The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. CoQ10 Plus N Restored Oxidative Stress-Related DNA Damage
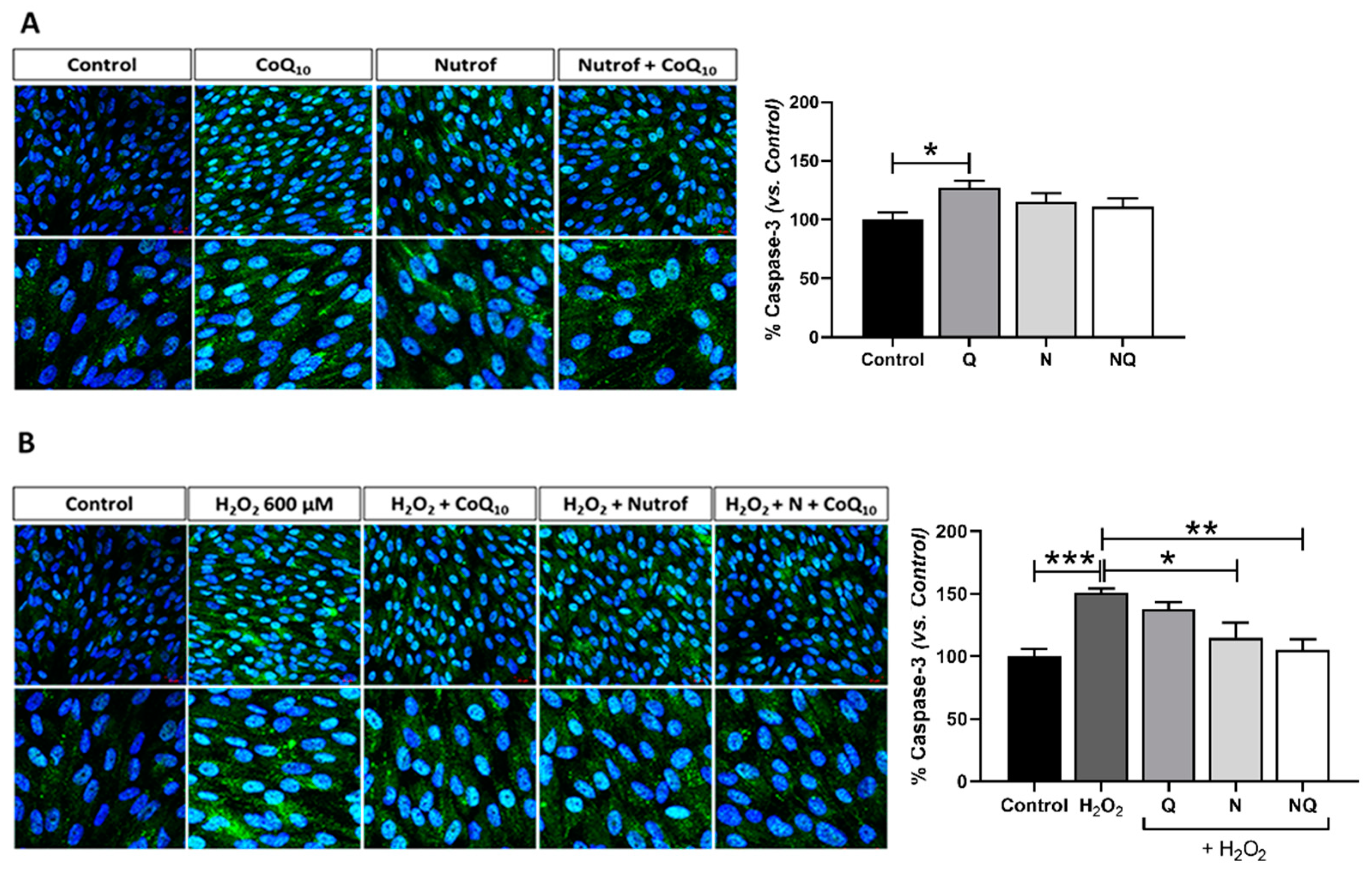
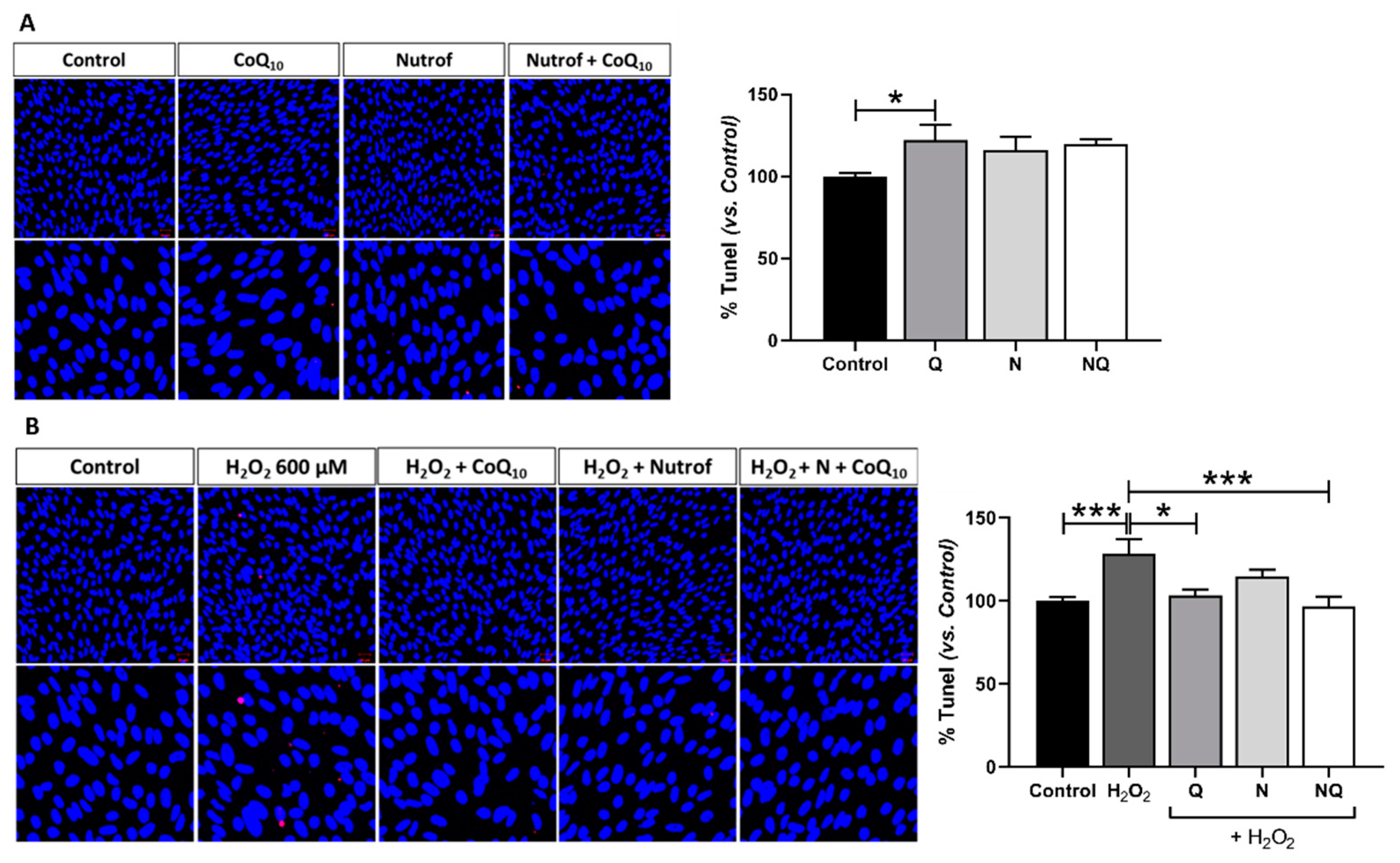
2.2. CoQ10 Plus N Protects from Early and Late Apoptosis Induced by Oxidative Stress
2.3. CoQ10 Reduces Caspase-1 Levels Increased by Oxidative Stress
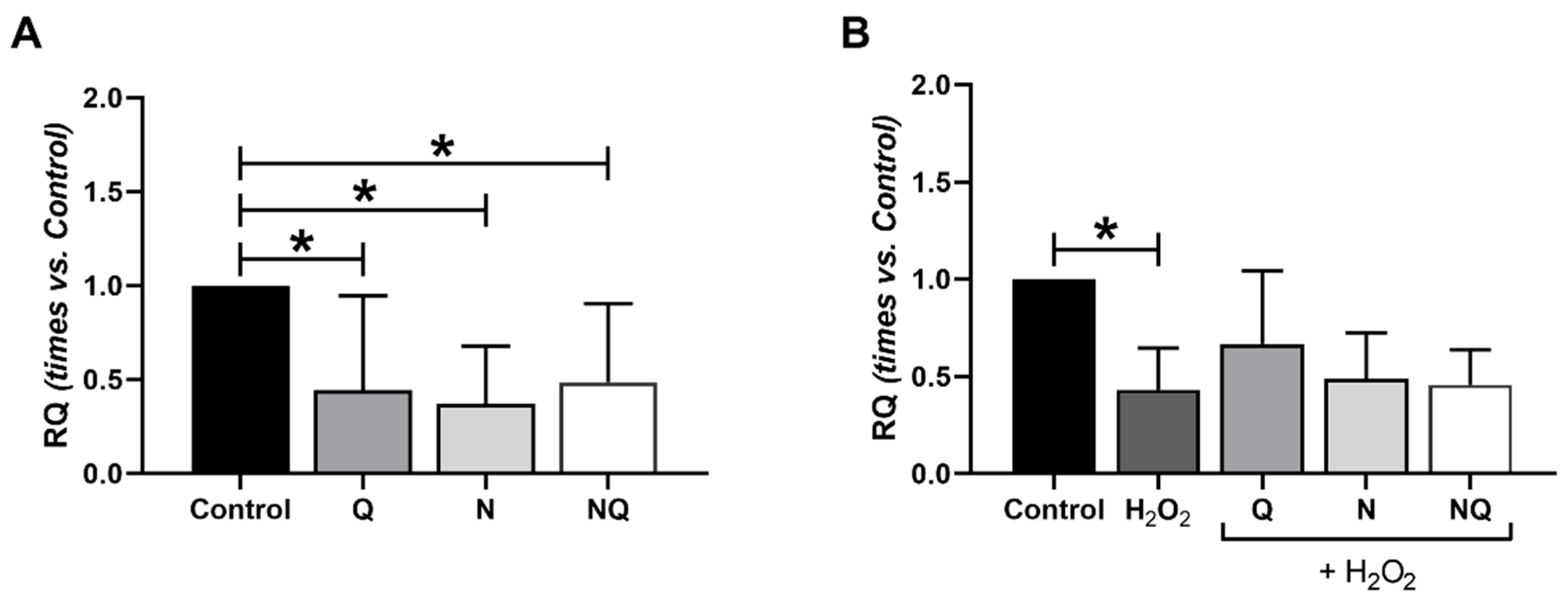
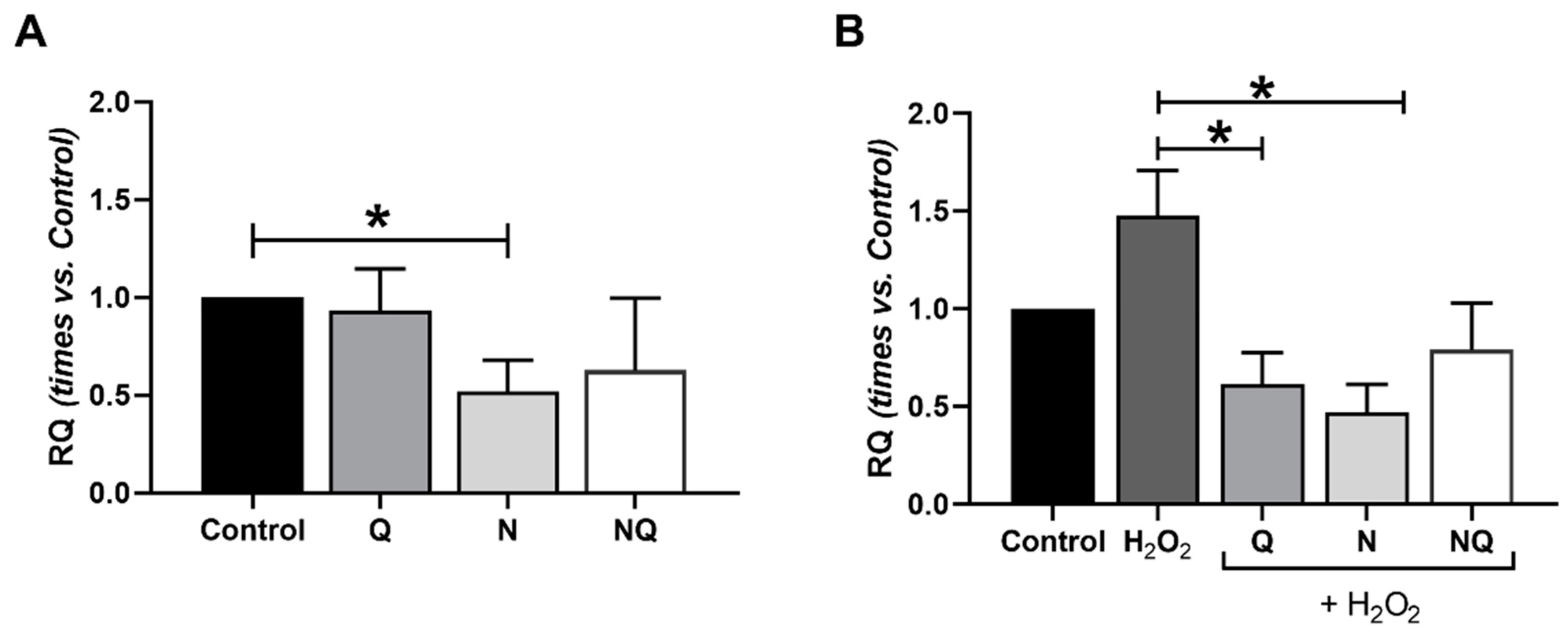
2.4. Interleukin (IL) 1β, Superoxide Dismutase 2 (SOD2) and Catalase (CAT) Gene Expression
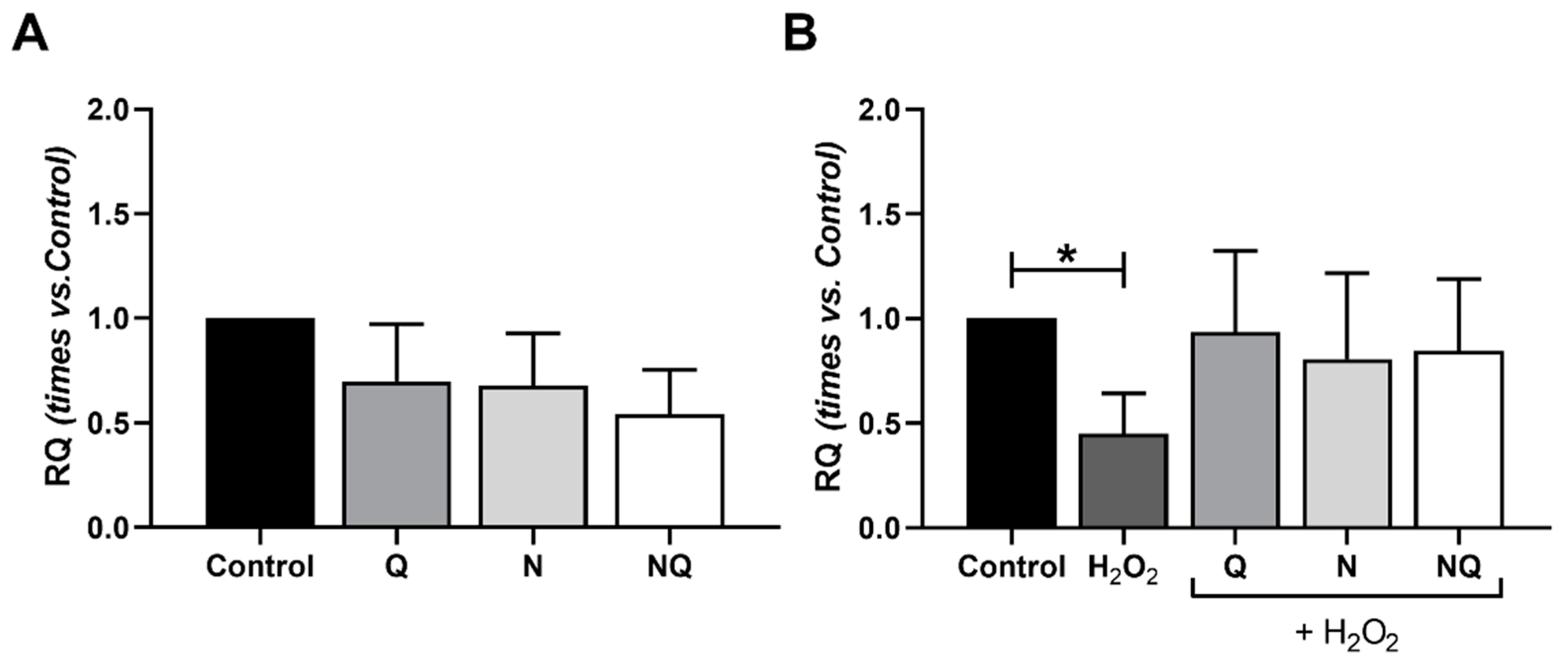
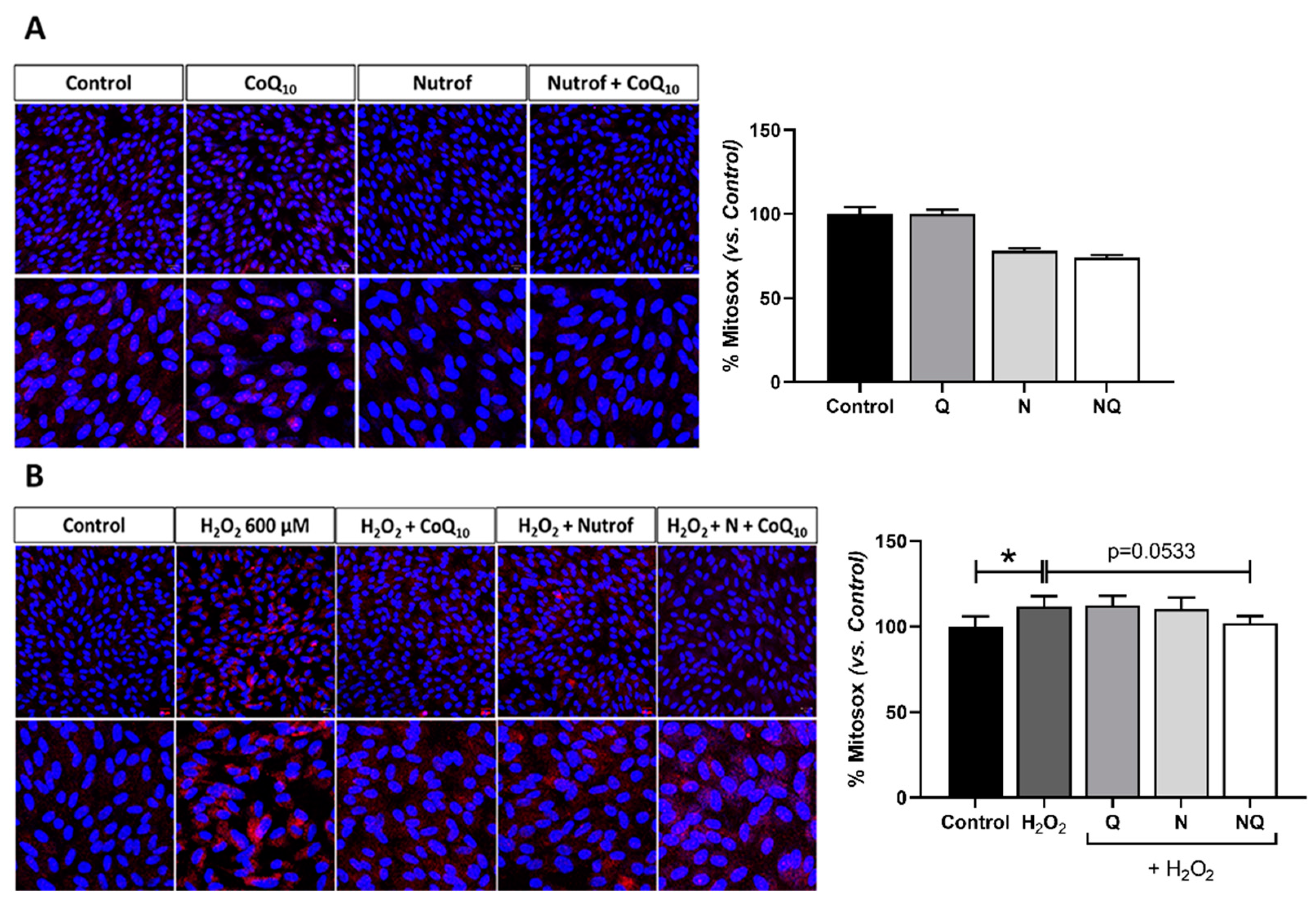
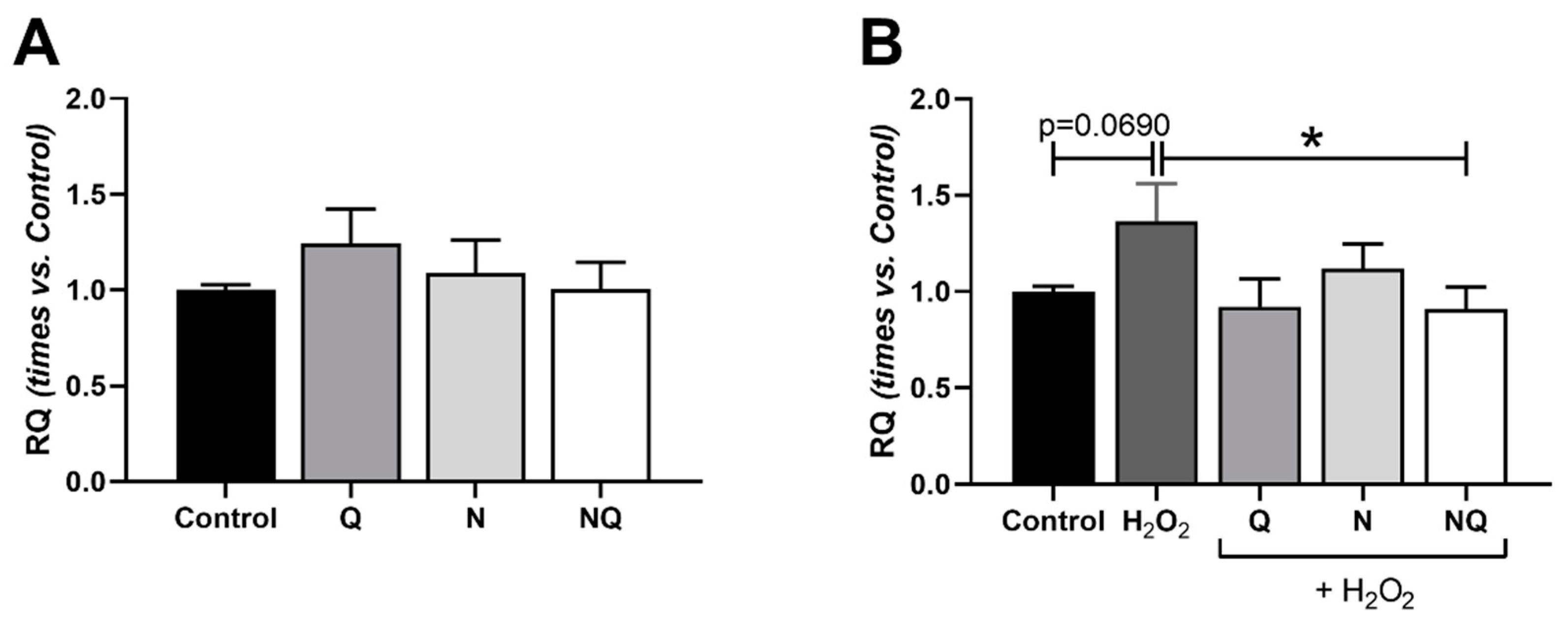
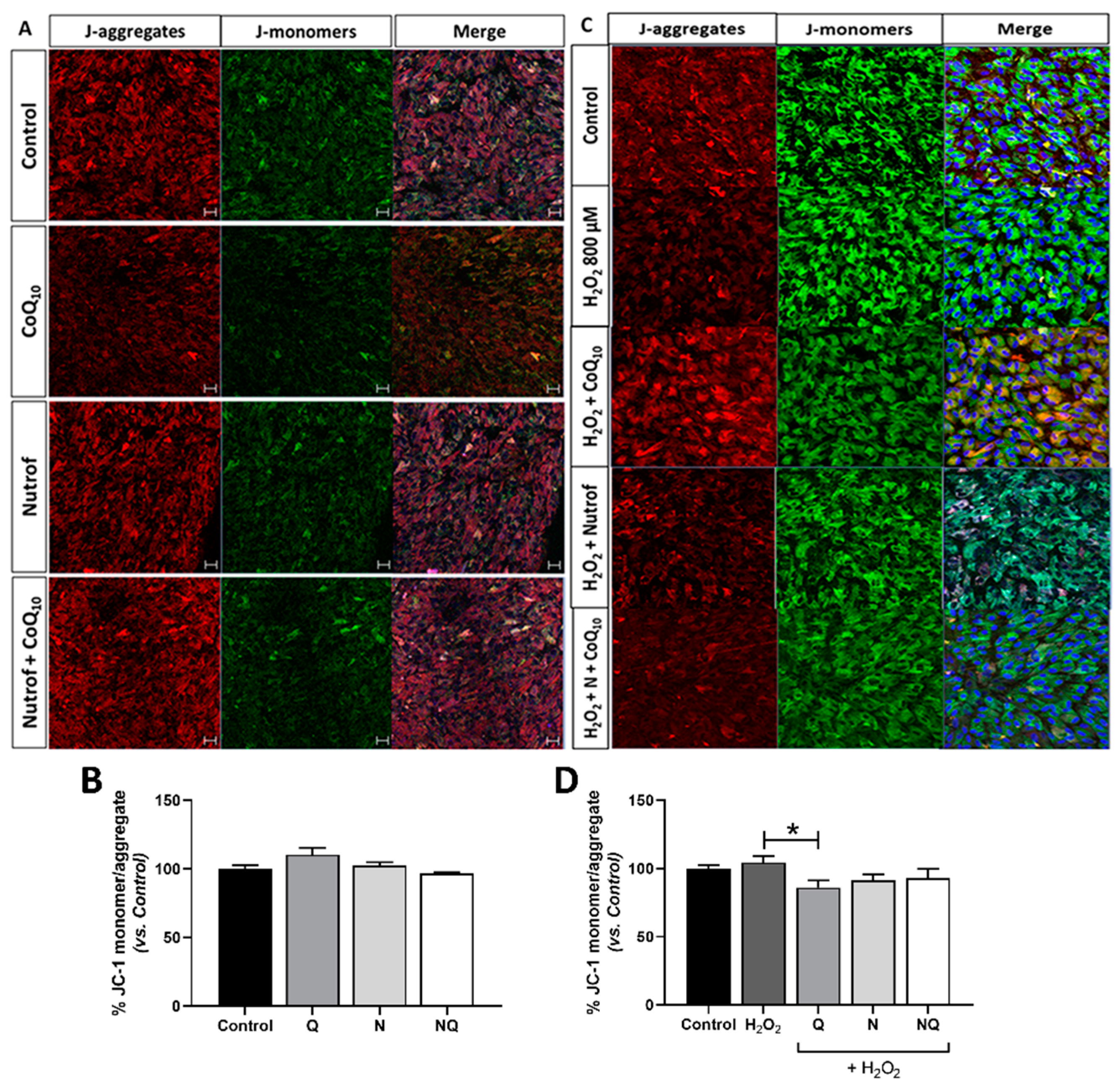
2.5. Mitochondrial Dysfunctionality and Damaged Mitochondrial DNA (mtDNA)
2.5.1. Mitochondrial Superoxide Production
2.5.2. Mitochondrial DNA (mtDNA) Amount
2.5.3. CoQ10 Decreases Mitochondrial Membrane Potential (mtΔψ) under Oxidative Stress Conditions
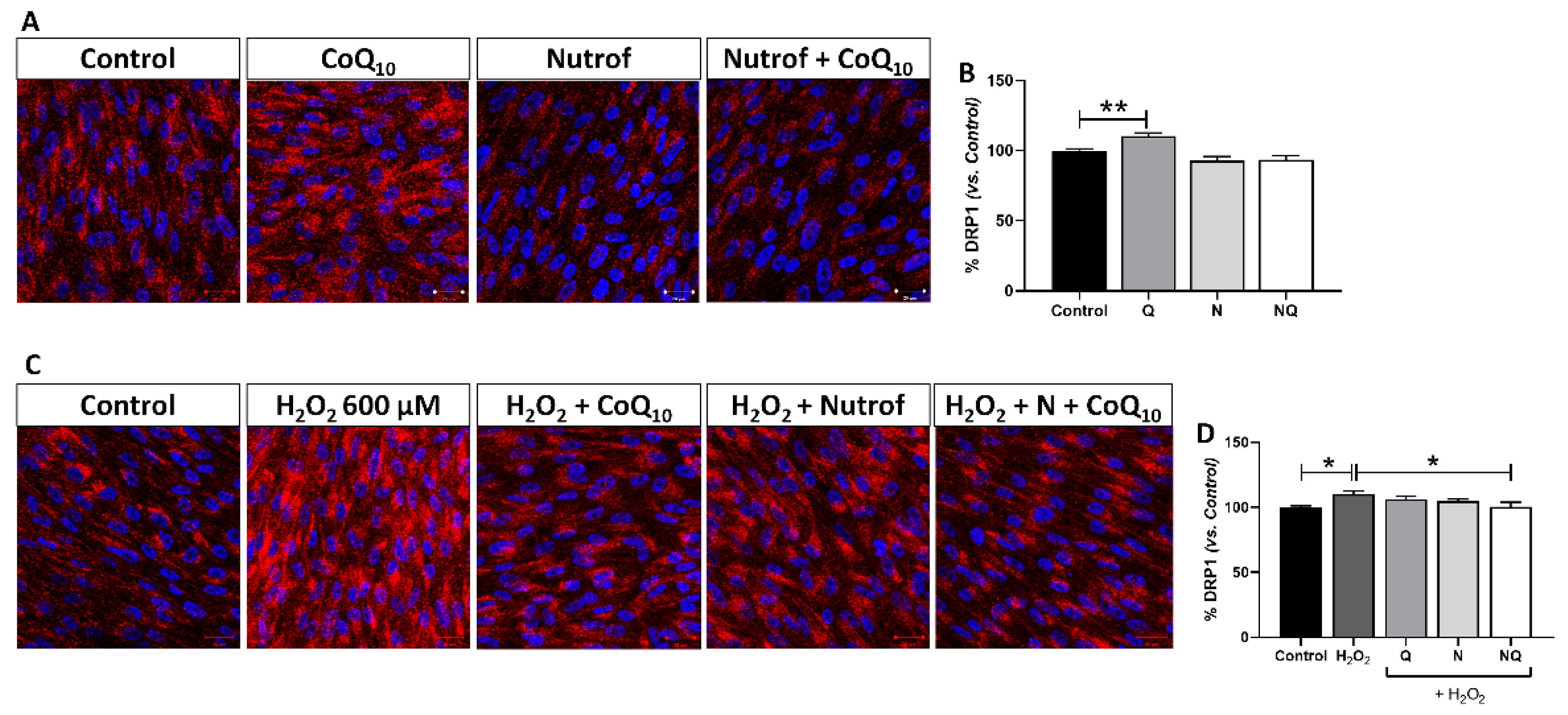
2.5.4. Mitochondrial Dysfunction Determined by DRP-1 Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Phenotypic Characterization by Flow Cytometry (FC) and Immunofluorescence
4.3. Cell Viability Determination after Oxidative Stress Induction and Treatments Application
4.4. Selection of the Oxidative Stress Conditions and Treatment Concentrations
4.5. Cell Apoptosis Evaluation by TUNEL and Caspase-3 Immunofluorescence
4.6. Analysis of Mitochondrial Function
4.6.1. Analysis for Membrane Mitochondrial Potential (mtΔψ)
4.6.2. Detection of Mitochondrial Superoxide Production Using MitoSOX
4.6.3. DRP-1 Immunofluorescence
4.6.4. Mitochondrial DNA Amount (mtDNA)
4.7. Measurement of 8-Hydroxidioxiguanosine (8-OHdG) under Oxidative Stress Conditions
4.8. DNA Multiplex Cytokine Analysis
4.9. RNA Analysis: Expression of IL-1β, SOD2, and CAT
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shankar, K.; Mehendale, H.M. Oxidative Stress. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 735–737. ISBN 9780123864550. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; de Viteri, M.S. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bulotta, R.M.; Oppedisano, F.; Bosco, F.; Scarano, F.; Nucera, S.; Guarnieri, L.; Ruga, S.; Macri, R.; Caminiti, R.; et al. Potential Properties of Natural Nutraceuticals and Antioxidants in Age-Related Eye Disorders. Life 2023, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Querques, G. Association of Focal Choroidal Excavation with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8542. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Maiello, M.; Lorenzi, M. Increased Expression of Basement Membrane Collagen in Human Diabetic Retinopathy. J. Clin. Investig. 