Systematic Review of Naturally Derived Substances That Act as Inhibitors of the Nicotine Metabolizing Enzyme Cytochrome P450 2A6
Abstract
:1. Introduction
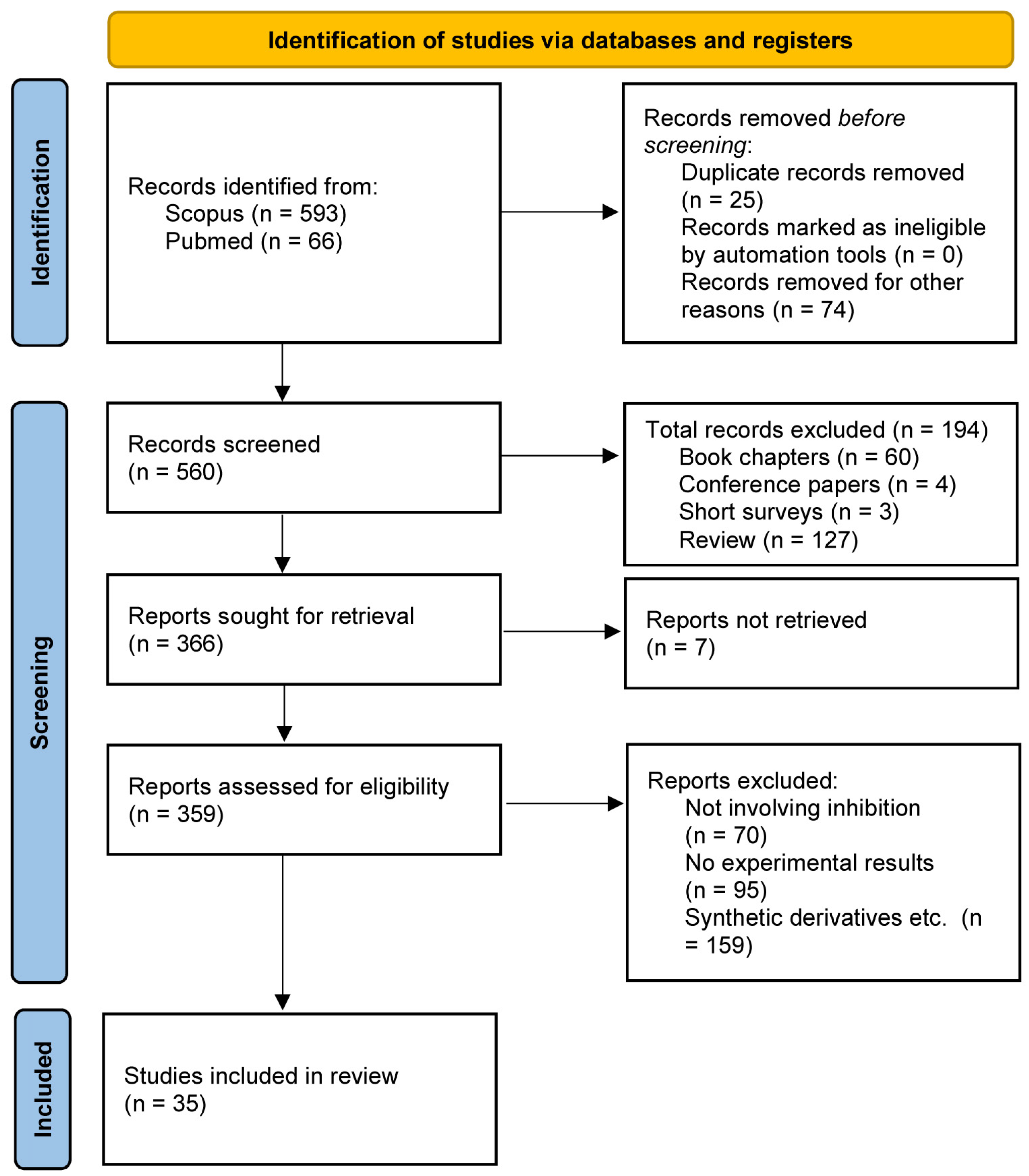
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Study Information Sources and Search Terms
2.4. Study Selection
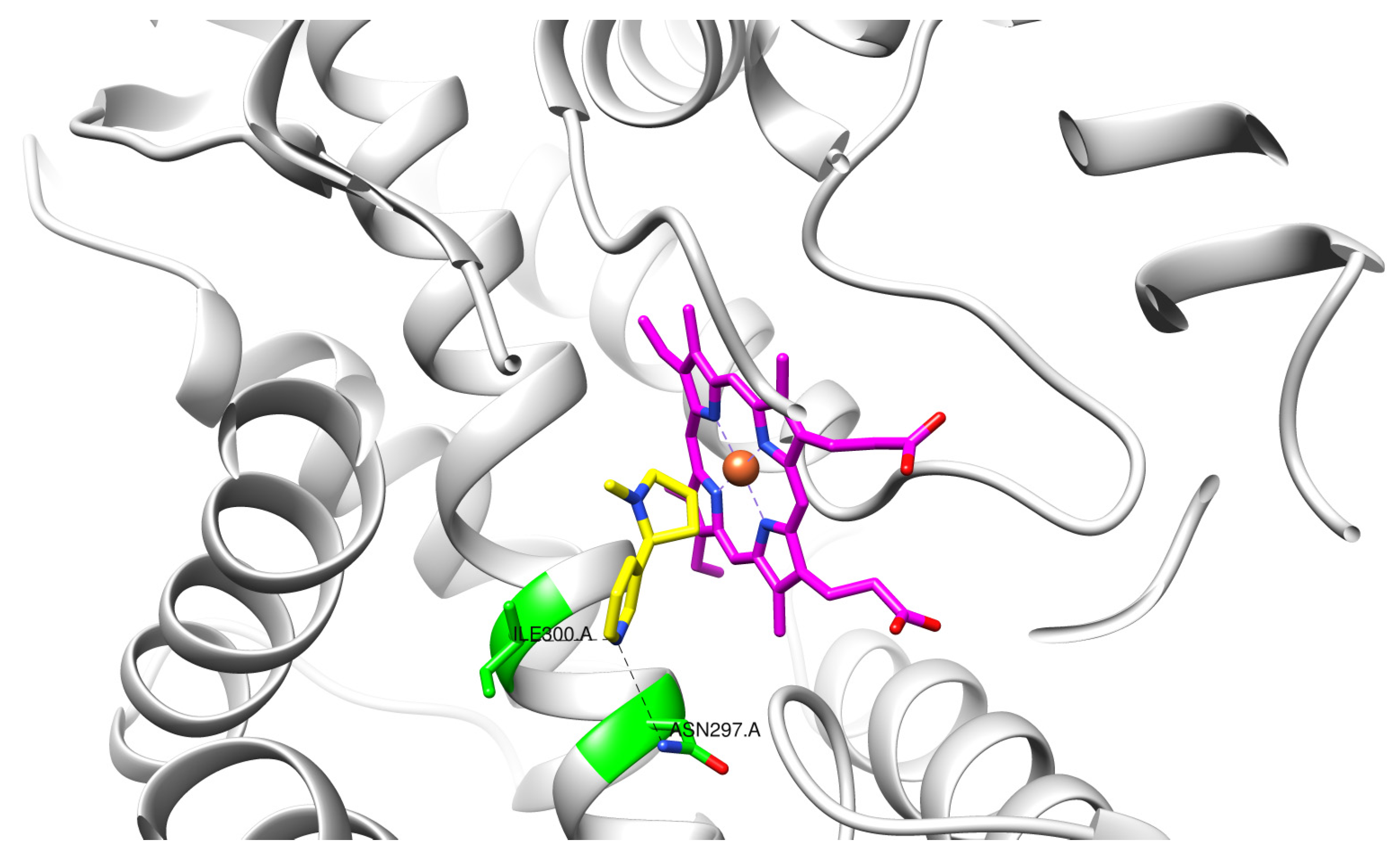
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Smoking. Our World Data 2023. 2023. Available online: https://ourworldindata.org/smoking (accessed on 5 March 2024).
- Statistics|Eurostat n.d. 2022. Available online: https://ec.europa.eu/eurostat/databrowser/product/page/HLTH_EHIS_SK1C (accessed on 4 March 2024).
- Dai, X.; Gil, G.F.; Reitsma, M.B.; Ahmad, N.S.; Anderson, J.A.; Bisignano, C.; Carr, S.; Feldman, R.; Hay, S.I.; He, J.; et al. Health effects associated with smoking: A Burden of Proof study. Nat. Med. 2022, 28, 2045–2055. [Google Scholar] [CrossRef]
- Kopa-Stojak, P.N.; Pawliczak, R. Comparison of the effects of active and passive smoking of tobacco cigarettes, electronic nicotine delivery systems and tobacco heating products on the expression and secretion of oxidative stress and inflammatory response markers. A systematic review. Inhal. Toxicol. 2024, 36, 1–15. [Google Scholar] [CrossRef]
- Wells, A.C.; Lotfipour, S. Prenatal nicotine exposure during pregnancy results in adverse neurodevelopmental alterations and neurobehavioral deficits. Adv. Drug Alcohol. Res. 2023, 3, 11628. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Harun, N.; Glover, M.; Folger, A.T.; Parikh, N.A.; Altaye, M.; Arnsperger, A.; Beiersdorfer, T.; Bridgewater, K.; et al.; Cincinnati Infant Neurodevelopment Early Prediction Study (CINEPS) Investigators Prenatal tobacco smoke exposure and risk for cognitive delays in infants born very premature. Sci. Rep. 2024, 14, 1397. [Google Scholar] [CrossRef]
- Hatsukami, D.K.; Luo, X.; Jensen, J.A.; al’Absi, M.; Allen, S.S.; Carmella, S.G.; Chen, M.; Cinciripini, P.M.; Denlinger-Apte, R.; Drobes, D.J.; et al. Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA 2018, 320, 880. [Google Scholar] [CrossRef]
- Froggatt, S.; Reissland, N.; Covey, J. The effects of prenatal cigarette and e-cigarette exposure on infant neurobehaviour: A comparison to a control group. EClinicalMedicine 2020, 28, 100602. [Google Scholar] [CrossRef]
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2016, 2016, CD008286. [Google Scholar] [CrossRef]
- De Santi, O.; Orellana, M.; Di Niro, C.A.; Greco, V. Evaluation of the effectiveness of cytisine for the treatment of smoking cessation: A systematic review and meta-analysis. Addiction 2024, 119, 649–663. [Google Scholar] [CrossRef]
- Deng, X.; Shang, X.; Guo, K.; Zhou, L.; Wang, Y.; Wu, Y.; Liang, S.; Fen, F.; Liu, W.; Wang, Z.; et al. Efficacy and safety of antidepressants for smoking cessation: A systematic review and network meta-analysis. Addict. Biol. 2023, 28, e13303. [Google Scholar] [CrossRef]
- Kim, S.S.; Prasad, A.; Nayak, M.M.; Chen, H.; Srisoem, C.; DeMarco, R.F.; Castaldi, P.; Cooley, M.E. Predictors of Nicotine Replacement Therapy Adherence: Mixed-Methods Research With a Convergent Parallel Design. Ann. Behav. Med. 2024, 58, kaae006. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018, 2019, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, A.; Chepkin, S.C.; Ye, W.; Fanshawe, T.R.; Bullen, C.; Hartmann-Boyce, J.; Livingstone-Banks, J.; Hajizadeh, A.; Lindson, N. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2023, 2023, CD013308. [Google Scholar] [CrossRef]
