Specific Biomarkers in Spinocerebellar Ataxia Type 3: A Systematic Review of Their Potential Uses in Disease Staging and Treatment Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Data Extraction and Management
2.3. Statistical Analysis of Neurofilament Light Chain (NfL) Levels
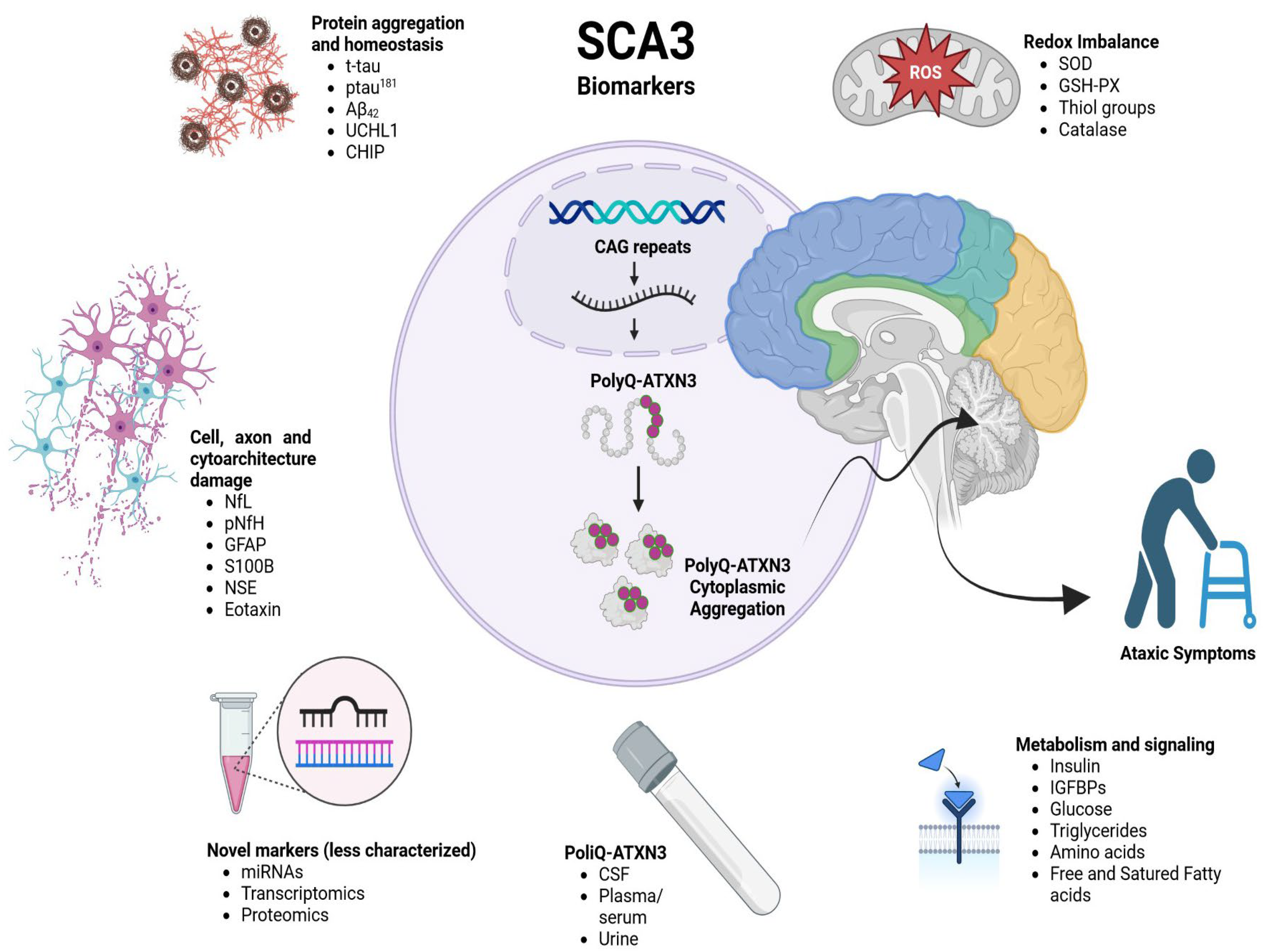
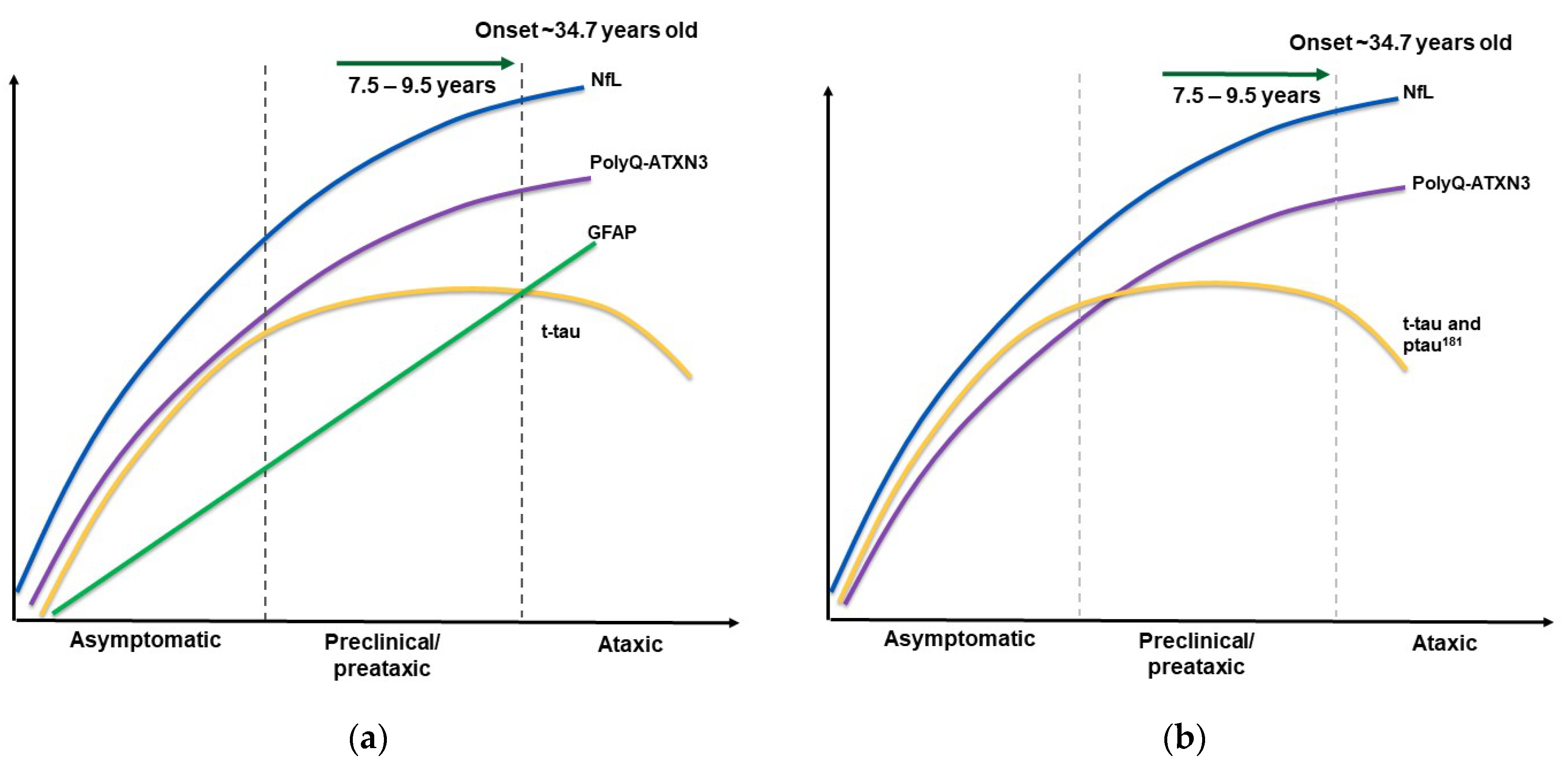
3. Results and Discussion
| Biomarker | Fluid | Controls | Preataxic/ Preclinical SCA3 Patients | Ataxic Patients | Associated Variables | Reference |
|---|---|---|---|---|---|---|
| PolyQ-ATXN3 | Plasma | 0.00 [0.00; 0.28] pg/µL (n = 34) | 0.84 [0.48; 1.68] pg/µL (n = 4) | 1.28 [0.03; 3.44] pg/µL (n = 41) | Perfect discrimination capacity between ataxic vs. controls. No clinical associations. No differences observed between presymptomatic and symptomatic. | [31] |
| 0.14 [0.1; 0.4] pg/mL (n = 15) | 53.80 [40.28; 63.37] pg/mL (n = 11) | 83.30 [55.38; 106.6] pg/mL (n = 45) | Positively correlated with SARA and negatively correlated with age of ataxia onset. | [35] | ||
| CSF | 0.00 [0.00; 0.04] pg/µL (median, n = 33) | 0.04 [0.04; 0.07] pg/µL (median, n = 4) | 0.13 [0.04; 0.46] pg/µL (median, n = 45) | Perfect discrimination between SCA3 patients and controls. No associations with other variables found. The median concentration was higher in symptomatic vs. asymptomatic. | [31] | |
| 0.11 [0.1; 0.4] pg/mL (n = 18) | Not reported. Preataxic levels were not different from ataxic (n = 5). | 5.48 [4.85; 6.67] pg/mL (n = 12) | CSF perfectly discriminated between controls and ataxic carriers. | [35] | ||
| Urine | Not reported | Not reported. | Not reported. PolyQ-ATXN3 is higher in symptomatic patients than in those with other types of ataxia and controls. | Mild correlation with plasma levels. | [36] | |
| NfL | Serum | Cohort B: 6.88 ± 2.72 pg/mL (n = 91) | 15.03 ± 7.49 pg/mL (n = 26) | 37.56 ± 13.47 pg/mL (n = 90) | Positively related to disease severity. Positively associated with SARA and ICARS. Negatively associated with cerebellar and brainstem volumes. | [37] |