1994, 93, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Khin, S.; Lieth, E.; Tarbell, J.M.; Gardner, T.W. Vascular Permeability in Experimental Diabetes Is Associated with Reduced Endothelial Occludin Content: Vascular Endothelial Growth Factor Decreases Occludin in Retinal Endothelial Cells. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, J.; Liu, L.; Sun, X.; Huang, J.; Dong, J. Drp1-Mediated Mitochondrial Fission Contributes to Baicalein-Induced Apoptosis and Autophagy in Lung Cancer via Activation of AMPK Signaling Pathway. Int. J. Biol. Sci. 2020, 16, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.; Youle, R.J.; Pike, R. Mitochondrial Fission and Fusion. Essays Biochem. 2010, 47, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, F.; Bergamini, C.; Lamperti, C.; Fato, R. The Roles of Coenzyme Q in Disease: Direct and Indirect Involvement in Cellular Functions. Int. J. Mol. Sci. 2022, 23, 128. [Google Scholar] [CrossRef]
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170172. [Google Scholar] [CrossRef]
- Yang, M.; Tsui, M.G.; Tsang, J.K.W.; Goit, R.K.; Yao, K.M.; So, K.F.; Lam, W.C.; Lo, A.C.Y. Involvement of FSP1-CoQ10-NADH and GSH-GPx-4 Pathways in Retinal Pigment Epithelium Ferroptosis. Cell Death Dis. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Donovan, T.; Yujiao, L.; Zhang, Q. Mitochondrial Targeted Antioxidant in Cerebral Ischemia. J. Neurol. Neurosci. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Klongpanichapak, S.; Govitrapong, P.; Sharma, S.K.; Ebadi, M. Attenuation of Cocaine and Methamphetamine Neurotoxicity by Coenzyme Q 10. Neurochem. Res. 2006, 31, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tohari, A.M.; Marcheggiani, F.; Zhou, X.; Reilly, J.; Tiano, L.; Shu, X. Therapeutic Potential of Co-Enzyme Q10 in Retinal Diseases. Curr. Med. Chem. 2017, 24, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.A.; Bovina, C.; Carella, G.; Genova, M.L.; Jansen, A.M.A.; Lenaz, G.; Brancato, R. Does Coenzyme Q10 Play a Role in Opposing Oxidative Stress in Patients with Age-Related Macular Degeneration? Ophthalmologica 2001, 215, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Bilen, H.; Keles, S.; Hakan Alp, H.; Keleş, M.S.; Yildirim, K.; Öndas, O.; Can Pinar, L.; Civelekler, M.; Baykal, O. Plasma Coenzyme Q10 Levels in Type 2 Diabetic Patients with Retinopathy. Int. J. Ophthalmol. 2013, 6, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.; Chang, K.T.; Lee, D.S. Peroxiredoxin 5 Prevents Amyloid-Beta Oligomer-Induced Neuronal Cell Death by Inhibiting ERK-Drp1-Mediated Mitochondrial Fragmentation. Free. Radic. Biol. Med. 2016, 90, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Recalde, S.; González-Zamora, J.; Bilbao-Malavé, V.; de Viteri, M.S.; Bezunartea, J.; Moreno-Orduña, M.; Belza, I.; Barrio-Barrio, J.; Fernandez-Robredo, P.; et al. Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin d and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration. Nutrients 2021, 13, 1423. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The Synergistic Effects of Nano-Curcumin and Coenzyme Q10 Supplementation in Migraine Prophylaxis: A Randomized, Placebo-Controlled, Double-Blind Trial. Nutr. Neurosci. 2021, 24, 317–326. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying Mechanisms of Synergistic Antioxidant Interactions during Lipid Oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- García-López, C.; García-López, V.; Matamoros, J.A.; Fernández-Albarral, J.A.; Salobrar-García, E.; de Hoz, R.; López-Cuenca, I.; Sánchez-Puebla, L.; Ramírez, J.M.; Ramírez, A.I.; et al. The Role of Citicoline and Coenzyme Q10 in Retinal Pathology. Int. J. Mol. Sci. 2023, 24, 5072. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, F.; Korb, C.A.; Stoffelns, B.; Pfeiffer, N.; Gericke, A. Recent Advances in Our Understanding of Age-Related Macular Degeneration: Mitochondrial Dysfunction, Redox Signaling, and the Complement System. Aging Dis. 2024, 1–41. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, V.M.; Chan, C.C. The Role of Anti-Inflammatory Agents in Age-Related Macular Degeneration (AMD) Treatment. Eye 2011, 25, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Madsen-Bouterse, S.A.; Mohammad, G.; Kanwar, M.; Kowluru, R.A. Role of Mitochondrial DNA Damage in the Development of Diabetic Retinopathy, and the Metabolic Memory Phenomenon Associated with Its Progression. Antioxid. Redox Signal. 2010, 13, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hazra, T.K.; Das, A.; Das, S.; Choudhury, S.; Kow, Y.W.; Roy, R. Oxidative DNA Damage Repair in Mammalian Cells: A New Perspective. DNA Repair 2007, 6, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sala, R.; Cagliero, E.; Lorenzi, M. Overexpression of Fibronectin Induced by Diabetes or High Glucose: Phenomenon with a Memory. Proc. Natl. Acad. Sci. USA 1990, 87, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Choo, P.P.; Woi, P.J.; Bastion, M.L.C.; Omar, R.; Mustapha, M.; Md Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. BioMed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef] [PubMed]
- Chepchumba Yego, D.J.F.E. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, H. Research Progress on Mitochondrial Dysfunction in Diabetic Retinopathy. Antioxidants 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, M.; Gawlowski, T.; Kocalis, H.; Scott, B.T.; Dillmann, W.H. Mitochondrial 8-Oxoguanine Glycosylase Decreases Mitochondrial Fragmentation and Improves Mitochondrial Function in H9C2 Cells under Oxidative Stress Conditions. Am. J. Physiol.-Cell Physiol. 2014, 306, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.H.; Willis, K.; Nwokwu, C.D.; Maynard, M.; Nestorova, G.G. Photon versus Proton Neurotoxicity: Impact on Mitochondrial Function and 8-OHdG Base-Excision Repair Mechanism in Human Astrocytes. NeuroToxicology 2021, 82, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.F.; Hurst, J.S.; Godley, B.F. Hydrogen Peroxide Stimulates Apoptosis in Cultured Human Retinal Pigment Epithelial Cells. Curr. Eye Res. 