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and nicotine use. Nat. Rev. Dis. Primer 2022, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mersha, A.G.; Eftekhari, P.; Bovill, M.; Tollosa, D.N.; Gould, G.S. Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: A protocol for systematic review and meta-analysis. BMJ Open 2020, 10, e039775. [Google Scholar] [CrossRef]
- Mersha, A.G.; Gould, G.S.; Bovill, M.; Eftekhari, P. Barriers and Facilitators of Adherence to Nicotine Replacement Therapy: A Systematic Review and Analysis Using the Capability, Opportunity, Motivation, and Behaviour (COM-B) Model. Int. J. Environ. Res. Public Health 2020, 17, 8895. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Chang, Y.; Rigotti, N.A.; Singer, D.E.; Levy, D.E.; Tyndale, R.F.; Davis, E.M.; Freiberg, M.S.; King, S.; Wells, Q.S.; et al. Can Treatment Support Mitigate Nicotine Metabolism-Based Disparities in Smoking Abstinence? Secondary Analysis of the Helping HAND 4 Trial. Nicotine Tob. Res. 2023, 25, 1575–1584. [Google Scholar] [CrossRef]
- Jones, S.K.; Wolf, B.J.; Froeliger, B.; Wallace, K.; Carpenter, M.J.; Alberg, A.J. Nicotine Metabolism Predicted by CYP2A6 Genotypes in Relation to Smoking Cessation: A Systematic Review. Nicotine Tob. Res. 2022, 24, 633–642. [Google Scholar] [CrossRef]
- Murphy, S.E. Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem. 2021, 296, 100722. [Google Scholar] [CrossRef]
- Benowitz, N.L. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef]
- Gorrod, J.W.; Schepers, G. Biotransformation of nicotine in mammalian systems. In Analytical Determination of Nicotine and Related Compounds and Their Metabolites; Elsevier: Amsterdam, The Netherlands, 1999; pp. 45–67. [Google Scholar] [CrossRef]
- Hosono, H.; Kumondai, M.; Maekawa, M.; Yamaguchi, H.; Mano, N.; Oda, A.; Hirasawa, N.; Hiratsuka, M. Functional Characterization of 34 CYP2A6 Allelic Variants by Assessment of Nicotine C-Oxidation and Coumarin 7-Hydroxylation Activities. Drug Metab. Dispos. 2017, 45, 279–285. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-H.; Han, S.; Lim, Y.-R.; Park, H.-G.; Chun, Y.-J.; Park, S.-W.; Kim, D. Directed-Evolution Analysis of Human Cytochrome P450 2A6 for Enhanced Enzymatic Catalysis. J. Toxicol. Environ. Health A 2014, 77, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bloom, A.J.; Baker, T.B.; Smith, S.S.; Piper, M.E.; Martinez, M.; Saccone, N.; Hatsukami, D.; Goate, A.; Bierut, L. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction 2014, 109, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Langlois, A.W.R.; El-Boraie, A.; Pouget, J.G.; Cox, L.S.; Ahluwalia, J.S.; Fukunaga, K.; Mushiroda, T.; Knight, J.; Chenoweth, M.J.; Tyndale, R.F.; et al. Genotyping, characterization, and imputation of known and novel CYP2A6 structural variants using SNP array data. J. Hum. Genet. 2023, 68, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.-A.; Tyndale, R. Variation in CYP2A6 Activity and Personalized Medicine. J. Pers. Med. 2017, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, T.; St.Helen, G.; Benowitz, N.L.; Tyndale, R.F. Effect of UGT2B10, UGT2B17, FMO3, and OCT2 genetic variation on nicotine and cotinine pharmacokinetics and smoking in African Americans. Pharm. Genom. 2017, 27, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Mwenifumbo, J.C.; Tyndale, R.F. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics 2007, 8, 1385–1402. [Google Scholar] [CrossRef] [PubMed]
- Omura, T. Forty Years of Cytochrome P450. Biochem. Biophys. Res. Commun. 1999, 266, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450; Ortiz De Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 523–785. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- DeVore, N.M.; Scott, E.E. Nicotine and 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone Binding and Access Channel in Human Cytochrome P450 2A6 and 2A13 Enzymes. J. Biol. Chem. 2012, 287, 26576–26585. [Google Scholar] [CrossRef]