| Cohort 1 (ESMI): 8.6 [5.7;11.7] pg/mL (n = 77) Cohort 2 (EuroSCA/RiSCA):19.4 [15.1:25.4] pg/mL (n = 48) | 29.1 [15.9:43.7] pg/mL (n = 8) 47.3 [25.5:78.0] pg/mL (n = 14) | 34.8 [28.3:47.0] pg/mL (n = 75) 85.5 [70.2:100.2] pg/mL (n = 27) | Positive correlation with age, CAG repeat length and longitudinal SARA score. Prediction of time to onset. Preconversion stage delineation. Differentiation between early and late preataxic stages. | [38] | ||
| 8.24 [5.92–10.84] pg/mL (n = 185) | 21.84 [18.37–23.45] pg/mL (n = 20) | 36.06 [30.04–45.90] pg/mL (n = 198) | Negative correlation with gray matter in left precentral gyrus and paracentral lobule, as well as mean diffusivity in widespread matter tracts. | [34] | ||
| 7.43 pg/mL (n = 14) | -- | 35.33 pg/mL (n = 20) | Positively correlated with SARA and ICARS, speech disorders and limbic kinetic function. | [39] | ||
| 10.24 ± 4.48 pg/mL (n = 19) | Not reported. | 34.92 ± 10.95 pg/mL(n = 20) | Optimal cut-off: 16.04 pg/mL. Baseline levels correlate with disease duration and SARA. Concentrations persisted after a 2-year follow-up. | [40] | ||
| Plasma | 11.20 [3.31: 33.56] pg/mL (n = 30) | 15.42 [11.05: 28.68] pg/mL (n = 4) | 30.1 [17.24: 69.9] pg/mL (n = 41) | Discrimination between SCA3 patients and controls. | [31] | |
| 2.31 [0.83] log pg/mL (n = 172) | 2.70 [0.47] log pg/mL (n = 23) | 3.26 [0.46] log pg/mL (n = 120) | Predictors: age and number of CAG repeats account for 4.2% variability in ataxic and 30.63% in preataxic. | [41] | ||
| 5.7 [4.3; 7.2] pg/mL (n = 39) | 19.8 [13.9; 27.3] pg/mL (n = 24) | 31.4 [26.4; 36.4] pg/mL (n = 64) | Correlation with disease duration, SARA, INAS and CCFS, as well as diplopia. | [23] | ||
| CSF | Cohort A 471.70 ± 210.40 pg/mL (n = 17) | Not reported. | 4262.00 ± 1762.00 pg/mL (n = 9) | Higher in manifest SCA3 patients. CSF was 102X higher than serum. | [37] | |
| 449 [137: 1512] pg/mL (n = 34) | 1352 [1019: 1398] pg/mL (n = 4) | 3569 [1413: 6837] pg/mL (n = 46) | NfL discriminates symptomatic SCA3 patients from asymptomatic carriers and controls. | [31] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S. Hereditary Ataxia Overview. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1138/ (accessed on 20 July 2024).
- Li, T.J.; Martins, S.; Peng, Y.; Wang, P.Z.; Hou, X.C.; Chen, Z.; Wang, C.R.; Tang, Z.L.; Qiu, R.; Chen, C.; et al. Is the High Frequency of Machado-Joseph Disease in China Due to New Mutational Origins? Front. Genet. 2019, 9, 740. [Google Scholar] [CrossRef]
