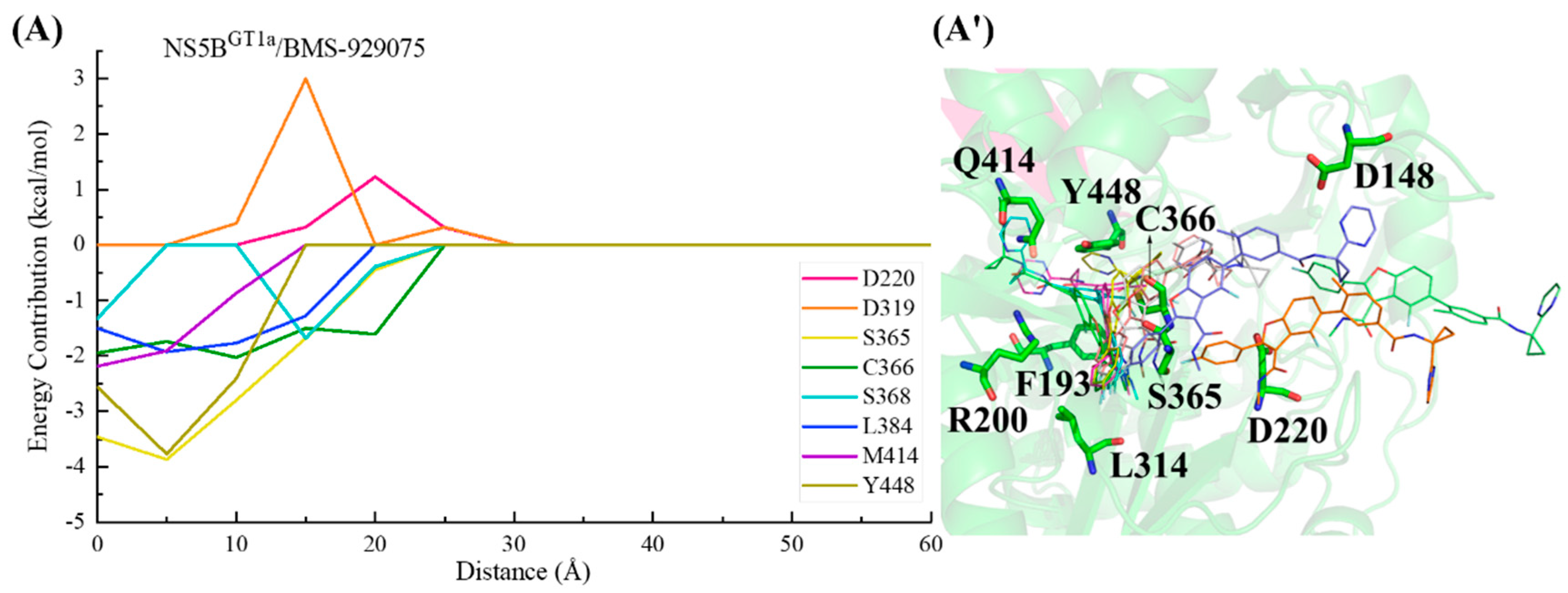
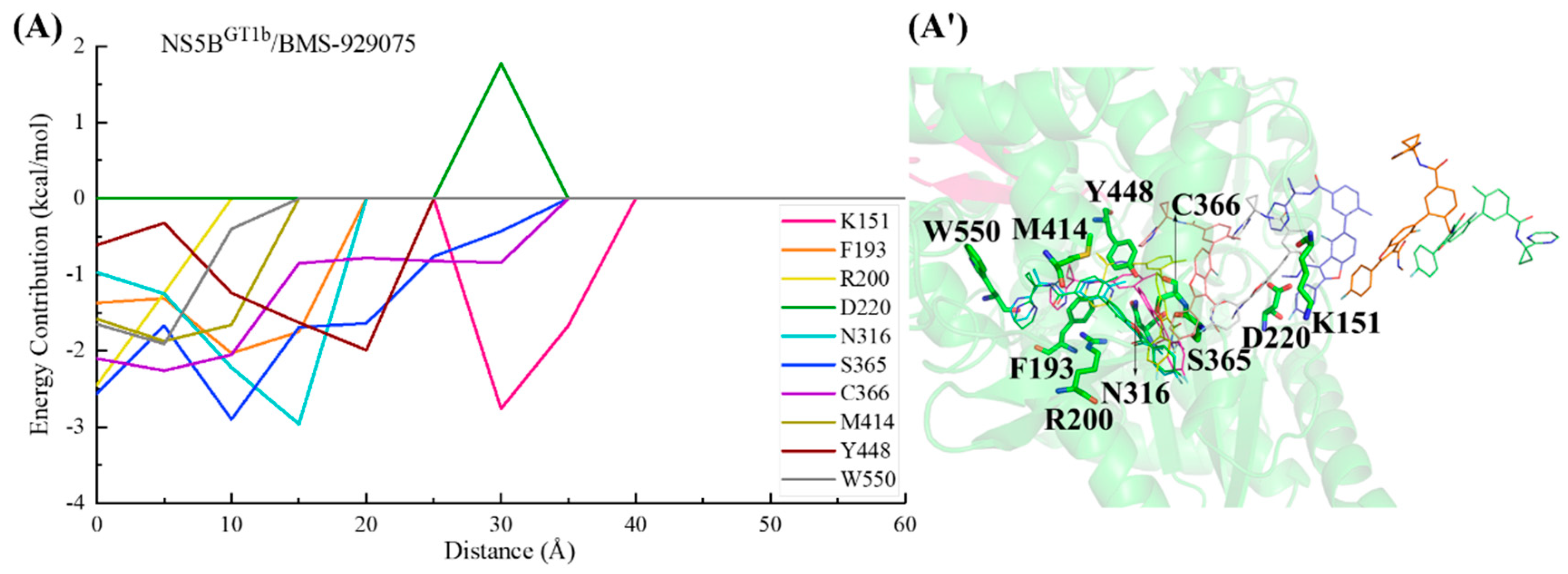
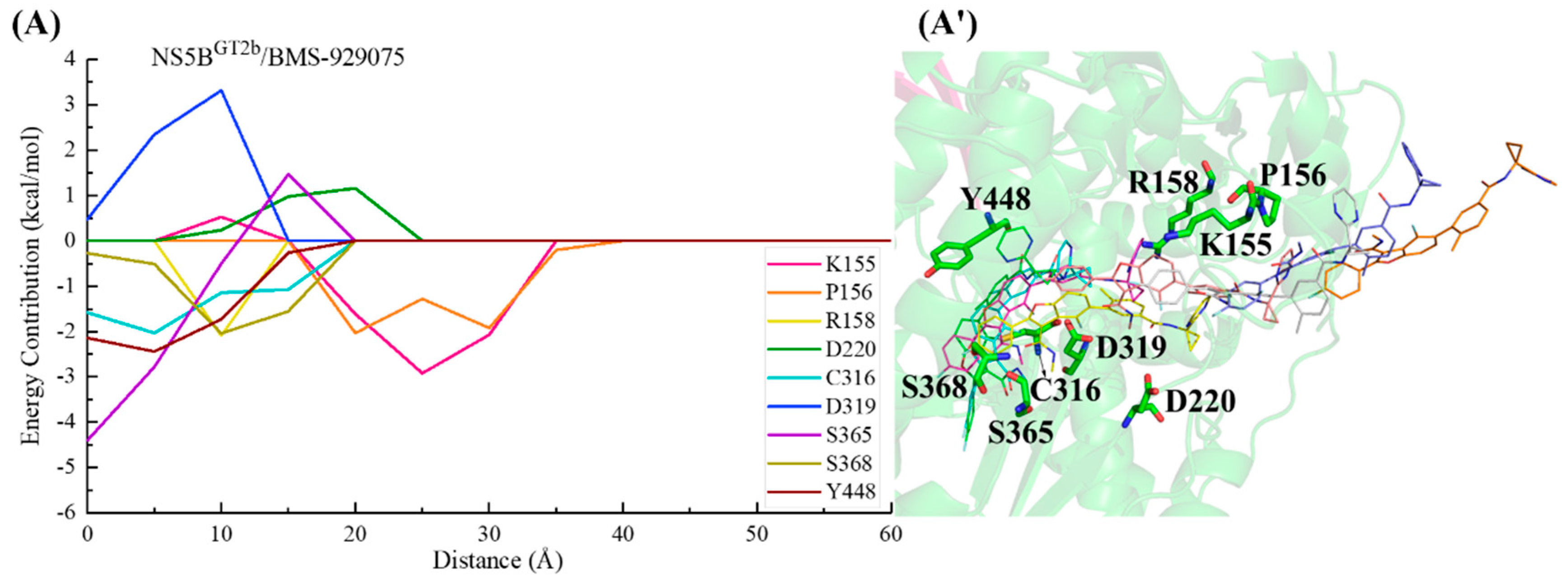
2.3.2. The Binding Modes of BMS-929075 with NS5B Polymerases
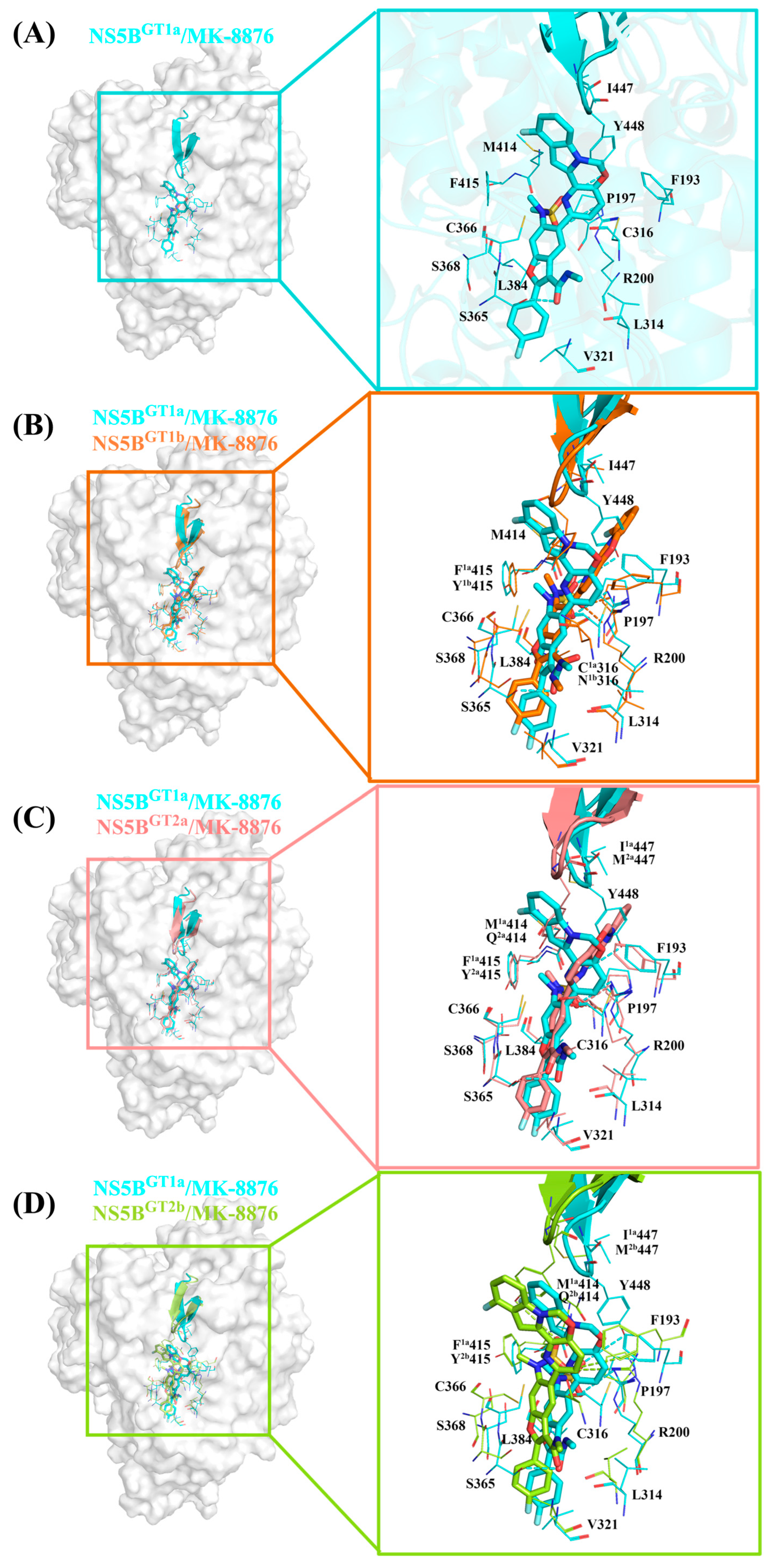
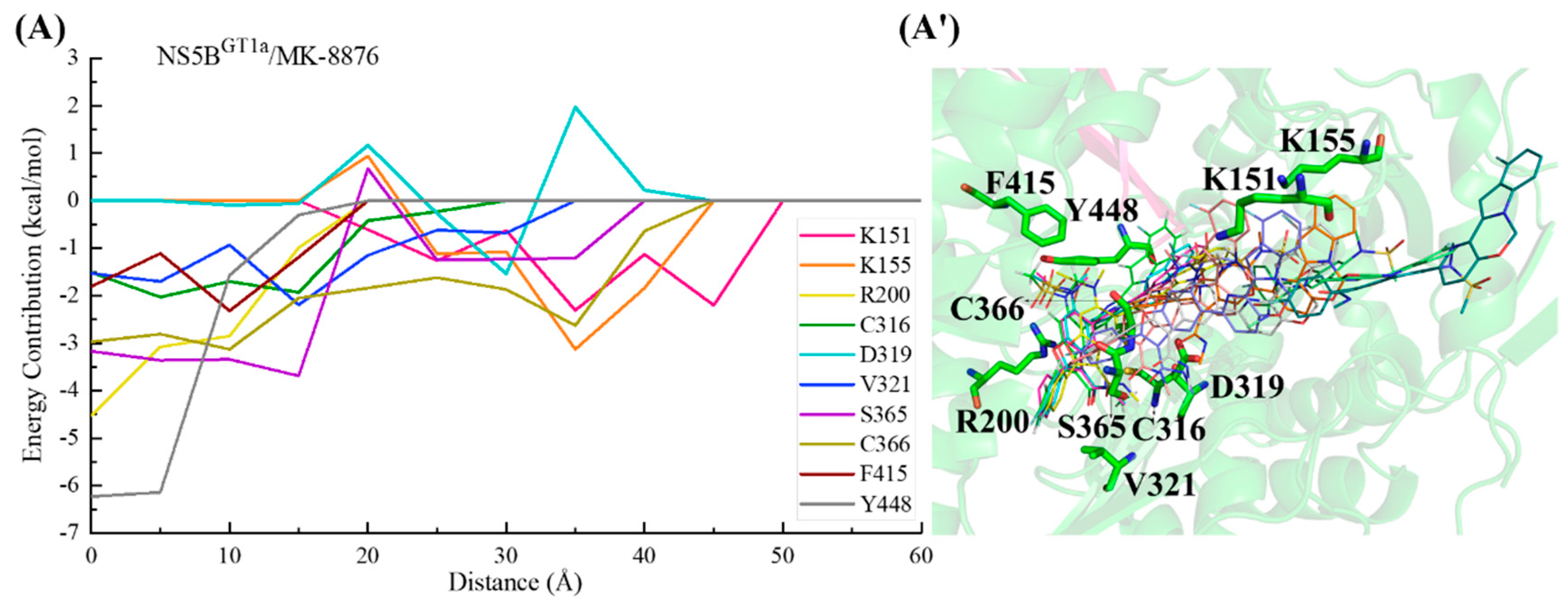
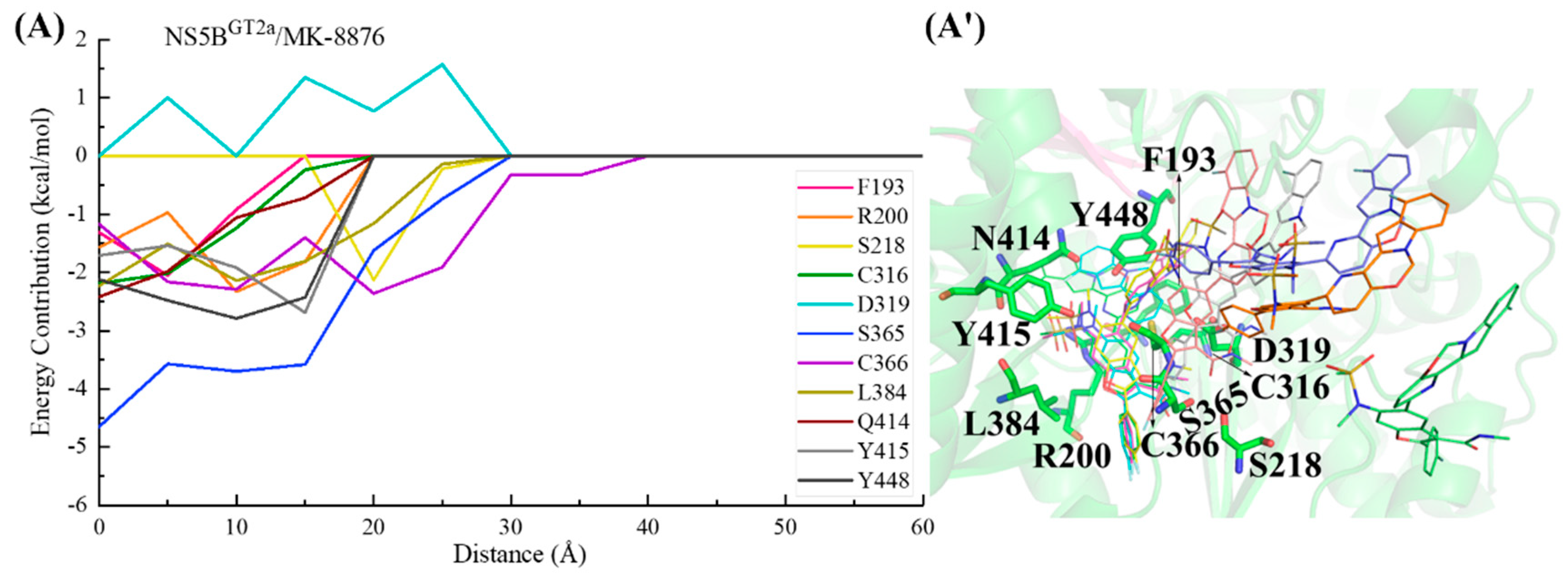
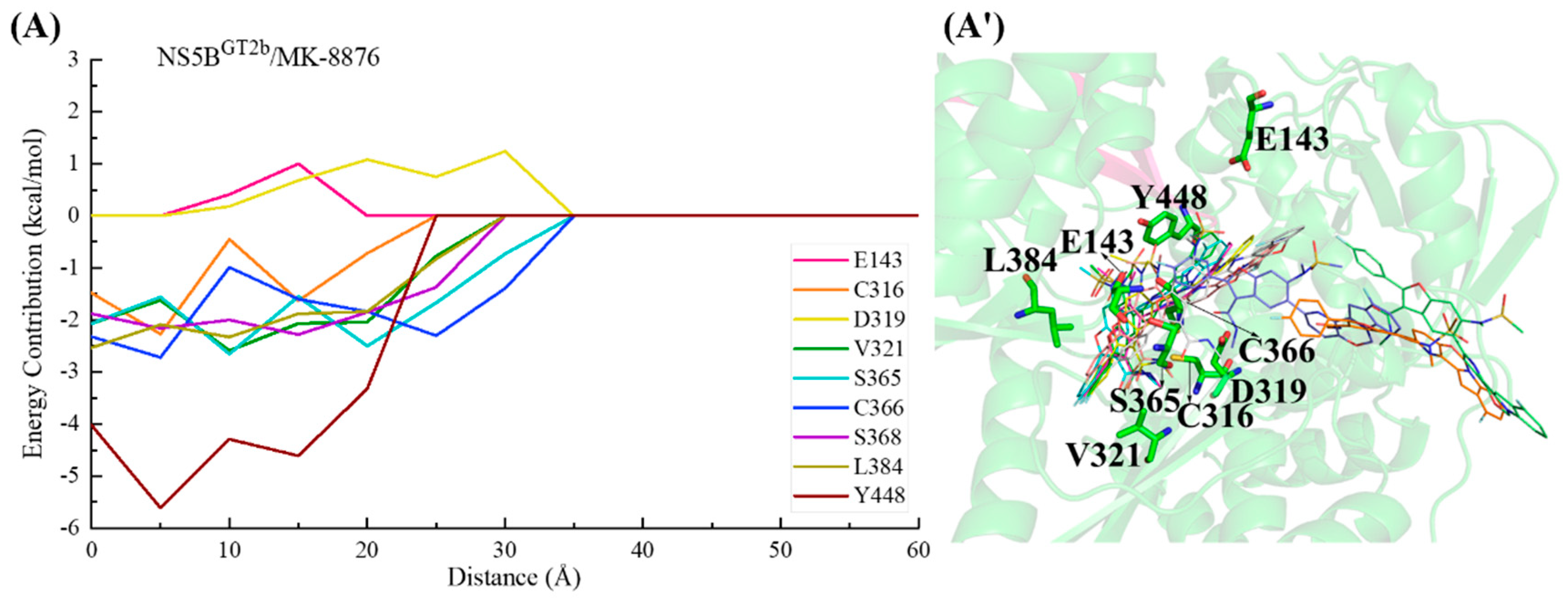
2.3.3. The Binding Modes of MK-8876 with NS5B Polymerases