- Sellers, E.M.; Tyndale, R.F.; Fernandes, L.C. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov. Today 2003, 8, 487–493. [Google Scholar] [CrossRef]
- Deng, X.; Pu, Q.; Wang, E.; Yu, C. Celery extract inhibits mouse CYP2A5 and human CYP2A6 activities via different mechanisms. Oncol. Lett. 2016, 12, 5309–5314. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishizono, N.; Kobayashi, D.; Yoshimura, T.; Wada, K.; Kobayashi, K.; Oda, K. Synthesis and biological evaluation of coumarin derivatives as selective CYP2A6 inhibitors. Bioorg. Med. Chem. Lett. 2023, 86, 129206. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-M.; Neslund, E.M.; Sun, D.; Tan, X. Methoxsalen Inhibits the Acquisition of Nicotine Self-Administration: Attenuation by Cotinine Replacement in Male Rats. Nicotine Tob. Res. 2024, ntae063. [Google Scholar] [CrossRef]
- Di, Y.; Chow, V.; Yang, L.-P.; Zhou, S.-F. Structure, Function, Regulation and Polymorphism of Human Cytochrome P450 2A6. Curr. Drug Metab. 2009, 10, 754–780. [Google Scholar] [CrossRef]
- Sellers, E. Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clin. Pharmacol. Ther. 2000, 68, 35–43. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Wang, Q.; Kuang, Y.; He, J.; Li, K.; Song, W.; Jin, H.; Qiao, X.; Ye, M. The prenylated phenolic natural product isoglycycoumarin is a highly selective probe for human cytochrome P450 2A6. Eur. J. Pharm. Sci. 2017, 109, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, C.; Zacharia, L.C. Ginkgo biloba L. flavonoids inhibit CYP 2A5; potential dietary supplement for nicotine replacement therapy enhancement. Nat. Prod. Res. 2022, 36, 4210–4214. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Oshiro, T.; Thomas, S.; Higa, A.; Black, S.; Todorovic, A.; Elbarbry, F.; Harrelson, J.P. Inactivation of CYP2A6 by the Dietary Phenylpropanoid trans-Cinnamic Aldehyde (Cinnamaldehyde) and Estimation of Interactions with Nicotine and Letrozole. Drug Metab. Dispos. 2016, 44, 534–543. [Google Scholar] [CrossRef]
- Rahnasto, M.; Raunio, H.; Poso, A.; Wittekindt, C.; Juvonen, R.O. Quantitative Structure−Activity Relationship Analysis of Inhibitors of the Nicotine Metabolizing CYP2A6 Enzyme. J. Med. Chem. 2005, 48, 440–449. [Google Scholar] [CrossRef]
- Roy, K.; Roy, P.P. Exploring QSAR and QAAR for inhibitors of cytochrome P450 2A6 and 2A5 enzymes using GFA and G/PLS techniques. Eur. J. Med. Chem. 2009, 44, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Gharaghani, S.; Khayamian, T.; Keshavarz, F. Docking, molecular dynamics simulation studies, and structure-based QSAR model on cytochrome P450 2A6 inhibitors. Struct. Chem. 2012, 23, 341–350. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- PubMed n.d. Available online: https://pubmed.ncbi.nlm.nih.gov/?db=PubMed (accessed on 24 April 2024).
- Scopus Preview—Scopus—Welcome to Scopus, n.d. Available online: https://www.scopus.com/home.uri (accessed on 24 April 2024).
- Von Weymarn, L.B.; Chun, J.A.; Hollenberg, P.F. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: Potential for chemoprevention in smokers. Carcinogenesis 2006, 27, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Srovnalova, A.; Vanduchova, A.; Svecarova, M.; Anzenbacherova, E.; Tomankova, V.; Anzenbacher, P.; Dvorak, Z. Effects of sulforaphane and its S- and R-enantiomers on the expression and activities of human drug-metabolizing cytochromes P450. J. Funct. Foods 2015, 14, 487–501. [Google Scholar] [CrossRef]