- Haberhausen, G.; Damian, M.S.; Leweke, F.; Muller, U. Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado-Joseph disease (MJD). J. Neurol. Sci. 1995, 132, 71–75. [Google Scholar] [CrossRef]
- Paulson, H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012, 103, 437–449. [Google Scholar] [PubMed]
- Kaczyńska, J.; Sitek, E.J.; Witkowski, G.; Rudzińska-Bar, M.; Janik, P.; Sławek, J.; Edwin, E.M.G.; Zielonka, D. Is deep brain stimulation effective in Huntington’s Disease?—A systematic literature review. Neurol. Neurochir. Pol. 2022, 56, 299–307. [Google Scholar] [CrossRef]
- Fujigasaki, H.; Uchihara, T.; Koyano, S.; Iwabuchi, K.; Yagishita, S.; Makifuchi, T.; Nakamura, A.; Ishida, K.; Toru, S.; Hirai, S.; et al. Ataxin-3 is translocated into the nucleus for the formation of intranuclear inclusions in normal and Machado-Joseph disease brains. Exp. Neurol. 2000, 165, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Paulson, H.L.; Perez, M.K.; Trottier, Y.; Trojanowski, J.Q.; Subramony, S.H.; Das, S.S.; Vig, P.; Mandel, J.L.; Fischbeck, K.H.; Pittman, R.N. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 1997, 19, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A.H. The Neuropathology of Spinocerebellar Ataxia Type 3/Machado-Joseph Disease. Adv. Exp. Med. Biol. 2018, 1049, 233–241. [Google Scholar] [PubMed]
- Schols, L.; Reimold, M.; Seidel, K.; Globas, C.; Brockmann, K.; Hauser, T.K.; Auburger, G.; Burk, K.; den Dunnen, W.; Reischl, G.; et al. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain 2015, 138 Pt. 11, 3316–3326. [Google Scholar] [CrossRef]
- Adanyeguh, I.M.; Henry, P.G.; Nguyen, T.M.; Rinaldi, D.; Jauffret, C.; Valabregue, R.; Emir, U.E.; Deelchand, D.K.; Brice, A.; Eberly, L.E.; et al. In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7. Mov. Disord. 2015, 30, 662–670. [Google Scholar] [CrossRef]