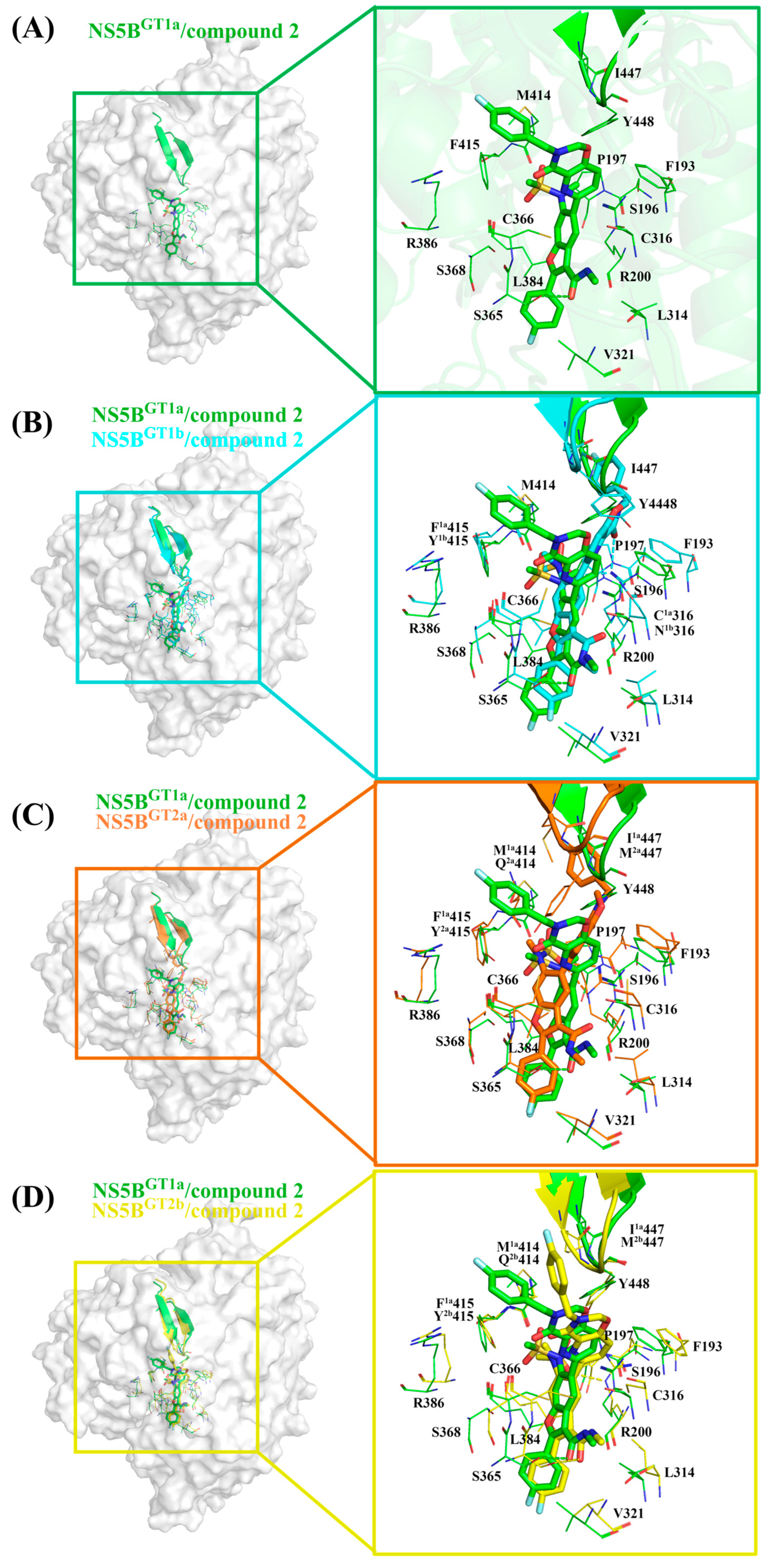
2.3.4. The Binding Modes of Compound 2 with NS5B Polymerases
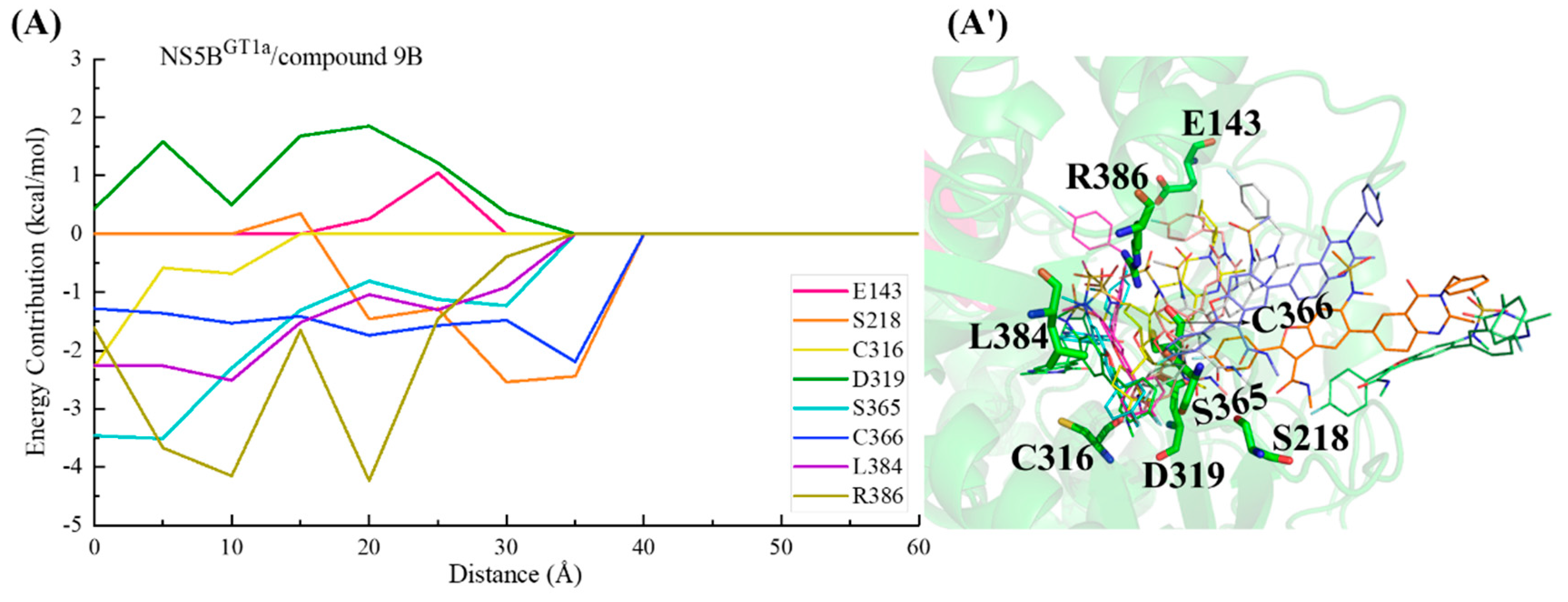
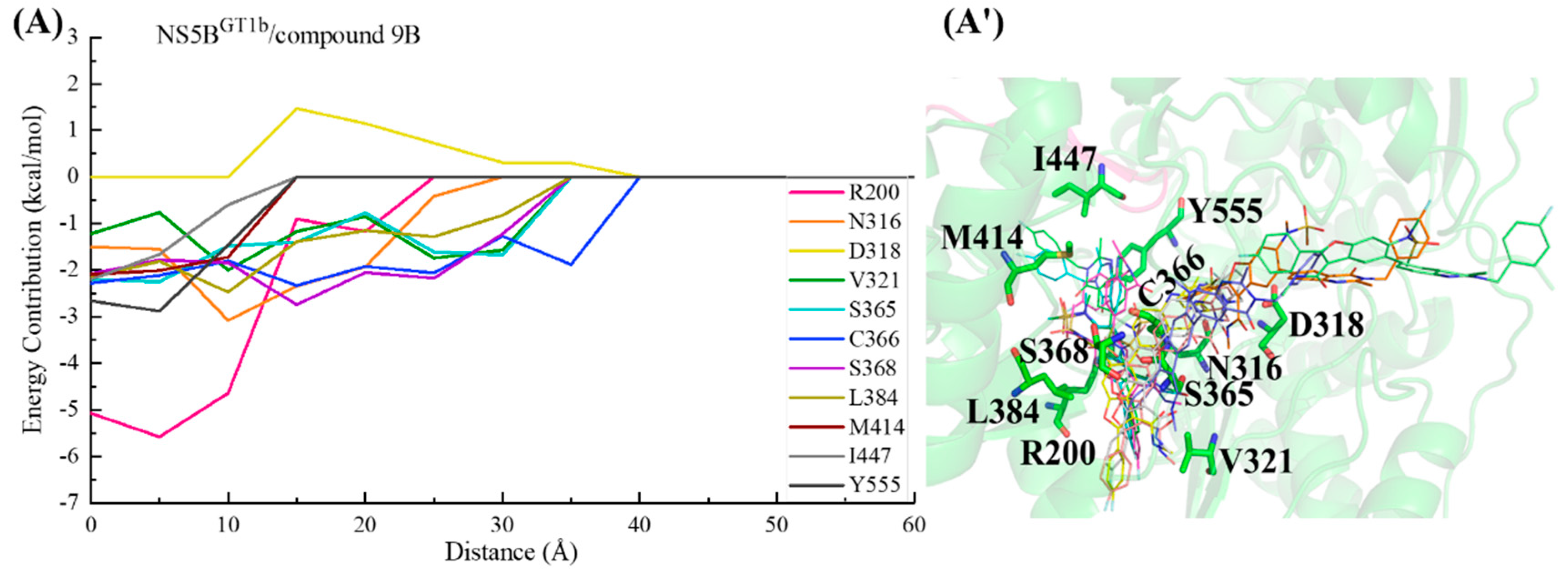
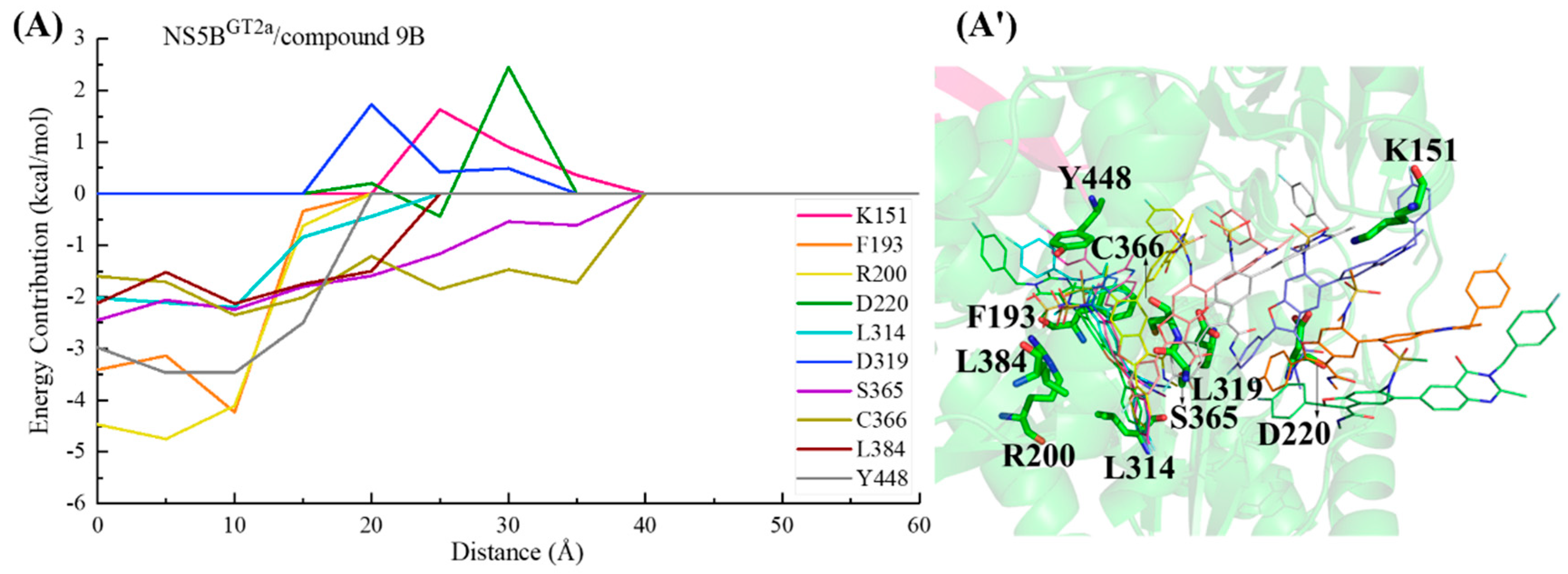
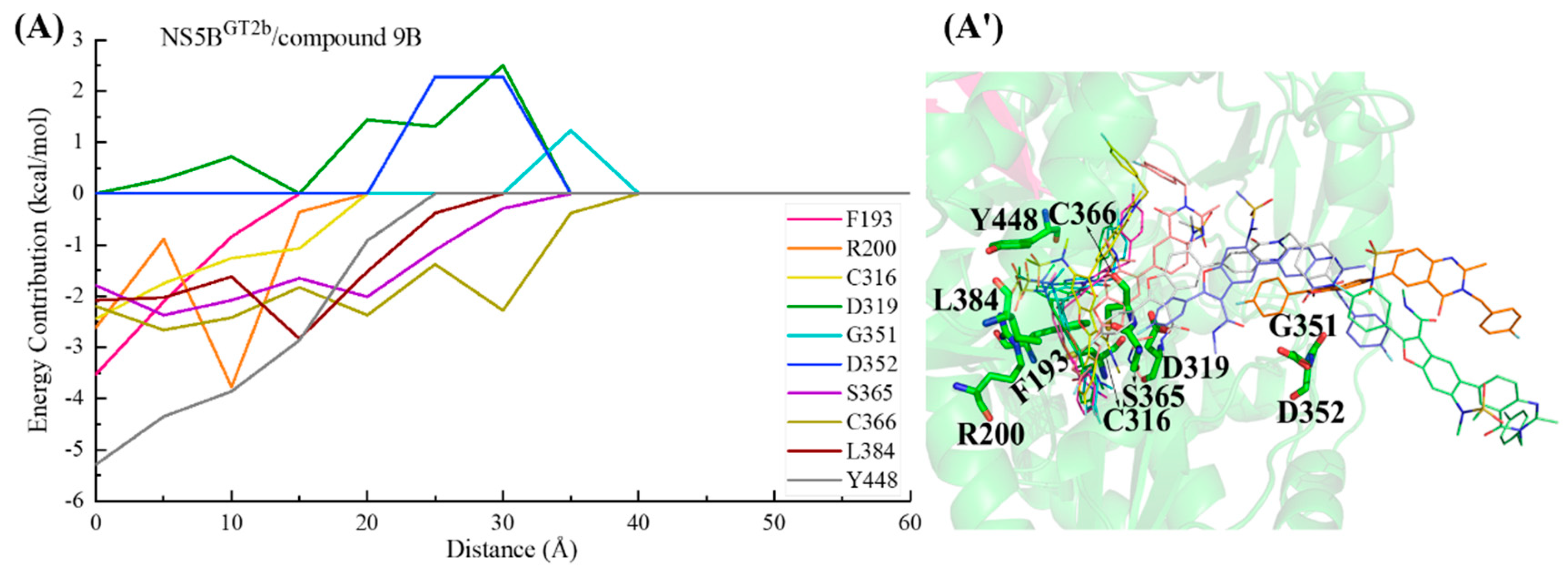
2.3.5. The Binding Modes of Compound 9B with NS5B Polymerases
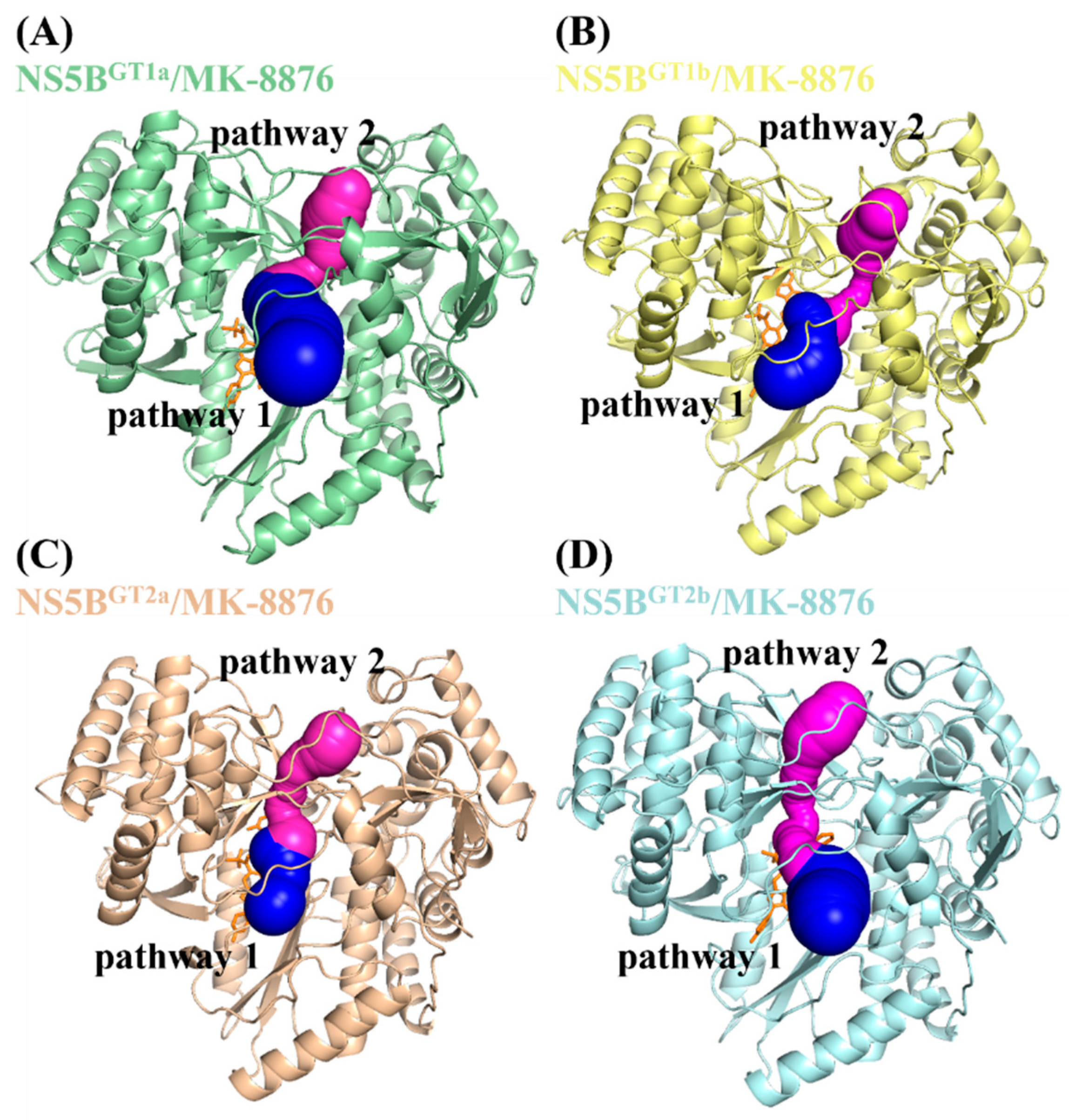
2.4. Identification of the Binding Pathway of Benzofuran Inhibitors to NS5B Polymerases