- Kramlinger, V.M.; Von Weymarn, L.B.; Murphy, S.E. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, β-nicotyrine and menthol. Chem. Biol. Interact. 2012, 197, 87–92. [Google Scholar] [CrossRef]
- Stephens, E.S.; Walsh, A.A.; Scott, E.E. Evaluation of Inhibition Selectivity for Human Cytochrome P450 2A Enzymes. Drug Metab. Dispos. 2012, 40, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ban, M.; Hirata, K.; Yamamoto, A.; Hara, Y.; Momose, Y. Involvement of CYP2A6 in the Formation of a Novel Metabolite, 3-Hydroxypilocarpine, from Pilocarpine in Human Liver Microsomes. Drug Metab. Dispos. 2007, 35, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kinonen, T.; Pasanen, M.; Gynther, J.; Poso, A.; Järvinen, T.; Alhava, E.; Juvonen, R.O. Competitive inhibition of coumarin 7-hydroxylation by pilocarpine and its interaction with mouse CYP 2A5 and human CYP2A6. Br. J. Pharmacol. 1995, 116, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; Honkakoski, P.; Hukkanen, J.; Raunio, H. Inhibition and induction of human cytochrome P450 enzymes: Current status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef]
- Bojić, M.; Kondža, M.; Rimac, H.; Benković, G.; Maleš, Ž. The Effect of Flavonoid Aglycones on the CYP1A2, CYP2A6, CYP2C8 and CYP2D6 Enzymes Activity. Molecules 2019, 24, 3174. [Google Scholar] [CrossRef] [PubMed]
- Tiong, K.H.; Yiap, B.C.; Tan, E.L.; Ismail, R.; Ong, C.E. In vitro modulation of naturally occurring flavonoids on cytochrome P450 2A6 (CYP2A6) activity. Xenobiotica 2010, 40, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Boonruang, S.; Prakobsri, K.; Pouyfung, P.; Srisook, E.; Prasopthum, A.; Rongnoparut, P.; Sarapusit, S. Inhibition of human cytochromes P450 2A6 and 2A13 by flavonoids, acetylenic thiophenes and sesquiterpene lactones from Pluchea indica and Vernonia cinerea. J. Enzyme Inhib. Med. Chem. 2017, 32, 1136–1142. [Google Scholar] [CrossRef]
- Draper, A.J.; Madan, A.; Parkinson, A. Inhibition of Coumarin 7-Hydroxylase Activity in Human Liver Microsomes. Arch. Biochem. Biophys. 1997, 341, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Skalicka-Wozniak, K.; Kruk-Slomka, M.; Wydrzynska-Kuzma, M.; Biala, G. In vivo modulation of the behavioral effects of nicotine by the coumarins xanthotoxin, bergapten, and umbelliferone. Psychopharmacology 2016, 233, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Ueng, Y.; Chen, C.; Chung, Y.; Liu, T.; Chang, Y.; Lo, W.; Murayama, N.; Yamazaki, H.; Souček, P.; Chau, G.; et al. Mechanism-based inhibition of cytochrome P450 (CYP)2A6 by chalepensin in recombinant systems, in human liver microsomes and in mice in vivo. Br. J. Pharmacol. 2011, 163, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Pouyfung, P.; Prasopthum, A.; Sarapusit, S.; Srisook, E.; Rongnoparut, P. Mechanism-based Inactivation of Cytochrome P450 2A6 and 2A13 by Rhinacanthus nasutus Constituents. Drug Metab. Pharmacokinet. 2014, 29, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Woo, M.M.; Flockhart, D.A.; Desta, Z. Inhibition of drug metabolizing cytochrome P450s by the aromatase inhibitor drug letrozole and its major oxidative metabolite 4,4′-methanol-bisbenzonitrile in vitro. Cancer Chemother. Pharmacol. 2009, 64, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Dou, T.; Wang, Z.; Wu, J.; Yang, L.; Zeng, S.; Deng, M.; Lü, M.; Liang, S. Inhibition of human cytochrome P450 2A6 by 7-hydroxycoumarin analogues: Analysis of the structure-activity relationship and isoform selectivity. Eur. J. Pharm. Sci. 2019, 136, 104944. [Google Scholar] [CrossRef]
- Fujita, K.; Kamataki, T. Screening of organosulfur compounds as inhibitors of human CYP2A6. Drug Metab. Dispos. Biol. Fate Chem. 2001, 29, 983–989. [Google Scholar]