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin d Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Totan, Y.; Yaǧci, R.; Bardak, Y.; Özyurt, H.; Kendir, F.; Yilmaz, G.; Şahin, Ş.; Tiǧ, U.Ş. Oxidative Macromolecular Damage in Age-Related Macular Degeneration. Curr. Eye Res. 2009, 34, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxidative Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, J.; Jiang, H.; Kong, J. Klotho Levels Are Decreased and Associated with Enhanced Oxidative Stress and Inflammation in the Aqueous Humor in Patients with Exudative Age-Related Macular Degeneration. Ocul. Immunol. Inflamm. 2022, 30, 630–637. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Cui, Y.; Chen, L.; Song, J.; Sun, L. Urinary 8-Hydroxydeoxyguanosine Levels in Diabetic Retinopathy Patients. Eur. J. Ophthalmol. 2008, 18, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Increased Mitochondrial DNA Damage and Down-Regulation of DNA Repair Enzymes in Aged Rodent Retinal Pigment Epithelium and Choroid. Mol. Vis. 2008, 14, 644–651. [Google Scholar] [PubMed]
- Kunitomo, M.; Yamaguchi, Y.; Kagota, S.; Otsubo, K. Beneficial Effect of Coenzyme Q10 on Increased Oxidative and Nitrative Stress and Inflammation and Individual Metabolic Components Developing in a Rat Model of Metabolic Syndrome. J. Pharmacol. Sci. 2008, 107, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Niklowitz, P.; Sonnenschein, A.; Janetzky, B.; Andler, W.; Menke, T. Enrichment of Coenzyme Q10 in Plasma and Blood Cells: Defense against Oxidative Damage. Int. J. Biol. Sci. 2007, 3, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jemiola-Rzeminska, M.; Kruk, J.; Skowronek, M.; Strzalka, K. Location of ubiquinone homologues in liposome membranes studied by fluorescence anisotropy of diphenyl-hexatriene and trimethylammonium-diphenyl-hexatriene. Chem. Phys. Lipids 1996, 79, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Littarru, G.P.; Stocker, R.; Alleva, R. Coenzyme Q10 enrichment decreases oxidative DNA damage in human lymphocytes. Free Radic. Biol. Med. 1999, 27, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Lulli, M.; Witort, E.; Papucci, L.; Torre, E.; Schiavone, N.; Dal Monte, M.; Capaccioli, S. Coenzyme Q10 Protects Retinal Cells from Apoptosis Induced by Radiation In Vitro and In Vivo. J. Radiat. Res. 2012, 53, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F. Superoxide Dismutases: Ancient Enzymes and New Insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed]