2.5. Analyses of the Binding Processes of Benzofuran Inhibitors to NS5B Polymerases
3. Materials and Methods
3.1. Preparation of Initial Models
3.2. Molecular Dynamics Simulations
3.3. Binding Free Energy Calculations and Energy Decomposition Analyses
3.4. Adaptive Steered Molecular Dynamics (ASMD) Simulations and Potential of Mean Force (PMF) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.K.; Chung, R.T. Overview of Direct-Acting Antiviral Drugs and Drug Resistance of Hepatitis C Virus. Nat. Rev. Microbiol. 2009, 7, 798–812. [Google Scholar] [CrossRef]
- Ganta, N.M.; Gedda, G.; Rathnakar, B.; Satyanarayana, M.; Yamajala, B.; Ahsan, M.J.; Jadav, S.S.; Balaraju, T. A review on HCV inhibitors: Significance of non-structural polyproteins. Eur. J. Med. Chem. 2018, 164, 576–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Y.; Zhu, J.; Zhao, S.; Qi, S.; Chen, X.; Zhang, H.; Ni, Z.; Zhou, Y.; Chen, G.; et al. The roles and mechanism of m6A RNA methylation regulators in cancer immunity. Biomed. Pharmacother. 2023, 163, 114839. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Fact Sheets: Hepatitis C. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 1 May 2023).