- Winters, B.R.; Kochar, T.K.; Clapp, P.W.; Jaspers, I.; Madden, M.C. Impact of E-Cigarette Liquid Flavoring Agents on Activity of Microsomal Recombinant CYP2A6, the Primary Nicotine-Metabolizing Enzyme. Chem. Res. Toxicol. 2020, 33, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Coates, S.; Bardhi, K.; Watson, C.; Muscat, J.E.; Lazarus, P. Inhibition of Nicotine Metabolism by Cannabidiol (CBD) and 7-Hydroxycannabidiol (7-OH-CBD). Chem. Res. Toxicol. 2023, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Maeda, C.; Yamamoto, I.; Watanabe, K. Differential inhibition of human cytochrome P450 2A6 and 2B6 by major phytocannabinoids. Forensic Toxicol. 2011, 29, 117–124. [Google Scholar] [CrossRef]
- Ueng, Y.-F.; Hsieh, C.-H.; Don, M.-J. Inhibition of human cytochrome P450 enzymes by the natural hepatotoxin safrole. Food Chem. Toxicol. 2005, 43, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.M.; Alshagga, M.A.; Alshawsh, M.A.; Ong, C.E.; Pan, Y. In vitro effects of 95% khat ethanol extract (KEE) on human recombinant cytochrome P450 (CYP)1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2E1, CYP2J2 and CYP3A5. Drug. Metab. Pers. Ther. 2022, 37, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, W.; Huang, X.; Yao, G.; Jia, Q.; Shen, J.; Ouyang, H.; Chang, Y.; He, J. HPLC-MS/MS Analysis of Aconiti Lateralis Radix Praeparata and Its Combination with Red Ginseng Effect on Rat CYP450 Activities Using the Cocktail Approach. Evid. Based Complement. Alternat Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Showande, J.S.; Igbinoba, S.I.; Kajula, M.; Hokkanen, J.; Tolonen, A.; Adegbolagun, O.M.; Fakeye, T.O. In vitro modulation of cytochrome P450 isozymes and pharmacokinetics of caffeine by extracts of Hibiscus sabdariffa Linn calyx. J. Basic. Clin. Physiol. Pharmacol. 2019, 30, 20180206. [Google Scholar] [CrossRef]
- Rodeiro, I.; José Gómez-Lechón, M.; Perez, G.; Hernandez, I.; Herrera, J.A.; Delgado, R.; Castell, J.V.; Teresa Donato, M. Mangifera indica L. Extract and Mangiferin Modulate Cytochrome P450 and UDP-Glucuronosyltransferase Enzymes in Primary Cultures of Human Hepatocytes. Phytother. Res. 2013, 27, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Begas, E.; Kilindris, T.; Kouvaras, E.; Tsioutsioumi, A.; Kouretas, D.; Asprodini, E.K. Dietary effects of Sideritis scardica “mountain tea” on human in vivo activities of xenobiotic metabolizing enzymes in healthy subjects. Food Chem. Toxicol. 2018, 122, 38–48. [Google Scholar] [CrossRef]
- Wan, X.; Ji, H.; Ma, H.; Yang, Z.; Li, N.; Chen, X.; Chen, Y.; Yang, H.; Wang, Z. Lycopene alleviates aflatoxin B 1 induced liver damage through inhibiting cytochrome 450 isozymes and improving detoxification and antioxidant systems in broiler chickens. Ital. J. Anim. Sci. 2022, 21, 31–40. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, M.; Shi, M.; Chen, J.; Zhang, X.; Yang, J.; Zhang, K.; Han, Y.; Guo, C. Effects of Danhong injection, a traditional Chinese medicine, on nine cytochrome P450 isoforms in vitro. Biomed. Chromatogr. 2019, 33, e4454. [Google Scholar] [CrossRef]
- Showande, S.J.; Fakeye, T.O.; Kajula, M.; Hokkanen, J.; Tolonen, A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs—Implication for herb–drug interactions. Food Sci. Nutr. 2019, 7, 44–55. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Gutmann, H.; Schiller, H.; James, A.-D.; Bouic, P.J.; Rosenkranz, B. The Potential of Sutherlandia frutescens for Herb-Drug Interaction. Drug Metab. Dispos. 2013, 41, 488–497. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Pelkonen, O.; Rautio, A.; Raunio, H.; Pasanen, M. CYP2A6: A human coumarin 7-hydroxylase. Toxicology 2000, 144, 139–147. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Anywar, G.; Muhumuza, E. Bioactivity and toxicity of coumarins from African medicinal plants. Front. Pharmacol. 2024, 14, 1231006. [Google Scholar] [CrossRef]