- Lima, M.; Raposo, M.; Ferreira, A.; Melo, A.R.V.; Pavao, S.; Medeiros, F.; Teves, L.; Gonzalez, C.; Lemos, J.; Pires, P.; et al. The Homogeneous Azorean Machado-Joseph Disease Cohort: Characterization and Contributions to Advances in Research. Biomedicines 2023, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Lema, Y.Y.; Gamo, N.J.; Yang, K.; Ishizuka, K. Trait and state biomarkers for psychiatric disorders: Importance of infrastructure to bridge the gap between basic and clinical research and industry. Psychiatry Clin. Neurosci. 2018, 72, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Noorasyikin, M.A.; Azizan, E.A.; Teh, P.C.; Waheeda, T.F.; Hajar, M.D.S.; Long, K.C.; Norlinah, M.I. Oral trehalose maybe helpful for patients with spinocerebellar ataxia 3 and should be better evaluated. Park. Relat. Disord. 2020, 70, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Coarelli, G.; Marcotulli, C.; Leonardi, L.; Piccolo, F.; Spadaro, M.; Frontali, M.; Ferraldeschi, M.; Vulpiani, M.C.; Ponzelli, F.; et al. Riluzole in patients with hereditary cerebellar ataxia: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015, 14, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Salci, Y.; Fil, A.; Keklicek, H.; Cetin, B.; Armutlu, K.; Dolgun, A.; Tuncer, A.; Karabudak, R. Validity and reliability of the International Cooperative Ataxia Rating Scale (ICARS) and the Scale for the Assessment and Rating of Ataxia (SARA) in multiple sclerosis patients with ataxia. Mult. Scler. Relat. Disord. 2017, 18, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Al-Shaikh, R.H.; Sulek, A.; Kasprzak, J.; Sławek, J.; Wszolek, Z.K. First report on spinocerebellar ataxia type 3 (Machado-Joseph disease) in Poland. Park. Relat. Disord. 2022, 105, 39–42. [Google Scholar] [CrossRef]
- Ristori, G.; Romano, S.; Visconti, A.; Cannoni, S.; Spadaro, M.; Frontali, M.; Pontieri, F.E.; Vanacore, N.; Salvetti, M. Riluzole in cerebellar ataxia A randomized, double-blind, placebo-controlled pilot trial. Neurology 2010, 74, 839–845. [Google Scholar] [CrossRef]
- Lei, L.F.; Yang, G.P.; Wang, J.L.; Chuang, D.M.; Song, W.H.; Tang, B.S.; Jiang, H. Safety and efficacy of valproic acid treatment in SCA3/MJD patients. Park. Relat. Disord. 2016, 26, 55–61. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Section: Spinocerebellar Ataxia Type 3 NCT03378414. Available online: https://clinicaltrials.gov/search?cond=sca3&viewType=Table (accessed on 24 April 2023).