- MatÉs, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, K.Y.; Shim, M.S.; Choi, S.H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W.K. Inhibition of Oxidative Stress by Coenzyme Q10 Increases Mitochondrial Mass and Improves Bioenergetic Function in Optic Nerve Head Astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.Y.; Shim, M.S.; Kim, S.Y.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 Ameliorates Oxidative Stress and Prevents Mitochondrial Alteration in Ischemic Retinal Injury. Apoptosis 2014, 19, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 Inhibits Glutamate Excitotoxicity and Oxidative Stress-Mediated Mitochondrial Alteration in a Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Deppe, L.; Muellerbuehl, A.M.; Tsai, T.; Erb, C.; Dick, H.B.; Joachim, S.C. Protection against Oxidative Stress by Coenzyme Q10 in a Porcine Retinal Degeneration Model. J. Pers. Med. 2024, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Ashtekar, A.; Huk, D.; Magner, A.; La Perle, K.M.D.; Boucai, L.; Kirschner, L.S. Alterations in Sod2-Induced Oxidative Stress Affect Endocrine Cancer Progression. J. Clin. Endocrinol. Metab. 2018, 103, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Gallo, R.; Monache, S.D.; Di Cola, M.; Alesse, E.; Amicarelli, F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: Possible role for meiotic kinases and survival factor BCL2. Biol. Reprod. 2006, 74, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Larriva, A.P.A.D.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Donoso, L.A.; Kim, D.; Frost, A.; Callahan, A.; Hageman, G. The Role of Inflammation in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2006, 51, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G. NLRP3 Inflammasome Blockade Inhibits VEGF-A-Induced Age-Related Macular Degeneration. Cell Rep. 2013, 4, 945–958. [Google Scholar] [CrossRef]
- Cao, R.; Farnebo, J.; Kurimoto, M.; Cao, Y. Interleukin-18 Acts as an Angiogenesis and Tumor Suppressor. FASEB J. 1999, 13, 2195–2202. [Google Scholar] [CrossRef]
- Doyle, S.L.; Campbell, M.; Ozaki, E.; Salomon, R.G.; Mori, A.; Kenna, P.F.; Farrar, G.J.; Kiang, A.S.; Humphries, M.M.; Lavelle, E.C.; et al. NLRP3 Has a Protective Role in Age-Related Macular Degeneration through the Induction of IL-18 by Drusen Components. Nat. Med. 2012, 18, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hartnett, M.E. Regulation of Signaling Events Involved in the Pathophysiology of Neovascular AMD. Mol. Vis. 2016, 22, 189–202. [Google Scholar]
- Agrawal, R.; Balne, P.K.; Wei, X.; Bijin, V.A.; Lee, B.; Ghosh, A.; Narayanan, R.; Agrawal, M.; Connolly, J. Cytokine Profiling in Patients with Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 376–382. [Google Scholar] [CrossRef]
- Fonteh, C.N.; Palestine, A.G.; Wagner, B.D.; Patnaik, J.L.; Mathias, M.T.; Mandava, N.; Baldermann, R.; Lynch, A.M. Sex Differences in RANTES (CCL5) in Patients With Intermediate Age-Related Macular Degeneration (AMD) and Controls with No AMD. Transl. Vis. Sci. Technol. 2022, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 8546423. [Google Scholar] [CrossRef] [PubMed]
