Alginate Oligosaccharides Enhance Antioxidant Status and Intestinal Health by Modulating the Gut Microbiota in Weaned Piglets
Abstract
:1. Introduction
2. Results
2.1. Supplementations with 500 mg/kg AOS Elevated Body Weight
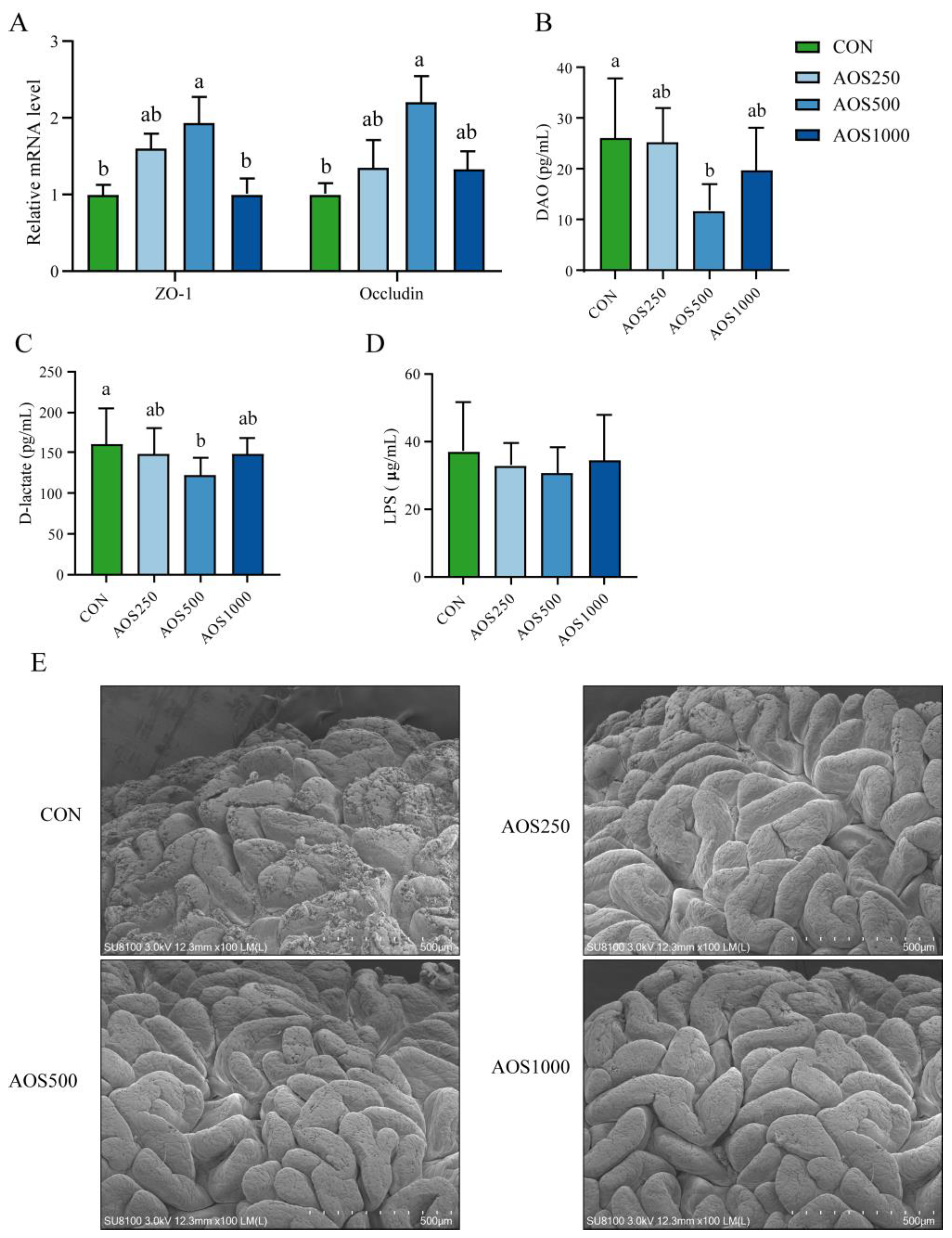
2.2. AOSs Enhanced the Intestinal Barrier Function
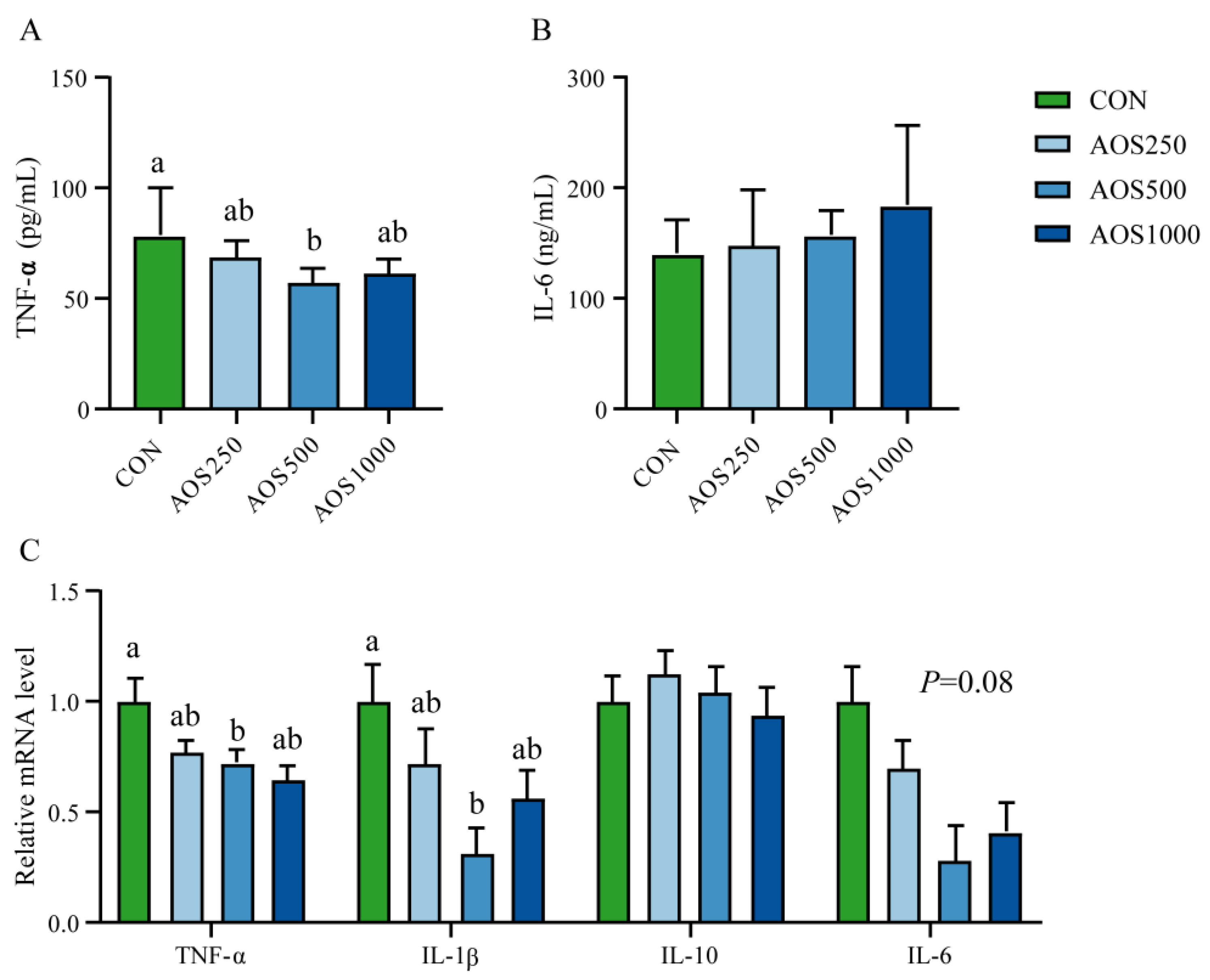
2.3. AOS Regulated Redox Status and Systemic Immunity
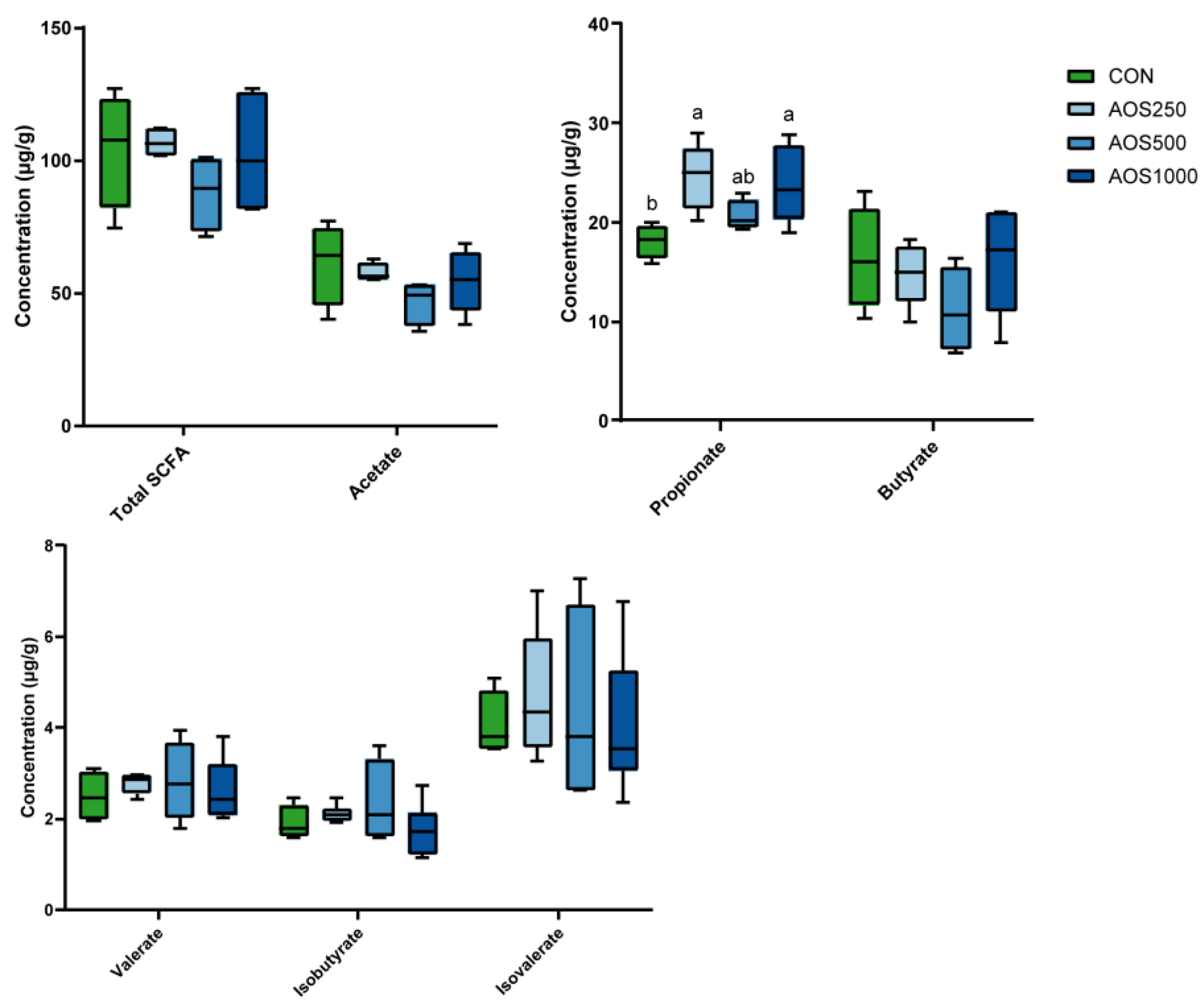
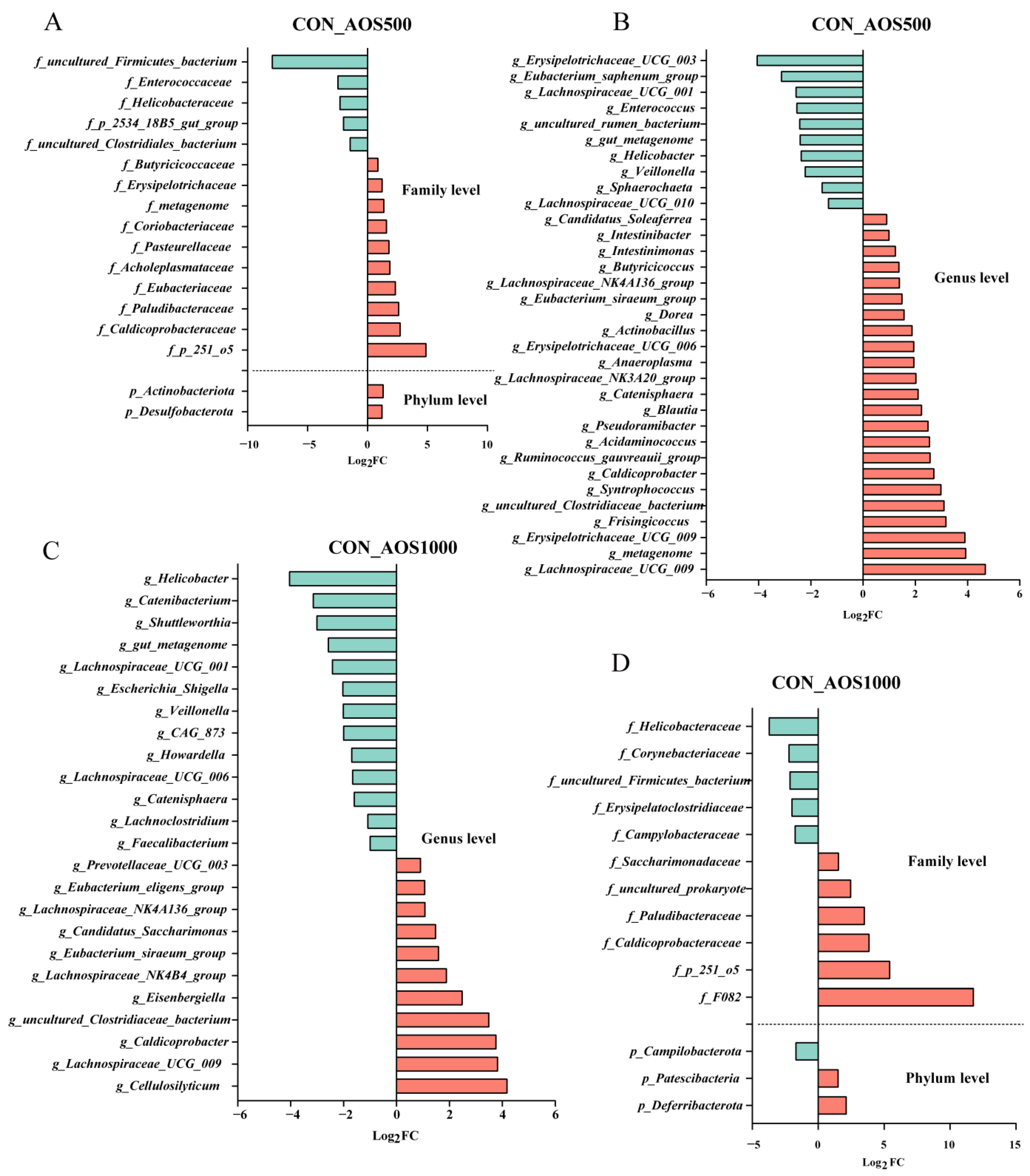
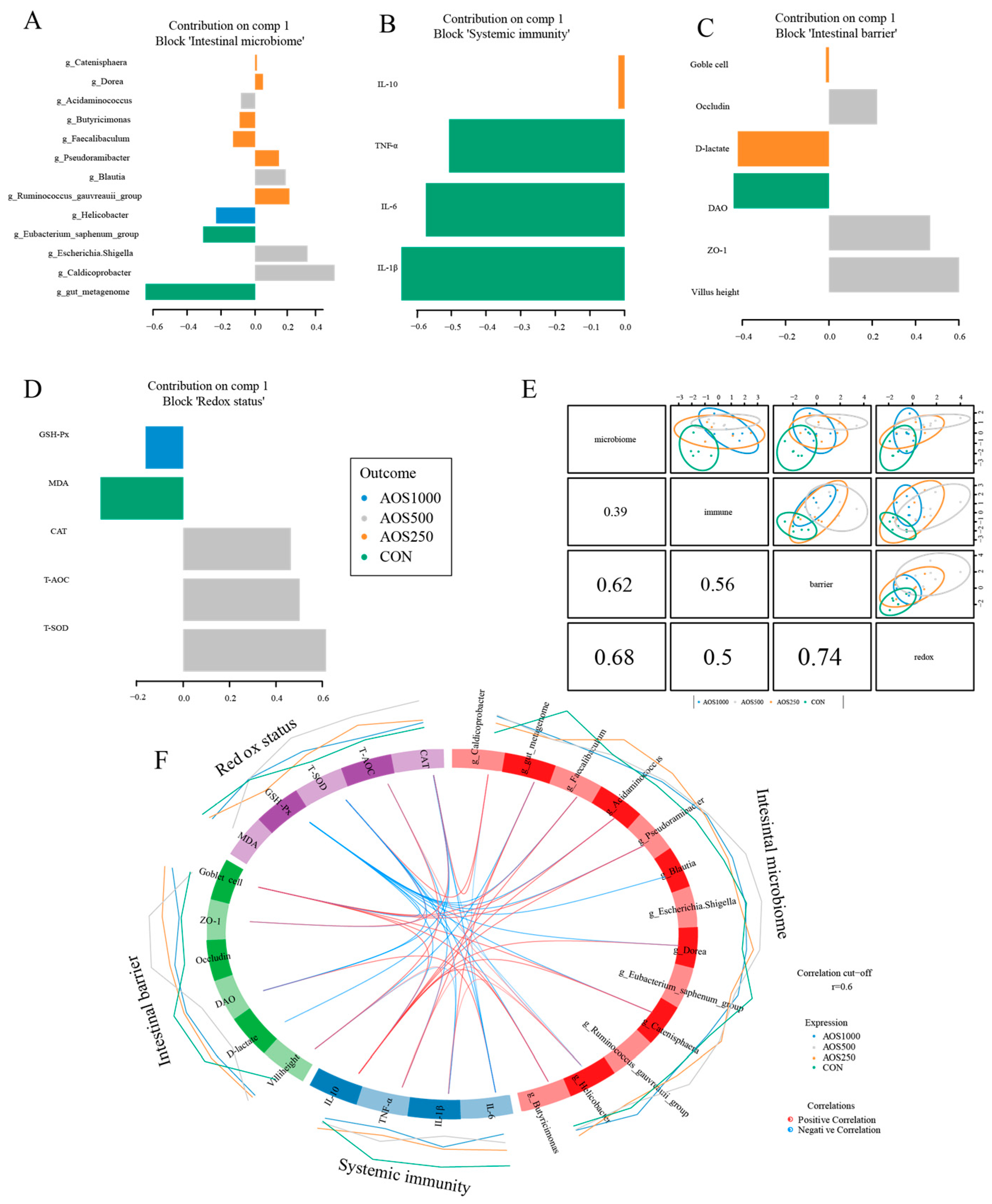
2.4. AOS Feeding Altered Microbial Composition of the Colonic Luminal Contents
2.5. Correlation Analysis between Intestinal Microbiota and Barrier Related Indicators
3. Discussion
4. Materials and Methods
4.1. Study Animals and Experimental Design
4.2. Sample Collection and Processing
4.3. Chemical Analysis
4.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.5. Intestinal Histological Evaluation
4.6. Biochemical and Immunological Parameters in Serum
4.7. 16S rRNA Gene Sequencing and Analysis
4.8. Quantitative Analysis of SCFAs
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Shim, W.Y.; Patel, S.K.S.; Gong, C.; Lee, J.K. Recent developments in antimicrobial growth promoters in chicken health: Opportunities and challenges. Sci. Total Environ. 2022, 834, 155300. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Rama Rao, S.V.; Hegde, N.; Williams, N.J.; Chatterjee, R.N.; Raju, M.; Reddy, G.N.; Kumar, V.; Phani Kumar, P.S.; Mallick, S.; et al. Effects of Dietary Antimicrobial Growth Promoters on Performance Parameters and Abundance and Diversity of Broiler Chicken Gut Microbiome and Selection of Antibiotic Resistance Genes. Front. Microbiol. 2022, 13, 905050. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, Y.; Cao, J. Dynamics impacts of oxytetracycline on growth performance, intestinal health and antibiotic residue of grouper in exposure and withdrawal treatment. Ecotoxicol. Environ. Saf. 2022, 247, 114203. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yang, X.; Lu, J.; Xu, X. Preparation and potential applications of alginate oligosaccharides. Crit. Rev. Food Sci. Nutr. 2022, 63, 10130–10147. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Cheong, K.L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2021, 62, 7703–7717. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Na, K.; Wei, J.; Zhang, L.; Guo, X. Alginate oligosaccharides: The structure-function relationships and the directional preparation for application. Carbohydr. Polym. 2022, 284, 119225. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Zhang, H.-f.; Yi, B.; Everaert, N. Alginate oligosaccharides preparation, biological activities and their application in livestock and poultry. J. Integr. Agric. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, S.; Song, Y.; Yuan, J.; Hu, S.; Chen, M.; Li, L. Alginate Oligosaccharide Alleviated Cisplatin-Induced Kidney Oxidative Stress via Lactobacillus Genus-FAHFAs-Nrf2 Axis in Mice. Front. Immunol. 2022, 13, 857242. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, Y.; Liu, M.; Chen, L.; Meng, Q.; Tang, X.; Wang, S.; Liu, L.; Li, L.; Shen, W.; et al. Single-cell RNA sequencing analysis reveals alginate oligosaccharides preventing chemotherapy-induced mucositis. Mucosal Immunol. 2020, 13, 437–448. [Google Scholar] [CrossRef]
- He, N.; Yang, Y.; Wang, H.; Liu, N.; Yang, Z.; Li, S. Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Lu, S.; Na, K.; Wei, J.; Tao, T.; Zhang, L.; Fang, Y.; Li, X.; Guo, X. Alginate oligosaccharide structures differentially affect DSS-induced colitis in mice by modulating gut microbiota. Carbohydr. Polym. 2023, 312, 120806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiong, B.; Chen, L.; Ge, W.; Yin, S.; Feng, Y.; Sun, Z.; Sun, Q.; Zhao, Y.; Shen, W.; et al. Rescue of male fertility following faecal microbiota transplantation from alginate oligosaccharide-dosed mice. Gut 2021, 70, 2213–2215. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Huang, Z.; Luo, J.; Luo, Y.; et al. Alginate oligosaccharide alleviates enterotoxigenic Escherichia coli-induced intestinal mucosal disruption in weaned pigs. Food Funct. 2018, 9, 6401–6413. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Gao, H.; Sun, F.; Lin, G.; Guo, Y.; Zhao, J. Molecular actions of different functional oligosaccharides on intestinal integrity, immune function and microbial community in weanling pigs. Food Funct. 2022, 13, 12303–12315. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; He, J. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs. Anim. Feed. Sci. Tech. 2017, 234, 118–127. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effect of sialyllactose on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia coli. J. Anim. Sci. Biotechnol. 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, Y.; Zhong, R.; Han, H.; Liu, L.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J. Anim. Sci. 2021, 99, skab183. [Google Scholar] [CrossRef] [PubMed]
- Okrathok, S.; Sirisopapong, M.; Mermillod, P.; Khempaka, S. Modified dietary fiber from cassava pulp affects the cecal microbial population, short-chain fatty acid, and ammonia production in broiler chickens. Poult. Sci. 2023, 102, 102265. [Google Scholar] [CrossRef]
- Frazer, L.C.; Good, M. Intestinal epithelium in early life. Mucosal Immunol. 2022, 15, 1181–1187. [Google Scholar] [CrossRef]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef]
- Xia, B.; Zhong, R.; Meng, Q.; Wu, W.; Chen, L.; Zhao, X.; Zhang, H. Multi-omics unravel the compromised mucosal barrier function linked to aberrant mucin O-glycans in a pig model. Int. J. Biol. Macromol. 2022, 207, 952–964. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, K.; Luan, Z.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Zha, A.; Tu, R.; Qi, M.; Wang, J.; Tan, B.; Liao, P.; Wu, C.; Yin, Y. Mannan oligosaccharides selenium ameliorates intestinal mucosal barrier, and regulate intestinal microbiota to prevent Enterotoxigenic Escherichia coli-induced diarrhea in weaned piglets. Ecotoxicol. Environ. Saf. 2023, 264, 115448. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Zhou, Y.; Li, X.; Xu, S.; Jin, Q.; Li, W. Bacillus amyloliquefaciens SC06 Relieving Intestinal Inflammation by Modulating Intestinal Stem Cells Proliferation and Differentiation via AhR/STAT3 Pathway in LPS-Challenged Piglets. J. Agric. Food Chem. 2024, 72, 6096–6109. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Xu, X.; Liu, Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim. Nutr. Zhongguo Xu Mu Shou Yi Xue Hui 2021, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C.; et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Luo, L.; Jia, W.; Xiao, J.; Huang, G.; Tian, G.; Li, J.; Xiao, Y. Serum diamine oxidase as a hemorrhagic shock biomarker in a rabbit model. PLoS ONE 2014, 9, e102285. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, Z.; Ji, R.; Liu, Y.; Zhao, H.; Liu, L.; Li, F. Chlorogenic acid improves growth performance of weaned rabbits via modulating the intestinal epithelium functions and intestinal microbiota. Front. Microbiol. 2022, 13, 1027101. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Xia, B.; Zhong, R.; Wu, W.; Luo, C.; Meng, Q.; Gao, Q.; Zhao, Y.; Chen, L.; Zhang, S.; Zhao, X.; et al. Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model. Microbiome 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Gao, Y.; Kan, R.; Ren, P.; Xue, C.; Kong, B.; Tang, Q. Alginate Oligosaccharides Prevent Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Modulating Gut Microbiota. Foods 2023, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mansbridge, S.C.; Liang, L.; Connerton, I.F.; Mellits, K.H. Galacto-Oligosaccharides Increase the Abundance of Beneficial Probiotic Bacteria and Improve Gut Architecture and Goblet Cell Expression in Poorly Performing Piglets, but Not Performance. Animals 2023, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zeng, Z.; Chen, G.; Dong, W.; Peng, Y.; Xu, W.; Sun, Y.; Zeng, X.; Liu, Z. Intracellular Polysaccharides of Aspergillus cristatus from Fuzhuan Brick Tea Leverage the Gut Microbiota and Repair the Intestinal Barrier to Ameliorate DSS-Induced Colitis in Mice. J. Agric. Food Chem. 2023, 71, 8023–8037. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, R.; Baumdick, M.E.; Sagebiel, A.F.; Kaufmann, M.; Mokry, M.; Klarenbeek, P.L.; Schaltenberg, N.; Steinert, F.L.; van Rijn, J.M.; Drewniak, A.; et al. Human Fetal TNF-α-Cytokine-Producing CD4(+) Effector Memory T Cells Promote Intestinal Development and Mediate Inflammation Early in Life. Immunity 2019, 50, 462–476.e8. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Liu, X.; Zhao, Y.; Gao, N.; Wu, Q.; Song, K.; Cui, Y.; Li, Y.; McDaniel, J.M.; McGee, S.; et al. Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice. Gastroenterology 2016, 151, 152–164.e11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Zhou, C.; Xu, Y.; Liu, D.; Fujiwara, N.; Kubota, N.; Click, A.; Henderson, P.; Vancil, J.; et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity 2022, 56, 58–77.e11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, S.; Wang, J.; Yin, P.; Liu, H.; Sun, C. Alginate oligosaccharides protect against fumonisin B1-induced intestinal damage via promoting gut microbiota homeostasis. Food Res. Int. 2022, 152, 110927. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Cui, C.; Wu, C.; Wang, J.; Ma, Z.; Zheng, X.; Zhu, P.; Wang, N.; Zhu, Y.; Guan, W.; Chen, F. Restored intestinal integrity, nutrients transporters, energy metabolism, antioxidative capacity and decreased harmful microbiota were associated with IUGR piglet’s catch-up growth before weanling. J. Anim. Sci. Biotechnol. 2022, 13, 129. [Google Scholar] [CrossRef]