- Maas, R.P.; van Gaalen, J.; Klockgether, T.; van de Warrenburg, B.P. The preclinical stage of spinocerebellar ataxias. Neurology 2015, 85, 96–103. [Google Scholar] [CrossRef]
- Paulson, H.; Shakkottai, V. Spinocerebellar Ataxia Type 3. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Tezenas du Montcel, S.; Petit, E.; Olubajo, T.; Faber, J.; Lallemant-Dudek, P.; Bushara, K.; Perlman, S.; Subramony, S.H.; Morgan, D.; Jackman, B.; et al. Baseline Clinical and Blood Biomarker in Patients with Preataxic and Early-Stage Disease Spinocerebellar Ataxia 1 and 3. Neurology 2023, 100, e1836–e1848. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Comparison of four heterogeneity measures for meta-analysis. J. Eval. Clin. Pract. 2020, 26, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.L.; Wang, S.; Chen, Z.; Peng, Y.; Wang, C.R.; Long, Z.; Peng, H.R.; Shi, Y.T.; Hou, X.; Lei, L.J.; et al. Blood Neurofilament Light Chain in Genetic Ataxia: A Meta-Analysis. Mov. Disord. 2022, 37, 171–181. [Google Scholar] [CrossRef]
- Wilke, C.; Bender, F.; Hayer, S.N.; Brockmann, K.; Schols, L.; Kuhle, J.; Synofzik, M. Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: A pilot study. J. Neurol. 2018, 265, 1618–1624. [Google Scholar] [CrossRef]
- Wang, H.; Wu, M.; Zhan, C.; Ma, E.; Yang, M.; Yang, X.; Li, Y. Neurofilament proteins in axonal regeneration and neurodegenerative diseases. Neural Regen. Res. 2012, 7, 620–626. [Google Scholar]
- Prudencio, M.; Garcia-Moreno, H.; Jansen-West, K.R.; Al-Shaikh, R.H.; Gendron, T.F.; Heckman, M.G.; Spiegel, M.R.; Carlomagno, Y.; Daughrity, L.M.; Song, Y.; et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3. Sci. Transl. Med. 2020, 12, eabb7086. [Google Scholar] [CrossRef]
- Shen, X.N.; Wu, K.M.; Huang, Y.Y.; Guo, Y.; Huang, S.Y.; Zhang, Y.R.; Chen, S.F.; Wang, H.F.; Zhang, W.; Cheng, W.; et al. Systematic assessment of plasma biomarkers in spinocerebellar ataxia. Neurobiol. Dis. 2023, 181, 106112. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Byrne, L.M.; Tortelli, R.; Johnson, E.B.; Wijeratne, P.A.; Arridge, M.; De Vita, E.; Alexander, D.C.; Tabrizi, S.J.; Schobel, S.; et al. Longitudinal Dynamics of Mutant Huntingtin and Neurofilament Light in Huntington’s Disease: The Prospective HD-CSF Study. Neurotherapeutics 2020, 17 (Suppl. S1), 1–54. [Google Scholar]
- Peng, Y.; Zhang, Y.; Chen, Z.; Peng, H.; Wan, N.; Zhang, J.; Tang, J.; Wang, P.; Xie, Y.; Cai, Q.; et al. Association of serum neurofilament light and disease severity in patients with spinocerebellar ataxia type 3. Neurology 2020, 95, e2977–e2987. [Google Scholar] [CrossRef] [PubMed]
- Hubener-Schmid, J.; Kuhlbrodt, K.; Peladan, J.; Faber, J.; Santana, M.M.; Hengel, H.; Jacobi, H.; Reetz, K.; Garcia-Moreno, H.; Raposo, M.; et al. Polyglutamine-Expanded Ataxin-3: A Target Engagement Marker for Spinocerebellar Ataxia Type 3 in Peripheral Blood. Mov. Disord. 2021, 36, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Jansen-West, K.R.; Hanna Al-Shaikh, R.; Carlomagno, Y.; Song, Y.; Dunmore, J.A.; LeDoux, M.S.; Friedman, J.H.; Pena, A.B.; Uitti, R.J.; et al. Urine levels of the polyglutamine ataxin-3 protein are elevated in patients with spinocerebellar ataxia type 3. Park. Relat. Disord. 2021, 89, 151–154. [Google Scholar] [CrossRef]
- Li, Q.F.; Dong, Y.; Yang, L.; Xie, J.J.; Ma, Y.; Du, Y.C.; Cheng, H.L.; Ni, W.; Wu, Z.Y. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol. Neurodegener. 2019, 14, 39. [Google Scholar] [CrossRef]
- Wilke, C.; Haas, E.; Reetz, K.; Faber, J.; Garcia-Moreno, H.; Santana, M.M.; van de Warrenburg, B.; Hengel, H.; Lima, M.; Filla, A.; et al. Neurofilaments in spinocerebellar ataxia type 3: Blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol. Med. 2020, 12, e11803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, Y.; Hu, Z.; Qiu, M.; Li, D.; Cai, Q.; Tao, C.; Lou, D.; Qi, L.; Chen, S.; et al. Association between Serum Neurofilament Light Chain and Neurochemistry Deficits in Patients with Spinocerebellar Ataxia Type 3. Cerebellum 2023, 23, 92–100. [Google Scholar] [CrossRef]