- Martinez, M.A.; Franco, S. Therapy Implications of Hepatitis C Virus Genetic Diversity. Viruses 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Ducancelle, A.; Le Guillou-Guillemette, H.; Gaudy, C.; Lunel, F. HCV virology and diagnosis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101626. [Google Scholar] [CrossRef] [PubMed]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype from Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef]
- Borgia, G.; Maraolo, A.E.; Nappa, S.; Gentile, I.; Buonomo, A.R. NS5B polymerase inhibitors in phase II clinical trials for HCV infection. Expert Opin. Investig. Drugs 2017, 27, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38, 7–13. [Google Scholar] [CrossRef]
- Warkad, S.D.; Nimse, S.B.; Song, K.-S.; Kim, T. HCV Detection, Discrimination, and Genotyping Technologies. Sensors 2018, 18, 3423. [Google Scholar] [CrossRef]
- Warkad, S.D.; Nimse, S.B.; Song, K.-S.; Kim, T. Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes. ACS Omega 2020, 5, 10794–10799. [Google Scholar] [CrossRef]
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M.J.T.L. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Alazard-Dany, N.; Denolly, S.; Boson, B.; Cosset, F.-L. Overview of HCV Life Cycle with a Special Focus on Current and Possible Future Antiviral Targets. Viruses 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, H.; Wang, Z.; Wang, Q. Progression of Antiviral Agents Targeting Viral Polymerases. Molecules 2022, 27, 7370. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Chotpatiwetchkul, W.; Phanus-Umporn, C.; Nantasenamat, C.; Charoenkwan, P.; Shoombuatong, W. StackHCV: A web-based integrative machine-learning framework for large-scale identification of hepatitis C virus NS5B inhibitors. J. Comput. Mol. Des. 2021, 35, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- McComas, C.C.; Palani, A.; Chang, W.; Holloway, M.K.; Lesburg, C.A.; Li, P.; Liverton, N.; Meinke, P.T.; Olsen, D.B.; Peng, X.; et al. Development of a New Structural Class of Broadly Acting HCV Non-Nucleoside Inhibitors Leading to the Discovery of MK-8876. ChemMedChem 2017, 12, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Kneteman, N.M.; Howe, A.Y.; Gao, T.; Lewis, J.; Pevear, D.; Lund, G.; Douglas, D.; Mercer, D.F.; Tyrrell, D.L.J.; Immermann, F. HCV796: A selective nonstructural protein 5B polymerase inhibitor with potent anti-hepatitis C virus activity in vitro, in mice with chimeric human livers, and in humans infected with hepatitis C virus. Hepatology 2009, 49, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Parcella, K.; Nickel, A.; Beno, B.R.; Sheriff, S.; Wan, C.; Wang, Y.-K.; Roberts, S.B.; Meanwell, N.A.; Kadow, J.F. Discovery and initial optimization of alkoxyanthranilic acid derivatives as inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2017, 27, 295–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, M.; Peng, E.; Huang, N.; Huang, Q.; Huq, A.; Lau, M.; Colonno, R.; Li, L. Discovery of novel potent HCV NS5B polymerase non-nucleoside inhibitors bearing a fused benzofuran scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.-S.; Beno, B.R.; Parcella, K.; Bender, J.A.; Grant-Young, K.A.; Nickel, A.; Gunaga, P.; Anjanappa, P.; Bora, R.O.; Selvakumar, K.; et al. Discovery of a Hepatitis C Virus NS5B Replicase Palm Site Allosteric Inhibitor (BMS-929075) Advanced to Phase 1 Clinical Studies. J. Med. Chem. 2017, 60, 4369–4385. [Google Scholar] [CrossRef]
- Liu, H.; Dai, X.; He, S.; Brockunier, L.; Marcantonio, K.; Ludmerer, S.W.; Li, F.; Feng, K.-I.; Nargund, R.P.; Palani, A. Design and evaluation of novel tetracyclic benzofurans as palm site allosteric inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2018, 29, 126104. [Google Scholar] [CrossRef]
- Xiao, D.; Dai, X.; Liu, H.; He, S.; Shi, Z.-C.; Ludmerer, S.W.; Li, F.; Nargund, R.; Palani, A. Multi-step parallel synthesis enabled optimization of benzofuran derivatives as pan-genotypic non-nucleoside inhibitors of HCV NS5B. Bioorg. Med. Chem. Lett. 2020, 30, 127004. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, H.; Wujieti, B.; Zhang, B.; Cui, W.; Chen, B.-Z. Insight into the drug resistance mechanisms of GS-9669 caused by mutations of HCV NS5B polymerase via molecular simulation. Comput. Struct. Biotechnol. J. 2021, 19, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.C.; Brown, J.A.; Thorpe, I.F. Allosteric inhibitors have distinct effects, but also common modes of action, in the HCV polymerase. Biophys. J. 2015, 108, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.L.; Iraci, N.; Manfroni, G.; Cecchetti, V. Allosteric Inhibition of the Hepatitis C Virus NS5B Polymerase: In Silico Strategies for Drug Discovery and Development. Future Med. Chem. 2011, 3, 1027–1055. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2014, 61, 77–87. [Google Scholar] [CrossRef]
- Hang, J.Q.; Yang, Y.; Harris, S.F.; Leveque, V.; Whittington, H.J.; Rajyaguru, S.; Ao-Ieong, G.; McCown, M.F.; Wong, A.; Giannetti, A.M.; et al. Slow Binding Inhibition and Mechanism of Resistance of Non-nucleoside Polymerase Inhibitors of Hepatitis C Virus. J. Biol. Chem. 2009, 284, 15517–15529. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.; Crosby, R.M.; Ellis, B.; Hamatake, R.; Hong, Z.; Johns, B.A.; Kahler, K.M.; Koble, C.; Leivers, A.; Leivers, M.R.; et al. Discovery of a Potent Boronic Acid Derived Inhibitor of the HCV RNA-Dependent RNA Polymerase. J. Med. Chem. 2013, 57, 1902–1913. [Google Scholar] [CrossRef]