- Su, W.; Jiang, Z.; Wang, C.; Zhang, Y.; Gong, T.; Wang, F.; Jin, M.; Wang, Y.; Lu, Z. Co-fermented defatted rice bran alters gut microbiota and improves growth performance, antioxidant capacity, immune status and intestinal permeability of finishing pigs. Anim. Nutr. Zhongguo Xu Mu Shou Yi Xue Hui 2022, 11, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Simões, I.C.M.; Teixeira, J.; Cagide, F.; Potes, Y.; Soares, P.; Carvalho, A.; Tavares, L.C.; Benfeito, S.; Pereira, S.P.; et al. Mitochondria-targeted anti-oxidant AntiOxCIN(4) improved liver steatosis in Western diet-fed mice by preventing lipid accumulation due to upregulation of fatty acid oxidation, quality control mechanism and antioxidant defense systems. Redox Biol. 2022, 55, 102400. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, Q.; Huang, C.; Shi, L.; Jahangir, A.; Pan, T.; Wei, X.; He, J.; Liu, W.; Shi, R.; et al. Ferric citrate-induced colonic mucosal damage associated with oxidative stress, inflammation responses, apoptosis, and the changes of gut microbial composition. Ecotoxicol. Environ. Saf. 2022, 249, 114364. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Duan, Q.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; He, J. Protective effect of sialyllactose on the intestinal epithelium in weaned pigs upon enterotoxigenic Escherichia coli challenge. Food Funct. 2022, 13, 11627–11637. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated astragalus polysaccharides on the immune response, antioxidant capacity, and intestinal microbiota composition of broilers. Poult. Sci. 2022, 101, 101996. [Google Scholar] [CrossRef]
- Wan, T.; Wang, Y.; He, K.; Zhu, S. Microbial sensing in the intestine. Protein Cell 2023, 14, 824–860. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Ralser, A.; Dietl, A.; Jarosch, S.; Engelsberger, V.; Wanisch, A.; Janssen, K.P.; Middelhoff, M.; Vieth, M.; Quante, M.; Haller, D.; et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 2023, 72, 1258–1270. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Associations of Gut Microbiota with Heat Stress-Induced Changes of Growth, Fat Deposition, Intestinal Morphology, and Antioxidant Capacity in Ducks. Front. Microbiol. 