- Coarelli, G.; Darios, F.; Petit, E.; Dorgham, K.; Adanyeguh, I.; Petit, E.; Brice, A.; Mochel, F.; Durr, A. Plasma neurofilament light chain predicts cerebellar atrophy and clinical progression in spinocerebellar ataxia. Neurobiol. Dis. 2021, 153, 105311. [Google Scholar] [CrossRef]
- Garcia-Moreno, H.; Prudencio, M.; Thomas-Black, G.; Solanky, N.; Jansen-West, K.R.; AL-Shaikh, R.H.; Heslegrave, A.; Zetterberg, H.; Santana, M.M.; de Almeida, L.P.; et al. Tau and neurofilament light-chain as fluid biomarkers in spinocerebellar ataxia type 3. Eur. J. Neurol. 2022, 29, 2439–2452. [Google Scholar] [CrossRef]
- Martier, R.; Sogorb-Gonzalez, M.; Stricker-Shaver, J.; Hubener-Schmid, J.; Keskin, S.; Klima, J.; Toonen, L.J.; Juhas, S.; Juhasova, J.; Ellederova, Z.; et al. Development of an AAV-Based MicroRNA Gene Therapy to Treat Machado-Joseph Disease. Mol. Ther. Methods Clin. Dev. 2019, 15, 343–358. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Peng, L.; Zhao, H.; Li, S.; Han, X.; Habimana, J.D.; Chen, Z.; Wang, C.; Peng, Y.; et al. CRISPR/Cas9 mediated gene correction ameliorates abnormal phenotypes in spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cells. Transl. Psychiatry 2021, 11, 479. [Google Scholar] [CrossRef]
- Ouyang, S.; Xie, Y.; Xiong, Z.; Yang, Y.; Xian, Y.; Ou, Z.; Song, B.; Chen, Y.; Xie, Y.; Li, H.; et al. CRISPR/Cas9-Targeted Deletion of Polyglutamine in Spinocerebellar Ataxia Type 3-Derived Induced Pluripotent Stem Cells. Stem Cells Dev. 2018, 27, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Gonsior, K.; Kaucher, G.A.; Pelz, P.; Schumann, D.; Gansel, M.; Kuhs, S.; Klockgether, T.; Forlani, S.; Durr, A.; Hauser, S.; et al. PolyQ-expanded ataxin-3 protein levels in peripheral blood mononuclear cells correlate with clinical parameters in SCA3: A pilot study. J. Neurol. 2021, 268, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Braga-Neto, P.; Felicio, A.C.; Hoexter, M.Q.; Pedroso, J.L.; Dutra, L.A.; Alessi, H.; Minett, T.; Santos-Galduroz, R.F.; da Rocha, A.J.; Garcia, L.A.; et al. Cognitive and olfactory deficits in Machado-Joseph disease: A dopamine transporter study. Park. Relat. Disord. 2012, 18, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Park. Dis. 2020, 2020, 2076942. [Google Scholar] [CrossRef]
- Oppo, V.; Melis, M.; Melis, M.; Tomassini Barbarossa, I.; Cossu, G. “Smelling and Tasting” Parkinson’s Disease: Using Senses to Improve the Knowledge of the Disease. Front. Aging Neurosci. 2020, 12, 43. [Google Scholar] [CrossRef]
- Pedroso, J.L.; Braga-Neto, P.; Felicio, A.C.; Minett, T.; Yamaguchi, E.; Prado, L.B.; Carvalho, L.B.; Dutra, L.A.; Hoexter, M.Q.; da Rocha, A.J.; et al. Sleep disorders in Machado-Joseph disease: A dopamine transporter imaging study. J. Neurol. Sci. 2013, 324, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Al-Shaikh, R.H.; Sulek, A.; Kasprzak, J.; Slawek, J.; Wszolek, Z.K. Spinocerebellar ataxia type 3 (Machado-Joseph disease). Pol. Arch. Intern. Med. 2022, 132, 16322. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.; Ruschil, C.; Abdelhak, A.; Wilke, C.; Maleska, A.; Kuhle, J.; Krumbholz, M.; Kowarik, M.C.; Schule, R. Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Biomarkers in Primary Progressive Multiple Sclerosis and Hereditary Spastic Paraplegia Type 4. Int. J. Mol. Sci. 2022, 23, 13466. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Lin, X.; Lu, T.; Deng, H.; Liu, C.; Yang, Y.; Chen, T.; Qin, Y.; Xie, X.; Xie, Z.; Liu, M.; et al. Serum neurofilament light chain or glial fibrillary acidic protein in the diagnosis and prognosis of brain metastases. J. Neurol. 2022, 269, 815–823. [Google Scholar] [CrossRef]