- Eastman, K.J.; Parcella, K.; Yeung, K.-S.; Grant-Young, K.A.; Zhu, J.; Wang, T.; Zhang, Z.; Yin, Z.; Beno, B.R.; Sheriff, S.; et al. The discovery of a pan-genotypic, primer grip inhibitor of HCV NS5B polymerase. MedChemComm 2017, 8, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, E.H.; Cellucci, A.; Bartholomew, L.; Mattu, M.; Barbato, G.; Ludmerer, S.W.; Graham, D.J.; Altamura, S.; Paonessa, G.; De Francesco, R.; et al. Structural Basis for Resistance of the Genotype 2b Hepatitis C Virus NS5B Polymerase to Site A Non-Nucleoside Inhibitors. J. Mol. Biol. 2009, 390, 1048–1059. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Sprenger, K.G.; Jaeger, V.W.; Pfaendtner, J. The General AMBER Force Field (GAFF) Can Accurately Predict Thermodynamic and Transport Properties of Many Ionic Liquids. J. Phys. Chem. B 2015, 119, 5882–5895. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision B.01; Gaussian: Wallingford, CT, USA, 2010. [Google Scholar]
- Kimura, S.R.; Rajamani, R.; Langley, D.R. Communication: Quantum polarized fluctuating charge model: A practical method to include ligand polarizability in biomolecular simulations. J. Chem. Phys. 2011, 135, 231101. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Mamatkulov, S.; Schwierz, N. Force fields for monovalent and divalent metal cations in TIP3P water based on thermodynamic and kinetic properties. J. Chem. Phys. 2018, 148, 074504. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Folescu, D.E.; Onufriev, A.V. A Closed-Form, Analytical Approximation for Apparent Surface Charge and Electric Field of Molecules. ACS Omega 2022, 7, 26123–26136. [Google Scholar] [CrossRef]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, F.; Zhao, B.; Yang, J.; Ferrara, J.; Sankaran, B.; Venkataram Prasad, B.; Kundu, B.B.; Phillips, G.N., Jr.; Gao, Y. Structural basis of the stereoselective formation of the spirooxindole ring in the biosynthesis of citrinadins. J. Comput. Chem. 2021, 12, 4158. [Google Scholar] [CrossRef] [PubMed]
- Naroğlu, S.S.; Timuçin, E. Comprehensive evaluation of the MM-GBSA method on bromodomain-inhibitor sets. Brief. Bioinform. 2020, 21, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, B.; Gao, Y.; Chen, Y.; Han, D.; Lu, J.; Liu, T.; Gao, Q.; Zhang, J.Z.; Wang, M. Design Two Novel Tetrahydroquinoline Derivatives against Anticancer Target LSD1 with 3D-QSAR Model and Molecular Simulation. Molecules 2022, 27, 8358. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, E.; Pavelka, A.; Benes, P.; Strnad, O.; Brezovsky, J.; Kozlikova, B.; Gora, A.; Sustr, V.; Klvana, M.; Medek, P.; et al. CAVER 3.0: A Tool for the Analysis of Transport Pathways in Dynamic Protein Structures. PLoS Comput. Biol. 2012, 8, e1002708. [Google Scholar] [CrossRef] [PubMed]
- Ozer, G.; Quirk, S.; Hernandez, R. Adaptive steered molecular dynamics: Validation of the selection criterion and benchmarking energetics in vacuum. J. Chem. Phys. 2012, 136, 215104. [Google Scholar] [CrossRef] [PubMed]
- Ozer, G.; Keyes, T.; Quirk, S.; Hernandez, R. Multiple branched adaptive steered molecular dynamics. J. Chem. Phys. 2014, 141, 064101. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Schulten, K. Calculating potentials of mean force from steered molecular dynamics simulations. J. Chem. Phys. 2004, 120, 5946–5961. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Lett. 1997, 78, 2690–2693. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; Delano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]










| System | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| NS5BGT1a/HCV-796 | −22.21 ± 7.41 | −59.56 ± 3.02 | 35.42 ± 5.30 | −7.50 ± 0.19 | 13.21 ± 9.11 | 27.93 ± 5.26 | −53.83 ± 4.20 |
| NS5BGT1b/HCV-796 | −41.68 ± 7.03 | −62.15 ± 2.95 | 46.79 ± 3.32 | −7.81 ± 0.14 | 5.11 ± 7.77 | 38.98 ± 3.31 | −64.85 ± 4.86 |
| NS5BGT2a/HCV-796 | −36.65 ± 5.27 | −61.27 ± 2.94 | 44.40 ± 3.90 | −7.92 ± 0.18 | 7.75 ± 6.56 | 36.48 ± 3.87 | −61.44 ± 3.62 |
| NS5BGT2b/HCV-796 | −43.44 ± 6.98 | −63.13 ± 3.21 | 46.67 ± 3.57 | −8.12 ± 0.13 | 3.23 ± 7.84 | 38.54 ± 3.56 | −68.03 ± 4.71 |
| NS5BGT1a/BMS-929075 | −15.33 ± 3.85 | −64.84 ± 2.71 | 36.43 ± 3.06 | −7.78 ± 0.21 | 21.10 ± 4.92 | 28.64 ± 3.05 | −51.53 ± 2.81 |
| NS5BGT1b/BMS-929075 | −32.10 ± 3.30 | −73.73 ± 3.14 | 50.57 ± 2.86 | −9.17± 0.22 | 18.47 ± 4.37 | 41.40 ± 2.81 | −64.43 ± 3.46 |
| NS5BGT2a/BMS-929075 | −33.57 ± 4.48 | −76.74 ± 3.02 | 54.33 ± 2.79 | −9.55 ± 0.14 | 20.76 ± 5.28 | 44.78 ± 2.78 | −65.53 ± 3.56 |
| NS5BGT2b/BMS-929075 | −10.80 ± 4.59 | −61.87 ± 3.26 | 32.49 ± 4.09 | −7.79 ± 0.28 | 21.69 ± 6.15 | 24.70 ± 4.07 | −47.97 ± 3.19 |
| NS5BGT1a/MK-8876 | −31.81 ± 5.67 | −75.47 ± 3.39 | 48.27 ± 3.20 | −8.81 ± 0.18 | 16.46 ± 6.51 | 39.46 ± 3.27 | −67.82 ± 4.34 |
| NS5BGT1b/MK-8876 | −39.49 ± 4.21 | −84.31 ± 3.09 | 57.78 ± 3.19 | −9.98 ± 0.16 | 18.29 ± 5.28 | 47.81 ± 3.18 | −75.99 ± 3.36 |
| NS5BGT2a/MK-8876 | −35.83 ± 3.76 | −83.43 ± 3.10 | 52.47 ± 2.87 | −9.88 ± 0.14 | 16.64 ± 4.73 | 42.59 ± 2.85 | −76.67 ± 3.49 |
| NS5BGT2b/MK-8876 | −28.53 ± 5.22 | −72.69 ± 3.43 | 46.69 ± 3.31 | −8.62 ± 0.24 | 18.16 ± 6.18 | 38.07 ± 3.27 | −63.15 ± 4.76 |
| NS5BGT1a/compound 2 | −21.63 ± 7.45 | −71.57 ± 5.82 | 48.06 ± 6.00 | −8.60 ± 0.56 | 26.43 ± 9.57 | 39.46 ± 5.97 | −53.74 ± 7.72 |