- Wu, T.-S.; Hsu, M.-Y.; Kuo, P.-C.; Sreenivasulu, B.; Damu, A.G.; Su, C.-R.; Li, C.-Y.; Chang, H.-C.; Chang, H.-C. Constituents from the Leaves of Phellodendron a murense var. w ilsonii and Their Bioactivity. J. Nat. Prod. 2003, 66, 1207–1211. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef]
- Song, X.; Porter, M.E.; Whitaker, V.M.; Lee, S.; Wang, Y. Identification of ethyl vanillin in strawberry (Fragaria × ananassa) using a targeted metabolomics strategy: From artificial to natural. Food Chem. X 2023, 20, 100944. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef]
- Paley, E.; Perry, G.; Sokolova, O. Tryptamine Induces Axonopathy and Mitochondriopathy Mimicking Neurodegenerative Diseases via Tryptophanyl-tRNA Deficiency. Curr. Alzheimer Res. 2013, 10, 987–1004. [Google Scholar] [CrossRef]
- Götz, M.E.; Sachse, B.; Schäfer, B.; Eisenreich, A. Myristicin and Elemicin: Potentially Toxic Alkenylbenzenes in Food. Foods 2022, 11, 1988. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Li, X.; Cournoyer, P.; Choudhuri, S.; Guo, L.; Chen, S. Toxicity of cannabidiol and its metabolites in TM3 mouse Leydig cells: A comparison with primary human Leydig cells. Arch. Toxicol. 2024. [CrossRef]
- Chen, S.; Li, Y.; Li, X.; Wu, Q.; Puig, M.; Moulin, F.; Gingrich, J.; Guo, L. Metabolism and liver toxicity of cannabidiol. J. Environ. Sci. Health Part C 2024, 1–17. [Google Scholar] [CrossRef]
- Neradugomma, N.K.; Drafton, K.; Mor, G.G.; Mao, Q. Marijuana-derived cannabinoids inhibit uterine endometrial stromal cell decidualization and compromise trophoblast-endometrium cross-talk. Reprod. Toxicol. 2019, 87, 100–107. [Google Scholar] [CrossRef]
- Marileo, A.M.; Lara, C.O.; Sazo, A.; Contreras, O.V.; González, G.; Castro, P.A.; Aguayo, L.G.; Moraga-Cid, G.; Fuentealba, J.; Burgos, C.F.; et al. Molecular Pharmacology of Gelsemium Alkaloids on Inhibitory Receptors. Int. J. Mol. Sci. 2024, 25, 3390. [Google Scholar] [CrossRef]
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthotoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2022, 36, 3805–3832. [Google Scholar] [CrossRef]
- Young, A.R.; Walker, S.L.; Kinley, J.S.; Plastow, S.R.; Averbeck, D.; Morlière, P.; Dubertret, L. Phototumorigenesis studies of 5-methoxypsoralen in bergamot oil: Evaluation and modification of risk of human use in an albino mouse skin model. J. Photochem. Photobiol. B 1990, 7, 231–250. [Google Scholar] [CrossRef]
- De Oliveira, R.T.; Da Silva Oliveira, J.P.; Da Silva, A.L.M.; Carrão Dantas, E.K.; Koblitz, M.G.B.; Bello, M.L.; Felzenszwalb, I.; Araújo-Lima, C.F.; Macedo, A.F. Vanilla from Brazilian Atlantic Forest: In vitro and in silico toxicity assessment and high-resolution metabolomic analysis of Vanilla spp. ethanolic extracts. Food Chem. 2024, 456, 139948. [Google Scholar] [CrossRef]
- Hossain, M.A.; Sohel, M.; Sultana, T.; Hasan, M.I.; Khan, M.S.; Kibria, K.M.K.; Mahmud, S.H.; Rahman, H. Study of kaempferol in the treatment of COVID-19 combined with Chikungunya co-infection by network pharmacology and molecular docking technology. Inform. Med. Unlocked 2023, 40, 101289. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.N.; De Sousa, D.P. Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxid. Med. Cell Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Goyal, N.; Do, C.; Sridhar, J.; Shaik, S.; Thompson, A.; Perry, T.; Carter, L.; Foroozesh, M. Design, Synthesis, and Biological Studies of Flavone-Based Esters and Acids as Potential P450 2A6 Inhibitors. Chem. Res. Toxicol. 2023, 36, 1973–1979. [Google Scholar] [CrossRef]