- Nacmias, B. Neurofilaments as Decay Rate Biomarker in Spinocerebellar Ataxia Type 1: Highlighting Key Questions of Application and Future Challenges. Neurology 2022, 98, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, N.; Shirani, A.; Salinas, V.H.; Tardo, L.; Hussain, R.Z.; Pitt, D.; Stuve, O. Clinical trials in multiple sclerosis: Past, present, and future. Neurol. Neurochir. Pol. 2022, 56, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.L.; Zhao, Y.M. Analysis of factors correlated with spinal clinically isolated syndrome conversion to multiple sclerosis. Neurol. Neurochir. Pol. 2022, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jasiak-Zatońska, M.; Pietrzak, A.; Wyciszkiewicz, A.; Więsik-Szewczyk, E.; Pawlak-Buś, K.; Leszczyński, P.; Kozubski, W.; Michalak, S.; Kalinowska-Łyszczarz, A. Different blood-brain-barrier disruption profiles in multiple sclerosis, neuromyelitis optica spectrum disorders, and neuropsychiatric systemic lupus erythematosus. Neurol. Neurochir. Pol. 2022, 56, 246–255. [Google Scholar] [CrossRef]
- Reddy, D.S.; Abeygunaratne, H.N. Experimental and Clinical Biomarkers for Progressive Evaluation of Neuropathology and Therapeutic Interventions for Acute and Chronic Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 11734. [Google Scholar] [CrossRef]
- Hou, X.; Gong, X.; Zhang, L.; Li, T.; Yuan, H.; Xie, Y.; Peng, Y.; Qiu, R.; Xia, K.; Tang, B.; et al. Identification of a potential exosomal biomarker in spinocerebellar ataxia Type 3/Machado-Joseph disease. Epigenomics 2019, 11, 1037–1056. [Google Scholar] [CrossRef]
- Raposo, M.; Hubener-Schmid, J.; Ferreira, A.F.; Vieira Melo, A.R.; Vasconcelos, J.; Pires, P.; Kay, T.; Garcia-Moreno, H.; Giunti, P.; Santana, M.M.; et al. Blood transcriptome sequencing identifies biomarkers able to track disease stages in spinocerebellar ataxia type 3. Brain 2023, 146, 4132–4143. [Google Scholar] [PubMed]
- Sihombing, N.R.B.; Winarni, T.I.; Belladonna, M.; Ediati, A.; Faradz, S.M. Dilemma of presymptomatic testing in children with history of late onset neurodegenerative spinocerebellar ataxia in Indonesia. J. Biomed. Transl. Res. 2022, 8, 6. [Google Scholar] [CrossRef]
- Velazquez-Perez, L.; Rodriguez-Labrada, R.; Rodriguez-Diaz, J.; Vazquez-Mojena, Y.; Medrano-Montero, J.; Estupinan-Rodriguez, A. Early Diagnosis in Spinocerebellar Ataxias: Prospects for Clinical Alterations and Ethical Dilemmas During Preclinical Trials. Mov. Disord. 2017, 32, 794. [Google Scholar]
- Chen, X.Y.; Huang, Z.Q.; Lin, W.; Li, M.C.; Ye, Z.X.; Qiu, Y.S.; Xia, X.Y.; Chen, N.P.; Hu, J.P.; Gan, S.R.; et al. Altered brain white matter structural motor network in spinocerebellar ataxia type 3. Ann. Clin. Transl. Neurol. 2023, 10, 225–236. [Google Scholar] [CrossRef]
- Ye, L.Q.; Li, X.Y.; Zhang, Y.B.; Cheng, H.R.; Ma, Y.; Chen, D.F.; Tao, Q.Q.; Li, H.L.; Wu, Z.Y. The discriminative capacity of CSF beta-amyloid 42 and Tau in neurodegenerative diseases in the Chinese population. J. Neurol. Sci. 2020, 412, 116756. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, C.; Huang, F.; Chen, Z.; Sun, Z.; Wang, J.; Tang, B.; Ashizawa, T.; Klockgether, T.; Jiang, H. High Serum GFAP Levels in SCA3/MJD May Not Correlate with Disease Progression. Cerebellum 2015, 14, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Tort, A.B.; Portela, L.V.; Rockenbach, I.C.; Monte, T.L.; Pereira, M.L.; Souza, D.O.; Rieder, C.R.; Jardim, L.B. S100B and NSE serum concentrations in Machado Joseph disease. Clin. Chim. Acta 2005, 351, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lei, L.; Shi, Y.; Wang, J.; Jiang, H.; Shen, L.; Tang, B. Serum concentrations of NSE and S100B in spinocerebellar ataxia type 3/Machado-Joseph disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 504–510. [Google Scholar] [PubMed]
- Hu, Z.W.; Yang, Z.H.; Zhang, S.; Liu, Y.T.; Yang, J.; Wang, Y.L.; Mao, C.Y.; Zhang, Q.M.; Shi, C.H.; Xu, Y.M. Carboxyl Terminus of Hsp70-Interacting Protein Is Increased in Serum and Cerebrospinal Fluid of Patients With Spinocerebellar Ataxia Type 3. Front. Neurol. 2019, 10, 1094. [Google Scholar] [CrossRef]