| NS5BGT1b/compound 2 | −39.79 ± 3.86 | −87.35 ± 3.15 | 57.53 ± 3.03 | −10.58 ± 0.18 | 17.74 ± 4.91 | 46.95 ± 3.02 | −80.18 ± 3.49 |
| NS5BGT2a/compound 2 | −47.00 ± 4.34 | −85.22 ± 2.95 | 64.66 ± 3.20 | −10.42 ± 0.17 | 17.66 ± 5.39 | 54.24 ± 3.17 | −77.99 ± 3.83 |
| NS5BGT2b/compound 2 | −32.81 ± 7.14 | −76.52 ± 3.86 | 47.25 ± 5.65 | −9.17 ± 0.30 | 14.44 ± 9.11 | 38.08 ± 5.71 | −71.24 ± 4.54 |
| NS5BGT1a/compound 9B | −13.92 ± 6.99 | −68.33 ± 4.02 | 38.72 ± 5.57 | −8.44 ± 0.36 | 24.8 ± 8.94 | 30.28 ± 5.49 | −51.97 ± 4.18 |
| NS5BGT1b/compound 9B | −36.08 ± 3.84 | −92.10 ± 3.18 | 56.97 ± 3.69 | −10.94 ± 0.17 | 20.89 ± 5.33 | 46.03 ± 2.66 | −82.14 ± 3.48 |
| NS5BGT2a/compound 9B | −44.38 ± 4.33 | −93.35 ± 3.34 | 63.32 ± 3.08 | −10.58 ± 0.16 | 18.94 ± 5.31 | 52.74 ± 3.07 | −84.98 ± 3.79 |
| NS5BGT2b/compound 9B | −31.47 ± 4.32 | −84.10 ± 3.45 | 51.52 ± 3.07 | −9.87 ± 0.16 | 20.05 ± 5.30 | 41.65 ± 3.05 | −73.91 ± 3.82 |
| System | Donor | Acceptor | Occupied (%) | Distance (Å) | Angle (deg) |
|---|---|---|---|---|---|
| NS5BGT1a/HCV-796 | Ser365@OG | HCV-796@O2 | 70.81 | 2.75 | 156.77 |
| Arg200@NH1 | HCV-796@O3 | 24.97 | 2.89 | 151.74 | |
| NS5BGT1b/HCV-796 | Tyr448@OH | HCV-796@O4 | 63.34 | 2.81 | 164.68 |
| Tyr415@OH | HCV-796@O3 | 52.39 | 2.76 | 160.03 | |
| Arg200@NH1 | HCV-796@O2 | 44.95 | 2.85 | 149.42 | |
| Asn316@ND2 | HCV-796@O2 | 25.90 | 2.87 | 156.13 | |
| NS5BGT2a/HCV-796 | Gln414@NE2 | HCV-796@O4 | 73.29 | 2.83 | 159.52 |
| Arg200@NH2 | HCV-796@O5 | 67.65 | 2.83 | 150.34 | |
| NS5BGT2b/HCV-796 | Arg200@NH2 | HCV-796@O5 | 75.10 | 2.82 | 150.20 |
| Arg200@NE | HCV-796@O5 | 52.91 | 2.86 | 149.51 | |
| Tyr415@OH | HCV-796@O3 | 37.17 | 2.76 | 160.12 | |
| NS5BGT1a/BMS-929075 | Ser365@OG | BMS-929075@O2 | 87.00 | 2.74 | 158.40 |
| NS5BGT1b/BMS-929075 | Arg200@NH1 | BMS-929075@O2 | 76.92 | 2.80 | 151.47 |
| NS5BGT2a/BMS-929075 | Gln414@NE2 | BMS-929075@O3 | 66.85 | 2.86 | 157.30 |
| Arg200@NH2 | BMS-929075@O3 | 59.63 | 2.85 | 150.75 | |
| NS5BGT2b/BMS-929075 | Ser365@OG | BMS-929075@O2 | 94.14 | 2.70 | 159.35 |
| NS5BGT1a/MK-8876 | Arg200@NH2 | MK-8876@O5 | 66.27 | 2.83 | 154.21 |
| Ser365@OG | MK-8876@O3 | 51.14 | 2.77 | 155.59 | |
| Tyr448@OH | MK-8876@O4 | 41.49 | 2.75 | 162.82 | |
| NS5BGT1b/MK-8876 | Arg200@NE | MK-8876@O5 | 59.05 | 2.86 | 152.30 |
| Arg200@NH2 | MK-8876@O5 | 56.52 | 2.85 | 149.85 | |
| NS5BGT2a/MK-8876 | Ser365@OG | MK-8876@O3 | 75.67 | 2.76 | 156.90 |
| Arg200@NH1 | MK-8876@O5 | 53.52 | 2.86 | 152.22 | |
| Arg200@NE | MK-8876@O5 | 45.36 | 2.88 | 153.68 | |
| NS5BGT2b/MK-8876 | Tyr448@OH | MK-8876@O4 | 69.77 | 2.76 | 161.14 |
| Arg200@NH2 | MK-8876@O5 | 25.69 | 2.82 | 150.93 | |
| Arg200@NH1 | MK-8876@O5 | 23.52 | 2.82 | 151.63 | |
| Ser365@OG | MK-8876@O3 | 4.60 | 2.79 | 152.98 | |
| NS5BGT1a/compound 2 | Ser365@OG | compound 2@O4 | 30.33 | 2.79 | 153.92 |
| NS5BGT1b/compound 2 | Arg200@NH1 | compound 2@O2 | 69.64 | 2.84 | 154.15 |
| Arg200@NH1 | compound 2@O6 | 57.19 | 2.86 | 151.57 | |
| NS5BGT2a/compound 2 | Arg200@NE | compound 2@O6 | 43.99 | 2.89 | 153.96 |
| Arg200@NH1 | compound 2@O6 | 35.68 | 2.86 | 150.18 | |
| NS5BGT2b/compound 2 | Tyr448@OH | compound 2@O5 | 81.31 | 2.75 | 160.11 |
| Arg200@NE | compound 2@O6 | 60.94 | 2.85 | 151.81 | |
| Ser365@OG | compound 2@O4 | 55.35 | 2.77 | 154.42 | |
| Arg200@NH2 | compound 2@O6 | 45.01 | 2.84 | 148.11 | |
| NS5BGT1a/compound 9B | Ser365@OG | compound 9B@O3 | 57.09 | 2.77 | 156.50 |
| NS5BGT1b/compound 9B | Arg200@NH1 | compound 9B@O3 | 47.58 | 2.83 | 146.21 |
| Asn316@ND2 | compound 9B@O3 | 20.86 | 2.85 | 161.70 | |
| NS5BGT2a/compound 9B | Arg200@NH1 | compound 9B@O5 | 63.50 | 2.84 | 149.45 |
| Arg200@NH1 | compound 9B@O1 | 36.93 | 2.82 | 145.36 | |
| NS5BGT2b/compound 9B | Tyr448@OH | compound 9B@O4 | 80.70 | 2.77 | 161.65 |
| Arg200@NH2 | compound 9B@O5 | 47.47 | 2.82 | 152.72 |
| System | Pathway 1 | Pathway 2 | ||||
|---|---|---|---|---|---|---|
| Bottleneck Radius (Å) | Length (Å) | Curvature (Å) | Bottleneck Radius (Å) | Length (Å) | Curvature (Å) | |
| NS5BGT1a/MK-8876 | 4.60 | 14.81 | 1.06 | 2.25 | 19.13 | 1.13 |
| NS5BGT1b/MK-8876 | 3.29 | 11.39 | 1.22 | 1.49 | 26.04 | 1.46 |
| NS5BGT2a/MK-8876 | 3.11 | 9.73 | 1.18 | 2.20 | 21.85 | 1.24 |
| NS5BGT2b/MK-8876 | 3.44 | 11.81 | 1.26 | 1.73 | 26.72 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Zhao, F.; Chen, Y.; Xue, Y.; Bao, K.; Chang, Y.; Lu, J.; Wang, M.; Liu, T.; Gao, Q.; et al. Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study. Int. J. Mol. Sci. 2024, 25, 8028. https://doi.org/10.3390/ijms25158028
Han D, Zhao F, Chen Y, Xue Y, Bao K, Chang Y, Lu J, Wang M, Liu T, Gao Q, et al. Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study. International Journal of Molecular Sciences. 2024; 25(15):8028. https://doi.org/10.3390/ijms25158028
Chicago/Turabian StyleHan, Di, Fang Zhao, Yifan Chen, Yiwei Xue, Ke Bao, Yuxiao Chang, Jiarui Lu, Meiting Wang, Taigang Liu, Qinghe Gao, and et al. 2024. "Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study" International Journal of Molecular Sciences 25, no. 15: 8028. https://doi.org/10.3390/ijms25158028
APA StyleHan, D., Zhao, F., Chen, Y., Xue, Y., Bao, K., Chang, Y., Lu, J., Wang, M., Liu, T., Gao, Q., Cui, W., & Xu, Y. (2024). Distinct Characteristic Binding Modes of Benzofuran Core Inhibitors to Diverse Genotypes of Hepatitis C Virus NS5B Polymerase: A Molecular Simulation Study. International Journal of Molecular Sciences, 25(15), 8028. https://doi.org/10.3390/ijms25158028