2019, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, D.; Ge, C.; Luo, X.; Du, L.; Zhang, X.; Hui, C. Combined effects of microplastics and chlortetracycline on the intestinal barrier, gut microbiota, and antibiotic resistome of Muscovy ducks (Cairina moschata). Sci. Total Environ. 2023, 887, 164050. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, K.; Tan, J.; Mao, Y. Alginate oligosaccharide alleviates aging-related intestinal mucosal barrier dysfunction by blocking FGF1-mediated TLR4/NF-κB p65 pathway. Phytomedicine 2023, 116, 154806. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xia, C.; Liu, N.; Chen, Z.; Zhou, Q.; Li, P. Lactobacillus plantarum ZJ316 alleviates ulcerative colitis by inhibiting inflammation and regulating short-chain fatty acid levels and the gut microbiota in a mouse model. Food Funct. 2023, 14, 3982–3993. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wei, K.; He, J.; Ding, N.; Hua, J.; Zhou, T.; Niu, F.; Zhou, G.; Shi, T.; et al. Review: Effect of gut microbiota and its metabolite SCFAs on radiation-induced intestinal injury. Front. Cell Infect. Microbiol. 2021, 11, 577236. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, Y.; Li, D.; Zhu, W.; Xiao, H.; Xie, M.; Xiong, Y.; Wu, J.; Ni, Q.; Zhang, M.; et al. Metagenome and metabolome insights into the energy compensation and exogenous toxin degradation of gut microbiota in high-altitude rhesus macaques (Macaca mulatta). npj Biofilms Microbiomes 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, X.; Liu, T.; Hu, B.; Yu, B.; Jiang, L.; Wu, Z.; Schroyen, M.; Liu, M. Alginate oligosaccharides improve hepatic metabolic disturbance via regulating the gut microbiota. Food Hydrocoll. 2024, 153, 109980. [Google Scholar] [CrossRef]
- ISO 9831:1998; Animal feeding stuffs, animal products, and faeces or urine –Determination of gross calorific value–Bomb calorimeter method. ISO 9831:1998(E). International Organization for Standardization: Geneva, Switzerland, 1998.