- Higashi, E.; Fukami, T.; Itoh, M.; Kyo, S.; Inoue, M.; Yokoi, T.; Nakajima, M. Human CYP2A6 Is Induced by Estrogen via Estrogen Receptor. Drug Metab. Dispos. 2007, 35, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Higashi, E.; Nakajima, M.; Katoh, M.; Tokudome, S.; Yokoi, T. Inhibitory Effects of Neurotransmitters and Steroids on Human CYP2A6. Drug Metab. Dispos. 2007, 35, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Tiong, K.H.; Yunus, N.A.M.; Yiap, B.C.; Tan, E.L.; Ismail, R.; Ong, C.E. Inhibitory Potency of 8-Methoxypsoralen on Cytochrome P450 2A6 (CYP2A6) Allelic Variants CYP2A6*15, CYP2A6*16, CYP2A6*21 and CYP2A6*22: Differential Susceptibility Due to Different Sequence Locations of the Mutations. PLOS ONE 2014, 9, e86230. [Google Scholar] [CrossRef] [PubMed]
- Prasopthum, A.; Pouyfung, P.; Sarapusit, S.; Srisook, E.; Rongnoparut, P. Inhibition effects of Vernonia cinerea active compounds against cytochrome P450 2A6 and human monoamine oxidases, possible targets for reduction of tobacco dependence. Drug Metab. Pharmacokinet. 2015, 30, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Boonruang, S.; Prakobsri, K.; Pouyfung, P.; Prasopthum, A.; Rongnoparut, P.; Sarapusit, S. Structure–activity relationship and in vitro inhibition of human cytochrome CYP2A6 and CYP2A13 by flavonoids. Xenobiotica 2019, 50, 630–639. [Google Scholar] [CrossRef]
- Koenigs, L.L.; Trager, W.F. Mechanism-Based Inactivation of P450 2A6 by Furanocoumarins. Biochemistry 1998, 37, 10047–10061. [Google Scholar] [CrossRef]
- Heo, J.-K.; Nguyen, P.-H.; Kim, W.; Phuc, N.; Liu, K.-H. Inhibitory Effect of Selaginellins from Selaginella tamariscina (Beauv.) Spring against Cytochrome P450 and Uridine 5′-Diphosphoglucuronosyltransferase Isoforms on Human Liver Microsomes. Molecules 2017, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Chen, Z.; Zhang, H.; Zhao, W. Inhibitory effects of cytochrome P450 enzymes CYP1A2, CYP2A6, CYP2E1 and CYP3A4 by extracts and alkaloids of Gelsemium elegans roots. J. Ethnopharmacol. 2015, 166, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, Y.; Kwak, T.H.; Yoo, H.H. Effects of β-lapachone, a new anticancer candidate, on cytochrome P450-mediated drug metabolism. Cancer Chemother. Pharmacol. 2013, 72, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, L.; Yang, Y.; Sun, W.; Xie, R.; Liu, X.; Wang, Q. In Vitro Inhibition and Induction of Human Liver Cytochrome P450 Enzymes by Gentiopicroside: Potent Effect on CYP2A6. Drug Metab. Pharmacokinet. 2013, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Lee, M.W.; Kim, Y.C.; Yun, C.-H.; Kim, D.-H. Mechanism-Based Inactivation of Cytochrome P450 2A6 by Decursinol Angelate Isolated from Angelica Gigas. Drug Metab. Dispos. 2007, 35, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Fujita, K.-I.; Yamazaki, Y.; Kamataki, T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. Mol. Mech. Mutagen. 2001, 479, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Guo, L.-Q.; Fukuda, K.; Ohta, T.; Yamazoe, Y. Inhibition Selectivity of Grapefruit Juice Components on Human Cytochromes P450. Arch. Biochem. Biophys. 2000, 378, 356–363. [Google Scholar] [CrossRef]
- Lim, S.Y.M.; Loo, J.S.E.; Alshagga, M.; Alshawsh, M.A.; Ong, C.E.; Pan, Y. In vitro and In silico studies of interactions of cathinone with human recombinant cytochrome P450 CYP(1A2), CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2E1, CYP2J2, and CYP3A5. Toxicol. Rep. 2022, 9, 759–768. [Google Scholar] [CrossRef]
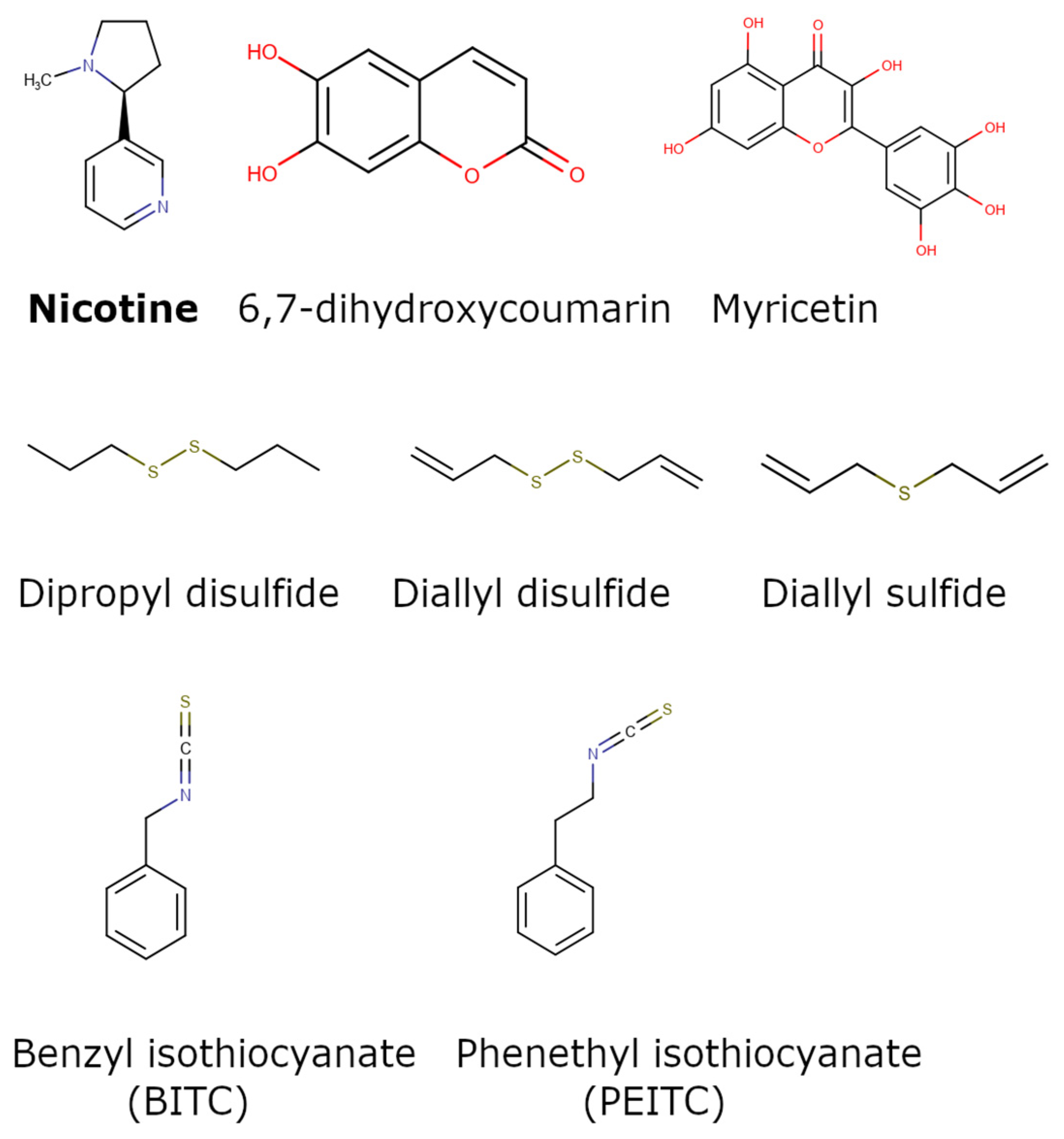