- Garber, D.; Zhu, S. Implications of Caspase 1/ Interleukin-1 Beta (IL-1β) Signaling and Hypoxia-Inducible Factor 1-Alpha (HIF-1α) on Diabetic Retinopathy Pathology. Cureus 2023, 15, e42479. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Pan, Y.; Jin, M.; Li, J.; Luo, Y.; Sun, X.; Li, G. Diabetic Retinopathy: Involved Cells, Biomarkers, and Treatments. Front. Pharmacol. 2022, 13, 953691. [Google Scholar] [CrossRef]
- Dabbaghi Varnousfaderani, S.; Musazadeh, V.; Ghalichi, F.; Kavyani, Z.; Razmjouei, S.; Faghfouri, A.H.; Ahrabi, S.S.; Seyyed Shoura, S.M.; Dehghan, P. Alleviating Effects of Coenzyme Q10 Supplements on Biomarkers of Inflammation and Oxidative Stress: Results from an Umbrella Meta-Analysis. Front. Pharmacol. 2023, 14, 1191290. [Google Scholar] [CrossRef]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of Coenzyme Q10 in Inflammation and Gene Expression. BioFactors 2008, 32, 179–183. [Google Scholar] [CrossRef]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of Coenzyme Q10 Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef]
- Bessler, H.; Bergman, M.; Blumberger, N.; Djaldetti, M.; Salman, H. Coenzyme Q10 Decreases TNF-α and IL-2 Secretion by Human Peripheral Blood Mononuclear Cells. J. Nutr. Sci. Vitaminol. 2010, 56, 77–81. [Google Scholar] [CrossRef]
- Schrier, S.A.; Falk, M.J. Mitochondrial Disorders and the Eye. Curr. Opin. Ophthalmol. 2011, 22, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef]
- Liang, F.Q.; Godley, B.F. Oxidative Stress-Induced Mitochondrial DNA Damage in Human Retinal Pigment Epithelial Cells: A Possible Mechanism for RPE Aging and Age-Related Macular Degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef]
- Nakazawa, H.; Ikeda, K.; Shinozaki, S.; Yasuhara, S.; Yu, Y.M.; Martyn, J.A.J.; Tompkins, R.G.; Yorozu, T.; Inoue, S.; Kaneki, M. Coenzyme Q10 Protects against Burn-Induced Mitochondrial Dysfunction and Impaired Insulin Signaling in Mouse Skeletal Muscle. FEBS Open Bio 2019, 9, 348–363. [Google Scholar] [CrossRef]
- Arend, N.; Wertheimer, C.; Laubichler, P.; Wolf, A.; Kampik, A.; Kernt, M. Idebenone Prevents Oxidative Stress, Cell Death and Senescence of Retinal Pigment Epithelium Cells by Stabilizing BAX/Bcl-2 Ratio. Ophthalmologica 2015, 234, 73–82. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yu, J.H.; Quan, Y.; Shin, Y.J.; Lee, K.E.; Kim, H.L.; Ko, E.J.; Chung, B.H.; Lim, S.W.; Yang, C.W. Therapeutic Potential of Coenzyme Q10 in Mitochondrial Dysfunction during Tacrolimus-Induced Beta Cell Injury. Sci. Rep. 2019, 9, 7995. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J.A. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free. Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-avila, M.; Vega, A.F.; Mata, M.D.; Pavon, A.D.; Alcocer-gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical Applications of Coenzyme Q10. Front. Biosci. 2014, 19, 619–633. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef] [PubMed]