- GB/T6432; Determination of crude protein in feeds-Kjeldahl method (GB/T 6432-2018). State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Xia, B.; Wu, W.; Zhang, L.; Wen, X.; Xie, J.; Zhang, H. Gut microbiota mediates the effects of inulin on enhancing sulfomucin production and mucosal barrier function in a pig model. Food Funct. 2021, 12, 10967–10982. [Google Scholar] [CrossRef]



| Diet | p Values | ||||||
|---|---|---|---|---|---|---|---|
| Item | CON | AOS250 | AOS500 | AOS1000 | Diet | Linear | Quadratic |
| Initial BW, kg | 7.78 ± 0.02 | 7.77 ± 0.02 | 7.80 ± 0.01 | 7.81 ± 0.02 | 0.453 | 0.771 | 0.120 |
| Day 14 BW, kg | 11.58 ± 0.15 | 12.12 ± 0.22 | 12.82 ± 0.48 | 12.04 ± 0.39 | 0.106 | 0.128 | 0.099 |
| Final BW, kg | 19.18 b ± 0.48 | 20.05 ab ± 0.36 | 22.35 a ± 1.06 | 20.29 ab ± 0.77 | 0.036 | 0.183 | 0.028 |
| Day 1–14 ADG, kg/day | 0.27 ± 0.01 | 0.31 ± 0.01 | 0.36 ± 0.03 | 0.30 ± 0.03 | 0.118 | 0.127 | 0.120 |
| Day 15–28 ADG, kg/day | 0.54 b ± 0.02 | 0.57 ab ± 0.03 | 0.68 a ± 0.04 | 0.59 ab ± 0.04 | 0.046 | 0.292 | 0.027 |
| Day 1–28 ADG, kg/day | 0.41 b ± 0.02 | 0.44 ab ± 0.01 | 0.52 a ± 0.04 | 0.45 ab ± 0.03 | 0.039 | 0.164 | 0.032 |
| Day 1–14 G:F | 1.56 ± 0.09 | 1.47 ± 0.05 | 1.53 ± 0.09 | 1.40 ± 0.07 | 0.523 | 0.674 | 0.558 |
| Day 15–28 G:F | 1.39 ± 0.14 | 1.50 ± 0.08 | 1.36 ± 0.04 | 1.49 ± 0.05 | 0.570 | 0.606 | 0.774 |
| Day 1–28 G:F | 1.45 ± 0.10 | 1.48 ± 0.06 | 1.42 ± 0.04 | 1.46 ± 0.04 | 0.906 | 0.796 | 0.665 |
| Day 1–14 ADFI, kg | 0.42 ± 0.03 | 0.45 ± 0.02 | 0.54 ± 0.05 | 0.42 ± 0.04 | 0.096 | 0.202 | 0.261 |
| Day 15–28 ADFI, kg | 0.75 ± 0.07 | 0.85 ± 0.06 | 0.92 ± 0.05 | 0.87 ± 0.04 | 0.224 | 0.175 | 0.114 |
| Day 1–28 ADFI, kg | 0.59 ± 0.05 | 0.65 ± 0.04 | 0.73 ± 0.04 | 0.64 ± 0.03 | 0.114 | 0.123 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Deng, X.; Zhao, Y.; Everaert, N.; Zhang, H.; Xia, B.; Schroyen, M. Alginate Oligosaccharides Enhance Antioxidant Status and Intestinal Health by Modulating the Gut Microbiota in Weaned Piglets. Int. J. Mol. Sci. 2024, 25, 8029. https://doi.org/10.3390/ijms25158029
Liu M, Deng X, Zhao Y, Everaert N, Zhang H, Xia B, Schroyen M. Alginate Oligosaccharides Enhance Antioxidant Status and Intestinal Health by Modulating the Gut Microbiota in Weaned Piglets. International Journal of Molecular Sciences. 2024; 25(15):8029. https://doi.org/10.3390/ijms25158029
Chicago/Turabian StyleLiu, Ming, Xiong Deng, Yong Zhao, Nadia Everaert, Hongfu Zhang, Bing Xia, and Martine Schroyen. 2024. "Alginate Oligosaccharides Enhance Antioxidant Status and Intestinal Health by Modulating the Gut Microbiota in Weaned Piglets" International Journal of Molecular Sciences 25, no. 15: 8029. https://doi.org/10.3390/ijms25158029