| Group Name | Scaffolds/Representative Structure | No. of Compounds | References |
|---|---|---|---|
| Isothiocyanates |  | 3 | [50,51] |
| Terpenes |  | 2 | [52,53] |
| Alkaloids | Diverse chemical group of organic nitrogen-containing bases | 5 | [52,53,54,55,56] |
| Flavonoids | 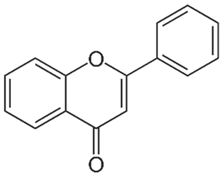 | 32 | [57,58,59] |
| Furanocoumarins/psoralen |  | 4 | [60,61,62] |
| 1,4-naphthoquinones | 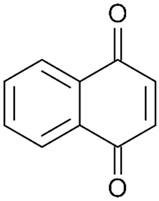 | 5 | [63] |
| Propylamines | 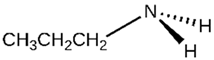 | 1 | [60] |
| Triazoles | 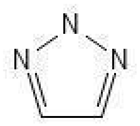 | 1 | [64] |
| Coumarins | 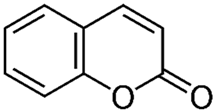 | 20 | [61,65] |
| Thiophenes | 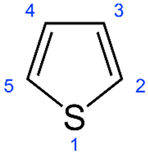 | 3 | [59] |
| Hirsutinolides | 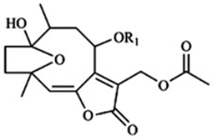 | 4 | [59] |
| Organosulfur compounds | Diverse group that contains structures with at least one –S atom connected to carbon atoms | 23 | [66] |
| Benzaldehydes |  | 2 | [53,67] |
| Cannabidiol derivatives | 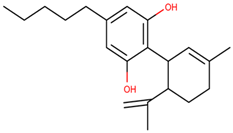 | 4 | [68,69] |
| Benzodioxoles |  | 1 | [70] |
| Reference | Exclusion Criteria | |
|---|---|---|
| 1 | [71] | Study reports only results from Khat ethanol extract. |
| 2 | [72] | Report about Aconiti Lateralis Radix Praeparata extract (in combination with red ginseng). |
| 3 | [73] | Study of CYP2A6 inhibition from the extracts of Hibiscus sabdariffa. No report of chemical structures and related data. |
| 4 | [61] | The specific study contains data only on in vivo modulation with no inhibitory information on the substances xanthotoxin/bergapten and umbelliferone |
| 5 | [74] | Study reporting the comparison between the plant extract from the mango tree and mangiferin (an ingredient derived from the plant) isolated and purified. Weak inhibition results |
| 6 | [75] | Study reporting the effect of a decoction from Sideritis scardica with no other inhibitory data provided. |
| 7 | [76] | Study presents data from mouse experiments but does not report any inhibitory data for CYP2A6 in the form of % inhibition, IC50 or Ki. |
| 8 | [77] | Study reports the CYP2A6 inhibition by Danhong injection (DHI) extract with no information on specific compounds that may act as inhibitors. |
| 9 | [78] | Study reports the inhibition of CYP2A6 from tropical medicinal herbs with no information on active ingredients. |
| 10 | [79] | Study reports the effect of a decoction from Sutherlandia frutescens but provides no information on specific compounds with inhibitory activity towards CYP2A6. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzoupis, H.; Papavasileiou, K.D.; Papatzelos, S.; Mavrogiorgis, A.; Zacharia, L.C.; Melagraki, G.; Afantitis, A. Systematic Review of Naturally Derived Substances That Act as Inhibitors of the Nicotine Metabolizing Enzyme Cytochrome P450 2A6. Int. J. Mol. Sci. 2024, 25, 8031. https://doi.org/10.3390/ijms25158031
Tzoupis H, Papavasileiou KD, Papatzelos S, Mavrogiorgis A, Zacharia LC, Melagraki G, Afantitis A. Systematic Review of Naturally Derived Substances That Act as Inhibitors of the Nicotine Metabolizing Enzyme Cytochrome P450 2A6. International Journal of Molecular Sciences. 2024; 25(15):8031. https://doi.org/10.3390/ijms25158031
Chicago/Turabian StyleTzoupis, Haralampos, Konstantinos D. Papavasileiou, Stavros Papatzelos, Angelos Mavrogiorgis, Lefteris C. Zacharia, Georgia Melagraki, and Antreas Afantitis. 2024. "Systematic Review of Naturally Derived Substances That Act as Inhibitors of the Nicotine Metabolizing Enzyme Cytochrome P450 2A6" International Journal of Molecular Sciences 25, no. 15: 8031. https://doi.org/10.3390/ijms25158031