- Brancato, R.; Schiavone, N.; Siano, S.; Lapucci, A.; Papucci, L.; Donnini, M.; Formigli, L.; Zecchi Orlandini, S.; Carella, G.; Carones, F.; et al. Prevention of Corneal Keratocyte Apoptosis after Argon Fluoride Excimer Laser Irradiation with the Free Radical Scavenger Ubiquinone Q10. Eur. J. Ophthalmol. 2000, 10, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Brancato, R.; Fiore, T.; Papucci, L.; Schiavone, N.; Formigli, L.; Orlandini, S.Z.; Gobbi, P.G.; Carones, F.; Donnini, M.; Lapucci, A.; et al. Concomitant Effect of Topical Ubiquinone Q10 and Vitamin E to Prevent Keratocyte Apoptosis after Excimer Laser Photoablation in Rabbits. J. Refract. Surg. 2002, 18, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yi, X.; Sá, R.D.C.D.S.e.; Zhang, Y.; Liu, K.; Xiao, F.; Zhong, C. CoQ10 Deficiency May Indicate Mitochondrial Dysfunction in Cr(VI) Toxicity. Int. J. Mol. Sci. 2017, 18, 816. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive Oxygen Speciesdependent Mitochondrial Dynamics and Autophagy Confer Protective Effects in Retinal Pigment Epithelial Cells against Sodium Iodate-Induced Cell Death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Zhang, D.; Wen, Y.; Zhang, L.; Xia, Y.; Chen, J.; Xie, C.; Zhu, H.; Tong, J.; et al. Long-Term Blue Light Exposure Impairs Mitochondrial Dynamics in the Retina in Light-Induced Retinal Degeneration In Vivo and In Vitro. J. Photochem. Photobiol. B Biol. 2023, 240, 112654. [Google Scholar] [CrossRef]
- Kim, D.; Sesaki, H.; Roy, S. Reduced Levels of Drp1 Protect against Development of Retinal Vascular Lesions in Diabetic Retinopathy. Cells 2021, 10, 1379. [Google Scholar] [CrossRef]
- Yasuda, H.; Nakamura, S.; Shimazawa, M. Mitochondrial Fission in Retinal Pigment Epithelium Contributes to Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3902. [Google Scholar]
- Li, H.N.; Zimmerman, M.; Milledge, G.Z.; Hou, X.L.; Cheng, J.; Wang, Z.H.; Li, P.A. Water-Soluble Coenzyme Q10 Reduces Rotenone-Induced Mitochondrial Fission. Neurochem. Res. 2017, 42, 1096–1103. [Google Scholar] [CrossRef]
- Oettinghaus, B.; D’Alonzo, D.; Barbieri, E.; Restelli, L.M.; Savoia, C.; Licci, M.; Tolnay, M.; Frank, S.; Scorrano, L. DRP1-Dependent Apoptotic Mitochondrial Fission Occurs Independently of BAX, BAK and APAF1 to Amplify Cell Death by BID and Oxidative Stress. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1267–1276. [Google Scholar] [CrossRef]