- De Assis, A.M.; Saute, J.A.M.; Longoni, A.; Haas, C.B.; Torrez, V.R.; Brochier, A.W.; Souza, G.N.; Furtado, G.V.; Gheno, T.C.; Russo, A.; et al. Peripheral Oxidative Stress Biomarkers in Spinocerebellar Ataxia Type 3/Machado-Joseph Disease. Front. Neurol. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.S.; da Silveira, A.F.; Trott, A.; Houenou, L.J.; Algarve, T.D.; Bello, C.; Lenz, A.F.; Manica-Cattani, M.F.; da Cruz, I.B.M. Association between Machado-Joseph disease and oxidative stress biomarkers. Mutat. Res. -Genet. Toxicol. Environ. Mutagen. 2013, 757, 99–103. [Google Scholar] [CrossRef]
- Da Silva Carvalho, G.; Saute, J.A.; Haas, C.B.; Torrez, V.R.; Brochier, A.W.; Souza, G.N.; Furtado, G.V.; Gheno, T.; Russo, A.; Monte, T.L.; et al. Cytokines in Machado Joseph Disease/Spinocerebellar Ataxia 3. Cerebellum 2016, 15, 518–525. [Google Scholar] [CrossRef]
- Yang, Z.H.; Shi, C.H.; Zhou, L.N.; Li, Y.S.; Yang, J.; Liu, Y.T.; Mao, C.Y.; Luo, H.Y.; Xu, G.W.; Xu, Y.M. Metabolic Profiling Reveals Biochemical Pathways and Potential Biomarkers of Spinocerebellar Ataxia 3. Front. Mol. Neurosci. 2019, 12, 159. [Google Scholar] [CrossRef]
- Saute, J.A.; da Silva, A.C.; Muller, A.P.; Hansel, G.; de Mello, A.S.; Maeda, F.; Vedolin, L.; Saraiva-Pereira, M.L.; Souza, D.O.; Arpa, J.; et al. Serum insulin-like system alterations in patients with spinocerebellar ataxia type 3. Mov. Disord. 2011, 26, 731–735. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, F.; Tang, B.; Li, J.; Wang, J.; Shen, L.; Xia, K.; Jiang, H. MicroRNA profiling in the serums of SCA3/MJD patients. Int. J. Neurosci. 2014, 124, 97–101. [Google Scholar] [CrossRef]
- Raposo, M.; Ramos, A.; Santos, C.; Kazachkova, N.; Teixeira, B.; Bettencourt, C.; Lima, M. Accumulation of Mitochondrial DNA Common Deletion Since The Preataxic Stage of Machado-Joseph Disease. Mol. Neurobiol. 2019, 56, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, V.; L’Italien, G.; Berman, R.; Beiner, M. Results from the Long-Term Open Label Extension Phase Analyses of BHV4157-201: A Phase IIb/III, Randomized, Double-blind, Placebo-controlled Trial of the Safety and Efficacy of Troriluzole in Adult Subjects with Spinocerebellar Ataxia. Neurology 2020, 94, 5308. [Google Scholar] [CrossRef]
- Zaltzman, R.; Elyoseph, Z.; Lev, N.; Gordon, C.R. Trehalose in Machado-Joseph Disease: Safety, Tolerability, and Efficacy. Cerebellum 2020, 19, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Section: Spinocerebellar Ataxia Type 3-BIIB132. Available online: https://clinicaltrials.gov/study/NCT05160558?cond=sca3&viewType=Table&page=2&rank=12 (accessed on 24 April 2023).
- Babacic, H.; Mehta, A.; Merkel, O.; Schoser, B. CRISPR-cas gene-editing as plausible treatment of neuromuscular and nucleotide-repeat-expansion diseases: A systematic review. PLoS ONE 2019, 14, e0212198. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.S.; Duarte-Silva, S.; Salgado, A.J.; Maciel, P. Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: An update. Neural Regen. Res. 2023, 18, 1203–1212. [Google Scholar] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef]
- Paul, G.; Anisimov, S.V. The secretome of mesenchymal stem cells: Potential implications for neuroregeneration. Biochimie 2013, 95, 2246–2256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Piña, A.E.; Pulido-Alvarado, C.C.; Dulski, J.; Wszolek, Z.K.; Magaña, J.J. Specific Biomarkers in Spinocerebellar Ataxia Type 3: A Systematic Review of Their Potential Uses in Disease Staging and Treatment Assessment. Int. J. Mol. Sci. 2024, 25, 8074. https://doi.org/10.3390/ijms25158074
Soto-Piña AE, Pulido-Alvarado CC, Dulski J, Wszolek ZK, Magaña JJ. Specific Biomarkers in Spinocerebellar Ataxia Type 3: A Systematic Review of Their Potential Uses in Disease Staging and Treatment Assessment. International Journal of Molecular Sciences. 2024; 25(15):8074. https://doi.org/10.3390/ijms25158074
Chicago/Turabian StyleSoto-Piña, Alexandra E., Caroline C. Pulido-Alvarado, Jaroslaw Dulski, Zbigniew K. Wszolek, and Jonathan J. Magaña. 2024. "Specific Biomarkers in Spinocerebellar Ataxia Type 3: A Systematic Review of Their Potential Uses in Disease Staging and Treatment Assessment" International Journal of Molecular Sciences 25, no. 15: 8074. https://doi.org/10.3390/ijms25158074