- Hoppe, U.; Bergemann, J.; Diembeck, W.; Ennen, J.; Gohla, S.; Harris, I.; Jacob, J.; Kielholz, J.; Mei, W.; Pollet, D.; et al. Coenzyme Q10, a Cutaneous Antioxidant and Energizer. BioFactors 1999, 9, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Papale, A.; Mannino, G.; Gualdi, L.; Balacco Gabrieli, C. Mitotropic Compounds for the Treatment of Age-Related Macular Degeneration: The Metabolic Approach and a Pilot Study. Ophthalmologica 2003, 217, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Kovacs, B.; Kovacs, I.; Schvöller, M.; Papale, A.; Balacco Gabrieli, C. Improvement of Visual Functions and Fundus Alterations in Early Age-Related Macular Degeneration Treated with a Combination of Acetyl-L-Carnitine, n-3 Fatty Acids, and Coenzyme Q10. Ophthalmologica 2005, 219, 154–166. [Google Scholar] [CrossRef] [PubMed]


| Processes | Markers | H2O2-RPE Cells | Antioxidant Treatment + H2O2 | ||
|---|---|---|---|---|---|
| CoQ10 | Nutrof | N + CoQ10 | |||
| Oxidative and DNA stress | 8-OHdG | ↑ * | - | - | p = 0.055 |
| SOD2 | ↓ *2 h | - | - | - | |
| CAT | ↓ *1 h | - | - | - | |
| Apoptosis | Caspase-3 | ↑ *** | - | * | ** |
| TUNEL | ↑ *** | * | - | *** | |
| Inflammation | TNF-α | Unchanged supernatants | * | - | - |
| ↓ Lysate | * | - | - | ||
| Caspase-1 | Unchanged supernatants | - | - | - | |
| ↑ Lysate | * | - | - | ||
| ILβ1 | ↓ **2 h | * | * | - | |
| RANTES | ↑ Lysate | - | - | - | |
| IL6, IL17A, IL18 | - | - | - | - | |
| IL12p70 | ↓ Lysate | p = 0.0634 | - | - | |
| Mitochondrial dysfunction | MitoSOX | ↑ * | - | - | p = 0.053 |
| mtDNA | ↑ p = 0.069 | - | - | * | |
| JC-1 | ↑ | * | - | - | |
| DRP-1 | ↑ * | - | - | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, M.; Recalde, S.; Bezunartea, J.; Moreno-Orduña, M.; Belza, I.; Chas-Prat, A.; Perugini, E.; Garcia-Layana, A.; Fernández-Robredo, P. The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 8070. https://doi.org/10.3390/ijms25158070
Hernandez M, Recalde S, Bezunartea J, Moreno-Orduña M, Belza I, Chas-Prat A, Perugini E, Garcia-Layana A, Fernández-Robredo P. The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells. International Journal of Molecular Sciences. 2024; 25(15):8070. https://doi.org/10.3390/ijms25158070
Chicago/Turabian StyleHernandez, Maria, Sergio Recalde, Jaione Bezunartea, Maite Moreno-Orduña, Idoia Belza, Ainara Chas-Prat, Elena Perugini, Alfredo Garcia-Layana, and Patricia Fernández-Robredo. 2024. "The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells" International Journal of Molecular Sciences 25, no. 15: 8070. https://doi.org/10.3390/ijms25158070
APA StyleHernandez, M., Recalde, S., Bezunartea, J., Moreno-Orduña, M., Belza, I., Chas-Prat, A., Perugini, E., Garcia-Layana, A., & Fernández-Robredo, P. (2024). The Scavenging Activity of Coenzyme Q10 Plus a Nutritional Complex on Human Retinal Pigment Epithelial Cells. International Journal of Molecular Sciences, 25(15), 8070. https://doi.org/10.3390/ijms25158070





